Abstract
Objective
To determine the prevalence of micronutrient deficiencies in children with intestinal failure as they transitioned from parenteral nutrition (PN) to enteral nutrition (EN).
Study design
We reviewed medical records of all patients with severe intestinal failure treated from 1999–2008 at a multidisciplinary intestinal rehabilitation program who had undergone micronutrient biochemical monitoring.
Results
The cohort of 30 children (mean age 5 yrs, range 2–9; 18 male) had median PN duration of 23 weeks (interquartile range [IQR], 13–34 wks). Median transition from PN to full EN lasted 12 weeks (IQR: 8–20 wks); during this transition, 42% of patients had at least one vitamin deficiency and 80% at least one mineral deficiency. After transition to 100% EN, 70% had at least one vitamin deficiency and 77% had at least one mineral deficiency, with the most common deficiencies being vitamin D (68%), zinc (67%), and iron deficiency (37%). After transition to 100% EN, multivariate analysis identified regular use of a multivitamin supplement (p=0.004) and intact ileocecal valve (p=0.02) as protective against the development of vitamin deficiencies, independent of bowel length, gestational age, and days on PN.
Conclusion
Children with intestinal failure exhibit a high prevalence of micronutrient deficiencies during intestinal rehabilitation. Regular monitoring and aggressive supplementation in children with intestinal failure is warranted.
Keywords: micronutrient deficiency, vitamin, mineral, intestinal failure, short bowel syndrome
Intestinal failure is marked by the body's inability to support growth and/or maintain fluid, electrolyte or nutrient homeostasis due to inadequate functional intestinal mass.1 Among patients with intestinal failure syndromes, the administration of parenteral nutrition (PN) is a life-saving intervention2 and PN is often necessary for growth and development until intestinal adaptation permits the transition from parenteral to full enteral feedings.3 Patients usually receive their full complement of macro- and micronutrients while on full PN support, but during the transition to full enteral nutrition (EN), patients must receive and absorb adequate micronutrients enterally in order to avoid deficiency.
Several micronutrients are critical components in the maintenance of gastrointestinal epithelial integrity and development4, and may also be important for the process of intestinal adaptation that follows massive resection. For example, in rat models of intestinal failure, vitamin A deficiency was noted to reduce crypt cell proliferation, epithelial migration rates, and increase apoptosis5, and zinc deficiency was associated with reduced mucosal protein and DNA content, as well as alkaline phosphatase activity.6 Because of the potential for vitamin and/or mineral malabsorption to occur with small intestinal resection, it is possible that poor micronutrient status may adversely effect human intestinal adaptation.
Previous data concerning the micronutrient status of patients with intestinal failure have been limited to cross-sectional case series of fewer than 10 patients each,7–9 and two case reports.10–11 To our knowledge, there are no studies that have followed the micronutrient status of children with intestinal failure from the beginning of the transition from PN to EN through to full EN tolerance. We therefore sought to determine the prevalence of micronutrient deficiencies from the beginning of PN-EN transition to full EN feeding in a cohort of children with intestinal failure. We also sought to assess the relationship of micronutrient deficiencies with demographic, clinical, and nutritional factors.
Methods
After obtaining approval from the Institutional Review Board at Children's Hospital Boston, data were retrospectively collected from the medical records of all eligible patients who attended the Center for Advanced Intestinal Rehabilitation, a multidisciplinary intestinal rehabilitation program, between May 1999 and January 2008. Inclusion criteria included age 0 to 18 years, severe short bowel syndrome (defined by history of PN use for ≥ 90 days) subsequent tolerance of full enteral nutrition (100% of nutrient and energy requirement via the gastrointestinal tract for >3 weeks), and documentation of at least three serum micronutrient concentrations during both the transition from PN to EN and during subsequent full EN tolerance. The micronutrients examined were vitamins A, B12, 25- OH D, E, and folate, plus the minerals copper, iron, selenium, and zinc. In addition, anemia with or without microcytosis was taken as an indication of possible or suspected iron deficiency, respectively. Excluded were those patients still receiving any amount of parenteral nutrition.
54 patients with severe short bowel syndrome were initially identified. Full enteral nutrition was achieved during the study period in 36 of 54 (67%) patients. Of these 36, 30 (83%) had documentation of micronutrient levels during both the transition from PN to EN and during subsequent full EN feeding. Data were abstracted from this group of 30 patients who had fulfilled all inclusion criteria. Clinical, demographic, surgical and anthropometric data (including height, weight, and head circumference) were recorded. Anthropometric z-scores—including weight-for-age, height-for-age, weight-for-height, BMI and head circumference—were calculated using the World Health Organization Anthro software12 and, in the case of premature birth, the Fenton preterm infant growth chart13 Percent bowel length was calculated according to a previously published formula.14 Data were collected from courses of PN received as inpatients throughout transition to full EN tolerance, and until normalization of nutrient deficiency (or no recorded nutritional values for > 6 months) while on full enteral nutrition.
Hemoglobin (Hgb) and mean corpuscular volume (MCV), were evaluated by Coulter's automated system. Hgb cutoff values for anemia were: 6 months-6 years, <10.5 g/dL; 7–12 years, <11.5 g/dL.15 Iron deficiency anemia was defined as MCV < 79 fL and Hb < 10.5 g/dL. Serum levels of folic acid and vitamin B12 were measured by chemiluminescence immunoassay (reference range of 4.2–20.0 ng/mL and 190–778 pg/mL, respectively). Iron was assessed by automated colorimetric method. Copper, selenium and zinc were measured by atomic absorption spectrophotometry. Reference ranges from the Children's Hospital, Boston Clinical Chemistry Laboratory were as follows: iron, 50 to 120 μg/dL; copper, 85 to 150 μg/dL; selenium, 23–190 μg/L and zinc 60–120 μg/dL. Vitamins A and E were assayed by high-performance liquid chromatography (reference ranges: vitamin A, 20–80 μg/dl; vitamin E 5.0 – 23.0 mg/L). 25 OH-vitamin D was measured by radioimmunoassay (reference range 30–74 ng/mL). C-reactive protein levels were estimated by nephelometry (reference range < 1 mg/L). If CRP was elevated, we did not consider the patient deficient in the following nutrients that have been found to be depressed during acute phase response: copper, iron, vitamin A and zinc.
While patients were receiving PN, they received the standard full complement of parenteral micronutrients.16 For this cohort, the multivitamin supplementation received was M.V.I. Pediatric (Hospira, Lakeforest, IL) for children < 11years old. Parenteral trace elements used were PediTrace (Fresenius Kabi, Bad Homburg, Germany), or supplemented as needed by the pharmacy. The concentration of vitamins in the M.V.I. Pediatric at 5 ml/day were as follows: 2300 IU vitamin A, 400 IU vitamin D (25-OH), 7 IU vitamin E, 200mcg vitamin K, 80 mg ascorbic acid, 1.2 mg thiamine, 1.4mg riboflavin, 17 mg niacin, 5 mg pantothenate, 1 mg pyridoxine, 1 mcg vitamin B12, 20 mcg biotin, and 140 mg folate. Standard full trace element dosing was zinc 2 mg/L (advanced to 3 mg/L in children < 2 kg), 60 mcg/L manganese, 200 mcg/L copper, and 2 mcg/L chromium. Selenium is not a standard part of trace element preparations and is generally added to PN if serum levels are low after 30 days of PN. Dosing of selenium begins at 2mcg/kg with a maximal daily dose of 40 mcg/day. While patients were being weaned from PN to enteral nutrition, they often received fewer than 7 days per week of parenteral multivitamins. Our schedule of monitoring micronutrient status generally followed our published standards17 which call for monthly measurements of selenium, copper, zinc, iron, carnitine, and vitamins A, D and E.
Because of the risk of toxicity of manganese and copper during cholestatic liver disease,3, 18 our practice was to provide ½ the usual dose of the trace elements in the PN whenever children had cholestasis (defined as serum direct bilirubin > 2 mg/dL) as well as more than 30 days of PN exposure. When this was done, a normal amount of zinc (2 mg/L for children > 2 kg or 3 mg/L for those < 2 kg) was added to the PN. Our general practice was also to provide oral multivitamin supplementation once a patient was fully weaned from PN. Due to their inclusion of zinc and water-miscible forms of fat-soluble vitamins, we usually prescribed AquADEK (1 to 2 ml of liquid or 1 to 2 gel tabs; Yasoo Health Inc., Morrisville, NC) or SourceCF (1 to 2 chewable tabs; Eurand Pharmaceuticals, Inc., Yardley PA).
Statistical methods
Demographic and clinical characteristics of patients were evaluated. Numerical variables were described using means with standard deviations (SDs) or medians with interquartile ranges (IQRs), and categorical variables were described using proportions. Numerical variables across groups were compared using appropriate parametric and nonparametric tests. Differences in the prevalence of micronutrient deficiencies during transition from PN to EN and after full transition to EN were assessed using Fisher exact test. Contingency tables were evaluated using Fisher exact test for comparing binary proportions such as presence or absence of deficiency and usage of multivitamin supplementation. Mann-Whitney U tests were performed to compare medians between patients with or without deficiency and those taking/not taking multivitamins. Associations between clinical and anthropometric variables and micronutrient deficiencies were tested by logistic regression. We performed multivariate logistic regression to identify independent predictors of vitamin and mineral deficiency during PN-EN transition and full EN.19 Linear regression was used to assess factors associated with the number of micronutrient deficiencies. Repeated measures analysis of variance (ANOVA) was applied to examine changes in anthropometic measures.20 Statistical analysis was performed using SPSS version 18.0 (SPSS Inc./IBM, Chicago, IL). Two-tailed P < .05 was considered statistically significant.
Results
The cohort consisted of 30 patients (age range 2.5 – 9 years old; mean 4.9 years; 18 boys and 12 girls) with intestinal failure (Table I). The most common etiology of intestinal failure was necrotizing enterocolitis (43% of patients), followed by volvulus (23%). Many patients had undergone intestinal resection, with mean (SD) measured residual small bowel length of 87 (55) cm, and most had had some evidence of hepatic dysfunction during their courses of PN. Most patients (63%) had an intact ileocecal valve. Median PN duration was 142 days (interquartile range [IQR], 100–235 days), and median follow up was 30 months (IQR: 21 – 68 months).
Table 1.
Baseline characteristics of 30 children with intestinal failure.
| Characteristics (n=30) | |
| Male sex, n (%) | 18 (60%) |
| Gestational age (wk), mean (SD) | 31 (5) |
| Birth weight, kg, mean (SD) | 2.15 (0.87) |
| Birth height, cm, mean (SD) | 45.8 (4.6) |
| Primary Diagnosis, n (%) | |
| NEC | 13 (43) |
| Volvulus | 7 (23) |
| Gastroschisis | 3 (10) |
| Intestinal Atresia | 4 (13) |
| Other | 3 (10) |
| Measured residual bowel length, cm mean (SD) n = 20 | 87 (55) |
| Mean percentage of expected small bowel length (SD) | 70 (39) |
| Presence of ileocecal valve, n (%) | 19 (63) |
| Duration of PN, days, median (IQR) | 142 (100–235) |
| Duration of transition from PN to full EN, days, median (IQR) | 83 (63–185) |
| Peak direct bilirubin before cessation of PN (mg/dL), median (IQR) | 5.3 (3.4–11.2) |
| Peak ALT before cessation of PN (U/L), median (IQR) | 157 (116–239) |
IQR = interquartile range; SD = standard deviation; NEC = necrotizing enterocolitis; PN = parenteral nutrition; ALT = alanine aminotransferase.
Patients demonstrated growth deceleration while transitioning from PN-EN to full EN. Table II shows that patients showed reduced weight (p<0.0001), height (p=0.04), and weight-for-length Z scores (p=0.01) during the transition from PN to EN.
Table 2.
Anthropometric indices at different phases of nutrition treatment in children with intestina failure.
| Anthropometric variable – mean (SD) | At PN to EN transition | At start of full EN | N | P value* |
|---|---|---|---|---|
| Weight-for-age Z-score | −1.19 (0.98) | −2.06 (1.35) | 26 | <0.0001 |
| Length-for-age Z score | −1.01 (1.64) | −1.62 (1.91) | 22 | 0.04 |
| Weight-for-length Z-score | −0.41 (1.92) | −1.31 (1.29) | 15 | 0.01 |
| Head Circumference Z-score | −0.73 (1.09) | −1.24 (2.05) | 17 | 0.62 |
PN = parenteral nutrition, EN = enteral nutrition
Paired t-tests were performed for evaluating changes in each variable between start of transition from PN to EN and start of full EN.
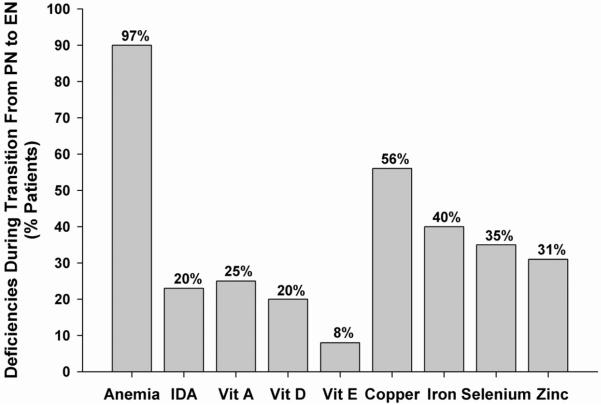
Figure 1 depicts the prevalence of micronutrient deficiencies while subjects were being transitioned from PN to EN. During this time, 97% (29/30) had anemia, with 20% (6/30) having suspected iron deficiency anemia. More than a third of all subjects had copper, iron or selenium deficiency, and more than one quarter had vitamin A or zinc deficiency. 33% of children had ≥ 1 vitamin and 80% had ≥ 1 mineral deficiency, with 50% of children having multiple micronutrient deficiencies.
Figure 1.
Prevalence of micronutrient deficiencies during transition from parenteral nutrition to enteral nutrition. Bar chart showing percentage of patients with deficiencies during transition from parenteral nutrition to enteral nutrition. IDA = iron deficiency anemia.
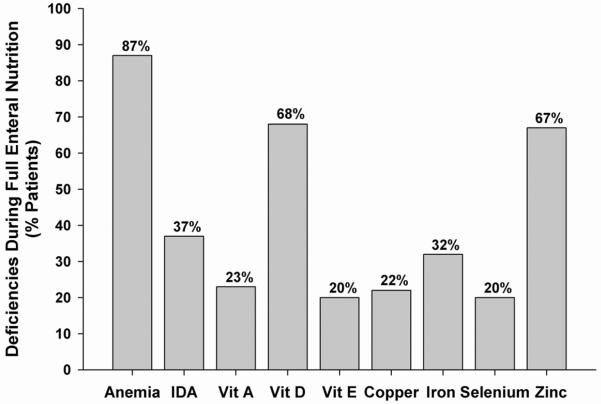
Figure 2 depicts the prevalence of micronutrient deficiencies after subjects had achieved full EN. Anemia affected 87% of subjects, and more than two-thirds of patients had vitamin D or zinc deficiency. 70% of children had ≥ 1 vitamin deficiency and 80% had ≥ 1 mineral deficiency, with 70% of children having multiple micronutrient deficiencies. Tables III and IV (available at www.jpeds.com) list the distributions and percentiles of the various micronutrients measured during the study's two time periods: during the transition from PN to EN (Table III) and after full EN was achieved (Table IV).
Figure 2.
Prevalence of micronutrient deficiencies during full enteral nutrition. Bar chart showing percentage of patients with deficiencies during full EN. IDA = iron deficiency anemia.
TABLE 3.
Mean, median, and percentile distributions of serum concentrations of vitamins, trace minerals, and Hb indices among children with intestinal failure during transition from PN to EN
| n1 | Mean | SD | 25% | Median | 75% | Normal ranges | |
|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dl) | 30 | 8.2 | 1.2 | 7.3 | 8.2 | 8.8 | > 10.5 |
| MCV (fL) | 30 | 86.3 | 6.5 | 80.4 | 86.3 | 90.7 | 79–89 |
| Vitamin A (mcg/dL) | 20 | 22.5 | 11.5 | 14.4 | 19.3 | 33.9 | 20–80 |
| Folate (ng/mL) | 3 | 13.4 | 12.4 | 5.9 | 6.5 | 27.7 | 4.2–20 |
| Vitamin B12 (pg/mL) | 3 | 1249 | 264 | 1023 | 1185 | 1539 | 190–778 |
| 25-OH Vitamin D (ng/mL) | 14 | 37.0 | 14.0 | 20 | 40 | 50 | 30–74 |
| Vitamin E (mg/dL) | 12 | 16.0 | 13.0 | 7.1 | 13 | 19.8 | 5–23 |
| Copper (mcg/dL) | 25 | 82.2 | 25.1 | 68 | 79.5 | 99 | 85–150 |
| Iron (mcg/dL) | 15 | 56 | 34.4 | 27 | 46 | 77 | 50–120 |
| Selenium (mcg/L) | 26 | 34.7 | 22.8 | 23 | 29 | 39 | 23–190 |
| Zinc (mcg/dL) | 26 | 69.1 | 26.1 | 53.5 | 64.5 | 83.5 | 60–120 |
Total sample size varies due to missing values or insufficient serum for analysis.
TABLE 4.
Mean, median, and percentile distributions of serum concentrations of vitamins, trace minerals, and Hb indices among children with intestinal failure during enteral nutrition
| n1 | Mean | SD | 25% | Median | 75% | Normal ranges | |
|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dl) | 30 | 9.9 | 1.3 | 8.5 | 9.9 | 11.2 | > 10.5 |
| MCV (fL) | 30 | 82.3 | 6.7 | 78 | 82.0 | 87.7 | 79–89 |
| Vitamin A (mcg/dL) | 30 | 26.6 | 8.5 | 21.4 | 27 | 30.8 | 20–80 |
| Folate (ng/mL) | 16 | 16.1 | 5.9 | 11.85 | 16 | 19.2 | 4.2–20 |
| Vitamin B12 (pg/mL) | 28 | 706 | 356 | 407.5 | 703 | 954.5 | 190–778 |
| 25-OH Vitamin D (ng/mL) | 28 | 25.9 | 14.0 | 19.5 | 22 | 28 | 30–74 |
| Vitamin E (mg/dL) | 30 | 7.0 | 2.8 | 5.2 | 6.1 | 8 | 5–23 |
| Copper (mcg/dL) | 23 | 107.1 | 36.4 | 73 | 115 | 135 | 85–150 |
| Iron (mcg/dL) | 25 | 55.2 | 27.5 | 40 | 50 | 74 | 50–120 |
| Selenium (mcg/L) | 20 | 81.3 | 65.7 | 22 | 74.5 | 104 | 23–190 |
| Zinc (mcg/dL) | 30 | 55.8 | 13.1 | 46 | 52 | 61 | 60–120 |
Total sample size varies due to missing values or insufficient serum for analysis.
When comparing the rates of fat-soluble vitamin deficiencies between the two sampling time frames, vitamin A deficiency was stable at 23 – 25%, but the prevalence of vitamin E deficiency rose from 8% of subjects to 20%, and that of vitamin D rose from 20% of children to 68%. When comparing mineral nutritional status, deficiencies of copper, iron and selenium were all less common after EN was reached, although the prevalence of zinc deficiency increased from 31% to 67%.
Although our standard practice is to routinely supplement children with intestinal failure with oral multivitamins, only 56% (17/30) children were documented to receive daily multivitamins after transition to full EN; the remaining 13 children either did not receive a multivitamin due to parental decision and/or were determined by the dietician to have adequate micronutrient intake based on dietary history. The median number of vitamin and mineral deficiencies for children receiving multivitamin supplementation was 1 (range 0 to 4) and the median number of vitamin and mineral deficiencies for children not receiving multivitamins was 3 (range, 1 to 5).
The presence of any micronutrient deficiency (vitamin or mineral) during the transition from PN to EN and after full EN tolerance was not associated with gestational age, diagnosis, bowel length, percent bowel length, presence of ileocecal valve, PN duration, peak values of direct bilirubin, ALT, or CRP (P > 0.2).
When analyzing the relationship between anthropometric z-scores and likelihood of developing vitamin or mineral deficiency, we found that during the PN-EN transition, children with greater height-for-age z-scores were less likely to have a mineral deficiency (P = 0.05). During full EN feeding, children with iron deficiency anemia had lower weight-for-height z-scores (mean (SD) −1.96 (1.05) vs. −0.68 (1.10), P = 0.01). All other anthropometric z-scores were not associated with increased likelihood of developing vitamin or mineral deficiency.
After weaning off PN, 21 of 30 patients had vitamin deficiency (70%). All 13 patients (100%) not taking multivitamin supplementation developed at least one vitamin deficiency compared with 8 of 17 (47%) who were taking multivitamins (P = .003, Fisher's exact test).
When analyzing predictors of vitamin deficiencies (separate from mineral deficiencies), univariate analysis indicated no significant difference in gestational age (p=0.91), bowel length (p=0.43) and days on PN (p=0.69) between patients with and without vitamin deficiency after full EN was achieved. However, univariate analysis using Fisher's exact test indicated a highly significant association between the use of multivitamin supplementation and lower rate of vitamin deficiency (p=0.003) and a statistical trend between intact ileocecal valve and lower rate of vitamin deficiency (p=0.08). Multivariate logistic regression analysis identified multivitamin supplementation (p=0.004) and intact ileocecal valve (p=0.02) as significant independent predictors of vitamin sufficiency, irrespective of gestational age (p=0.21), bowel length (p=0.58) or duration of PN (p=0.55).
Discussion
We report a high prevalence of micronutrient deficiencies in children with intestinal failure at two distinct periods: 1) transition from parenteral nutrition to enteral nutrition and, 2) during long-term follow-up after transition to full enteral nutrition. We found a high frequency of biochemical deficiencies in a majority of the indices that we assessed, including low levels in hemoglobin, MCV, vitamin A, 25-OH vitamin D, vitamin E, copper, iron, selenium and zinc. During the transition from PN to full EN, 33% of children had ≥ 1 vitamin and 80% had ≥ 1 mineral deficiency, with 50% of children having multiple micronutrient deficiencies. After cessation of PN and transition to full EN feeding, 70% of patients had ≥ 1 vitamin deficiency and 80% had ≥ 1 mineral deficiency, with 70% of children having multiple micronutrient deficiencies. In addition, we noted that children weaned from parenteral nutrition exhibited significant reduction in standard anthropometric measures of weight, height, and weight for height Z scores.
Our finding that micronutrient abnormalities are present after transition to full enteral nutrition in children with intestinal failure is consistent with previous studies, although in general we found higher prevalences of deficiencies. In nine children in the U.S. with intestinal failure who had transitioned to full enteral tolerance, Leonberg et al assessed growth and serum levels of several micronutrients.7 They reported low serum levels of vitamin B12 (2/9), vitamin D (7/9), vitamin E (2/9), folate (1/9) as well as vitamin A, ferritin, and zinc (one child each). Gonzalez et al studied 10 children in Argentina with intestinal failure after they had transitioned to full EN, and assessed anthropometric measurements, hemoglobin values, iron, zinc, copper, folate and vitamin B12.8 They found four children with macrocytosis, two with anemia and low serum levels of vitamin B12 (1/10), folate (4/10 children), and ferritin (2/10). Wu et al assessed hemoglobin, albumin, prealbumin, electrolytes, iron, zinc, copper, vitamin A, beta-carotene, vitamin E in 9 children with intestinal failure in China who had weaned off PN.9 The authors report no children with anemia, although one presented with macrocytosis and another had microcytosis. Low serum concentrations of zinc (3/8), iron (1/8), vitamin A (2/8) vitamin E (4/8) cases, and beta-carotene (2/8) were also reported. Duro et al described a child with short bowel syndrome but normal somatic growth who had transitioned to full EN, but had presented after 3 years with low hemoglobin, as well as deficiencies of vitamins A, D, E, B12 and zinc.10 Ordonez et al described a child with congenital short bowel syndrome who weaned off PN, and had persistent low zinc levels, but normal growth.11
Our results add to this previous literature by its longitudinal design and evaluation of risk factors for micronutrient deficiencies. By evaluating the evolution of micronutrient status over time, we documented that after reaching full enteral nutrition, the prevalence of deficiencies of vitamin A, vitamin E and zinc was higher than when children were receiving partial PN. In addition, our longitudinal data showed that height and weight were not maintained, suggesting a possible growth-limiting effect from these important micronutrient deficiencies. Zinc deficiency is a well-recognized risk factor for linear growth delay21 and our data identified mineral deficiencies, including iron and zinc, as being associated with poorer growth.
We also found that an intact ileocecal valve (and by extension, greater length of undamaged ileum) and regular multivitamin supplementation appeared to reduce the occurrence of micronutrient deficiencies in children with intestinal failure. First, after transitioning to full EN, the prevalence of vitamin deficiency was 47% among those receiving multivitamin supplementation, compared with 100% among those not receiving supplements (P < .003). Second, using multivariate analysis, we found that, independent of several other factors (gestational age, bowel length, and duration of PN), intact ileocecal valve (P <0.05) and multivitamin supplementation (P < 0.01) were both associated with lower risk of developing any vitamin deficiency during full EN tolerance.
Our findings concerning the importance of the ileocecal valve and multivitamin use are consistent with the known pathophysiology of conditions leading to intestinal failure. The terminal ileum plays an important role in fat and fat-soluble vitamin absorption, and resection of the ileocecal valve suggests resection of part of the ileum and reduced surface area for vitamin A, D, E and K absorption.22 Cholestatic liver disease also affected a number of our subjects. Cholestasis can further reduce fat-soluble vitamin absorption due to decreased intraluminal bile salt concentrations that are less than the critical micellar level.23 A higher prevalence of vitamin D and E deficiencies during full EN, compared with partial PN, also suggests that steatorrhea is an important contributing factor to these deficiencies. Steatorrhea can be associated with calcium and zinc malabsorption as well24, and we observed a high rate of zinc deficiency during full enteral nutrition.
Our study is limited by its small sample size and retrospective design. In addition, it is possible that a selection bias might have resulted in patients with confirmed deficiencies early in their course be more likely to undergo biochemical assessment subsequently, thereby increasing the chances of detecting deficiencies. We should note, however, that our criteria of at least three biochemical assessments during both PN transition and full EN only excluded 6 of 36 possible subjects. In addition, the mean (SD) number of biochemical assessments that our subjects underwent during PN-EN transition was 5.3 (2.4) and during full EN was 8.5 (1.2). This suggests that biochemical monitoring was nearly universal in our practice, regardless of prior results. Larger, prospective studies would certainly be helpful to confirm these findings, however.
Two important considerations emerge from these data. First, although the rationale for the amount of micronutrient supplementation during PN use in children has been made empirically15, 25 it is generally assumed that standard multivitamin and trace element preparations meet the nutrient requirements for fully parenterally fed infants and children. Our findings of a high rate of multiple micronutrient deficiencies even though partial PN is provided suggest that some of these may have existed even earlier in the course of intestinal rehabilitation. The adequacy of parenteral micronutrient supplementation guidelines may therefore need to be reviewed.
Second, although the use of a micronutrients supplementation was found to be associated with a lower risk of deficiencies, 47% (8/17) patients on multivitamin supplementation still developed at least one biochemical abnormality. Clinicians still need to rely on biochemical assessment of micronutrient status because, as we have shown the identification of clinical factors, including extent of bowel loss, are not helpful to diagnose micronutrient deficiencies. Although we showed that children with higher height-for-age z-scores were less likely to have a mineral deficiency during PN-EN transition and that children with iron deficiency anemia had lower weight-for-height z-scores, anthropometric measures alone cannot be relied upon to screen for micronutrient deficiencies.
Micronutrient deficiencies are common throughout the course of intestinal rehabilitation; whether these deficiencies adversely impact the course of rehabilitation and gastrointestinal function is unknown, but animal data suggest this possibility. Aggressive micronutrient supplementation, combined with strict surveillance of nutrition status in patients with intestinal failure, is warranted.
Acknowledgments
We thank Kathleen Gura, PharmD, and Megan Brenn, RD, for helpful discussions about the use and content of parenteral multivitamins, as well as the staff of the Center for Advanced Intestinal Failure at Children's Hospital Boston for their expert patient care.
Supported in part by NIH 1K24HD058795 and the Howard Hughes Medical Institute Medical Student Fellowship (to C.Y.).
List of Abbreviations
- ALT
alanine aminotransferase
- CRP
c-reactive protein
- EN
Enteral nutrition
- Hgb
Hemoglobin
- IFALD
Intestinal failure-associated liver disease
- IDA
Iron deficiency anemia
- IQR
Interquartile range
- MCV
Mean corpuscular volume
- PN
Parenteral nutrition
- SD
Standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.O'Keefe SJ. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol. 2006;4(1):6–10. doi: 10.1016/j.cgh.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Wilmore DW, Dudrick SJ. Growth and development of an infant receiving all nutrients exclusively by vein. JAMA. 1968;203:860–864. [PubMed] [Google Scholar]
- 3.Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006;130:S70–7. doi: 10.1053/j.gastro.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 4.Duggan C, Gannon J, Walker WA. Protective nutrients and functional foods for the gastrointestinal tract. Am J Clin Nutr. 2002;75:789–808. doi: 10.1093/ajcn/75.5.789. [DOI] [PubMed] [Google Scholar]
- 5.Swartz-Basile DA, Wang L, Tang Y, Pitt HA, Rubin DC, Levin MS. Vitamin A deficiency inhibits intestinal adaptation by modulating apoptosis, proliferation, and enterocyte migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G424–G432. doi: 10.1152/ajpgi.00524.2002. [DOI] [PubMed] [Google Scholar]
- 6.Vanderhoof JA, Park JH, Grandjean CJ. Effect of zinc deficiency on mucosal hyperplasia following 70% bowel resection. Am J Clin Nutr. 1986;44:670–7. doi: 10.1093/ajcn/44.5.670. [DOI] [PubMed] [Google Scholar]
- 7.Leonberg BL, Chuang E, Eicher P, Tershakovec AM, Leonard L, Stallings VA, et al. Long-term growth and development in children after home parental nutrition. J Pediatr. 1998;132(3 Pt 1):461–6. doi: 10.1016/s0022-3476(98)70021-6. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez HF, Perez NB, Malpeli A, Martinez MI, Del Buono B, Viteri FE. Nutrition and immunological status in long-term follow up of children with short bowel syndrome. J Parenter Enteral Nutr. 2005;29:186. doi: 10.1177/0148607105029003186. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Tang Q, Feng Y, Huang J, Tao Y, Wang Y, Cai W, Shi C. Nutrition assessment in children with short bowel syndrome weaned off parenteral nutrition: a long-term follow-up study. J Pediatr Surg. 2007;242:1372–1376. doi: 10.1016/j.jpedsurg.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Duro D, Jaksic T, Duggan C. Multiple micronutrient deficiencies in a child with short bowel syndrome and normal somatic growth. J Pediatric Gastroenterol and Nutr. 2008;46:461–464. doi: 10.1097/MPG.0b013e3181373b91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ordonez P, Sondheimer JM, Fidanza S, Wilkening G, Hoffenberg EJ. Long-term outcome of a patient with congenital short bowel syndrome. J Pediatric Gastroenterol and Nutr. 2006;42:576–580. doi: 10.1097/01.mpg.0000189360.84169.da. [DOI] [PubMed] [Google Scholar]
- 12.WHO Anthro 2010 June; from http://www.who.int/childgrowth/software/en/
- 13.Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003 Dec 16;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duro D, Kalish A, Johnston P, Jaksic T, McCarthy M, Martin C, et al. Risk factors for intestinal failure in infants with necrotizing enterocolitis: a Glaser Pediatric Research Network study. J Pediatr. 2010;157(2):203–208. doi: 10.1016/j.jpeds.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrman RE, Kliegman RM, Jenson HB, editors. Nelson Textbook of Pediatrics. 17th edition Elsevier Science; Philadelphia: 2004. Laboratory medicine, drug therapy, and reference tables; pp. 2398–421. [Google Scholar]
- 16.Greene HL, Hambidge KM, Schanler R, Tsang RC. Guidelines for the use of vitamins, trace elements, calcium, magnesium, and phosphorus in infants and children receiving total parenteral nutrition: report of the Subcommittee on Pediatric Parenteral Nutrient Requirements from the Committee on Clinical Practice Issues of the American Society for Clinical Nutrition. Am J Clin Nutr. 1988;48:1324–42. doi: 10.1093/ajcn/48.5.1324. [DOI] [PubMed] [Google Scholar]
- 17.Collier SB, Gura K, Richardson D, Duggan C. Parenteral nutrition. In: Hendricks KM, Duggan C, editors. Manual of Pediatric Nutrition. 4th ed. BC Decker, Inc.; 2005. [Google Scholar]
- 18.Slicker J, Vermilyea S. Pediatric Parenteral Nutrition: Putting the Microscope on Macronutrients and Micronutrients. Nutr Clin Pract. 2009;24:481. doi: 10.1177/0884533609339073. [DOI] [PubMed] [Google Scholar]
- 19.Katz MH. Multivariable analysis: a practical guide for clinicians. 2nd ed. Cambridge University Press; New York: 2006. pp. 137–57. [Google Scholar]
- 20.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Linear, logistic, survival, and repeated measures models. Springer; New York: 2005. Regression methods in biostatistics; pp. 291–322. [Google Scholar]
- 21.Rivera JA, Hotz C, Gonzalez-Cossio T, Neufeld L, Garcia-Guerra A. The effect of micronutrient deficiencies on child growth: a review of results from community-based supplementation trials. J Nutr. 2003;133:4010S–4020S. doi: 10.1093/jn/133.11.4010S. [DOI] [PubMed] [Google Scholar]
- 22.Jeejeehoy K. Short bowel syndrome: a nutritional and medical approach. CMAJ. 2002;166(10) [PMC free article] [PubMed] [Google Scholar]
- 23.Strople J, Lovell G, Heubi J. Prevalence of Subclinical Vitamin K Deficiency in Cholestatic Liver Disease. J Pediatr Gastroenterol Nutr. 2009 Jun 3; doi: 10.1097/MPG.0b013e31819a61ff. [DOI] [PubMed] [Google Scholar]
- 24.Ovesen L, Chu R, Howard L. The influence of dietary fat on jejunostomy output in patients with severe short bowel syndrome. Am J Clin Nutr. 1983;38(2):270–7. doi: 10.1093/ajcn/38.2.270. [DOI] [PubMed] [Google Scholar]
- 25.Task Force for the Revision of Safe Practice for Parenteral Nutrition: Mirtallo J, Canada T, Johnson D, Kumpf V, Petersen C, Sacks G, Seres D, Guenter P. Safe practices for parenteral nutrition. JPEN J Parenter Enteral Nutr. 2004;28S:S52–S57. doi: 10.1177/0148607104028006s39.