Abstract
HIV-1-associated neurocognitive disorders (HAND), associated with infection and activation of mononuclear phagocytes (MP) in the brain, occur late in disease. Infected/activated MP initiate neuroinflammation activating glial cells and ultimately disrupting neuronal function. Astrocytes secrete tissue inhibitor of metalloproteinase (TIMP)-1 in response to neural injury. Altered TIMP-1 levels are implicated in several CNS diseases. CCAAT enhancer binding protein β (C/EBP), a transcription factor, is detected in rodent brains in response to neuroinflammation, implicating it in Alzheimer’s, Parkinson’s and HIV-1-associated neurocognitive disorders (HAND). Here, we report that C/EBPβ mRNA levels are elevated and its isoforms differentially expressed in total brain tissue lysates of HIV-1-infected and HIV-1 encephalitis patients. In vitro, HAND-relevant stimuli synergistically induce C/EBPβ nuclear expression in human astrocytes through 7 days of stimulations. Overexpression of C/EBPβ increases TIMP-1 promoter activity, mRNA and protein levels in human astrocytes activated with IL-1β. Knockdown of C/EBPβ with siRNA decreases TIMP-1 mRNA and protein levels. These data suggest C/EBPβ isoforms are involved in complex regulation of astrocyte TIMP-1 production during HIV-1 infection; however, further studies are required to completely understand their role during disease progression.
Keywords: HIV-1-associated dementia, TIMP-1, C/EBPβ, Astrocyte, Neuroinflammation
Introduction
Human immunodeficiency virus (HIV)-1 infects approximately 33 million people worldwide and 40-70% of these have associated complications in the CNS. The most severe form of HIV-1-associated neurocognitive disorder (HAND) is HIV-1-associated dementia (HAD); dementia associated with reactive astrogliosis and neuronal dysfunction/death (Lindl et al. 2010). While the advent of antiretroviral therapy (ART) has transformed HIV-1 infection into a manageable, chronic condition and lowered the incidence of HAD, the prevalence of HAD has increased due to the extended life span of HIV-1 infected individuals (Lindl et al. 2010). Here, we investigate the expression of CCAAT enhancer binding protein (C/EBP) β during HIV-1 infection and its regulation of astrocyte tissue inhibitor of metalloproteinase (TIMP)-1 production.
Astrocytes, important homeostatic regulators in the brain, are activated during HAD and display an altered gene expression profile during neuroinflammation (Sofroniew 2005, Laird et al. 2008, Faulkner et al. 2004, Sofroniew & Vinters 2010, Heales et al. 2004, Yadav & Collman 2009). Although the cellular expression of TIMP-1 in the CNS varies with disease state (Rivera et al. 1997, Jaworski 2000, La Fleur et al. 1996, Bugno et al. 1999), astrocytes are major producers of this (Pagenstecher et al. 1998, Jaworski 2000, Suryadevara et al. 2003) physiological antagonist of matrix metalloproteinases (MMPs) that stimulates cellular proliferation and inhibits apoptosis (Ould-yahoui et al. 2009, Jourquin et al. 2005, Hornebeck 2003). TIMP-1 is a multifunctional molecule that may affect pathology in multiple ways besides MMP inhibition, and this places an impetus on understanding astrocyte TIMP-1 expression contributes to overall levels during HAD. TIMP-1 expression levels are dysregulated in HAD and other CNS pathologies (Gardner & Ghorpade 2003). Reduced TIMP-1 expression in the CSF and brain tissues of HAD patients was previously reported (Gardner & Ghorpade 2003, Suryadevara et al. 2003), but the mechanism causing this deficit is unknown. In vitro, astrocyte activation with the HIV-relevant stimulus, interleukin (IL)-1β, was shown to initially increase TIMP-1 expression, which decreased with long-term stimulation (Suryadevara et al. 2003). Although, the TIMP-1:MMP balance is implicated in several CNS pathologies (Yong et al. 1998) and the TIMP-1 knockout mice showed impaired learning and memory, the mechanism for this is unknown and is likely multifaceted (Chaillan et al. 2006, Hornebeck 2003, Lorenzl et al. 2003, Lorenzl et al. 2002, Lorenzl et al. 2008). TIMP-1 expression is well studied in several model systems, but the distinct mechanisms controlling short-term versus long-term regulation in astrocytes are incompletely understood. The 1.7 kb sequence upstream of exon 1 and part of intron 1 contain numerous regulatory elements including five CCAAT boxes (Phillips et al. 1999, Clark et al. 1997).
Several transcriptional regulators are known to be upregulated in astrocytes during neuroinflammation (Sofroniew & Vinters 2010, Brambilla et al. 2009, Brambilla et al. 2005, Herrmann et al. 2008, Abraham et al. 2006, Chen et al. 2008, Gris et al. 2007, Panenka et al. 2001). Recently, it was reported that the transcription factor C/EBPβ is expressed in rodent astrocytes in response to inflammatory stimuli (Ejarque-Ortiz et al. 2007, Albertini et al. 1998) and is involved in many cellular processes of the CNS (Sterneck & Johnson 1998, Cortes-Canteli et al. 2004, Menard et al. 2002, Nadeau et al. 2005, Cardinaux et al. 2000, Ejarque-Ortiz et al. 2007, Sandhir & Berman 2010, Alberini et al. 1994, Yukawa et al. 1998). Although, C/EBPβ is a prolific transcription factor that is expressed in microglia and neurons (Ejarque-Ortiz et al. 2007, Sterneck & Johnson 1998), it is unclear what proportion each of these cell types contributes to C/EBPβ expression in the brain. It is likely that C/EBPβ plays a distinct role in regulating the response of each cell type during neuroinflammation. However, astrocytes are well-accepted major producers of brain TIMP-1, thus, in this study, we focus on the role of C/EBPβ in astrocytes (Pagenstecher et al. 1998, Crocker et al. 2006). Alternative start site initiation results in a single C/EBPβ mRNA being translated into three isoforms; 42 kDa, 40 kDa and 20 kDa. The two larger isoforms have transcriptional activation properties, while the 20 kDa isoform is a transcriptional silencer (Sears & Sealy 1994). C/EBPβ functions by dimerizing with other factors to regulate transcription (Sears & Sealy 1994). In this study, we hypothesize that C/EBPβ is expressed in astrocytes during HAND and contributes to the initial increase in TIMP-1 production by astrocytes, and possibly to the following dysregulation of TIMP-1 that we reported from in vitro studies and clinical cases (Suryadevara et al. 2003).
Here, we show that C/EBPβ is differentially expressed in the brains of HIV-1-infected (HIV+) and HIV encephalitis (HIVE) brain specimens. We report that primary human astrocytes express C/EBPβ in response to HAND-relevant stimuli and the transcription factor contributes to the complex regulation of astrocyte TIMP-1. Overall, this work identifies another level of regulation for astrocyte TIMP-1 production by C/EBPβ in the human brain during HIV-infection. These findings may have broader implications in many other neuroinflammatory CNS pathologies.
Experimental Procedures
Preparation of human brain lysates
Brain lysates were prepared from specimens obtained from the NNTC, Center for Neurovirology and Neurodegenerative Disorders brain bank and Rapid Autopsy Program at the University of Nebraska Medical Center as previously described by Suryadevara et al. (Suryadevara et al. 2003). Protein concentration was determined by bicionconic acid method as suggested by the manufacturer (Pierce, Rockford, IL).
Isolation, cultivation, and activation of human astrocytes
Human astrocytes were isolated from first- and early second-trimester aborted specimens obtained from the Birth Defects Laboratory, University of Washington, Seattle; in full compliance with the ethical guidelines of the NIH, University of Washington and University of North Texas Health Science Center. Astrocytes were isolated from specimens as described by Gardner (Gardner et al. 2006). Astrocyte activation was achieved by stimulating with IL-1β, tumor necrosis factor (TNF)-α and/or HIV-1JR-FL for various time periods. All treatment conditions were derived empirically through testing a range of concentrations for maximal activation of astrocytes. Astrocytes were treated with HIVJR-FL at 6000 counts reverse transcriptase activity/ml/minute and IL-1β was used at 20 ng/ml. The concentrations used were in the range described in the current literature (Dhar et al. 2006, Gardner et al. 2006, Suryadevara et al. 2003, Liu et al. 1996). Furthermore, an in vivo correlate, mouse models utilizing adenovirus-driven IL-1β overexpression in the brain, achieved expression levels around 10 ng/mg total protein 7 days post-injection (Ferrari et al. 2006) or a mean of 41 ng in the whole striatum 8 days post-injection, (Ferrari et al. 2004) respectively. During prolonged activation, cells received a medium exchange every 4 days with the original treatment concentrations. De novo TIMP-1 synthesis was measured in activated astrocytes by blocking translation with 10 μg/ml cycloheximide (Sigma Chemicals, St. Louis, MO) for 2, 8, and 24 h. Data presented is representative of a minimum of three independent experiments with two or more independent donors.
RNA Isolation and RT2PCR
RNA from activated astrocytes was extracted (Qiagen, Alameda, CA) and reverse-transcribed into cDNA as per the manufacturer’s instructions (PE Applied Biosystems, Inc., Foster City, CA). TaqMan 5′ nuclease real time (RT2) PCR assays were performed using an ABI Prism 7500 sequence-detection system (PE Applied Biosystems, Inc.). The following TaqMan Gene Expression Assay primers were used: TIMP-1 (C/N: Hs99999139_m1), C/EBPβ (C/N: Hs00270923_s1) and glyceraldehyde phosphate dehydrogenase (GAPDH; C/N: 4310859). The reactions were carried out at 48°C for 30 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Samples were analyzed in triplicate. All experiments and analyses meet the minimum standard guidelines for fluorescence-based RT2PCR experiment. One-way ANOVA was used to analyze RT2PCR data.
Western Blot
Astrocytes were cultured as adherent monolayers in 75 cm2 flasks at a density of 8 × 106 cells per flask. The following day, cells were treated with IL-1β (20 ng/ml). Cells were lysed, and nuclear extracts were isolated at 72 and 168 h post IL-1β treatment using nuclear extraction reagent (Fisher, Waltham, MA, USA). Equal amounts of protein (30 μg/lane from astrocytes and 160 μg/lane from brain lysates) were resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and subsequently transferred to a nitrocellulose membrane using i-Blot (Invitrogen, Carlsbad, CA, USA). The membrane was incubated with anti-C/EBPβ (Santa Cruz Biotechnology, Santa Cruz, CA, USA; C/N: C-19 at a dilution of 1:200 for astrocyte lysates and 1:40 for brain lysates), and then in secondary antibody at a concentration of 1:5,000. β-actin was used as a loading control.
Measurement of TIMP-1 protein and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) levels
TIMP-1 levels in astrocyte supernatants were measured using a commercially available ELISA kit (R&D Systems, Minneapolis, MN). The MTT assay was performed at appropriate time points according to the method originally described by Manthrope (Manthrope et al. 1986). The ELISA determinations yielded quantities of protein in units of ng/ml, and were normalized to MTT values. Results were analyzed with GraphPad Prism 4.0 using one-way ANOVA with Newman Keul’s post-test for multiple comparisons.
TIMP-1 promoter constructs and C/EBPβ-expressing plasmids
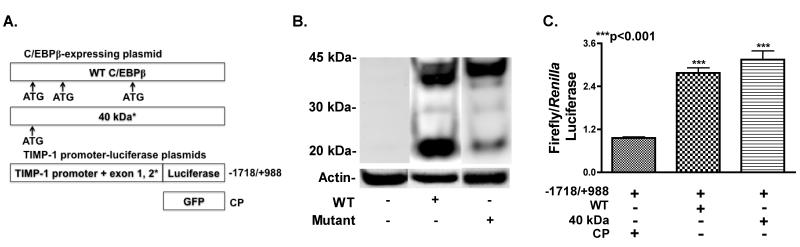
A pTIMP-1-luc construct was used to measure TIMP-1 promoter activity in transfected astrocytes. The −1718/+988 portion of the TIMP-1 sequence was cloned into the pGL3-Basic reporter vector (Promega, Madison, WI) and kindly provided by Dr. Ian Clark at the University of East Anglia, UK (Clark et al. 1997). The wild type (WT) C/EBPβ-expressing plasmid expresses the 42 kDa, 40 kDa and 20 kDa isoforms, while the mutated plasmid has a mutation in the 3′ translation start sites and predominantly expresses the 42 kDa isoform. Dr. Calkhoven kindly provided the C/EBPβ-expressing plasmids from Leibniz Institute for Age Research, Fritz Lipman Institute in Germany.
Transfection of primary human astrocytes with luciferase reporter constructs
Astrocytes were cultured as adherent monolayers in a 48-well plate at a density of 0.15 × 106 cells per well. The following day, cells were transfected with 1.5 μg of total DNA. Total DNA consisted of a mixture of the pTIMP-1-luc and the simian vacuolating virus promoter-driven Renilla luciferase (pRL-SV40, Promega) plasmids with or without C/EBPβ-expressing plasmids, or a control plasmid (CP). We used lipofectamine reagent to transfect the plasmid per the manufacturers instructions (Invitrogen, Carlsbad, CA). Untreated and mock-transfected controls were maintained for comparison. Mock controls were transfected without plasmid. At 2 days post-transfection promoter activity was measured as luciferase activity in cell extracts using the Dual-Glo Luciferase Assay System per the manufacturers instructions (Promega). Luciferase activity was determined as a ratio of firefly to Renilla luciferase using the Tecan Infinite Pro 200 luminometer (Tecan, Mannedorf, Switzerland). Renilla luciferase activity was thus used as an internal control. All data were analyzed with GraphPad Prism 4.0 in triplicate using one-way ANOVA with Newman Keul’s post-test for multiple comparisons unless otherwise specified. Data presented is representative of a minimum of three independent experiments with two or more independent donors.
Transfection of primary human astrocytes with siRNA
Astrocytes were resuspended in nucleofector transfection reagent (Lonza, Walkersville, MD) at a concentration of 80 × 106 cells/ml and transfected with short interfering (si) C/EBPβ (GGCCCUGAGUAAUCGCUUA - 100 nM) or siCON (Dharmacon, Lafayette, CO) as per the manufacturer’s instructions. Cells were then cultured in 25 cm2 flasks and allowed to recover for 24 h prior to treatment with IL-1β (20 ng/ml).
Statistical analyses
Statistical analyses were carried out using GraphPad Prism 4.0 software, with one-way analysis of variance (ANOVA) and Newman-Keuls post-test for multiple comparisons. Significance was set at p<0.05 and data represents means +/− standard error of the mean (S.E.M.). Data presented is representative of a minimum of three independent experiments with two or more independent donors.
Results
C/EBPβ and TIMP-1 expression in HIV-1-infected individuals
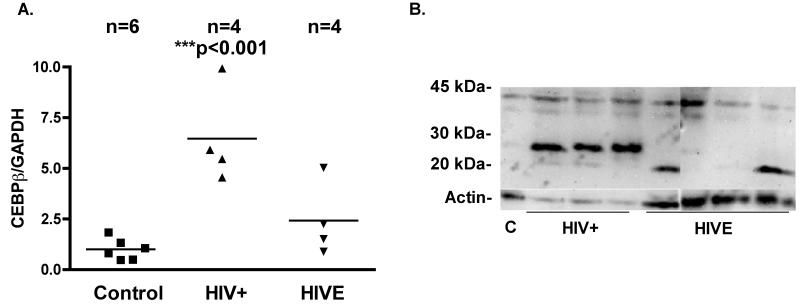
C/EBPβ is a prolific transcription factor that regulates gene expression during inflammation (Sterneck & Johnson 1998, Cortes-Canteli et al. 2004, Menard et al. 2002, Nadeau et al. 2005, Cardinaux et al. 2000, Ejarque-Ortiz et al. 2007, Sandhir & Berman 2010, Alberini et al. 1994, Yukawa et al. 1998). To determine C/EBPβ expression patterns in the brain of HIV-1-infected patients, we performed RT2PCR (n = 14) and western blotting (n = 8) using total brain tissue lysates from the frontal cortex of control, HIV+ and HIVE patients. The control samples used for RT2PCR were made up of four females and two males (37 - 52); of the HIV-infected patients, seven were male and one was female, (25 - 55) and all had progressed to AIDS. Of the AIDS patients, four showed signs of encephalitis and the remaining four showed no signs of CNS pathology. There were no detectable signs of opportunistic infection in the CNS of any patients studied. C/EBPβ mRNA levels were significantly higher in HIV+ patients compared to HIVE and control patients (Fig. 1 A). Low levels C/EBPβ mRNA and protein were detected in the brain tissues from the control patients. All HIV-1-infected patients showed expression of at least one isoform of C/EBPβ. C/EBPβ was detected in the brain lysates of three HIV+ patients, in which we detected a 42 kDa isoform and an unreported band around 25 kDa (Fig. 1 B). The 20 kDa isoform was detected in two brain lysates from HIVE patients. Overall, this work shows that C/EBPβ expression is markedly increased in the brains of these HIV-1-infected individuals compared to uninfected controls, C/EBPβ mRNA and protein levels are lower in the brain tissues of HIVE compared to HIV+ patients. Furthermore, the expression pattern of C/EBPβ isoforms varies between HIV+ and HIVE patients. A 42 kDa isoform and an unreported band at 25 kDa were detected in HIV+ tissues, while a 20 kDa isoform was detected in HIVE patients.
Fig. 1. C/EBPβ mRNA levels are elevated in the brain tissues from HIV+ patients and correlate with TIMP-1 mRNA.
C/EBPβ 42 kDa and 20 kDa isoforms are detectable in brain lysates of HIV+ and HIVE patients. Total RNA isolated from brains of control, HIV+ and HIVE patients was reverse transcribed to cDNA and subjected to RT2PCR for C/EBPβ and TIMP-1. A. C/EBPβ mRNA levels were significantly (***p<0.001) higher in samples from HIV+ patients compared to control or HIVE patients. B. Low levels of the 42 kDa isoform were detected in lysates from control patients whereas increased 42 kDa, a light band at 20 kDa and a strong band at 25 kDa was detected in all samples from HIV+ patients.
HIV-relevant stimuli induce C/EBPβ expression in human astrocytes
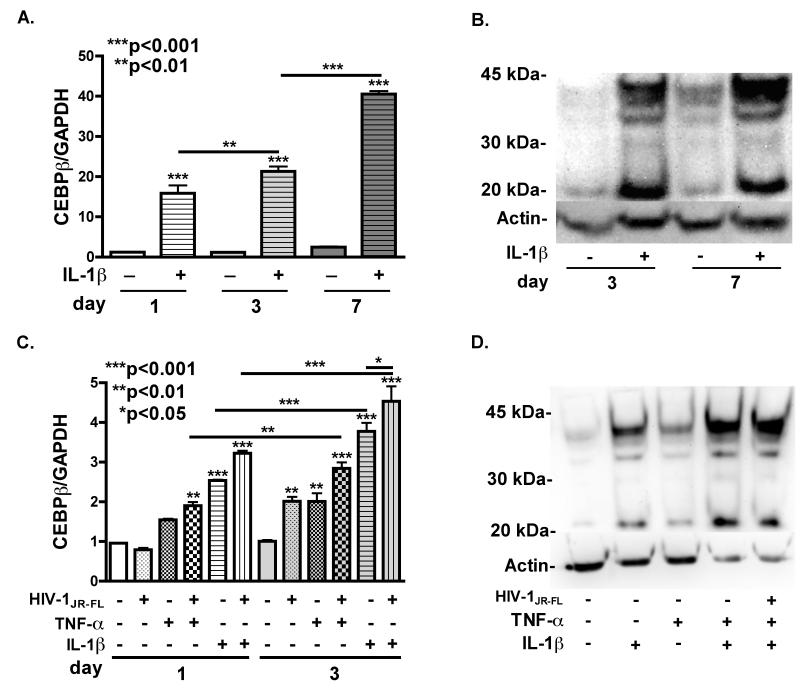
Cultured human astrocytes were used to determine if HIV-relevant stimuli induce C/EBPβ expression. We treated astrocytes with IL-1β (20 ng/ml) for day 1, 3 and 7, and then collected total RNA and protein (Fig. 2 A). C/EBPβ mRNA was significantly (p<0.001) increased in IL-1β-treated astrocytes compared to untreated controls at all time points. C/EBPβ mRNA was increased 1.4-fold in IL-1β-treated astrocytes at day 3 (p<0.01) and increased 2.5-fold further at day 7 (***p<0.001), (Fig. 2 A). Normalized levels of C/EBPβ mRNA in control astrocytes were not significantly different at any time-point. We performed immunoblotting for C/EBPβ in day 3 and day 7 nuclear extracts from IL-1β-treated astrocytes as described in the experimental procedures (Fig. 2 B). C/EBPβ levels were increased in IL-1β-treated astrocytes compared to untreated controls at day 3 and day 7. To determine if HAND-relevant stimuli, other than IL-1β are capable of inducing C/EBPβ expression, we treated astrocytes with HIVJR-FL (6000 counts RT activity/ml/minute), IL-1β (20 ng/ml) and TNF-α (20 ng/ml), alone, or in combination for 1 and 3 days. IL-1β and TNF-α both induced increases in C/EBPβ mRNA compared to control, and led to a further increase through day 3 of stimulation. Treatment with HIVJR-FL enhanced the IL-1β- and TNF-α-mediated increase in C/EBPβ mRNA. Treatment with TNF-α or HIVJR-FL alone had no significant effect on C/EBPβ mRNA levels at day 1, but resulted in a 2-fold (p<0.01) increase in at day 3 compared to untreated controls. HIVJR-FL combined with TNF-α further enhanced the induction of C/EBPβ mRNA to 3-fold, compared to untreated controls at day 3. Treatment with IL-1β significantly (p<0.001) increased C/EBPβ mRNA levels at day 1 and day 3 compared to untreated controls. We isolated protein from HIV-relevant stimuli-treated astrocytes two days post-treatment, and performed western blot for C/EBPβ (Fig. 2 D). Protein levels were markedly increased in IL-1β-, TNF-α-, IL-1β + TNF-α- and HIVJR-FL + IL-1β + TNF-α-treated astrocytes compared to untreated controls. Treatment with TNF-α alone had less effect than IL-1β alone, and combinations IL-1β + TNF-α and HIVJR-FL + IL-1β + TNF-α resulted in the most robust increase in C/EBPβ protein expression. Taken together, both C/EBPβ mRNA and protein levels are robustly increased in response to HIV-relevant stimuli.
Fig. 2. HAND-relevant stimuli induce C/EBPβ expression induced in human astrocytes.
A. C/EBPβ transcripts were measured in mRNA isolated from primary human astrocytes treated with IL-1β (20 ng/ml) for 1, 3 or 7 days. C/EBPβ mRNA expression was significantly increased compared to untreated at 1, 3 and 7 days (***p<0.001). C/EBPβ mRNA continued to increase, as levels at 3 days were significantly higher than at 1 (**p<0.01) and levels at 7 days were significantly higher than at 3 (***p<0.001). B. C/EBPβ protein levels were assayed by immunoblotting. C/EBPβ protein levels were increased compared to untreated controls at 3 and 7 days. C. C/EBPβ transcripts were measured in total RNA isolated from primary human astrocytes treated with IL-1β (20 ng/ml), TNF-α (20 ng/ml) and/or HIVJR-FL (6000 counts RT activity/ml/minute) for 1 or 3 days. C/EBPβ mRNA expression was significantly increased compared to untreated control at 1 and 3 days in IL-1β– and TNF-α- treated astrocytes (***p<0.001). D. Primary human astrocytes were treated with IL-1β (20 ng/ml), TNF-α (20 ng/ml) and or HIVJR-FL (6000 counts RT activity/ml/minute) for 2 days and then cell lysate was immunoblotted for C/EBPβ. C/EBPβ protein levels were increased compared to untreated controls in all samples. Data represents means +/− standard error of the mean (S.E.M.) in at least three independent experiments in at least two independent donors.
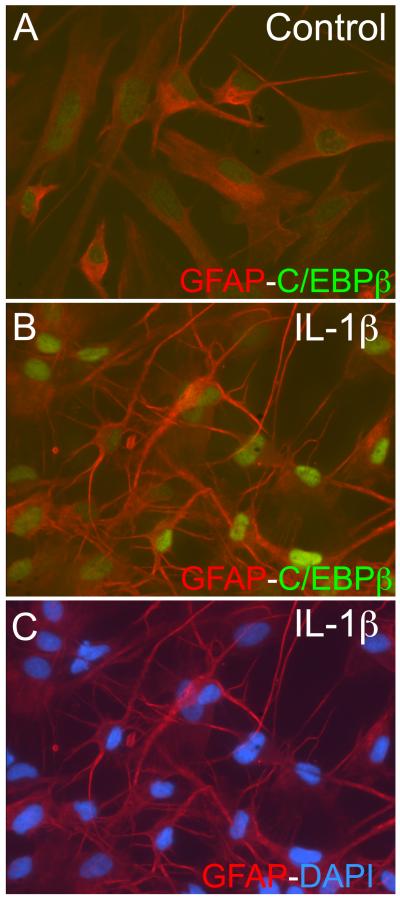
We treated astrocytes with IL-1β for 48 h, fixed and co-localized glial fibrillary acidic protein (GFAP) as an astrocyte-specific marker (red) with C/EBPβ (green) in activated human astrocytes (Fig. 3 A). In control cells, GFAP is present throughout the cell body of the astrocytes. Control astrocytes have a larger cell body, (Fig. 3 A) while activated astrocytes have extensive processes protruding from their soma and more dense staining of GFAP (Fig. 3 B). Low levels of C/EBPβ are present in the nuclei of control human astrocytes compared to activated astrocytes, where the green signal from the nuclei is markedly enhanced. Together with the mRNA and protein expression data represented earlier, this confirms that C/EBPβ expression is increased and localized to the nucleus of astrocytes in response to HAND-relevant stimuli.
Fig. 3. C/EBPβ localizes to the nucleus in response to IL-1β.
A. Untreated astrocytes were stained for GFAP (red) and C/EBPβ (green). Red signal is detected throughout the cytoplasm and green is localized to the nucleus. B. In IL-1β-treated astrocytes C/EBPβ (green) is localized to the nucleus and demonstrates greater intensity as compared to control astrocytes. C. DAPI staining in IL-1β-treated astrocytes. All pictures were taken at 200x and data represents at least three independent experiments in at least two independent donors.
TIMP-1 production de novo
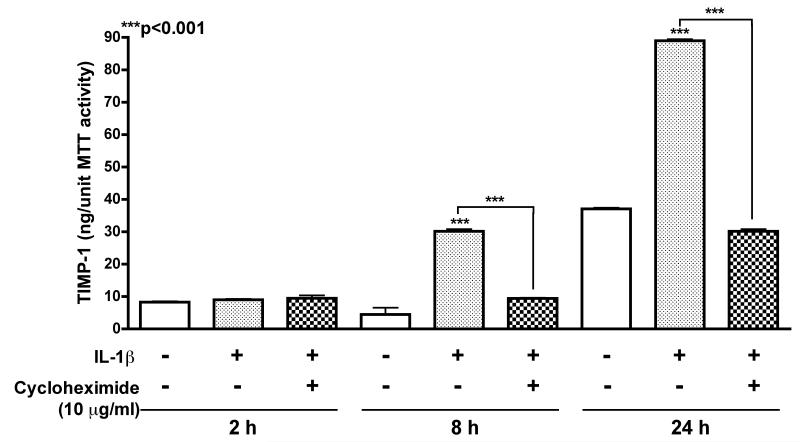
Prior to verifying the specific effects of C/EBPβ on TIMP-1 promoter regulation, we first evaluated whether TIMP-1 is synthesized de novo or instead released from intracellular stores upon activation. Human astrocytes were treated with translation inhibitor, cycloheximide at 10 μg/ml in conjunction with 20 ng/ml IL-1β for 2, 8 or 24 h (Fig. 4). TIMP-1 levels normalized to MTT units demonstrated that cycloheximide significantly inhibited TIMP-1 production following 8 and 24 h of IL-1β activation (***p<0.001). These data show that protein synthesis is necessary for TIMP-1 upregulation in acutely activated astrocytes and that TIMP-1 is synthesized de novo in astrocytes. Hence regulation of mRNA transcription may serve as a promising target to increase expression.
Fig. 4. De novo synthesis of astrocyte-TIMP-1.
Cycloheximide at 10 μg/ml was applied to astrocytes in conjunction with 20 ng/ml IL-1β for 2, 8 or 24 h. TIMP-1 levels in astrocyte supernatants were measured by ELISA and normalized to MTT activity. Cycloheximide significantly inhibited TIMP-1 production following 8 and 24 h of IL-1β activation, (***p<0.001) compared to untreated controls. Data represents means +/− S.E.M. of TIMP-1 protein levels determined in duplicate, in at least three independent experiments and two independent donors.
C/EBPβ overexpression increases TIMP-1 promoter activity, mRNA and protein levels
We used a panel of plasmids that overexpress C/EBPβ isoforms, in combination with luciferase-expressing plasmids driven by the TIMP-1 promoter (Fig. 5 A). Two C/EBPβ overexpression plasmids were used. The WT with three translation start sites leading to the production of three isoforms of C/EBPβ (42 kDa, 40 kDa and 20 kDa), and the mutant plasmid in which the two 3′ translation start sites are mutated to allow for predominant expression of the 42 kDa isoform. The −1718/+988 region of the TIMP-1 promoter was used to drive expression of the firefly luciferase gene. The −1718/+988 region contains five CCAAT sites possibly involved in promoter activity (Clark et al. 1997). We transfected human astrocytes, by nucleofection and isolated nuclear extracts 48 h post recovery. We resolved nuclear extracts from primary human astrocytes transfected with WT (lane 1) and mutant (lane 2) plasmids, and performed western blot for C/EBPβ (Fig. 5 B). Bands for all three isoforms were detected in the astrocytes transfected with WT, whereas the mutant transfected astrocytes expressed predominantly the 42 kDa isoform. Next, we cotransfected astrocytes with pTIMP-1-luc (−1718/+988) and CP, WT or mutant plasmids (Fig. 5 C). Cotransfection with the WT mutant plasmid significantly increased activity from the −1718/+988 region of the TIMP-1 promoter 2.6- and 3.0-fold, respectively, compared to cotransfection with CP (***p<0.001). Overall, overexpression of C/EBPβ in primary human astrocytes significantly increases TIMP-1 promoter activity.
Fig. 5. Overexpression of C/EBPβ WT and mutant C/EBPβ increases TIMP-1 promoter activity.
A. Constructs used. B. Primary human astrocytes were transfected with WT or mutant by nucleofection or Lipofectamine. WT expresses the C/EBPβ mRNA that has 3 translation start sites; generating 2 large (42 kDa and 40 kDa) isoforms from the first two start sites and one smaller 20 kDa isoform from the most 3′ start site. The mutant has a mutation in the two 3′ translation start sites, and predominately generates the 42 kDa isoform. C. Cotransfection with WT or LAP* with pTIMP-1-luc (−1718/+988) significantly (**p<0.01 and ***p<0.001, respectively) increased activity. Data represent means +/− S.E.M. of luciferase activity in triplicate, in at least three independent experiments and at least two independent donors.
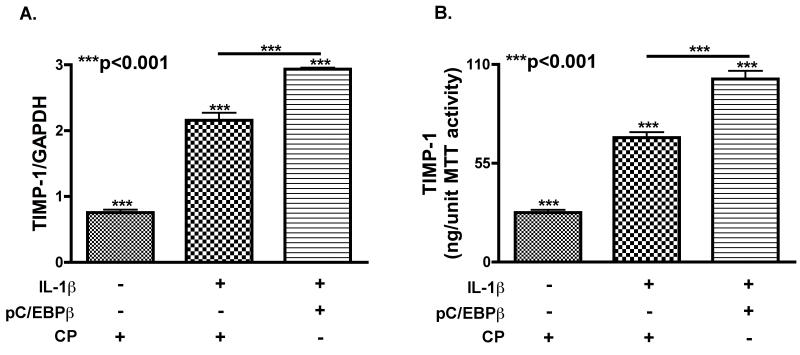
We transfected human astrocytes with C/EBPβ WT or CP by nucleofection, and activated with IL-1β for 24 h to determine if overexpressing C/EBPβ would increase the initial astrocyte TIMP-1 response. Maximum C/EBPβ overexpression was achieved between 24 and 48 h post transfection in time course studies. We collected supernatant and RNA one-day post-treatment. (Fig. 6 A) IL-1β treatment significantly increased (***p<0.001) TIMP-1 mRNA compared to untreated cells. Overexpressing C/EBPβ significantly increased TIMP-1 mRNA 1.4-fold compared to the CP-transfected astrocytes with IL-1β treatment (***p<0.001). (Fig. 6 B) Overexpressing C/EBPβ increased (***p<0.001) TIMP-1 secretion from activated astrocytes by 1.5-fold compared to CP-transfected-activated astrocytes. These data confirm that overexpressing C/EBPβ enhances TIMP-1 production in response to IL-1β.
Fig. 6. Overexpression of WT C/EBPβ increases TIMP-1 expression.
Astrocytes were transfected with plasmids overexpressing WT or CP nucleofection, treated with IL-1β and total mRNA and supernatant was collected 48 h post transfection. A. TIMP-1 mRNA levels were significantly (***p<0.001) increased in astrocytes transfected with WT as compared to CP-transfected cells. Data represents means +/− standard error of mean of TIMP-1 mRNA levels determined in triplicate in at least three independent experiments and two independent donors. B. TIMP-1 protein levels were significantly (***p<0.001) increased in the supernatant of astrocytes transfected with WT as compared to CP-transfected cells. Data represents means +/− S.E.M. of TIMP-1 levels determined in duplicate, in at least three independent experiments and two independent donors.
C/EBPβ knockdown decreases TIMP-1 mRNA and protein expression
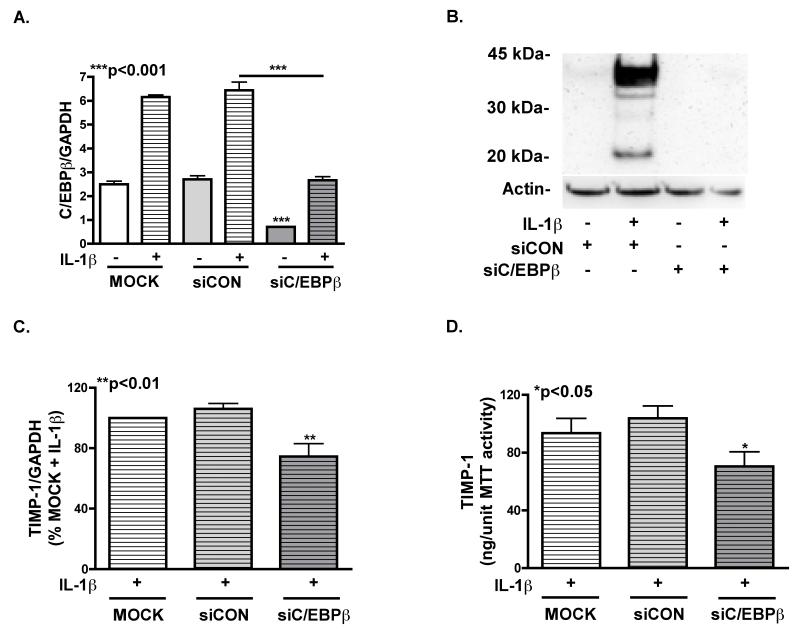
siC/EBPβ was used to evaluate the effect of C/EBPβ knockdown on TIMP-1 mRNA and protein levels in activated astrocytes (Fig. 7 A). We achieved 65% knockdown (***p<0.001) of C/EBPβ mRNA through 72 h in astrocytes transfected with siC/EBPβ, compared to mock-transfected cells. We isolated nuclear extracts from astrocytes transfected with siC/EBPβ, siCON and mock four days post-transfection, and found undetectable C/EBPβ levels in siC/EBPβ-transfected astrocytes compared to siCON-transfected cells (Fig. 7 B). Analysis of total RNA from siC/EBPβ-transfected astrocytes showed significant downregulation in TIMP-1 mRNA (**p<0.01) compared to siCON- or mock-transfected cells (Fig. 7 C). TIMP-1 levels supernatants from siC/EBPβ–transfected astrocytes were significantly lower (*p<0.05) than those from siCON- or mock-transfected cells (Fig. 7 D). Human astrocytes transfected with siC/EBPβ expressed approximately 30% less TIMP-1 mRNA and protein compared to siCON transfected astrocytes. These data illustrate that knockdown of C/EBPβ by siRNA results in downregulation of astrocyte TIMP-1 at the mRNA and protein levels.
Fig. 7. Knockdown of C/EBPβ with siRNA decreases TIMP-1 expression.
C/EBPβ mRNA was measured in primary human astrocytes 3 days after transfection with siC/EBPβ or siCON by nucleofection. A. C/EBPβ mRNA expression was significantly (***p<0.001) decreased in untreated and IL-1β-treated astrocytes. B. 4 days after transfection cell lysates were immunoblotted for C/EBPβ. C/EBPβ protein was detected in siCON-transfected astrocytes, but not in those transfected with siC/EBPβ. C. TIMP-1 mRNA expression was significantly decreased (**p<0.01) at 3 days in astrocytes transfected with siC/EBPβ compared to mock or siCON transfected astrocytes. D. TIMP-1 was measured using ELISA 3 days post transfection. Supernatant from astrocytes transfected with siC/EBPβ had significantly less (*p<0.05) TIMP-1 protein than controls. Data represents means +/− S.E.M. of TIMP-1 mRNA and protein levels determined in duplicate, in at least three independent experiments and two independent donors.
Discussion
Antiretroviral therapy (ART) has transformed HIV-1-infection into a chronic-manageable illness in the industrialized world; however, HAND represents a group of complications that are increasingly prevalent and require much investigation (Yadav & Collman 2009). Focus is shifting from the dysfunctional neurons to glia as an important contributing factor to neuroinflammation and to pathologies of the CNS like HAND. Here, we focus on astrocytes, the most abundant cell in the CNS, and their production of TIMP-1 in response to injury. Previously, we reported that TIMP-1 production is dysregulated in HIV-infected brain specimens, and in vitro experiments showed astrocytes upregulate TIMP-1 when acutely activated with IL-1β; however, long-term IL-1β exposure was shown to downregulate TIMP-1 production (Gardner et al. 2006, Suryadevara et al. 2003). In these studies, we sought to delineate the mechanism of astrocyte TIMP-1 dysregulation in the HIV infected brain. We report that total C/EBPβ mRNA and protein levels are higher in the brains of HIV+ patients compared to control individuals. Confirming similar results from rodent models, HAND-relevant stimuli is shown to induce C/EBPβ nuclear expression in human astrocytes, and this response is enhanced by co-stimulation with HIVJR-FL. Lastly, overexpression of C/EBPβ increases TIMP-1 production while knockdown of C/EBPβ decreases TIMP-1 production in human astrocytes in response to the HAND-relevant stimulus, IL-1β.
Astrocytes react to neural injury by changing gene expression that in turn leads to changes in cellular function, morphology and replication (Sofroniew & Vinters 2010). C/EBPβ regulates inflammatory responses in multiple cell types (Akira et al. 1990) through promoter regulation; the TIMP-1 promoter harbors five sequences predicted to bind C/EBPβ. Therefore, we focused on characterizing C/EBPβ expression in the brain during HIV infection and its involvement in injury-response and astrocyte TIMP-1 production. As C/EBPβ is highly expressed in glia during inflammation (Sterneck & Johnson 1998, Cortes-Canteli et al. 2004, Menard et al. 2002, Nadeau et al. 2005, Cardinaux et al. 2000, Ejarque-Ortiz et al. 2007, Sandhir & Berman 2010, Alberini et al. 1994, Yukawa et al. 1998), it was exciting to detect C/EBPβ upregulation at the mRNA and protein level in HIV-infected brain specimens. Our finding that C/EBPβ mRNA and protein levels were highest in HIV+ brains and higher in HIVE than control brains suggests that C/EBPβ may be involved in the initiation of neuroinflammation following HIV infection rather than a consequence of it. However, our data are derived from total brain tissue lysates, thus, the source of C/EBEβ in these brain tissue specimens likely represents expression by cell other than astrocytes as well. The presence of transcription-activating C/EBPβ isoforms in HIV patients versus the expression of the 20 kDa isoform detected in HIVE patients could represent a switch that contributes to TIMP-1 dysregulation during HAD. Low expression of the 42 kDa isoform of C/EBPβ was detected in control patients, and mRNA levels were minimal.
It is established that human astrocytes are morphologically and functionally more complex than their counterparts in rodents; therefore, we aimed to confirm that the C/EBPβ response is conserved in human astrocytes following activation with HAND-relevant stimuli (Oberheim et al. 2009). To our knowledge, this is the first report of C/EBPβ expression in primary human astrocytes in response to IL-1β. Interestingly, C/EBPβ expression showed additional significant increase when costimulated with HIV-1JR-FL, and other HAND-relevant stimuli. This phenomenon however, requires further investigation. C/EBPβ expression continues to rise through seven days of stimulation, which suggests a prolonged contribution to changes in astrocyte gene expression during inflammation. Along with other reports, the current study supports the idea that C/EBPβ could be an integral part of the regulatory mechanism in a general glial response to neuroinflammation (Sterneck & Johnson 1998, Cortes-Canteli et al. 2004, Menard et al. 2002, Nadeau et al. 2005, Cardinaux et al. 2000, Ejarque-Ortiz et al. 2007, Sandhir & Berman 2010, Alberini et al. 1994, Yukawa et al. 1998). We focused on IL-1β stimulation of human astrocytes for the remainder of the studies, as it is a prototypical inflammatory cytokine associated with HAND.
In our previous studies, we identified a robust increase in astrocyte TIMP-1 mRNA and protein production response to HAND-relevant stimuli (Gardner et al. 2006). Here, we show that TIMP-1 is produced de novo upon stimulation with HAND-relevant stimuli rather than released from intracellular stores. This suggests that transcriptional regulation may be a promising therapeutic target to enhance astrocyte TIMP-1 production. Regulation of TIMP-1 through the promoter has been studied and is very complex (Dean & Clark 1999, Dean et al. 2000, Fassina et al. 2000, Clark et al. 1997). Among other elements, the TIMP-1 promoter harbors five C/EBPβ binding sites (Clark et al. 1997). As we predicted, overexpression of wild-type C/EBPβ enhanced TIMP-1 promoter activity, and overexpression of the 42 kDa isoform alone showed further enhancement. This could be explained by the transcriptional silencing activity of the 20 kDa isoform. Overexpression of C/EBPβ increased TIMP-1 promoter activity, mRNA and protein expression in human astrocytes treated with HAND relevant stimulus, IL-1β. Knockdown of C/EBPβ resulted in the opposite effect on TIMP-1 expression; decreasing mRNA and protein levels in response to IL-1β activation. Overall, these studies suggest that C/EBPβ contributes to regulating TIMP-1 production activated astrocytes. Thus, identifying TIMP-1 as one of genes regulated by C/EBPβ may provide a target for increasing TIMP-1 expression during neuroinflammation. It is noteworthy that C/EBPβ knockdown did not completely ablate TIMP-1 production in response to IL-1β, however, overexpression of CEBPβ did enhance TIMP-1 production. These data support the hypothesis that C/EBPβ binds to some or all of the five CCAAT sites in the TIMP-1 promoter to regulate transcription, but this is most likely not the sole factor influencing transcription. It is possible that C/EBPβ regulates the transcription of other factors that influence TIMP-1 expression. Additional studies are necessary to determine other contributors for TIMP-1 transcription, to identify C/EBPβ binding partners and the precise binding site for C/EBPβ in the TIMP-1 promoter.
Neuroinflammation contributes to HAND, but the precise mechanism required to transition from activated and infected glia in the brain to dysfunctional neurons, is not fully understood (Yadav & Collman 2009, Borjabad et al. 2009). Here, we show that HIV-infected patients, not yet suffering from cognitive deficits or any CNS pathology, express C/EBPβ known to mediate inflammatory responses. C/EBPβ detected in control patients is very low. Patients with signs of cognitive deficits or CNS pathology also express C/EBPβ; although they show different pattern of isoform expression. This links observations about C/EBPβ to HIV infection of the CNS, and, most importantly, to pathology in humans. However, neurons and microglia also express C/EBPβ. Previously, we reported decreased TIMP-1 protein in the CSF and brain of HIV and HIVE compared to control patients (Suryadevara et al. 2003). Taken together with the C/EBPβ isoforms detected in the eight patient samples, a shift in C/EBPβ isoform expression may contribute to dysregulation of TIMP-1 during HIV infection. Further studies, using a large and well characterized cohort of patient samples, will be useful in determining if the different C/EBPβ isoforms are expressed at different stages of HIV infection, the cell types in which they are expressed, if this contributes to dysregulation of TIMP-1 during disease, and if the unidentified 25 kDa isoform plays a role. There is no evidence, in the literature or our studies, that C/EBPβ or TIMP-1 expression is influenced by gender.
Exciting new findings are revealing alternative roles for secreted TIMP-1 as essential for maintaining homeostasis by altering extracellular matrix architecture and acting as a growth factor (Ould-yahoui et al. 2009, Jourquin et al. 2005, Hornebeck 2003). These new findings support a need for studies that culminate in the understanding of the mechanisms regulating this tightly controlled gene. TIMP-1 promoter regulation has been extensively studied, but nothing is known about the regulation of astrocyte TIMP-1 (Clark et al. 1997). This study opens the door to discovering additional functions for C/EBPβ in the CNS as well as another mechanism potentially regulating astrocyte TIMP-1 production during neuroinflammation. Further studies are needed to delineate the roles of C/EBPβ in the CNS, and the regulation of TIMP-1 during neuroinflammation.
Acknowledgments
This publication was made possible from NIH funding through the NIMH and NINDS Institutes by the following grants: Manhattan HIV Brain Bank: U01MH083501, R24MH59724 Texas NeuroAIDS Research Center U01MH083507, R24 NS45491 National Neurological AIDS Bank 5U01MH083500, NS 38841 California NeuroAIDS Tissue Network U01MH083506, R24MH59745 Statistics and Data Coordinating Center U01MH083545, N01MH32002. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NNTC or NIH. This work was supported by 2R01NS048837-06 from NINDS to A. Ghorpade and a fellowship from NIA T32 AG020494 to J. A. Fields. Dr. Calkhoven kindly provided the C/EBPβ-expressing plasmids from Leibniz Institute for Age Research, Fritz Lipman Institute in Germany.
iThe abbreviations used are
- HAND
HIV-1-associated neurocognitive disorders
- HAD
HIV-1 associated dementia
- ART
antiretroviral therapy
- C/EBPβ
CCAAT enhancer binding protein β
- TIMP
tissue inhibitor of metalloproteinase
- MMP
matrix metalloproteinase
- CSF
cerebrospinal fluid
- AP
activator protein
- WT
wild type C/EBPβ-expressing plasmid
- CP
control plasmid
- kDa
kiloDalton
- HIV+
HIV-1-infected
- HIVE
HIV-1 encephalitis
- RT2PCR
real time PCR
- cDNA
complementary DNA
- GAPDH
glyceraldehyde-phosphate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- GFAP
glial fibrillary acidic protein
- pTIMP-1-luc
TIMP-1 promoter-driven firefly luciferase
- pRL-SV40
simian vacuolating virus promoter-driven Renilla luciferase
- siC/EBPβ
C/EBPβ specific short interfering RNA
- siCON
nonspecific control short interfering RNA
Footnotes
The authors have no conflicts of interest to disclose that could have inappropriately influenced, or be perceived to influence, their work.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Albertini JJ, Sujka SK, Helal MA, Seigne JD, Lockhart JL. Adenocarcinoma in a continent colonic urinary reservoir. Urology. 1998;51:499–500. doi: 10.1016/s0090-4295(97)00640-7. [DOI] [PubMed] [Google Scholar]
- Borjabad A, Brooks AI, Volsky DJ. Gene Expression Profiles of HIV-1-Infected Glia and Brain: Toward Better Understanding of the Role of Astrocytes in HIV-1-Associated Neurocognitive Disorders. J Neuroimmune Pharmacol. 2009 doi: 10.1007/s11481-009-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Persaud T, Hu X, et al. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–2640. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugno M, Witek B, Bereta J, Bereta M, Edwards DR, Kordula T. Reprogramming of TIMP-1 and TIMP-3 expression profiles in brain microvascular endothelial cells and astrocytes in response to proinflammatory cytokines. FEBS Lett. 1999;448:9–14. doi: 10.1016/s0014-5793(99)00323-3. [DOI] [PubMed] [Google Scholar]
- Cardinaux JR, Allaman I, Magistretti PJ. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000;29:91–97. [PubMed] [Google Scholar]
- Chaillan FA, Rivera S, Marchetti E, Jourquin J, Werb Z, Soloway PD, Khrestchatisky M, Roman FS. Involvement of tissue inhibition of metalloproteinases-1 in learning and memory in mice. Behav Brain Res. 2006;173:191–198. doi: 10.1016/j.bbr.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chai H, Wang X, et al. Soluble CD40 ligand induces endothelial dysfunction in human and porcine coronary artery endothelial cells. Blood. 2008;112:3205–3216. doi: 10.1182/blood-2008-03-143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IM, Rowan AD, Edwards DR, Bech-Hansen T, Mann DA, Bahr MJ, Cawston TE. Transcriptional activity of the human tissue inhibitor of metalloproteinases 1 (TIMP-1) gene in fibroblasts involves elements in the promoter, exon 1 and intron 1. Biochem J. 1997;324(Pt 2):611–617. doi: 10.1042/bj3240611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Canteli M, Wagner M, Ansorge W, Perez-Castillo A. Microarray analysis supports a role for ccaat/enhancer-binding protein-beta in brain injury. J Biol Chem. 2004;279:14409–14417. doi: 10.1074/jbc.M313253200. [DOI] [PubMed] [Google Scholar]
- Crocker S, Whitmire J, Frausto R, Chertboonmuang P, Soloway P, Whitton J, Campbell I. Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. Am J Pathol. 2006;169:2104–2116. doi: 10.2353/ajpath.2006.060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G, Clark IM. Transcriptional regulation of the human tissue inhibitor of metalloproteinases-1: mapping transcriptional control in intron-1. Ann N Y Acad Sci. 1999;878:510–511. doi: 10.1111/j.1749-6632.1999.tb07711.x. [DOI] [PubMed] [Google Scholar]
- Dean G, Young DA, Edwards DR, Clark IM. The human tissue inhibitor of metalloproteinases (TIMP)-1 gene contains repressive elements within the promoter and intron 1. J Biol Chem. 2000;275:32664–32671. doi: 10.1074/jbc.275.42.32664. [DOI] [PubMed] [Google Scholar]
- Dhar A, Gardner J, Borgmann K, Wu L, Ghorpade A. Novel role of TGF-beta in differential astrocyte-TIMP-1 regulation: implications for HIV-1-dementia and neuroinflammation. J Neurosci Res. 2006;83:1271–1280. doi: 10.1002/jnr.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejarque-Ortiz A, Medina MG, Tusell JM, Perez-Gonzalez AP, Serratosa J, Saura J. Upregulation of CCAAT/enhancer binding protein beta in activated astrocytes and microglia. Glia. 2007;55:178–188. doi: 10.1002/glia.20446. [DOI] [PubMed] [Google Scholar]
- Fassina G, Ferrari N, Brigati C, Benelli R, Santi L, Noonan DM, Albini A. Tissue inhibitors of metalloproteases: regulation and biological activities. Clin Exp Metastasis. 2000;18:111–120. doi: 10.1023/a:1006797522521. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari CC, Depino AM, Prada F, Muraro N, Campbell S, Podhajcer O, Perry VH, Anthony DC, Pitossi FJ. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004;165:1827–1837. doi: 10.1016/S0002-9440(10)63438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari CC, Godoy M. C. Pott, Tarelli R, Chertoff M, Depino AM, Pitossi FJ. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol Dis. 2006;24:183–193. doi: 10.1016/j.nbd.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gardner J, Borgmann K, Deshpande MS, Dhar A, Wu L, Persidsky R, Ghorpade A. Potential mechanisms for astrocyte-TIMP-1 downregulation in chronic inflammatory diseases. J Neurosci Res. 2006;83:1281–1292. doi: 10.1002/jnr.20823. [DOI] [PubMed] [Google Scholar]
- Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J Neurosci Res. 2003;74:801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris P, Tighe A, Levin D, Sharma R, Brown A. Transcriptional regulation of scar gene expression in primary astrocytes. Glia. 2007;55:1145–1155. doi: 10.1002/glia.20537. [DOI] [PubMed] [Google Scholar]
- Heales SJ, Lam AA, Duncan AJ, Land JM. Neurodegeneration or neuroprotection: the pivotal role of astrocytes. Neurochem Res. 2004;29:513–519. doi: 10.1023/b:nere.0000014822.69384.0f. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornebeck W. Down-regulation of tissue inhibitor of matrix metalloprotease-1 (TIMP-1) in aged human skin contributes to matrix degradation and impaired cell growth and survival. Pathol Biol (Paris) 2003;51:569–573. doi: 10.1016/j.patbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Jaworski DM. Differential regulation of tissue inhibitor of metalloproteinase mRNA expression in response to intracranial injury. Glia. 2000;30:199–208. doi: 10.1002/(sici)1098-1136(200004)30:2<199::aid-glia9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Jourquin J, Tremblay E, Bernard A, et al. Tissue inhibitor of metalloproteinases-1 (TIMP-1) modulates neuronal death, axonal plasticity, and learning and memory. Eur J Neurosci. 2005;22:2569–2578. doi: 10.1111/j.1460-9568.2005.04426.x. [DOI] [PubMed] [Google Scholar]
- La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med. 1996;184:2311–2326. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-Associated Neurocognitive Disorder: Pathogenesis and Therapeutic Opportunities. J Neuroimmune Pharmacol. 2010 doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao M-L, Brosnan CF, Lee SC. Expression of Type II Nitric Oxide Synthase in Primary Human Astrocytes and Microglia. American Association of Immunologists. 1996:3569–3576. [PubMed] [Google Scholar]
- Lorenzl S, Albers DS, LeWitt PA, Chirichigno JW, Hilgenberg SL, Cudkowicz ME, Beal MF. Tissue inhibitors of matrix metalloproteinases are elevated in cerebrospinal fluid of neurodegenerative diseases. J Neurol Sci. 2003;207:71–76. doi: 10.1016/s0022-510x(02)00398-2. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF. Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson’s disease. Exp Neurol. 2002;178:13–20. doi: 10.1006/exnr.2002.8019. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Buerger K, Hampel H, Beal MF. Profiles of matrix metalloproteinases and their inhibitors in plasma of patients with dementia. Int Psychogeriatr. 2008;20:67–76. doi: 10.1017/S1041610207005790. [DOI] [PubMed] [Google Scholar]
- Manthrope M, Fagnani R, Skaper SD, Varon S. An automated colorimetric microassay for neurotrophic factors. Dev Brain Res. 1986;25:191–198. doi: 10.1016/s0006-8993(86)80227-x. [DOI] [PubMed] [Google Scholar]
- Menard C, Hein P, Paquin A, et al. An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron. 2002;36:597–610. doi: 10.1016/s0896-6273(02)01026-7. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Hein P, Fernandes KJ, Peterson AC, Miller FD. A transcriptional role for C/EBP beta in the neuronal response to axonal injury. Mol Cell Neurosci. 2005;29:525–535. doi: 10.1016/j.mcn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ould-yahoui A, Tremblay E, Sbai O, et al. A new role for TIMP-1 in modulating neurite outgrowth and morphology of cortical neurons. PLoS One. 2009;4:e8289. doi: 10.1371/journal.pone.0008289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci. 2001;21:7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BW, Sharma R, Leco PA, Edwards DR. A sequence-selective single-strand DNA-binding protein regulates basal transcription of the murine tissue inhibitor of metalloproteinases-1 (Timp-1) gene. J Biol Chem. 1999;274:22197–22207. doi: 10.1074/jbc.274.32.22197. [DOI] [PubMed] [Google Scholar]
- Rivera S, Tremblay E, Timsit S, Canals O, Ben-Ari Y, Khrestchatisky M. Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J Neurosci. 1997;17:4223–4235. doi: 10.1523/JNEUROSCI.17-11-04223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Berman NE. Age-dependent response of CCAAT/enhancer binding proteins following traumatic brain injury in mice. Neurochem Int. 2010;56:188–193. doi: 10.1016/j.neuint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears RC, Sealy L. Multiple forms of C/EBP beta bind the EFII enhancer sequence in the Rous sarcoma virus long terminal repeat. Mol Cell Biol. 1994;14:4855–4871. doi: 10.1128/mcb.14.7.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterneck E, Johnson PF. CCAAT/enhancer binding protein beta is a neuronal transcriptional regulator activated by nerve growth factor receptor signaling. J Neurochem. 1998;70:2424–2433. doi: 10.1046/j.1471-4159.1998.70062424.x. [DOI] [PubMed] [Google Scholar]
- Suryadevara R, Holter S, Borgmann K, Persidsky R, Labenz-Zink C, Persidsky Y, Gendelman HE, Wu L, Ghorpade A. Regulation of tissue inhibitor of metalloproteinase-1 by astrocytes: Links to HIV-1 dementia. Glia. 2003;44:47–56. doi: 10.1002/glia.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4:430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- Yukawa K, Tanaka T, Tsuji S, Akira S. Expressions of CCAAT/Enhancer-binding proteins beta and delta and their activities are intensified by cAMP signaling as well as Ca2+/calmodulin kinases activation in hippocampal neurons. J Biol Chem. 1998;273:31345–31351. doi: 10.1074/jbc.273.47.31345. [DOI] [PubMed] [Google Scholar]