Abstract
Tools permitting the isolation of live pancreatic cell subsets for culture and/or molecular analysis are limited. To address this, we developed a collection of monoclonal antibodies with selective surface labeling of endocrine and exocrine pancreatic cell types. Cell type labeling specificity and cell surface reactivity were validated on mouse pancreatic sections and by gene expression analysis of cells isolated using FACS. Five antibodies which marked populations of particular interest were used to isolate and study viable populations of purified pancreatic ducts, acinar cells, and subsets of acinar cells from whole pancreatic tissue or of alpha or beta cells from isolated mouse islets. Gene expression analysis showed the presence of known endocrine markers in alpha and beta cell populations and revealed that TTR and DPPIV are primarily expressed in alpha cells whereas DGKB and GPM6A have a beta cell specific expression profile.
Keywords: Antibody, FACS, beta cell, alpha cell, acinar, duct
1. Introduction
The development of strategies for the de novo generation of beta cells, the enhancement of in situ beta cell proliferation, and/or the reprogramming of other adult cells to serve as beta surrogates requires a sufficient understanding of the regulation of beta cell identity. Experimental tools that allow the convenient isolation of specific endocrine cell subsets from mice - whether wild type or compound transgenics - and the selective comparison of their gene expression profile to that of other defined cell types are in short supply. A transcriptional profile of beta cells from different genetic environments, for comparison with candidate progenitors and reference populations of mature pancreatic cells, will be particularly useful.
Markers of adult pancreatic cell types have been comprehensively identified, but detection of the expression of these genes nearly always requires cell fixation. In certain cases, however, the consistent physical properties of the cell type(s) have been used to facilitate viable isolation. For beta cells, a naturally high intracellular zinc ion concentration has been exploited using the low-toxicity membrane permeable fluorescent dye Newport Green (NG) in combination with orthogonal scatter gating (Lukowiak et al., 2001). Enrichment of insulin expression among NG+ progeny of differentiated embryonic stem (ES) cells has also been observed (Narushima et al., 2005). In addition, certain transgenic mice incorporating marker genes driven by promoters associated with known cell types have proven quite useful. Mouse insulin promoter-green fluorescence protein (MIP-GFP) transgenic animals (Hara et al., 2003) have aided the identification and isolation of pancreatic islets and beta cells, and the more recently derived GluCre-ROSA26EYFP mice (Quoix et al., 2007) may facilitate the convenient isolation of alpha cells. However, a comprehensive collection of transgenic animals with pancreatic cell lineage-restricted marker gene expression has not yet been assembled – and the costs of maintaining or back-crossing additional mouse lines are significant.
Viable cell isolation by antibody labeling has been instrumental in the characterization of functional cell subsets of hematopoietic, neural, and other cell types (Lawson et al., 2007; Maric and Barker, 2004; Swart et al., 2005). Excluding the well-studied hematopoietic field, however, the introduction of new cell lineage markers has been disappointingly elusive. Recently, we reported the development of a collection of antibodies marking human endocrine and exocrine pancreatic cell populations (Dorrell et al., 2008b). Although these have proven useful for the isolation and study of important human cell types, these reagents do not work on mouse cells.
In this report we describe the development and application of novel tools for the study of murine pancreatic biology. These antibodies allow the isolation of duct and acinar cells (and subsets thereof) from “bulk” pancreatic tissue. When applied to mouse islet samples, alpha and beta cells can be selectively marked and purified. Expression analysis of these populations reveals striking differences between alpha and beta cells including the alpha cell specificity of transthyretin (TTR) and dipeptidyl peptidase 4 (DPPIV) and the selective expression of diacylglycerol kinase beta (DGKB) and glycoprotein M6A (GPM6A) in beta cells. The ability to conveniently isolate viable exocrine and endocrine populations should facilitate the study of these important cell populations.
2. Materials and methods
2.1 Tissue sources and pancreatic cell isolation
Animal care and immunization procedures were performed in accordance with the institutional review committee at Oregon Health & Science University. BALB/cBy, 129/S3, and NOD.Cg-Prkdcscid Tg(Ins1-EGFP/GH1)14Hara/Sz (“MIP-GFP”) mice were obtained from the Jackson Laboratory. F344 rats were acquired from Charles River Laboratories. Adult pancreatic tissue was collected from c129/S3 mice aged 2–4 months. For the optimal preparation of a single cell suspension of whole pancreas tissue, a modified perfusion digest was first employed. This involved cannulation of the portal vein and the sequential administration of Ca2+/Mg2+-free EBSS (4’; Gibco) followed by a digest solution containing 0.1 mg/ml Collagenase XI (12’; Sigma-Aldrich) in regular EBSS at 2.5 ml/minute. The organ was then removed and manually dispersed using forceps and pipetting action (a p1000 minipipetter with a clipped disposable tip). Remaining solid tissue was subjected to in vitro dissociation (30’) with a solution of 2.5 mg/ml Collagenase D (plus 0.1 mg/ml Dnase I and trypsin/chymotrypsin inhibitor [all Sigma-Aldrich]). Fully dissociated cells were collected after perfusion (fraction 1) and after in vitro dissociation (fraction 2) by passage through a 40 µm cell strainer (BD Falcon) and stored without further enzyme exposure. For immunofluorescent screening, pancreata were collected from wild type or MIP-GFP mice (also aged 2–4 months), embedded in Tissue-tek cryomatrix (Sakura, Tokyo, Japan), and stored at −80°C.
2.2 Antibody production
A modified subtractive immunization protocol (Williams et al., 1992) was employed. Specifically, a F344 rat was pre-immunized with undesirable antigens including partially dispersed whole-pancreatic tissue, fetal bovine serum (FBS), and small amounts of collagenase. Cyclophosphamide (Sigma-Aldrich; 51 mg/kg) was then injected IP after 24h and 48h to eliminate B lymphocytes reacting against these antigens. Immunization of purified mouse islets was performed intraperitoneally (IP) on day 19 (dose #1) and day 38 (boost) after the initial treatment. On day 42, the rats were sacrificed and their spleens were harvested. Splenocytes were fused with SP2/0 Ag14 myeloma cells and successfully fused clones were selected by growth in methylcellulose-containing HAT medium (Stem Cell Technologies Inc., Vancouver, Canada). Approximately 500 isolated clones were transferred to liquid media in 96w plates; supernatants were collected for screening by immunofluoresence (IF) on sections of mouse pancreas and by flow cytometry on dispersed viable mouse pancreatic cells. Clones of particular interest were cryopreserved and passaged to larger culture flasks for increased supernatant production.
2.3 Immunofluorescent imaging
Cryosections (5 µm) of mouse pancreas were prepared using a Reichert 2800 Frigocut cryostat (Reichert Scientific Instruments) and fixed with acetone (5’) at −20°C. After drying, slides were stored at −86°C for up to three months. Labeling was performed with 50 µl of 1:20 diluted hybridoma supernatant (30’). Slides were then washed in DPBS and labeled (20’) with 50 µl of secondary antibody solution (1:200 dilution of Cy3-conjugated goat anti-rat IgG [Jackson ImmunoResearch, West Grove PA]) and 2% FBS [Hyclone]). DPBS was used for a final wash and storage of the slides prior to evaluation with a Zeiss Axioskop 2 plus (Carl Zeiss, Jenna, Germany). For dual antibody labeling, rabbit anti-mouse CK19 or rabbit anti-mouse amylase was included in the primary antibody labeling and Alexa488-conjugated goat anti-rabbit IgG (Invitrogen) was added for the secondary antibody labeling. All sections were mounted in a solution containing 10% glycerol and 4% N-propyl gallate (Sigma-Aldrich) with 0.001% Hoechst 33342 as a nuclear label. Antibodies specific for mouse DGKB (Abgent Inc., San Diego, CA), GPM6a (MBL International Corp., Woburn, MA), DPPIV/CD26 (BD Biosciences) and TTR (Lifespan Biosciences, Seattle, WA) were used on mouse pancreatic cryosections fixed with 10% formalin and detected using the secondary antibodies described above.
2.4 Flow cytometry and FACS
Dissociated cells were resuspended at 1x106 cells/ml in DMEM + 2% FBS + 0.1 mg/ml trypsin/chymotrypsin inhibitor prior to the addition of hybridoma supernatant (at a 1:20 dilution) and storage at 4°C (for 30’). After a wash with cold DPBS, the cells were resuspended in DMEM + 2% FBS containing a 1:200 dilution of APC-conjugated goat anti-rat secondary antibody adsorbed against mouse serum proteins (Jackson Immunoresearch, cat# 712-136-153). After another wash, cells were resuspended in DMEM + 5% rat serum (Serotec) and held on ice (10’) to block the secondary antibody. A final incubation with Alexa488-conjugated anti-CD45 and Alexa488-anti-CD11b/Mac1 (Invitrogen) facilitated exclusion gating of hematopoietic cells. Propidium iodide staining was used to label dead cells for exclusion. Cells were analyzed and sorted with a Cytopeia inFluxV-GS (Becton-Dickenson, Franklin Lakes, NJ); FSC:PW gating was used to exclude cell doublets from analysis or collection.
2.5 RNA isolation and qRT-PCR
For molecular analysis, populations of interest were sorted directly into Trizol LS (Invitrogen). RNA was collected after chloroform extraction, glycogen-assisted isopropanol precipitation, and a 70% ethanol wash. First strand cDNA synthesis was generated by MMLV reverse transcriptase and random oligonucleotide primers (Invitrogen). Gene expression was quantified by qRT-PCR using a Bio-Rad iQ5 thermocycler with a single-color MyiQ detection system. All reactions were performed in 45 cycles (15” @ 95°C, 20” @ 68°C, 20” @ 72°C). Reaction mixtures included 1.5u Platinum Taq DNA polymerase (Invitrogen), 2.5 mM MgCl2, 10 µM 5’ and 3’ primers, 10 mM dNTPs and 0.5x SYBR green. All primers were designed and tested to specifically amplify cDNA products of RNA encoding Pancreatic amylase 2 (5’: TGGCGTCAAATCAGGAACATGG, 3’: GGCTGACAAAGCCCAGTCATCA), Glycoprotein 2 (5’: AGGAGCCGAAGTGTTGCTTCCA, 3’: TCACGTTGGTTTGGGCATCTGT), CK19 (5’: GGACCCTCCCGAGATTACAACCA, 3’: GCCAGCTCCTCCTTCAGGCTCT), Cystic Fibrosis Transmembrane Receptor (5’: TCTCAGCCTTCTGTGGCCTTGG, 3’: TCCGGGTCATTTTCAGCTCCAC), von Willenbrand factor (5’: TGTGGGCTGTGCGGTGATTTTA, 3’: TGGGAGGAGATGCCCGTTTACA), Insulin 1* (5’: AGACCTTGGCGTTGGAGGTGGCCCG, 3’: GCAGAGGGGTGGGGCGGGTCGAG), Insulin 2* (5’: CCTGCCCCTGCTGGCCCTGCTCT, 3’: CCCGGGCCTCCACCCAGCTCCA), Glucagon (5’: ACCTGGACTCCCGCCGTGCCCA, 3’: TCGCCTTCCTCGGCCTTTCACCAGCC), Somatostatin* (5’: TGGCTGCGCTCTGCATCGTCCTGGCT, 3’: TGACGGAGTCTGGGGTCCGAGGGCG), Pdx1* (5’: GCGGTGGGGGCGAAGAGCCGGA, 3’: GACGCCTGGGGGCACGGCACCT), TTR (5’: GCGGAGTCTGGAGAGCTGCACGGGCT, 3’: TGGGCTGAGCAGGGCTGCGATGGT), DPPIV (5’: GGCCCTGCGTGCTACTTCCTGGCTCG, 3’: ACGTCCTGCGCGGCTGCTCTGC), DGKB (5’: GCCCGCTCTTCTTTCAGGTGGT, 3’: GGTGGATCACTTCTGGGGAGCA), GPM6A (5’: GTGGCAGATGTGTGAGCGCTTG, 3’: TGTCACAATCCCAAACTGACGCA), and GAPDH* (5’: AAGGTCGGTGTGAACGGATTTGG, 3’: CGTTGAATTTGCCGTGAGTGGAG). Except where noted (asterisks), each amplicon spans a >2kb intron. Where possible, one primer lies on an intron-exon boundary to further minimize amplification of contaminating genomic DNA. Cycle threshold values were recorded as baseline corrected curve-fitted values and reported as normalized values relative to housekeeping gene GAPDH.
3. Results
3.1 Generation of monoclonal antibodies by negative selection
To favor the production of antibodies with selective reactivity against cells of interest, a subtractive immunization strategy was employed (Williams et al., 1992). A F344 rat was pre-immunized with murine hematopoietic cells and FCS followed by cyclophosphamide treatment to ablate reactive lymphoid cells. Subsequent immunizations of this rat and a negative selection-free control animal used enriched preparations of murine islet cells (500 islets per animal per immunization). The resulting hybridoma supernatants were screened for selective activity against acetone-fixed mouse pancreas tissue sections and surface reactivity on viable isolated pancreatic and islet cell preparations. Table 1 lists the clones that were selected for full characterization.
Table 1.
Monoclonal antibodies recognizing surface antigens on subsets of mouse pancreas cells. Antibody labeling specificities are classified using tissue section labeling and both whole pancreas and islet-specific flow cytometry.
| Marker ID | Hybridoma | Isotype | Classification |
|---|---|---|---|
| MPdi1 | MIC1-1C3 | rat IgG2a | duct, pan-islet* |
| MPxi1 | MIC1-6A2 | rat IgG2a | acinar, alpha cells* |
| MPx1 | MIC0-2A6 | rat IgG2a | acinar |
| MPx2 | MIC1-6B10 | rat IgG2a | acinar subset |
| MPx3 | Panc216 | rat IgG2a | acinar subset |
The accessible cell types labeled by these antibodies depend on the tissue source used.
3.2 Selective labeling of distinct mouse pancreatic cell subsets
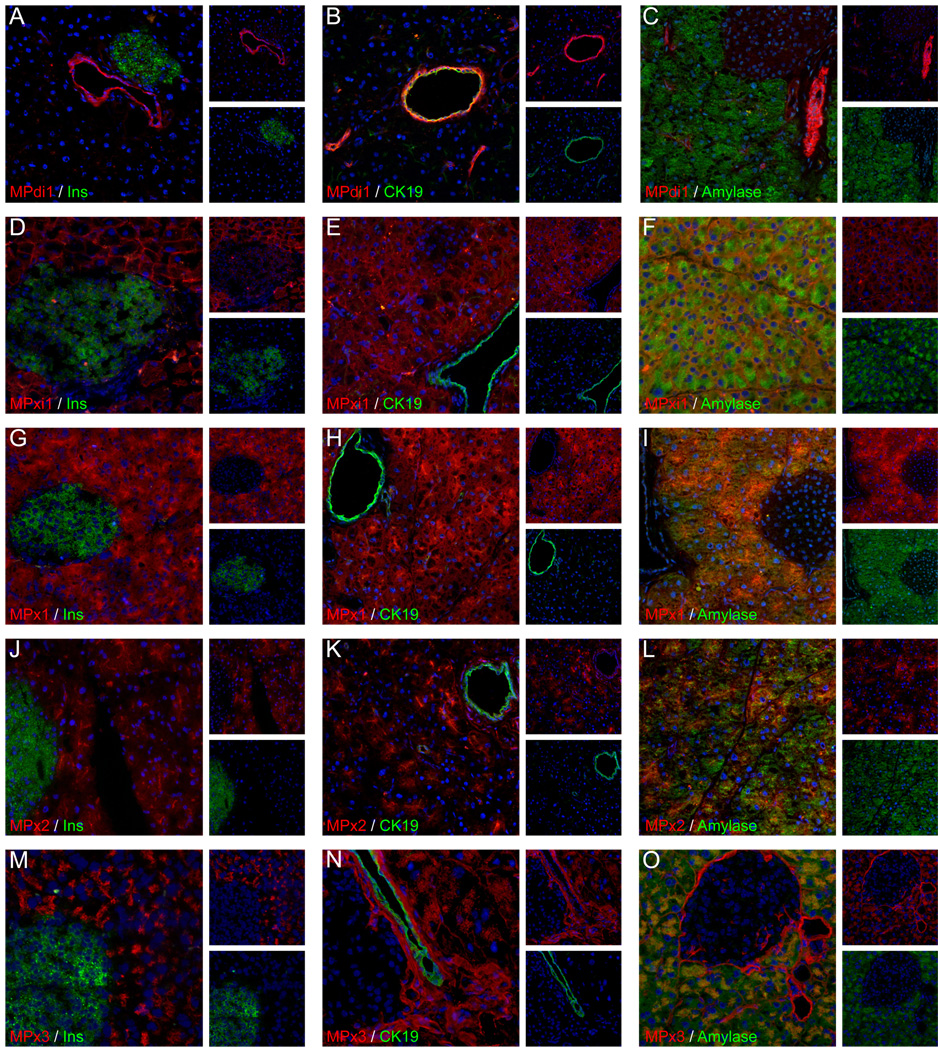
Determination of the specific cell types recognized by each antibody was first made by co-labeling with lineage-specific markers. Monoclonal antibody MPdi1 labels pancreatic ducts, showing no detectable co-labeling with insulin-expressing islet cells (Fig. 1a) or amylase-expressing acinar cells (Fig. 1c). All CK19+ duct cells are labeled by MPdi1 (Fig. 1b), with a predominantly basal localization that contrasts with the lumenal localization of the cytokeratin. We have previously shown that this antibody has duct-specific labeling in the liver (Dorrell et al., 2008b), and may therefore label ducts in all mouse tissues. Both MPxi1 and MPx1 are pan-acinar, with labeling that overlaps amylase (Fig. 1f,i) but not insulin (Fig. 1d,g) or CK19 (Fig. 1e,h). These two pan-acinar markers are distinguished by subcellular localization; MPxi1 label is concentrated at the acinar cell surface, whereas MPx1 labeling is uniform. MPx2 marks a large subset of acinar cells (Fig. 1l) with a predominantly apical pattern but not islet (Fig. 1j) or duct (Fig. 1k) cells. Finally, MPx3 exhibits a more complex labeling pattern including an acinar subset and connective tissue (Fig. 1m–o).
Fig. 1.
Cell type specific labeling of mouse pancreatic tissue. Acetone-fixed mouse pancreas cryosections were co-labeled with supernatant from a hybridoma line with pancreatic cell subset specificity and with a known cell type marker. In each case the experimental rat anti-mouse antibody is visualized using Cy3-conjugated anti-rat IgG (red) and the reference marker with Alexa488-conjugated anti-rabbit IgG (green); nuclei were labeled with Hoechst 33342 (blue).
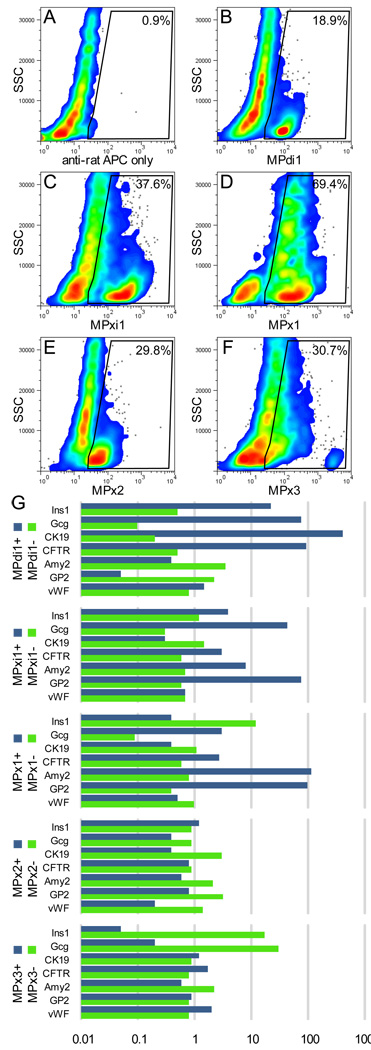
Because these antibodies recognize cell surface antigens, it was possible to isolate the labeled subpopulations of viable cells by FACS for further study. As illustrated in Figure 2a–f, distinct subsets of pancreatic acinar and ductal cells were labeled by each antibody. For these experiments, tissue preparation was restricted to in vitro collagenase digestion to avoid the dispersal and collection of islet-resident endocrine cells. Gene expression within the FACS-sorted populations as assessed by qRT-PCR (relative to unsorted cells; Fig. 2g), generally reflected the identities predicted by histology. MPdi1+ cells exhibited strong enrichment for duct-associated mRNA (CK19, CFTR) and depletion of acinar mRNA (amylase, GP2 [glycoprotein 2]) relative to their MPdi1− counterparts, whereas MPx1+ cells show the opposite pattern. MPxi1+ cells also showed an acinar gene expression profile, albeit with much stronger enrichment for GP2 than for amylase. MPx2 and MPx3 each labeled approximately half of the total acinar cell population. It was unclear what distinguished MPx2/3+ acinar cells from MPx2/3− acinar cells (other than antibody reactivity. Both populations contained comparable levels of amylase and GP2. Although slight enrichment of insulin and glucagon mRNA was observed in MPdi1+ and MPxi1+ cells, the absolute amount of endocrine-associated gene expression was very low in these populations (consistent with the exclusion of islets during cell preparation).
Fig. 2.
FACS analysis and isolation of live pancreatic cell populations. Cells isolated by enzymatic whole-pancreas preparation were labeled with the indicated primary hybridoma supernatant and detected with APC-conjugated anti-rat IgG. Excluded events include dead cells (propidium iodide positive), hematopoietic cells (Alexa488-CD45-positive), and debris/cell clusters/erythrocytes (FSC/SSC restriction). The indicated gate for positive events was defined based on the negative control (A). qRT-PCR results (G) are expressed as the ratio of the normalized Ct (vs. GAPDH) of each population to that of dispersed but unsorted mouse pancreas cells. Each value is the calculated mean ΔCt of three amplifications each of two different sorted populations.
3.3 Isolation of islet cell subsets by antibody labeling
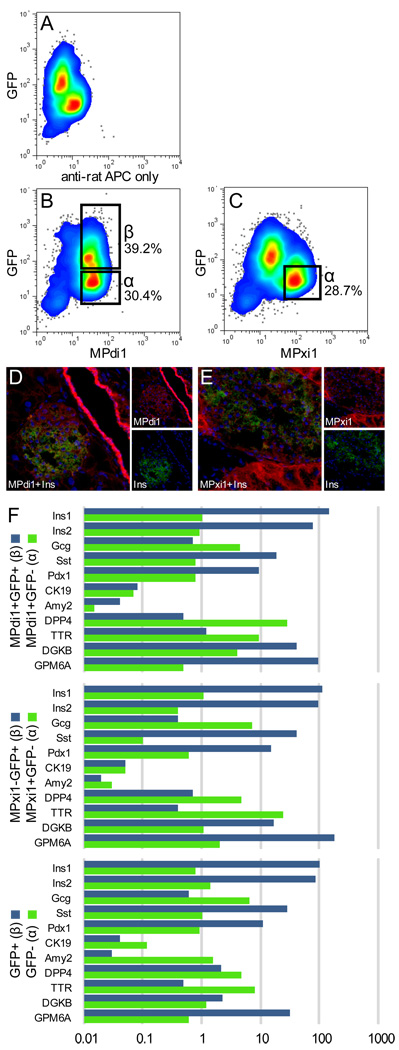
Although islet cell labeling was not observed in our original examination of fixed tissue sections, the RNA analyses of FACS-sorted cell populations suggested that a subset of these antibodies might nonetheless be useful for the isolation of live endocrine cells. To test this, islets were isolated from MIP-GFP transgenic mice and dispersed by trypsin to obtain a highly enriched endocrine cell population. FACS observations recorded after labeling these cells with the antibody panel are shown in Figure 3. MPdi1 (Fig. 3b) labeled both alpha (GFP−) and beta (GFP+) islet cells at comparable levels. MPxi1, however, strongly labeled alpha cells only; beta cells were dim/negative for this marker (Fig. 3c). The other antibodies did not recognize islet cells, and gave results comparable to the negative control (data not shown). To account for the seeming discrepancy between tissue section labeling (Fig.1 a,d) and islet cell binding by MPdi1 and MPxi1, longer exposures of the labeled sections were taken. As shown in Figure 3 (d,e), dim islet labeling was observed in each case with a sufficiently lengthy exposure.
Fig. 3.
Islet cell subpopulations can be isolated using MPdi1 and MPxi1. Trypsin-treated pancreatic islets were labeled with primary hybridoma supernatant and APC-conjugated anti-rat IgG. The subpopulations exhibiting islet-positive labeling (B–C) are shown compared to the negative control (A). Tissue section labeling using MPdi1 (D) and MPxi1 (E) was detected by a 5-fold overexposure of Cy3 fluorescence. qRT-PCR results (F) are expressed as the ratio of the normalized Ct (vs. GAPDH) of each population to that of dispersed but unsorted islet cells. Each value is the calculated mean ΔCt of two different amplifications each of two different sorted populations.
To confirm the molecular identity of these antibody-defined islet populations, qRT-PCR was used to survey the expression of several islet-associated genes (Fig. 3f). Antibody-mediated enrichment of insulin and Pdx1 and depletion of glucagon mRNA was comparable to or better than that achieved with FACS isolation using GFP+ alone, although co-enrichment of somatostatin was observed in every case. Thus, although alpha and beta cells were efficiently separated, delta cells were present in the beta cell fraction. In order to take advantage of the ability to separate endocrine cells subpopulations for RNA analysis, a group of candidate genes reported by BioGPS (http://biogps.gnf.org) to have higher expression in human islets than whole human pancreas was evaluated in murine islet cell subsets. Among these, four genes (Dgkb, Gpm6a, Dppiv and Ttr) were found to have dramatically different expression levels in alpha and beta cells. The expression of Dgkb and Gpm6a was found to be considerably higher in beta cell fractions MPdi1+GFP+ (10-fold and 196-fold) and MPxi1−GFP+ (16-fold and 89-fold) than the corresponding alpha cell fractions. Conversely, DPPIV and TTR were elevated in alpha cell fractions MPdi1+GFP− (58-fold and 8-fold) and MPxi1+GFP− (7-fold and 60-fold). Differential expression of these genes suggests that they may perform specialized functions in beta or alpha cells, respectively.
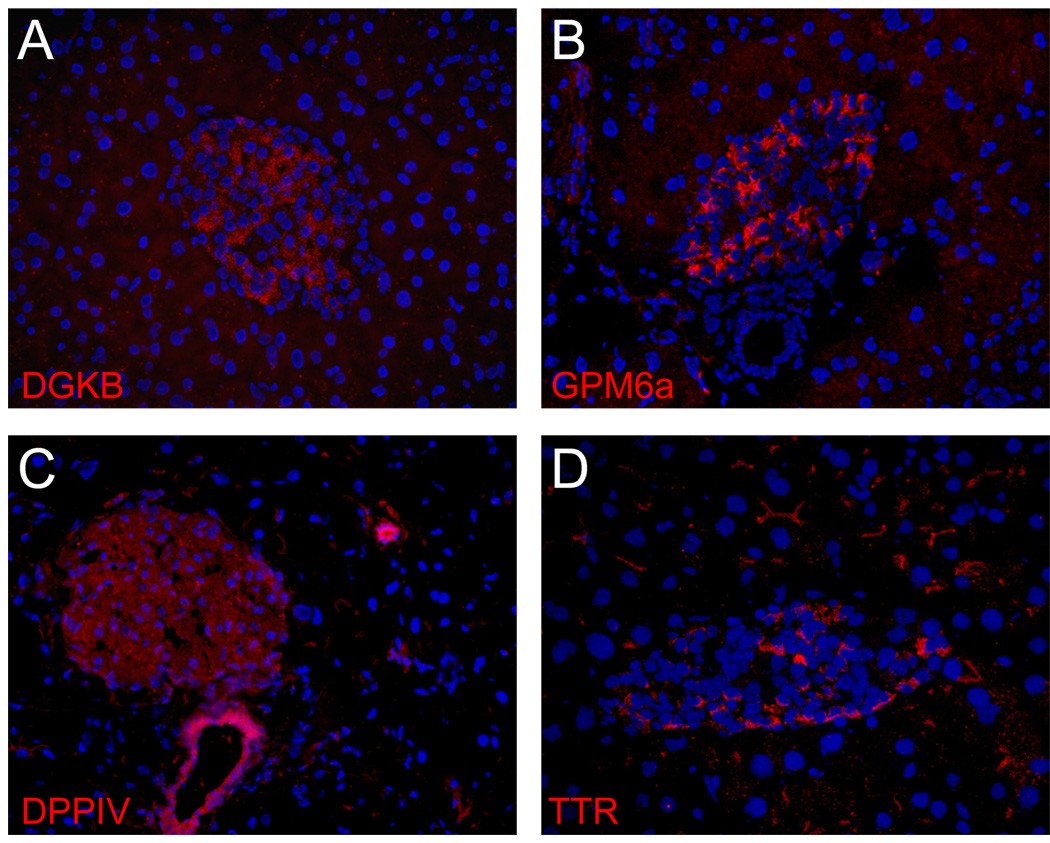
Immunofluorescent detection of DGKB, GPM6a, DPPIV and TTR was performed on mouse pancreatic tissue sections are illustrated in Figure 4. Both DGKB (Fig. 4a) and GPM6a (Fig. 4b) were detected on a large subset of islet cells consistent with beta cell-specific expression. DPPIV (Fig. 4c) was observed on both alpha and beta cells within islets, but the most intense labeling was actually observed on duct cells. The endocrine subtype labeling of TTR protein (Fig. 4d) is found on cells at the islet periphery, consistent with the localization of alpha cells in rodents. Thus, the detection of DGKB, GPM6a, DPPIV and TTR protein in tissue was in general agreement with the differential mRNA patterns observed in isolated endocrine cells.
Fig. 4.
Detection of DGKB, GPM6a, DPPIV and TTR in mouse pancreatic tissue. Formalin-fixed mouse pancreatic cryosections containing multiple islets were labeled with the indicated antibody and detected using a Cy3-conjugated anti-rabbit (A, D) or anti-rat (B, C) secondary antibody (red). Nuclei were labeled with Hoechst 33342 (blue).
3.4 Developmental dynamics of cell subset detection
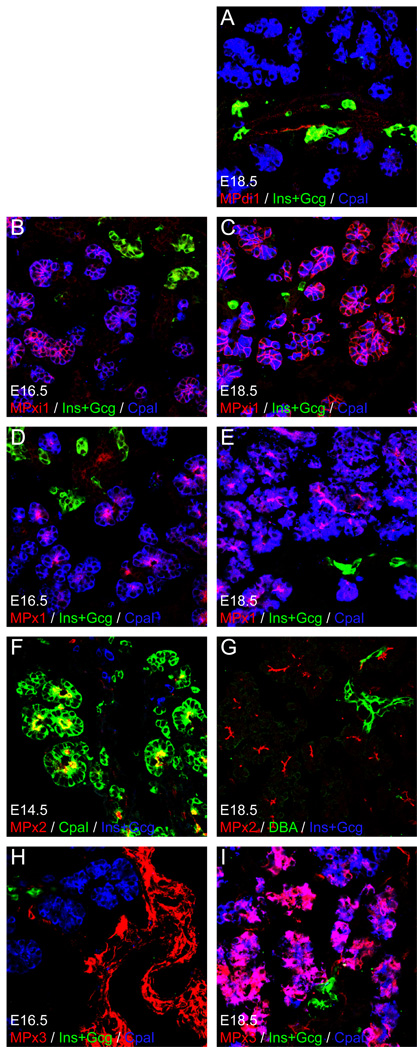
To determine our novel surface markers could label fetal cells during pancreatic cell fate specification, sections of E14.5-E18.5 pancreatic tissue were examined. Figure 5a shows labeling of E18.5 tissue with MPdi1. Labeling was weak at this developmental stage, but duct cells are recognized and endocrine cells are dimly labeled; carboxypeptidase I (CpaI) positive acinar cells were not. Both MPxi1 and MPx1 exclusively labeled CpaI+ acinar cells at E16.5 and E18.5 (Fig. 5b–e). MPx2 labeled the majority of acinar cells with a strong apical localization at E14.5 (Fig. 5f) and E18.5 (Fig. 5g). A more dynamic behavior was observed with MPx3. At E16.5, the label was restricted to mesenchyme and CpaI+ acinar cells were unlabeled (Fig. 5h). By E18.5, however, a substantial percentage of the CpaI+ cells were MPx3+, indicating that the expression of this antigen was a comparatively late developmental event.
Fig. 5.
Cell type specific labeling of fetal pancreas. Sections of E14.5-E18.5 mouse pancreas were sectioned, labeled, and scanned by confocal microscopy. Experimental rat anti-mouse antibodies are visualized using Cy3-conjugated anti-rat IgG (red). Acinar cells are labeled with anti-Carboxypeptidase A1 (CpaI; blue in A–E, H, I and green in F), endocrine cells are marked by a combination of antibodies recognizing insulin and glucagon (Ins+Gcg; green in A–E, H, I and blue in F, G), and in one panel duct cells are highlighted with Dolichos Biflorus Agglutinin (DBA; green in G) Each image shows a 1 µm virtual section combining these three labels.
4. Discussion
The investigation of pancreatic endocrinology and stem cell biology has not been adequately matched by reagents and tools from the field of mouse genetics. Transgenic animals with useful marker properties (e.g. MIP-GFP (Hara et al., 2003)) have proven useful, but investigators of pancreatic endocrine and exocrine biology still lack a comprehensive collection of transgenic animals with useful cell-lineage restricted marker expression. In this report we describe the development and characterization of tools for the isolation and study of different mouse pancreatic cell subpopulations
The ability to selectively isolate pancreatic exocrine populations should support studies of adult pancreatic progenitors. In part because endocrine cells arise from duct structures during development, pancreatic ducts have long been regarded as a possible location for adult stem cells (Xia et al., 2009). Cultures derived from partially purified mouse pancreatic duct material (Kikugawa et al., 2009; Noguchi et al., 2006) or fully purified human pancreatic duct cells (Dorrell et al., 2008a) have been shown to yield insulin-expressing cells (particularly after gene transfer of endocrine-associated transcription factors). Comparable studies using FACS-purified mouse duct cells will allow detailed molecular analysis of this reprogramming event. A recent report has shown that acinar cells can also give rise to beta-like insulin expressing cells after reprogramming by viral gene transfer (Zhou et al., 2008). Although this fate conversion process produces cells very similar in appearance and function to islet-resident beta cells, the relatively low frequency of reprogramming suggests that a subset of acinar cells might be more malleable than the general population. The isolation of pancreatic acinar cells with MPx1 and subsets of these cells using MPx2 and MPx3 will permit more detailed study of the reprogramming process.
The development of cell surface lineage markers present on both developing and mature cell types will also be a resource to guide the programmed differentiation of ES/iPS cells (Gadue et al., 2009). In addition to non-invasive monitoring of the differentiation process, such markers should permit recovery of successfully differentiated cells from a mixed population even when rare, as has been used for in vitro differentiated hepatocytes (Basma et al., 2009).
Although viable beta cells can be isolated from MIP-GFP transgenic mice on the basis of insulin promoter-driven fluorescence, we observed that antibody labeling provided more efficient beta cell recovery by FACS. The ability to collect pure populations of alpha cells (defined either as MPxi1+ or GFP-MPdi1+ cells from MIP-GFP mouse islets) allowed a survey of gene expression differences between these two key endocrine cell subsets. The expression of TTR has been observed to be elevated in type 2 diabetic islets and this has been suggested to be a result of changes in amyloid (Westermark and Westermark, 2008). Our observation that TTR may be more prevalent in alpha cells in mice could imply that that a proportionate loss of beta cells explains this phenomenon. DPPIV is an important membrane-resident peptidase responsible for degrading key hormones including glucagon-like peptide-1 (GLP-1), gastric inhibitory polypeptide (GIP), and insulin-like growth factor 1 (IGF-1) (Flatt et al., 2008). The observation that it is expressed much more abundantly in alpha than beta cells may help to guide the development of therapeutic DPPIV antagonists. GPM6A is a cell surface resident glycoprotein expressed on central nervous system neurons (Michibata et al., 2008). Further experimentation will determine whether detection of this protein in the pancreas can serve as a measure of beta cell mass. Finally, the xpression of DGKB in pancreatic islets has not been previously described, but diacyl- and acylglycerol concentrations and the activity of associated lipases have been described as important for beta cell function (Fex and Mulder, 2008). Beta cell-specific DGKB expression suggests a potential role for the modulation of diacylglycerol second messenger activity by phosphorylation in insulin secretion. Recently, the DGKB locus was linked to type 2 Diabetes in a meta-analysis of genome-wide studies on glucose metabolism (Dupuis et al.). Our observations reinforce the idea that the function of this gene is important for glucose homeostasis; as a beta cell-specific cell surface-localized protein, it may be an attractive therapeutic target.
Acknowledgements
We gratefully acknowledge the hard work of Jean Leif, Michael Bates, and Elaine Norowski who prepared the mouse islet samples used in this study. The advice of Dr. Soren Impey regarding qRT-PCR primer design was also very useful.
This work was supported by the Beta Cell Biology Consortium with grant U01 DK072477.
Abbreviations
- CK19
cytokeratin 19
- CFTR
Cystic Fibrosis Transmembrane Receptor
- MafA
Musculoaponeurotic fibrosarcoma oncogene homolog A
- GP2
Glycoprotein 2
- Pdx1
pancreatic and duodenal homeobox factor 1
- HNF4α
hepatocyte nuclear factor 4 alpha
- TTR
transthyretin
- DPPIV
Dipeptidyl peptidase 4
- DGKB
diacylglycerol kinase beta
- GPM6A
glycoprotein M6A
- IGF-1
insulin-like growth factor 1
- CpaI
carboxypeptidase I
- IF
immunofluorescence
- FACS
Fluorescence-activated cell sorter
- APC
allophycocyanin
- MIP
mouse insulin promoter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Duality of interest
OHSU has commercially licensed part of the technology disclosed herein (MPdi1); authors C.D., P.R.S. and M.G. are inventors of this reagent. This potential conflict of interest is reviewed and managed by OHSU. The other authors disclose no conflicts.
References
- Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Abraham SL, Lanxon-Cookson KM, Canaday PS, Streeter PR, Grompe M. Isolation of major pancreatic cell types and long-term culture-initiating cells using novel human surface markers. Stem Cell Res. 2008a;1:183–194. doi: 10.1016/j.scr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR, Grompe M. Surface markers for the murine oval cell response. Hepatology. 2008b;48:1282–1291. doi: 10.1002/hep.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fex M, Mulder H. Lipases in the pancreatic beta-cell: implications for insulin secretion. Biochem Soc Trans. 2008;36:885–890. doi: 10.1042/BST0360885. [DOI] [PubMed] [Google Scholar]
- Flatt PR, Bailey CJ, Green BD. Dipeptidyl peptidase IV (DPP IV) and related molecules in type 2 diabetes. Front Biosci. 2008;13:3648–3660. doi: 10.2741/2956. [DOI] [PubMed] [Google Scholar]
- Gadue P, Gouon-Evans V, Cheng X, Wandzioch E, Zaret KS, Grompe M, Streeter PR, Keller GM. Generation of monoclonal antibodies specific for cell surface molecules expressed on early mouse endoderm. Stem Cells. 2009;27:2103–2113. doi: 10.1002/stem.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. Am J Physiol Endocrinol Metab. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- Kikugawa R, Katsuta H, Akashi T, Yatoh S, Weir GC, Sharma A, Bonner-Weir S. Differentiation of COPAS-sorted non-endocrine pancreatic cells into insulin-positive cells in the mouse. Diabetologia. 2009;52:645–652. doi: 10.1007/s00125-009-1260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowiak B, Vandewalle B, Riachy R, Kerr-Conte J, Gmyr V, Belaich S, Lefebvre J, Pattou F. Identification and purification of functional human beta-cells by a new specific zinc-fluorescent probe. J Histochem Cytochem. 2001;49:519–528. doi: 10.1177/002215540104900412. [DOI] [PubMed] [Google Scholar]
- Maric D, Barker JL. Neural stem cells redefined: a FACS perspective. Mol Neurobiol. 2004;30:49–76. doi: 10.1385/MN:30:1:049. [DOI] [PubMed] [Google Scholar]
- Michibata H, Okuno T, Konishi N, Wakimoto K, Kyono K, Aoki K, Kondo Y, Takata K, Kitamura Y, Taniguchi T. Inhibition of mouse GPM6A expression leads to decreased differentiation of neurons derived from mouse embryonic stem cells. Stem Cells Dev. 2008;17:641–651. doi: 10.1089/scd.2008.0088. [DOI] [PubMed] [Google Scholar]
- Narushima M, Kobayashi N, Okitsu T, Tanaka Y, Li SA, Chen Y, Miki A, Tanaka K, Nakaji S, Takei K, et al. A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol. 2005;23:1274–1282. doi: 10.1038/nbt1145. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Xu G, Matsumoto S, Kaneto H, Kobayashi N, Bonner-Weir S, Hayashi S. Induction of pancreatic stem/progenitor cells into insulin-producing cells by adenoviral-mediated gene transfer technology. Cell Transplant. 2006;15:929–938. doi: 10.3727/000000006783981431. [DOI] [PubMed] [Google Scholar]
- Quoix N, Cheng-Xue R, Guiot Y, Herrera PL, Henquin JC, Gilon P. The GluCre-ROSA26EYFP mouse: a new model for easy identification of living pancreatic alpha-cells. FEBS Lett. 2007;581:4235–4240. doi: 10.1016/j.febslet.2007.07.068. [DOI] [PubMed] [Google Scholar]
- Swart B, Salganik MP, Wand MP, Tinckam K, Milford EL, Drbal K, Angelisova P, Horejsi V, Macardle P, Bailey S, et al. The HLDA8 blind panel: findings and conclusions. J Immunol Methods. 2005;305:75–83. doi: 10.1016/j.jim.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Westermark GT, Westermark P. Transthyretin and amyloid in the islets of Langerhans in type-2 diabetes. Exp Diabetes Res. 2008;2008:429274. doi: 10.1155/2008/429274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CV, Stechmann CL, McLoon SC. Subtractive immunization techniques for the production of monoclonal antibodies to rare antigens. Biotechniques. 1992;12:842–847. [PubMed] [Google Scholar]
- Xia B, Zhan XR, Yi R, Yang B. Can pancreatic duct-derived progenitors be a source of islet regeneration? Biochem Biophys Res Commun. 2009;383:383–385. doi: 10.1016/j.bbrc.2009.03.114. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]