Abstract
Objective
To assess the continuity of care and outcome of pediatric HIV prevention, testing, and treatment services, focusing on early infant diagnosis with DNA PCR.
Design
A retrospective observational cohort.
Methods
Maternal HIV antibody, infant HIV DNA PCR test results, and outcome data from HIV-infected infants from the prevention of mother-to-child transmission, early infant diagnosis, and pediatric HIV treatment programs operating in Lilongwe, Malawi between 2004 and 2008 were collected, merged and analyzed.
Results
Of the 14,669 pregnant women who tested HIV antibody positive, 7,875 infants (53.7%) received HIV DNA PCR testing. One thousand eighty-four infants (13.8%) were HIV-infected. 320 (29.5%) children enrolled into pediatric HIV care, with 202 (63.1%) at the Baylor Center of Excellence. Among these, antiretroviral therapy was initiated on 110 infants (54.5%) whose median age was 9.1 months (interquartile range, 5.4 to 13.8) and a median of 2.5 months (interquartile range, 1.4 to 9.2) after HIV clinic registration. Sixty-nine HIV-infected infants (34.2%) died or were lost by December 2008. Initiation of antiretroviral therapy increased the likelihood of survival seven-fold (odds ratio, 7.1; 95% confidence interval, 3.68 -13.70).
Conclusions
Separate programs for maternal and infant HIV prevention and care services demonstrated high attrition rates of HIV-exposed and HIV-infected infants, elevated levels of mother-to-child transmission, late infant diagnosis, delayed pediatric antiretroviral therapy initiation, and high HIV-infected infant mortality. Antiretroviral therapy increased HIV-infected infant survival, emphasizing the urgent need for improved service coordination and strategies that increase access to infant HIV diagnosis, improve patient retention, and reduce antiretroviral therapy initiation delays.
Keywords: Africa, antiretroviral therapy, early infant diagnosis, HIV testing, pediatric HIV, prevention of mother-to-child transmission, Malawi
Background
High rates of vertical HIV transmission, late diagnosis of pediatric HIV infection, and high early pediatric HIV-related mortality continue despite the scale-up of prevention of mother-to-child transmission (PMTCT), early infant diagnosis (EID) with DNA PCR, and pediatric antiretroviral therapy (ART) [1][2]. Inaccessibility and inadequate utilization of HIV services results in large numbers of unidentified, HIV-exposed and HIV-infected children. Globally, an estimated 420,000 new pediatric HIV infections occur annually, with the mortality of untreated infants approaching 50% by two years of age [3][4].
Malawi, a sub-Saharan African country with over 14 million people [5], reports an antenatal HIV prevalence of 15% in Lilongwe [6], and estimates that 90,000 HIV-infected children are living within the country [3]. Although PMTCT programs have expanded nationally such that 90% of facilities have antenatal care (ANC) testing services available as of the March 2010, low enrollment and high attrition rates pose challenges [7-9]. A national ART scale-up initiated in 2004 improved adult HIV treatment coverage to approximately 45% of eligible; however, pediatric HIV care has lagged [10]. Specifically, children account for 9% of HIV-infected individuals ever started on ART in Malawi, well below recommendations [11-13].
While previous studies described PMTCT, EID, and pediatric HIV care in isolation [14-17], few studies have assessed the continuum of mother-infant HIV service utilization. This study retrospectively reviewed data from the PMTCT, EID, and pediatric HIV care sites operating in Lilongwe since 2004 in order to determine the numbers of infants tested for HIV, to quantify the timing of infant diagnosis, clinic enrollment, and treatment initiation, and to analyze the outcome of HIV-infected infants found at ART clinics. Based upon this evaluation, we offer recommendations to address identified gaps in PMTCT and EID provision.
Materials and Methods
Study Setting and Population
ANC testing
The University of North Carolina (UNC) Project in Malawi implemented a PMTCT program in 2002 at Bwaila Hospital, the largest government ANC facility in Lilongwe District and three Lilongwe district health centers (Area 18, Area 25 and Kawale) [7]. Nurses issued an identification number, collected patient information, and conducted HIV antibody tests on all women at the initial ANC visit. Subsequently, HIV-infected women had a CD4 count analysis. All HIV infected women with WHO stage 3 or 4 disease or a CD4 count < 250 cells were ART eligible and referred for care. HIV-infected ART ineligible pregnant women were given single dose nevirapine and advised to take the medication during labor, as well as to administer a single dose to their newborn infant, per national guidelines.
EID and follow-up of exposed infants
Infant plasma HIV DNA PCR testing at six weeks of age began in 2004 at ANC clinics as part of the UNC Project PMTCT Program [7]. In mid-2007, EID, a Ministry of Health (MOH) program utilizing dried blood spot HIV DNA PCR testing of HIV-exposed infants, was implemented in 7 pilot sites nationally including the 4 ANC sites in Lilongwe, the Baylor College of Medicine-Abbott Fund Children's Clinical Centre of Excellence (COE), and at Kamuzu Central Hospital (KCH), a regional referral facility. All PCR testing used the Roche Amplicor.
In the EID program, infants of known HIV-infected mothers received DNA PCR testing at six weeks of age, and mothers were instructed to return in one month to receive the results. If the baby was DNA PCR negative but breastfeeding within six weeks of the test, the baby was defined as HIV-exposed. All HIV-exposed infants were recommended to return to an EID clinic or the Baylor COE on a monthly basis to receive cotrimoxazole preventive therapy (CPT). At 6 months post-partum, mothers were advised to wean their child from breastfeeding if they met World Health Organization (WHO) weaning criteria. Six weeks after breastfeeding cessation a second, definitive HIV DNA PCR test was conducted on the infant, with results available after one month. If DNA PCR negative, the baby was considered HIV-uninfected, CPT was discontinued, and the infant was discharged. If the infant became HIV-infected at any time, the child was verbally referred to an ART clinic. Although the child's HIV status was noted in their health passport, the EID program did not document referrals.Date of diagnosis was defined as the date of a patient's first positive DNA PCR test.
The Breastfeeding, Antiretroviral and Nutrition (BAN) study began in April 2004 and also included infant DNA PCR testing. Infants born to HIV-infected study participants were tested at birth, 2, 12, 28, and 48 weeks of age, with HIV-infected infants referred for treatment [18].
Non-routine inpatient pediatric HIV testing at KCH was introduced in 2006. In early 2008, the Baylor program implemented a routine opt-out HIV testing system for hospitalized children at KCH, which included DNA PCR [16].
Pediatric HIV Care
Lighthouse Trust first offered pediatric ART at KCH in 2002. The KCH pediatrics department operated a pediatric HIV clinic at KCH from 2004 until the opening of the Baylor COE clinic in late 2006. Another Lighthouse clinic opened near Bwaila Hospital, also late in 2006, providing a family centered approach although infants less than 18 months were referred to the Baylor COE. Pediatric ART was also offered at the Area 18, Area 25 and Kawale district health centers included in this study starting in early 2007. Registration data from Baylor COE is electronic while the health centers utilized paper ART registers and master cards. ART eligibility in Malawi includes any child who has a WHO stage 3 or 4 condition or CD4 percentage below age thresholds [19]. From mid-2008, universal ART was available for children under 12 months of age [20]. First line ART for children was stavudine/lamivudine/nevirapine.
Study design and data collection
We conducted a retrospective review of patient registers and electronic records of all pregnant women HIV tested at four UNC-affiliated ANC clinics to determine the potential number of HIV-exposed and HIV-infected children born and tested since 2004. Among the identified infected infants, we conducted an analysis of characteristics and outcomes. Routine MOH data from ANC registers, ART clinic registers and HIV testing records from the four UNC-affiliated ANC clinics and KCH were reviewed (Table 1). From 2004 until mid 2007 when PMTCT/EID services were operated by UNC project, ANC nurses documented HIV-infected infant data in logbooks and triplicate referral forms. The referral form contained maternal and infant information. One copy remained in the ANC records and two were given to the patient. One patient copy was for the ART clinic and the other was to be returned to the UNC Project to confirm patient enrollment into care. Prior to the EID program, HIV DNA PCR testing was performed in the UNC Project laboratory and entered into an electronic information system. After transition of the EID program to the MOH, the EID program utilized dried blood spot DNA PCR testing in the KCH laboratory only. Data officers electronically entered maternal PMTCT information and data of all patients enrolled in pediatric HIV clinics. The pediatric HIV clinics at the district health centers maintained registers and mastercards for all HIV-exposed and infected children starting in 2007. Additionally, the Baylor COE maintains an electronic record of all enrolled children. This study was approved by the Malawi National Health Sciences Research Committee, the University of North Carolina and the Baylor College of Medicine Institutional Review Boards. Written or verbal consent was not required since this evaluation used existing data sources.
Table 1. Sources of HIV-infected infant DNA PCR test results.
| Data Source | Site | Dates |
|---|---|---|
| Infant HIV test logbooks | ANC clinics | February 2004 – April 2007 |
| Referral logbooks | ANC clinics | January 2005 – December 2008 |
| BAN and PMTCT electronic laboratory results | Bwaila ANC clinic | August 2004 – December 2008 |
| EID electronic database | ANC clinics and Baylor COE | April 2007 – December 2008 |
| EID logbooks and master cards | ANC clinics | April 2007 – December 2008 |
| Baylor COE electronic database | Baylor COE | October 2004 – December 2008 |
| ART treatment registers | District Health Centers | January 2007- December 2008 |
| Pediatric HIV clinic registers | District Health Centers | February 2007- December 2008 |
ART, antiretroviral therapy, BAN, Breastfeeding Antiretroviral and Nutrition study, PMTCT, Prevention of Mother to Child Transmission; EID, early infant diagnosis; ANC, antenatal clinic; COE, Centre of Excellence
Data Analysis
We merged all infant testing information into one database, and removed any duplicates. We then linked each infant to their mother's ANC data using the PMTCT and BAN identification numbers. Our combined mother and infant data was linked to Baylor COE data using several identifiers. We required at least two matching factors and the absence of conflicting factors to link the data. After ensuring for accuracy, we created a final table devoid of identifiers and used SAS 9.2 (Cary, NC) for all analyses.
Pediatric outcome analysis was restricted to the children identified at the COE due to the availability of an electronic database. All ART clinic outcomes conformed to the Malawi national ART program; including: alive, died, lost to follow-up (LTFU), and transferred out. When assessing mortality, we performed two sets of analyses: one in which LTFU and Transferred out were censored and another where the outcomes LTFU and Died, and Transferred Out and Alive were merged, respectively. We used bivariate analyses to determine the relationship between survival outcome and the timing of diagnosis, presentation to care, treatment initiation, and WHO stage at presentation. Differences in overall survival probability curves were generated using cox-proportional hazards models and displayed using Kaplan-Meier plots according to ART status initiation status.
Results
Of the 14,669 pregnant women who tested HIV antibody positive, there were 7,875 infant HIV DNA PCR test results found, representing just over half of the HIV-exposed infants identified by maternal records from the sampled ANC clinics (Table 2). Most HIV DNA PCR tests were conducted through routine services 5506/7875 (70%) with the remainder of testing through the BAN study. Of the infants DNA PCR tested, 13.8% were HIV-infected, of whom less than one out of three enrolled into HIV care. Sixty-three percent of the infants traced to care were found at the Baylor COE, with the remainder receiving care at the district health centers (Table 2, Figure 1). The 6,794 HIV-exposed infants that were not HIV tested or successfully traced account for potentially 938 HIV-infected, untreated infants using this cohort's 13.8% vertical HIV transmission rate.
Table 2. Summary of HIV-exposed and HIV-infected infant tracing from antenatal clinic to pediatric ART Clinic, 2004-2008.
| Women HIV antibody tested | 101,251 |
| HIV-infected women, n/N (%) | 14,669/101,251 (14.5%) |
| HIV-infected women receiving any PMTCT prophylaxis† | 14,579/14,669 (99.2%) |
| ART (stavudine/lamivudine/nevirapine) | 1,284/14,669 (8.8%) |
| Single dose NVP | 13,295/14,669 (90.6%) |
| HIV-exposed infants receiving single dose NVP† | 6,930/14,669 (47.2%) |
| Infants HIV DNA PCR tested, n/N (%) | 7,875/14,669 (53.7%) |
| HIV-infected infants, n/N (%) | 1,084/7,875 (13.8%) |
| HIV-infected infants traced to an ART clinic, n/N (%) | 320/1,084 (29.5%) |
| HIV-infected infants traced to the Baylor COE, n/N (%) | 202/320 (63.1%) |
ART, antiretroviral; DNA, Deoxyribonucleic nucleic acid; PCR, polymerase chain reaction, COE, Center of Excellence
Received at ANC clinic, ingestion not verified.
Figure 1.
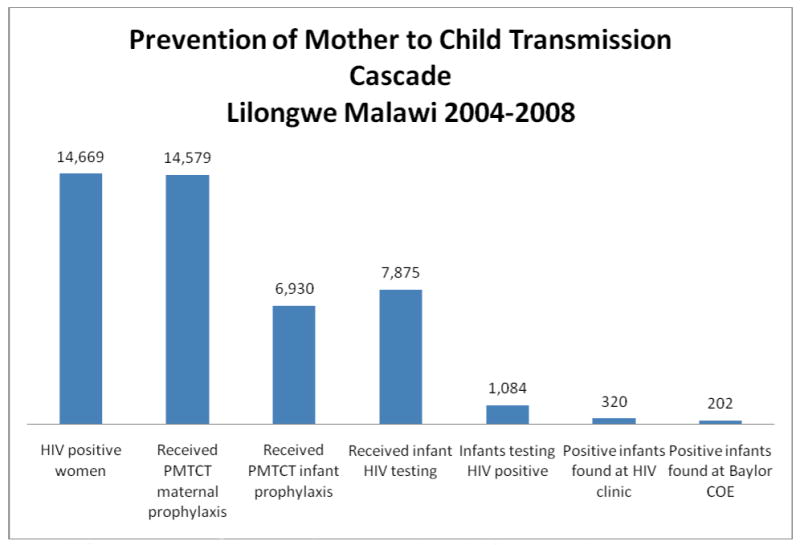
Cascade of PMTCT and Pediatric HIV services in Lilongwe Malawi, 2004-2008.
PMTCT- Prevention of Mother to Child Transmission. COE. Centre of Excellence
Of the 1,084 HIV-infected infants, 320 (29.5%) were traced to an ART clinic and 764 confirmed infected children were not linked to care. Among children arriving at the Baylor COE, nearly 72% were identified as outpatients, with the remainder inpatients at the time of referral (Table 3). Infants referred from outpatient clinics were diagnosed and enrolled into care at significantly younger ages than infants referred while hospitalized. Overall, the median age at diagnosis was 3.0 months for all patients, 2.0 months for infants referred from outpatient facilities, and 10.2 months for inpatient referrals (Table 3). Outpatient referrals enrolled into ART clinics a median of 1.4 (IQR 0.8-2.5) months after diagnosis.
Table 3.
Characteristics of HIV-infected infants traced to the Baylor COE stratified by point of entry.
| All Infants (%) | Outpatients (%) | Inpatients (%) | P-Value | |
|---|---|---|---|---|
| Total clients traced,1 n/N (%) | 202 (100.0) | 145/202 (71.8) | 57/202 (28.2) | NS |
| Females, n/N (%) | 108/202 (53.0) | 80/145 (55.2) | 28/57 (49.1) | NS |
| WHO stage at clinic enrollment, n/N (%) | ||||
| 1 | 107/202 (53.0) | 90/145 (62.1) | 17/57 (29.8) | NS |
| 2 | 19/202 (9.4) | 11/145 (7.6) | 8/57 (14.0) | NS |
| 3 | 48/202 (23.8) | 29/145 (20.0) | 19/57 (33.3) | NS |
| 4 | 13/202 (6.4) | 7/145 (4.8) | 6/57 (10.5) | NS |
| Not available | 15/202 (7.4) | 8/145 (5.5) | 7/57 (12.3) | NS |
| ART initiation, n/N (%) | 110/202 (54.5) | 83/145 (57.2) | 27/57 (47.4) | NS |
| Age < 12 months at ART initiation, n/N (%) | 77/110 (70.0) | 65/83 (78.3) | 12/27 (44.4) | NS |
| Outcome, n/N (%) | ||||
| Alive | 120/202 (59.4) | 88/145 (60.7) | 32/57 (56.1) | NS |
| Death | 43/202 (21.3) | 27/145 (18.6) | 16/57 (38.6) | NS |
| Loss to follow-up | 26/202 (12.9) | 20/145 (13.8) | 6/57 (10.5) | |
| Transferred out | 13/202 (6.4) | 10/145 (6.9) | 3/57 (5.3) | NS |
| Age at diagnosis (mos, median (IQR)) | 3.0 (0.5-8.6) | 2.0 (0.2-3.9) | 10.2 (6.5-15.7) | <0.001 |
| Age at enrollment (mos, median (IQR)) | 5.5 (2.7 – 10.0) | 4.2 (2.3-7.7) | 10.0 (6.5-15.7) | <0.001 |
| ART initiators | All (N=110) | Outpatient (N=83) | Inpatient (N=27) | P-Value |
|---|---|---|---|---|
| Age at ART initiation (mos, median (IQR)) | 9.1 (5.4-13.8) | 8.3 (5.0-11.5) | 12.7 (9.8-16.6) | 0.009 |
| Time from enrollment to ART initiation (mos, median (IQR)) | 2.5 (1.4 – 5.2) | 2.5 (1.2 – 5.6) | 2.5 (1.5 – 5.2) | NS |
| Time on ART (mos, median (IQR)) | 7.4 (3.8 – 13.1) | 7.8 (4.5 – 15.2) | 5.4 (3.1 – 9.6) | 0.0204 |
ART, antiretroviral therapy; mos, months; IQR, interquartile range; SD, standard deviation; NS, not significant, COE, Center of Excellence
Nineteen traced infants were excluded from outcomes analysis due to incomplete documentation.
As of December 2008, only about half of the 221 infants who we successfully traced to the Baylor COE had initiated ART, a median of 2.5 months after clinic registration (Table 3). The median duration on ART was 7.4 months. Outpatient referrals began treatment at a younger age compared to inpatient referrals; 8.3 versus 12.7 months. While we observed a reduction in the delay to ART initiation each year, the proportion of infants starting ART did not increase (Table 4). ART initiators were not significantly younger than non-initiators at diagnosis (5.1 versus 6.9 months, p= 0.062), however they were younger at enrollment (6.4 versus 8.7 months, p= 0.010).
Table 4. Changes in Time to ART initiation among HIV-infected infants at Baylor COE by year.
| Year | % of ANC attendees tested for HIV | Infants enrolled | Infants initiated on ART (% of total enrolled) | Mean (SD) time from enrollment to ART initiation (months) |
|---|---|---|---|---|
| 2005 | 93% | 9 | 5 (55.6) | 10.0 (11.6) |
| 2006 | 99% | 23 | 15 (65.2) | 7.3 (6.2) |
| 2007 | 99.5% | 72 | 43 (59.7) | 4.2 (3.4) |
| 2008 | 99.8% | 98 | 47 (48.0) | 2.4 (1.6) |
ART, antiretroviral therapy; COE, Center of Excellence; SD, standard deviation
Sixty-nine HIV-infected infants in care at the COE died or were lost to follow up (34.2%) by December 2008, with slightly more than one-third of these events (34.8%) occurring within the first 3 months (Figure 1). Antiretroviral therapy increased the likelihood of survival seven-fold (odds ratio, 7.1; 95% confidence interval, 3.68 -13.70; p<0.0001). We did not find significant associations between survival and age at diagnosis, time from diagnosis to enrollment, time from enrollment to ART initiation, or advanced WHO stage at presentation. The findings were similar in our sensitivity analysis that censored for LTFU and Transfer Out (data not shown).
Discussion
Large numbers of HIV-exposed infants in Lilongwe, Malawi did not access HIV testing. Among HIV-infected infants, only 29.5% successfully enrolled into facilities providing pediatric HIV services, with mortality rates remaining high despite successful linkage to a care facility. Overall, this suggests PMTCT, EID, and pediatric ART services are not sufficiently integrated, resulting in high default rates, elevated levels of vertical HIV transmission, late infant HIV diagnosis, delayed pediatric ART initiation, and high HIV-infected infant mortality.
Other studies evaluating PMTCT programs have previously reported high attrition rates after initial maternal HIV diagnosis [21] [22] [23]. Our findings expand on this by highlighting key components of PMTCT care from the infant's perspective, as well as the high mortality rate of ART naïve, HIV-infected infants [24] [25]. It is important to note that our results likely under-estimate infant mortality as we reported only the outcome of patients who enrolled in HIV care facilities.
We only traced 320 HIV-infected infants to a pediatric ART clinic, with a majority found at the Baylor COE, likely due to the gradual uptake of pediatric ART services at health centers during this time period. The high attrition can be partly attributed to the absence of one facility providing the full spectrum of maternal and infant HIV services on a daily basis, along with the relative cost of transportation for a mostly impoverished patient population. HIV-infected mothers may receive ANC and maternity at one location yet pediatric services at Baylor COE were on a different campus. Prior studies have demonstrated the cost of transport as a barrier to the retention of HIV-infected patients in care, and it is likely that it similarly impedes infant HIV test result follow-up and subsequent program attrition, particularly when care for the mother and infant are not at the same facility [26][27][8]. The lack of consistent patient identification, coupled with the disjointed, multi-facility provision of HIV services detrimentally affects retention. Suboptimal referral systems prevented tracing across the facilities and determining drop-out points. For example, verbal referral systems prevented determining if patients failed to collect PCR results or failed to report to clinic after being provided with results.
The identified patients are likely representative of HIV-infected Malawian infants that enter care in Lilongwe, as this study was generated from operational data from all sites implementing service during this time period. We acknowledge that some additional infants may have been tested or arrived to care and were not captured due incompatible patient identification numbers and suboptimal referral documentation. Missing infant test records, lost results, and illegible referral forms challenged data collection efforts, overall program monitoring, and patient and healthcare provider navigation of the maternal-child healthcare system. Additionally, our data sources were not robust enough to identify women who may have had HIV sero-conversion after their first ANC visit. Our findings may not be representative of areas with lower HIV prevalence such as more rural locations or those with less partner support. The additional activities support by the BAN study and the Baylor community outreach programs likely increased the number of children accessing services.
At the time of our evaluation, EID was only available in 7 sites nationally. Currently, EID has expanded to 14 of 28 districts covering 24% of PMTCT sites. However, given there are only 3 PCR labs which still rely on manual techniques, the results of dried blood spot DNA PCR testing are only available after one month. With expansion of the program, delays of four months have been observed (HIV unit, Ministry of Health). We observed inefficiencies in the EID system that affected test result availability, notably the unreliable transportation of test results from the central laboratory to the clinical facility. Point of care infant HIV testing, including rapid DNA PCR assays, bed-side p24 antigen testing, and quantitative reverse transcriptase activity analyses [27] [28][29][30] could dramatically improve EID systems by facilitating the immediate referral of HIV-infected infants, eliminating the need for a separate visit to receive results. Alternatively, the novel use of cell phones to transmit results from central labs to external clinics may improve communication of lab results in Malawi.[31].
While ART improved infant survival, we noted delays in ART initiation by several months after enrollment. The delay in ART initiation was influenced by the treatment guidelines of the period, which recommended ART initiation for all HIV-infected infants based upon clinical and/or immunologic criteria [19]. The WHO and MOH modified their recommendations for ART eligibility to include all HIV-infected infants younger than 12 months in mid-2008 after data demonstrated efficacy of this strategy [20] [32]. This policy change likely reduced the time to ART initiation that we observed in our cohort. Importantly, our findings of high mortality in patients not initiating ART further support these guidelines.
Delays in ART initiation may also stem from Baylor affiliated HIV clinics pre-ART education requirements. While the Malawi ART program requires only a single pre-ART education, one guardian and an understanding of the implications of ART [19], the Baylor COE requires two pre-ART education sessions for caregivers and identification of a second caregiver prior to initiating infant ART. While ART adherence education is a critical component of successful pediatric ART treatment [33], it must be balanced with the importance of timely ART initiation.
While the integration of PMTCT, EID, and pediatric HIV care programs has been a common challenge throughout resource limited settings [34][17], our findings suggest that the following five recommendations may improve PMTCT and pediatric HIV services.
Recommendation 1
ART initiation in infants should be treated as medically urgent, given the rapid disease progression and high mortality observed within this age group in the absence of ART. Following the WHO guidelines, all HIV-infected infants under 12 months of age should begin ART as soon as is feasible, with referral and care systems taking the necessary steps to accommodate this urgency. Combining HIV education and ART adherence training into enrollment visits or during hospitalization may allow ART initiation to occur earlier with well-adjusted and informed guardians.
Recommendation 2
In lieu of restructuring the delivery of maternal and infant HIV services into one facility, we recommend that a universal patient identification system be nationally implemented to allow tracing of mother-infant pairs between PMTCT, EID, and pediatric HIV facilities. Combined with standardized documentation within health passports, a universal identification system would facilitate communication between PMTCT, EID, and pediatric HIV sites and could help confirm arrival of referred patients. Moreover, such a system would benefit tuberculosis control, immunization, and other national health programs. Novel tracing strategies may ensure that mother-infant pairs are receiving the full continuum of HIV services. One example, is the Baylor Tingathe outreach program which uses community health workers to track mother infant pairs starting from the mother's diagnosis at antenatal care until there is either a definitive negative diagnosis for the infant or positive diagnosis and confirmed entry into clinical care with ART initiation. Preliminary results from this program have demonstrated improved utilization of services and a dramatic decrease in loss-to-follow-up of mothers and infants. [35, 36].
Recommendation 3
We recommend routine provider initiated infant HIV antibody testing at all immunization visits, starting at the sixth week of life in settings where HIV exposure documentation does not exist or tracking mother-infant pairs is not feasible. While high proportions of Malawian women deliver outside of formal healthcare facilities, most infants routinely access immunization clinics [33]. Using a strategy of HIV antibody screening of all infants attending the six-week immunization visit, followed by DNA PCR testing of those with positive HIV antibody test results, has been demonstrated to be high yield, feasible, and acceptable in South Africa [37,38]. The expansion of this strategy would be an effective utilization of an existing, well functioning healthcare service to provide early post-natal identification of HIV-exposed infants.
Recommendation 4
Routine inpatient pediatric HIV testing provides another critical avenue for the identification of HIV-exposed and HIV-infected children who may have been missed in the early post-natal period, or who acquired HIV later in infancy via breastfeeding. This strategy has been demonstrated as a highly productive, viable, and acceptable approach in Malawi [16], Zambia [39], and Uganda [40]. Furthermore, this is an effective strategy to diagnose previously unidentified HIV-infected mothers as well as re-identify defaulted HIV-exposed and HIV-infected children [16].
Recommendation 5
Infant HIV DNA PCR test results should be reported in two weeks or less if ART initiation hinges on the result in order to prevent excessive infant mortality. Where DNA PCR testing is not yet available or results are severely delayed, we recommend utilizing a clinical diagnostic algorithm to determine ART eligibility in HIV antibody positive infants. Development of a valid, rapid point of care assay for infant HIV infection remains a high priority for PMTCT and pediatric treatment programs.
While these recommendations should be interpreted within the context of national PMTCT, EID, and pediatric HIV treatment programs, as well as in light of current PMTCT regimens [42], they can likely be generalized given the HIV epidemiologic and program similarities between sub-Saharan African countries. The implementation of these recommendations would therefore represent a significant advancement for pediatric HIV prevention and care programs throughout generalized HIV-epidemic, resource-limited settings.
Conclusion
This study demonstrated a high attrition rate of HIV-exposed and HIV–infected infants between maternal and infant HIV care services, elevated levels of mother-to-child transmission, delayed diagnosis and late ART initiation in HIV-infected infants, and high subsequent mortality rates in HIV-infected, ART naïve infants enrolled into pediatric HIV care in urban Malawi. Our findings suggest that establishing integrated PMTCT, EID, and pediatric HIV care services along with implementing strategies to improve patient retention and access to earlier infant testing and ART initiation would greatly improve outcomes for HIV-infected infants in generalized epidemic, resource-constrained settings.
Figure 2.
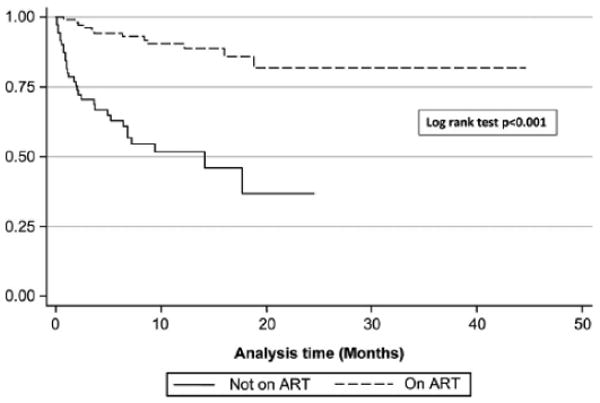
Kaplan Meier Survival Analysis of 202 children attending the Baylor Center of Excellence in Lilongwe Malawi. ART, antiretroviral therapy.
Acknowledgments
This study was supported by the Doris Duke Charitable Foundation Clinical Research Fellow Program, the Fogarty International Center of the National Institutes of Health (D43 TW01036), the University of North Carolina Center for AIDS research (#P30 AI50410), the Centers for Disease Control and Prevention (1U48DP001944), The Elizabeth Glaser Pediatric AIDS Foundation and the National Institutes of Health (R24 TW007988) through the Fogarty International Center and International Clinical Research Fellows Program at Vanderbilt University.
Footnotes
Previously presented in abstract form at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention 2009, Cape Town, South Africa, Abstracts WEPED218 and WEPDD103.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNICEF . Children and AIDS. Third Stocktaking Report 2008. New York, USA: UNICEF; 2008. [Google Scholar]
- 2.UNICEF . Towards universal access: scaling up priority HIV services for women and children in the health sector- progress report 2008. New York, USA: UNICEF; 2008. United Nations Children's Fund, Joint United Nations Programme on HIV/AIDS and the World Health Organization. [Google Scholar]
- 3.UNICEF . AIDS Epidemic Update 2007. Geneva: UNAIDS; 2007. [Google Scholar]
- 4.Chilongozi D, Wang L, Brown L, Taha T, Valentine M, Emel L, et al. Morbidity and mortality among a cohort of human immunodeficiency virus type 1-infected and uninfected pregnant women and their infants from Malawi, Zambia, and Tanzania. Pediatr Infect Dis J. 2008;27:808–14. doi: 10.1097/INF.0b013e31817109a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Central Intelligence Agency . The World Factbook 2009. Washington, DC: Central Intelligence Agency; 2009. [Google Scholar]
- 6.Malawi Ministry of Health . National AIDS Commission: 2006 report on HIV/AIDS in Malawi. Lilongwe, Malawi: Ministry of Health and Population, Malawi; 2006. [Google Scholar]
- 7.Moses A, Zimba C, Kamanga E, Nkhoma J, Maida A, Martinson F, et al. Prevention of mother-to-child transmission: program changes and the effect on uptake of the HIVNET 012 regimen in Malawi. AIDS. 2008;22:83–87. doi: 10.1097/QAD.0b013e3282f163b5. [DOI] [PubMed] [Google Scholar]
- 8.Manzi M, Zachariah R, Teck R, Buhendwa L, Kazima J, Bakali E, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Trop Med Int Health. 2005;10:1242–50. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 9.Malawi Ministry of Health. Department of HIV and AIDS . In: Quarterly report of the Antiretroviral Treatment Programme in Malawi with results up to March 2010. Department of HIV and AIDS MoH, editor. Lilongwe: 2010. [Google Scholar]
- 10.Lowrance D, Filler S, Makombe S, Harries A, Aberle-Grasse J, Hochgesang M, et al. Assessment of a national monitoring and evaluation system for rapid expansion of antiretroviral treatment in Malawi. Trop Med Int Health. 2007;12:377–81. doi: 10.1111/j.1365-3156.2006.01800.x. [DOI] [PubMed] [Google Scholar]
- 11.Makombe S, Libamba E, Mhango E, de Ascurra Teck O, Aberle-Grasse J, Hochgesang M, et al. Who is accessing antiretroviral therapy during national scale-up in Malawi? Trans R Soc Trop Med Hyg. 2006;100:975–9. doi: 10.1016/j.trstmh.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Malawi Ministry of Health . ART Public and Private Sector 2007 Q4 Report. Lilongwe, Malawi: Ministry of Health and Population, Malawi; 2007. [Google Scholar]
- 13.Malawi Ministry of Health . HIV Unit Annual Report 2008. Lilongwe, Malawi: Ministry of Health and Population HIV Unit, Malawi; 2008. [Google Scholar]
- 14.Spensley A, Sripipatana T, Turner AN, Hoblitzelle C, Robinson J, Wilfert C, et al. Preventing mother-to-child transmission of HIV in resource-limited settings: the Elizabeth Glaser Pediatric AIDS Foundation experience. Am J Public Health. 99:631–637. doi: 10.2105/AJPH.2007.114421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies MA, Keiser O, Technau K, Eley B, Rabie H, van Cutsem G, et al. Outcomes of the South African National Antiretroviral Treatment Programme for children: the IeDEA Southern Africa collaboration. S Afr Med J. 2009;99:730–737. [PMC free article] [PubMed] [Google Scholar]
- 16.McCollum ED, Preidis GA, Kabue MM, Singogo EB, Mwansambo C, Kazembe PN, et al. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PLoS ONE. 2010;5:e9626. doi: 10.1371/journal.pone.0009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherutich P, Inwani I, Nduati R, Mbori-Ngacha D. Optimizing paediatric HIV care in Kenya: challenges in early infant diagnosis. Bull World Health Organ. 2008;86:155–60. doi: 10.2471/BLT.07.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33. doi: 10.1016/j.cct.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malawi Ministry of Health . Treatment of AIDS: Guidelines for the use of antiretroviral therapy in Malawi. 2nd. Lilongwe, Malawi: Ministry of Health and Population HIV Unit, Malawi; 2006. [Google Scholar]
- 20.Malawi Ministry of Health . Treatment of AIDS: Guidelines for the use of antiretroviral therapy in Malawi. 3rd. Lilongwe, Malawi: Ministry of Health and Population HIV Unit, Malawi; 2008. [Google Scholar]
- 21.Sherman GG, Jones SA, Coovadia AH, Urban MF, Bolton KD. PMTCT from research to reality: results from a routine service. S Afr Med J. 2004;94:289–292. [PubMed] [Google Scholar]
- 22.Ginsburg AS, Miller A, Wilfert CM. Diagnosis of Pediatric Human Immunodeficiency Virus Infection in Resource-Constrained Settings. Pediatr Infect Dis J. 2006;25:1057–1064. doi: 10.1097/01.inf.0000243157.16405.f0. [DOI] [PubMed] [Google Scholar]
- 23.Stringer EM, Ekouevi DK, Coetzee D, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304:293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 24.Dunn D, HIV Paediatric Prognostic Markers Collaborative Study Group Short-term risk of disease progression in HIV-1-infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta-analysis. Lancet. 2003;362:1605–1611. doi: 10.1016/s0140-6736(03)14793-9. [DOI] [PubMed] [Google Scholar]
- 25.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zachariah R, Harries AD, Manzi M, Gomani P, Teck R, Phillips M, et al. Acceptance of anti-retroviral therapy among patients infected with HIV and tuberculosis in rural Malawi is low and associated with cost of transport. PLoS ONE. 2006;1:e121. doi: 10.1371/journal.pone.0000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jangam SR, Yamada DH, McFall SM, Kelso DM. Rapid, point of care extraction of human immunodeficiency virus type 1 proviral DNA from whole blood for detection by real-time PCR. J Clin Microbiol. 2009 Aug;47:2363–8. doi: 10.1128/JCM.r00092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knuchel MC, Jullu B, Shah C, Tomasik Z, Stoeckle MP, Speck RF, et al. Adaptation of the ultrasensitive HIV-1 p24 antigen assay to dried blood spot testing. J Acquir Immune Defic Syndr. 2007;44:247–53. doi: 10.1097/QAI.0b013e31802c3e67. [DOI] [PubMed] [Google Scholar]
- 29.Braun J, Plantier JC, Hellot MF, Tuaillon E, Gueudin M, Damond F, et al. A new quantitative HIV load assay based on plasma virion reverse transcriptase activity for the different types, groups and subtypes. AIDS. 2003;17:331–336. doi: 10.1097/00002030-200302140-00006. [DOI] [PubMed] [Google Scholar]
- 30.Ou CY, Yang H, Balinandi S, Sawadogo S, Shanmugam V, Tih PM, et al. Identification of HIV-1 infected infants and young children using real-time RT PCR and dried blood spots from Uganda and Cameroon. J Virol Methods. 2007;144:109–114. doi: 10.1016/j.jviromet.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Nadim Mahmud, Joce Rodriguez, Nesbit Josh. Mobiles in Malawi: A text message-based intervention to bridge the patient-physician gap in the rural developing world. The American Medical student Association's International Health Journal. 2010;6(1) Available at: http://www.globalpulsejournal.com.
- 32.Luzuriaga K, McManus M, Mofenson L, Britto P, Graham B, Sullivan JL, et al. A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med. 2004;350:2471–2480. doi: 10.1056/NEJMoa032706. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson O, Mellins C, Dolezal C, Brackis-Cott E, Abrams EJ. HIV treatment-related knowledge and self-efficacy among caregivers of HIV-infected children. Patient Educ Couns. 2006;61:405–10. doi: 10.1016/j.pec.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 34.KIDS-ART-LINC Collaboration Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–31. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed S, Kim Maria, Kanjelo Kondwani, Nanthuru Debora, Taimu Thomas, Ndovie Lughano, Mwasabi Innocent, Msiska Damson, Bowa Elson, Kazembe Peter, Kline Mark. Using Community Health Workers to Improve Identification, Enrollment Into Care, and Outcomes for HIV-Exposed Infants at the Kawale Health Centre in Lilongwe, Malawi. AIDS 2010 Conference. Abstract WEPE0842. [Google Scholar]
- 36.Ahmed S, Kim M, Kanjelo K, Taimu T, Kabue M, Kazembe P, Kline M. Using Community Health Workers to Improve Identification and Early Referral to Care of HIV-Infected Children. International AIDS Society Conference, Cape Town 2009. Abstract TUPED090. [Google Scholar]
- 37.UNICEF . UNICEF Country Profile: Malawi. New York, USA: UNICEF; 2006. [Google Scholar]
- 38.Rollins N, Little K, Mzolo S, Horwood C, Newell ML. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: The case for universal screening. AIDS. 2007;21:1341–1347. doi: 10.1097/QAD.0b013e32814db7d4. [DOI] [PubMed] [Google Scholar]
- 39.Rollins N, Mzolo S, Moodley T, Esterhuizen T, van Rooyen H. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–1857. doi: 10.1097/QAD.0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]
- 40.Kankasa C, Carter RJ, Briggs N, Bulterys M, Chama E, Cooper ER, et al. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51:202–208. doi: 10.1097/qai.0b013e31819c173f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wanyenze RK, Nawavvu C, Ouma J, Namale A, Colebunders R, Kamya MR. Provider-initiated HIV testing for paediatric inpatients and their caretakers is feasible and acceptable. Trop Med Int Health. 2010;15:113–119. doi: 10.1111/j.1365-3156.2009.02417.x. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization . Rapid Advice: Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva: WHO; 2009. [PubMed] [Google Scholar]


