Abstract
A 3T study is presented, comparing the ability of two 1H spectroscopy pulse sequences, Carr Purcell PRESS (CPRESS) (TE=45ms) and conventional PRESS (TE=35ms), to separate between groups of 20 normal control (NC) and 20 mild cognitive impairment (MCI) subjects. Both sequences showed higher myo-Inositol (mI) and mI/N-acetyl-aspartate (NAA) levels in the posterior cingulate gyrus of the MCI subjects. The increased intra-subject repeatability of mI and mI/NAA CPRESS measurements (~6% vs. ~9% for PRESS) translated into decreased intra-class variability. A 22% intra-class mI PRESS variability reduced to 16% for CPRESS, and an 18% intra-class mI/NAA PRESS variability reduced to 12% for CPRESS for the group of NC subjects. Similar results were observed for the MCI subjects. Decreased intra-class variability led to improved separation between NC and MCI subjects (p=0.017 for PRESS and p<0.0001 for CPRESS mI/NAA, the best NC/MCI discriminant for each method). 75% sensitivity at 80% specificity was demonstrated by mI/NAA CPRESS measurements in separating NC from MCI subjects. High correlations were also observed between subject performance on a number of neuropsychological tests (probing verbal memory, visuoconstruction performance and visual motor integration) and the mI/NAA ratio; higher correlation coefficients (with stronger statistical significance) were consistently evident for CPRESS than for PRESS data.
Keywords: mild cognitive impairment, MR spectroscopy, MRS, PRESS, Carr Purcell PRESS, CPRESS, 3T, myo-inositol
Introduction
Mild cognitive impairment (MCI) is generally regarded as a risk state for developing dementia of the Alzheimer type; at autopsy, prospective or retrospective studies show that ~60% of the MCI cases have evidence of amyloid plaques and neurofibrillary tangles, indicative of (or typical of) Alzheimer’s disease (AD). Yearly rates of progression from MCI to AD are estimated as 12% to 16% (1,2), compared to 1–2% conversion in normal individuals, leading some researchers to propose MCI as early stage AD (3). While no gold standard or single test exist for diagnosing MCI, current diagnosis is based on evaluation by a physician following the clinical criteria for MCI diagnosis defined by Petersen and the Mayo Alzheimer Disease Center (2,4).
MRI and magnetic resonance spectroscopy (MRS) have been suggested as alternative non-invasive means to the lengthy neuropsychological tests to detect early changes in brain neurochemistry associated with MCI, as well as to follow disease progression. It is generally agreed that the single, most sensitive measure to detect MCI, besides neuropsychological testing, is the one based on measurement of normalized, regional brain volumes, such as hippocampus or enthorhinal cortex (5,6). Although methods based on volumetric measurements appear to be more sensitive than MRS based methods, they can only show slow changes in the brain, and are thus limited in their ability to show response to therapy. In contrast, MRS offers measures of disease which reflect the underlying biochemical structure of tissue, which is much more likely to change on a fast time scale in response to disease or treatment. Consequently, optimizing spectroscopy acquisition techniques to increase the sensitivity/specificity of MRS based methods to changes due to disease is very compelling.
The MRS finding most commonly reported in the literature associated with MCI is an increase in myo-inositol (mI) and its ratio to creatine (Cr) or N-acetyl-aspartate (NAA)(7–9). Myo-Inositol is a cyclic sugar alcohol of glial origin (10); its (relatively little understood) role in the brain include being a requirement for cell growth, an osmolite, and a storage form for glucose (11). The mechanism by which the MCI disease process causes a mI concentration increase is presently unclear (12).
While Cr and NAA have prominent singlet resonances (at 3.03 and 3.91ppm for Cr, and at 2.01ppm for NAA) that can be detected in most MRS acquisitions, and at most echo times, mI has six coupled protons, with the two prominent multiplets being a doublet-of-doublet centered at 3.52ppm and a triplet at 3.61ppm (13). Evolution under J coupling leads to significant signal loss at long echo times for this metabolite. Consequently, the mI signal is usually detected in a clinical setting using short echo-time (TE) Point RESolved Spectroscopy (PRESS) acquisitions at 1.5 or 3T (14). PRESS is one of the sequences most commonly available on MRI scanners produced by all manufacturers, which was optimized for producing high signal to noise ratio (SNR) spectra with low levels of artifacts. However, it has not been optimized for individual metabolite detection. A Carr-Purcell PRESS (CPRESS) sequence was suggested in the past as a better alternative for detection of coupled metabolites (15). Moreover, a recent simulation study comparing the repeatability and accuracy of a large number of acquisition strategies for improved mI detection (16) has found that CPRESS is consistently better than the typical short TE PRESS currently used for the diagnosis of MCI. It is the aim of the current study to further validate the improved precision of CPRESS in mI detection in a population of normal elderly volunteers and MCI patients; following positive results of this initial test, a comparison between the diagnostic capability of the typical short TE PRESS and the CPRESS acquisition in detecting disease in a larger cohort of MCI patients and age-matched normal controls was performed.
Methods
Subjects
Twenty MCI patients and twenty healthy, elderly volunteers participated in the study. Subjects gave informed consent before the study, which was conducted with the approval of the Institutional Human Research Review Committee. Both patients and control subjects were assessed by: standard neurological examination, activities and instrumental activities of daily living (ADL/IADL), Clinical Dementia Rating Scale (CDR) (17), Mini Mental Status Examination (MMSE) (18), and a brief neuropsychological (NP) battery which included measures of recent memory/new learning ability (Wechlser Memory Scale – Revised: (19); Hopkins Verbal Learning Test-Revised, (20), language (Boston Naming Test, (21); Controlled Oral Word Association Test, (22); Category Fluency Test, (23), visuoconstruction (WAIS-III; Block Design (19), attention (WAIS-III; Digit Span), visual-motor integration (WAIS-III Digit Symbol, Trail Making Test – Part A, (24) and executive functioning (Trail Making Test – Part B, Clock Drawing Test). Normalized NP test scores were computed where appropriate, and used in the statistical correlation analysis described below. Premorbid intellectual ability was estimated utilizing the NART-R, (25). Subjects were screened for depression utilizing the Geriatric Depression Scale (26).
Diagnostic assignment was made by the study’s neurologist based on data from the clinical examination, rating scales and neuropsychological testing. With regard to the later, subjects were classified as meeting criteria for Amnestic MCI if they performed at least 1.5 standard deviations below the age/intelligence quotient adjusted mean on at least 1 measure of memory functioning, with preserved general cognitive functioning
MCI subjects were recruited from clinics associated with Albany Medical Center. Participants met criteria for MCI defined by Petersen and the Mayo Alzheimer Disease Center (2,4) as diagnosed by one of the investigators. All MCI subjects included in the study were at least amnestic, some with deficits in other domains such as visual-spatial and executive functioning. MCI subjects consisted of 11 men and 9 women, with a mean age of 68 years, and a standard deviation (SD) of 9.3 years. The average MMSE score for the MCI patients was 28.7, with a SD of 1.13.
The healthy elderly subjects enrolled in the study were recruited from the community and caregivers of the MCI patients. These subjects consisted of 10 men and 10 women, with an average age of 66.5 years and a SD of 9.7 years. The average MMSE score for normal controls was 29.75, with a SD of 0.56.
To validate the hypothesis that higher precision in mI and mI/NAA measurements is observed with a CPRESS than with a short TE PRESS acquisition, the first five MCI subjects and the first five normal controls recruited were scanned three times in a day, using the same acquisition protocol and anatomical voxel location. The subjects were removed from the scanner between each of the 3 daily sessions. During each of three scanning sessions, data was collected from the same voxel using both PRESS and CPRESS. Center frequency, shimming and water suppression was re-optimized for each session. Following validation of improved mI and mI/NAA precision for the CPRESS sequence, as evidenced by decreased coefficients of variation for this metabolite and metabolite ratio, the remaining 30 subjects to be enrolled in a study (15 NC’s and 15 MCI patients) underwent a single scanning session, using an identical protocol as the first subjects.
MRI/MRS protocol
All subjects were scanned on the same 3T MRI instrument (GE Healthcare, Waukesha, WI), using a quadrature birdcage coil, and all scans used the same protocol. The protocol consisted of a scout acquisition sequence to obtain the location and spatial orientation of the head. A fast, whole brain axial localizer was then acquired, followed by two spectroscopy acquisitions, from the same 2×2×4cm voxel (with the longest dimension in the anterior-posterior direction). The voxel location was chosen in the posterior cingulate gyrus of each subject, a site associated with MCI (8). No significant white matter hyperintensities were observed in either the MCI or the NC subjects, therefore no efforts to correct for such potential biases were attempted. Additionally, no correction for varying contributions of white and gray matter to the spectroscopy voxel was performed.
Two sets of MRS acquisitions were acquired during each scanning session, with their order randomized to prevent un-intended biases. One of the acquisitions was a PRESS acquisition with TE=35ms. The second acquisition was a CPRESS acquisition with TE=45ms. Briefly, PRESS uses a slice selective excitation pulse and two slice-selective refocusing pulses of (all spatially selective in orthogonal directions). For CPRESS, each of the two slice selective refocusing pulses of PRESS is replaced by a pair of 180° pulses with the same slice-selection gradients; the second pulse in the pair is used to rewind the quadratic phase induced by a first one. The diagrams and timings of the two sequences used in this work are identical to the ones described elsewhere (16). 128 averages, with a repetition time of TR=2s were used for each of the two sequences, leading to a total scanning time for each MRS acquisition of ~5min. Automated optimization of gradient shimming (consistently yielding spectra with linewidths of 6–7Hz), transmitter pulse power, and water suppression were used for both sequences. The transmitter frequency was also automatically adjusted, being centered on the water resonance. The slice selective RF excitations, however, were offset to the middle of the spectrum (~1.7ppm from the water resonance).
Data analysis
Both PRESS and CPRESS spectra were quantified using version 6.2 of LCModel (27). For both sequences, fitting was performed between 0 and 4ppm, using basis sets simulated using the GAMMA libraries (28). Quantitation was based on the reciprocity principle and signal calibration was performed with a 50mM NAA phantom scan prior to the patient scans. For each of the sequences, the scaling of the spectra (the parameter fcalib in LCModel) accounted for the difference in transverse relaxation time of the NAA calibration phantom (380ms) and the average T2 measured in our clinical setting in a cohort of normal elderly volunteers in a posterior cingulate gyrus voxel (254ms) (29). The metabolite concentrations reported here were not corrected for transverse relaxation, due to introduction of larger errors (30). Moreover, a 2s TR value may weigh reported concentration and concentration ratios by the longitudinal relaxation rate of the metabolites. Consequently, the metabolite concentrations and concentration ratios (representing the weighting factors for the metabolite basis sets computed by LCModel at the end of the fitting process (27)) are not absolute and may not be identical with e.g. HPLC results.
One-way ANOVA was performed for each of the pulse sequences and each metabolite concentration/concentration ratio in a univariate analysis to identify the factor which best discriminates between MCI and NC subjects. Post-hoc corrections were not performed; however, post-hoc adjustments for multiple comparisons could be made by comparing p-values to a Bonferroni adjusted threshold. Discriminant analysis was also performed for each of the acquisition sequences, using as predictor the measure best separating the 2 groups for each acquisition approach as identified above. Correlation analysis, aimed to understand the relationships between the best CPRESS and PRESS MRS measures and the results of NP tests, was also performed. All statistical analysis was done using SPSS 19 (IBM, Chicago, IL).
Receiver operating characteristic curves (ROC’s) were generated using ROCKIT (software available at http://www-radiology.uchicago.edu/krl/KRL_ROC/software_index.htm) for the factor which discriminated best between the two groups of subjects, for each of the sequences. ROCKIT uses maximum likelihood estimation to fit a binormal ROC curve to the continuously-distributed MRS data. It also calculates the statistical significance of differences between ROC index estimates and parameters. On the basis of a bivariate binormal model, it allows for comparison of 2 paired, partially paired, or unpaired datasets with regard to differences in the areas under the two estimated binormal ROC curves.
Results
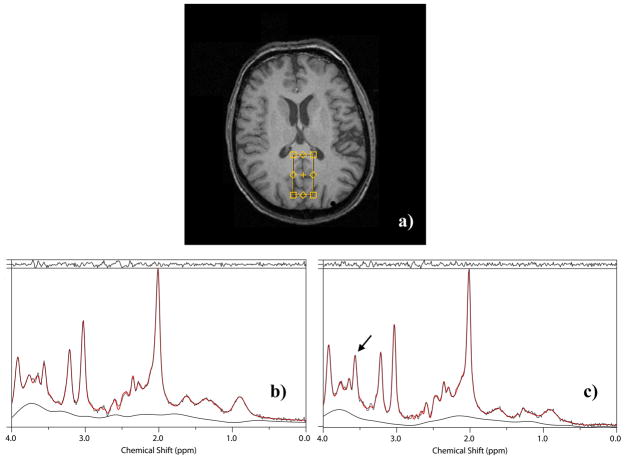
Figure 1 presents the location and typical spectra (experimental data, fitted spectra and fitted baselines) acquired from the same voxel of an elderly normal volunteer. Figure 1a presents a typical voxel location, Figure 1b the PRESS spectrum, and Figure 1c the CPRESS spectrum, displayed between 0 and 4ppm. Note the higher visibility of the main mI peak, indicated by an arrow in the CPRESS spectrum.
Figure 1.
a) Typical voxel location for the 1H MRS acquisitions. Typical b) PRESS and c) CPRESS spectra from the same voxel in a normal human volunteer. The arrow in Fig. 1c points to the increased mI signal in the CPRESS acquisition.
Table 1 presents the average intra-day, intra-individual coefficient of variation (CV) for the mI concentration and the mI/NAA concentration ratio for the five NC and MCI subjects, each scanned three times in a day using both PRESS and CPRESS. Consistent with previous reports (16), both mI and mI/NAA ratios show higher precision with a CPRESS acquisition, warranting the continuation of the study.
Table 1.
Average intra-day, intra-individual coefficient of variation (CV) for mI concentration and mI/NAA concentration ratio for the five normal control (NC) and mild cognitive impairment (MCI) subjects, each scanned three times in a day using both PRESS and CPRESS.
| mI | ||
|---|---|---|
| PRESS | CPRESS | |
| sigma [%] (NC) | 7.8 | 5.6 |
| sigma [%] (MCI) | 10.2 | 6.8 |
| average | 9.0 | 6.2 |
| mI/NAA | ||
| PRESS | CPRESS | |
| sigma [%] (NC) | 7.8 | 6.6 |
| sigma [%] (MCI) | 10.6 | 4.9 |
| average | 9.2 | 5.8 |
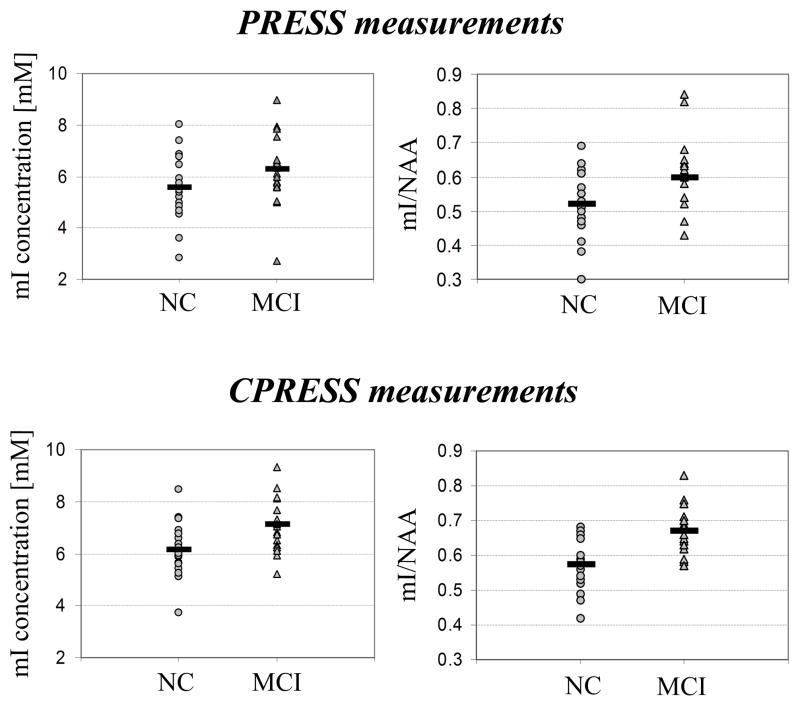
Figure 2 displays graphs with the mI and mI/NAA ratios for all 40 subjects scanned, using both PRESS and CPRESS. Note a few interesting features of these graphs:
Figure 2.
mI and mI/NAA ratios for the MCI and NC subjects, measured using PRESS and CPRESS.
An overall ~10% increase in the average mI and mI/NAA signal levels are seen when data is acquired with CPRESS compared to PRESS, for both NC and MCI subjects. This increase is not evident in all subjects (becoming noticeable, in fact, only when data from multiple subjects is averaged together), and cannot be reproduced when data is acquired using PRESS and CPRESS from a (37°C) brain phantom. We believe that this may be an artifact due to the (~30%) increased SNR of the mI signal quantified from the CPRESS acquisition. Similar findings, reporting changing metabolite concentrations in vivo as a function of SNR were previously reported in the literature (31,32), and are likely due to the increased capability of the fitting routines to determine more accurate baseline signals (and more accurate metabolite levels) as the information content (i.e. the SNR) of a spectrum increases.
The higher precision of the CPRESS sequence in measuring mI levels, evident in the lower intra-session, intra-volunteer CV’s shown in Table 1, also translates in lower intra-class variability. Within the NC group, a 22% within class mI PRESS variability reduces to 16% for CPRESS, and an 18% within class mI/NAA PRESS variability reduces to 12% for CPRESS. The same trend is observed for MCI subjects, where mI variability reduces from 21% to 14%, and mI/NAA variability from 17% to 10% when transitioning from PRESS to CPRESS. The reduced variability of CPRESS measurements is mostly due to data compaction. The errors in measuring metabolite concentrations with both acquisition strategies, however, lead to a number of “crossings” when plotting paired PRESS/CPRESS measurements (data not shown). Note also that the improved capability of CPRESS to measure the mI concentration is not related to an accidental mismatch in voxel size or profile between the PRESS and CPRESS acquisitions. Although CPRESS uses two additional refocusing pulses, which may alter voxel dimension or profile, imaging of the PRESS and CPRESS MRS voxels indicate that the energy within the intended volume differs by less that 10% between the two acquisitions, with no significant ripples present in either of the two approaches.
Statistical comparisons between metabolite concentrations/concentration ratios in the normal and MCI groups studied reveal the following:
When metabolites where quantified from PRESS spectra, the best separation between the MCI and control groups was offered, in the significance order, by the following measures: increased mI/NAA (p=0.017), increased mI/Cr (p=0.027) and increased mI (p=0.08).
Similarly, when metabolite concentrations from spectra acquired using CPRESS were determined, the best separation between the MCI and control group was offered, in the significance order, by: increased mI/NAA (p<0.0001), increased mI/Cr (p=0.001) and increased mI (p=0.005).
No other significant differences between other metabolite concentrations and concentration ratios were observed. Significantly, and consistent with previous literature reports (14), no changes in NAA levels are seen between the NC and MCI groups with either PRESS or CPRESS (p>0.7). This confirms that neuronal dysfunction, as highlighted by the NAA concentration levels measured through 1H MRS, is only visible late in the evolution from normal to demented status, and is essentially an AD, but not an MCI hallmark.
Discriminant analysis, performed to assess whether CPRESS is indeed superior to short TE PRESS in distinguishing MCI patients from normal controls, was done with mI/NAA as discriminant function for each of the two sequences. Classification using cross-validation indicated that 64% of the cases were classified correctly using PRESS, while 77% of the cases were classified correctly using CPRESS.
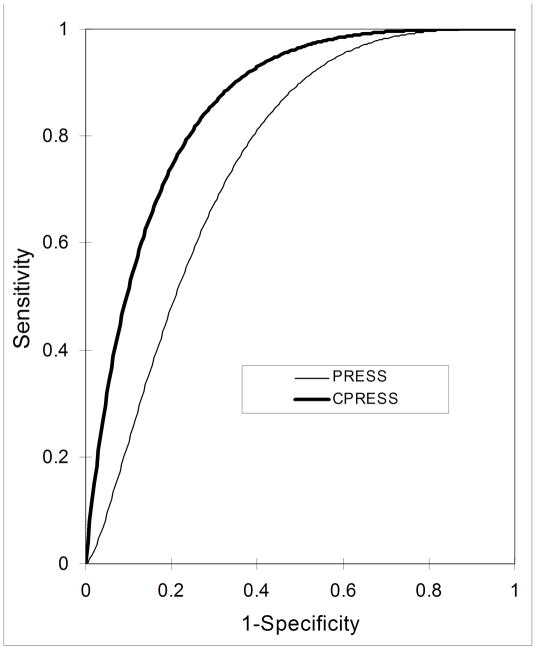
ROC curves of the mI/NAA ratios acquired using both sequences were also computed and compared. Figure 3 displays the ROC curves for mI/NAA PRESS and the mI/NAA CPRESS ratios. The ROC curves are binormal fits to the experimental data points. As evident from the graph, and confirmed by the statistical analysis, CPRESS performs better at identifying MCI patients: the area under the mI/NAA CPRESS curve is 0.84, and is significantly larger than the 0.72 area under the mI/NAA PRESS curve (p=0.03). At 80% specificity, the sensitivities of mI/NAA measurements in discriminating NC from MCI subjects, using CPRESS and PRESS acquisitions are 75% and 48%, respectively.
Figure 3.
Conventional binormal ROC curves, obtained from the mI/NAA metabolite ratios using CPRESS (thick line) and PRESS (thin line).
Table 2 displays the correlations between the mI/NAA PRESS and CPRESS data and the NP measures showing the strongest correlation with the MRS data. In this table, WAISB-SS represents the scaled score for Wexler Adult Intelligence Scale (3rd edition) -Block design, HVLT-DRS the normalized T-score for the Hopkins Verbal Learning Test- Delayed Recall, and TMT SS the scaled score for the Trail Making Test (part A). Note that stronger correlations between the NP scores and CPRESS data exist than between NP scores and PRESS data, underlying the fact that CPRESS may provide more precise overall mI measurements.
Table 2.
Correlation coefficients and p-values reflecting the relationship between the best PRESS and CPRESS measures for separating NC’s from MCI subject and the NP scores that most strongly correlate with the MRS data.
| CPRESS mI/NAA | PRESS mI/NAA | HVLT DRS | TMT SS | WAISB SS | ||
|---|---|---|---|---|---|---|
| CPRESS mI/NAA | Pearson Corr. Sig. (2-tailed) |
1 | .865 .000 |
−.448 .004 |
−.509 .002 |
−.538 .000 |
| PRESS mI/NAA | Pearson Corr. Sig. (2-tailed) |
.865 .000 |
1 | −.292 .071 |
−.450 .007 |
−.394 .013 |
| HVLT DRS | Pearson Corr. Sig. (2-tailed) |
−.448 .004 |
−.292 .071 |
1 | .552 .001 |
.309 .056 |
| TMT SS | Pearson Corr. Sig. (2-tailed) |
−.509 .002 |
−.450 .007 |
.552 .001 |
1 | .412 .014 |
| WAISB SS | Pearson Corr. Sig. (2-tailed) |
−.538 .000 |
−.394 .013 |
.309 .056 |
.412 .014 |
1 |
Discussion and Conclusions
A comparison was presented between the performance of two pulse sequences, short TE PRESS and CPRESS in distinguishing MCI patients from normal controls. Our initial hypothesis, that the improved precision of CPRESS in measuring mI levels will result in better capability of this sequence to identify MCI patients, was confirmed by the lower ANOVA CPRESS p-values and by the higher area under the curve for the CPRESS measurements.
While the increase in mI and mI/NAA levels evidenced by both PRESS and CPRESS are not novel facts (many studies have previously pointed out such increases in MCI), the increased sensitivity of the CPRESS method is notable. There is still overlap between mI and mI/NAA levels for the two populations studied (Figure 2), indicating that it is still impossible to distinguish an MCI patient solely based on these measurements. The choice of a better pulse sequence, however, can dramatically decrease the number of subjects enrolled in a clinical trial that would, e.g. monitor the effect of a treatment in MCI subjects. For example, assuming 10% change in the mI levels due to treatment, and 6% measurement variability for CPRESS (compared with 9% for PRESS)—see Table 1, a decrease in the number of subjects to be enrolled in each of the treated and untreated groups from 23 to 11 would be obtained, should a short TE PRESS sequence be replaced with CPRESS. It is expected for these trends (better repeatability of CPRESS mI and mI/NAA measurements, and improved CPRESS MCI detection performance) to hold at any one site where PRESS and CPRESS are compared; comparison of absolute metabolite concentrations and concentration ratios between sites may require cross-calibration procedures similar to the ones described in prior work (33).
While Carr Purcell PRESS was initially proposed (15), and later validated (16,34) as a good solution for measuring the concentration of coupled metabolites in the brain, such as myo-inositol, glutamate or glutamine, its performance in measuring the concentration of the usual singlets in the brain (NAA, Cr and Cho) is also comparable to the performance of short TE PRESS. For the subjects enrolled in our study, Cramer Rao Lower Bounds and coefficients of variation for the 3 singlets mentioned above are all in the low 3% range for both short TE PRESS and CPRESS, indicating that CPRESS may be simply a better overall solution than PRESS for MRS acquisitions. Note that up to 60% higher power deposition will be seen when using the CPRESS sequence compared to PRESS; the longer repetition times used in MRS scans to limit the impact of T1 on metabolite concentration measurements, however, should render the use of CPRESS feasible in most clinical situations; all of our exams were performed within the first SAR level (3W/kg) and resulted in no power monitor warnings.
High correlations were evidenced between the MRS measures (and CPRESS in particular) and a number of NP test scores. Analysis of the known brain regions involved in the tasks required by the NP tests is consistent with the correlations seen in Table 2. For example, the intersection of brain anatomical regions involved in verbal memory tasks (probed here by the HVLT test) (hippocampus and PCG (35)), and in visuoconstruction tasks (probed here by WAISB) (bilateral occipital-parietal-posterior temporal visual association cortices, the PCG, and a number of other brain regions- not including the hippocampus (36)) is exactly the PCG, probed by our MRS exam. Remarkably, though, the correlation between the increased mI (and constant NAA) concentrations seen the MRS exam (thought to reflect mostly glial cell health (13))- and the decreased performance of MCI subjects in NP tests (reflective of neuronal health) indicate a deep interconnection between neuronal and glial functions in the PCG.
The diagnosis of MCI using standard clinical evaluation tests following the criteria for MCI diagnosis defined by Petersen and the Mayo Alzheimer Disease Center (2,4) is known to be about 90% accurate. Unfortunately, none of the currently available MRI or MRS based approaches can attain or surpass this threshold. A past comparative study assessing the capability of a number of MRI or MRS based approaches to separate MCI from control subjects (14) indicates the best measure distinguishing the two groups is MR based volumetry, attaining 79% sensitivity at 80% specificity. This measure was shown to be considerably superior to short TE PRESS or measurements of apparent diffusion coefficients in separating the two groups. Our results, indicating very similar performance of the CPRESS approach with the hippocampal volumetric measurements, are extremely promising. While not improving diagnostic performance, CPRESS measurements and quantification are much simpler than volumetric techniques. They require a single 5 minutes scan, and minimal, automated post-processing. By comparison, volumetric measurements involve extended scan times (needed to achieve the high spatial resolution required for segmenting small anatomical structures), and significant manual or semi-automated post-processing, to segment out the desired structures. Moreover, volumetric measurements can only perceive anatomical changes due to disease or treatment that occur 6–12 months from an initial scan. By comparison, MRS based methods highlight biochemical processes, which can happen- and which it may be feasible to detect- on a much shorter time scale. Consequently, the similar performance of volumetric based measurements to CPRESS based-acquisitions in separating the NC from MCI subjects, coupled to the reduced scan time, ease of quantification, and capability of detecting earlier changes due to disease or treatment renders the latter much better suited for use in clinical trials.
Acknowledgments
NIH 1R21NS054303-01A2
References
- 1.Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, Stern Y, Devanand DP. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(5):758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Conceptual Overview. In: Petersen RC, editor. Mild cognitive impairment. New York: Oxford University Press; 2003. pp. 1–14. [Google Scholar]
- 5.Wolf H, Hensel A, Kruggel F, Riedel-Heller SG, Arendt T, Wahlund LO, Gertz HJ. Structural correlates of mild cognitive impairment. Neurobiol Aging. 2004;25(7):913–924. doi: 10.1016/j.neurobiolaging.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala EL, Vainio P, Vanninen R, Partanen K, Soininen H. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25(3):303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 7.Chantal S, Braun CM, Bouchard RW, Labelle M, Boulanger Y. Similar 1H magnetic resonance spectroscopic metabolic pattern in the medial temporal lobes of patients with mild cognitive impairment and Alzheimer disease. Brain Res. 2004;1003(1–2):26–35. doi: 10.1016/j.brainres.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 8.Kantarci K, Jack CR, Jr, Xu YC, Campeau NG, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catani M, Cherubini A, Howard R, Tarducci R, Pelliccioli GP, Piccirilli M, Gobbi G, Senin U, Mecocci P. (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12(11):2315–2317. doi: 10.1097/00001756-200108080-00007. [DOI] [PubMed] [Google Scholar]
- 10.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15(3–5):289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 11.Ross BD. Biochemical considerations in 1H spectroscopy. Glutamate and glutamine; myo-inositol and related metabolites. NMR Biomed. 1991;4(2):59–63. doi: 10.1002/nbm.1940040205. [DOI] [PubMed] [Google Scholar]
- 12.Ross BD, Bluml S, Cowan R, Danielsen E, Farrow N, Tan J. In vivo MR spectroscopy of human dementia. Neuroimaging Clin N Am. 1998;8(4):809–822. [PubMed] [Google Scholar]
- 13.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Kantarci K, Xu Y, Shiung MM, O’Brien PC, Cha RH, Smith GE, Ivnik RJ, Boeve BF, Edland SD, Kokmen E, Tangalos EG, Petersen RC, Jack CR., Jr Comparative diagnostic utility of different MR modalities in mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14(4):198–207. doi: 10.1159/000066021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennig J, Thiel T, Speck O. Improved sensitivity to overlapping multiplet signals in in vivo proton spectroscopy using a multiecho volume selective (CPRESS) experiment. Magn Reson Med. 1997;37(6):816–820. doi: 10.1002/mrm.1910370603. [DOI] [PubMed] [Google Scholar]
- 16.Hancu I. Which pulse sequence is optimal for myo-inositol detection at 3T? NMR Biomed. 2009;22(4):426–435. doi: 10.1002/nbm.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Folstein M, Folstein S, McHugh PR. Mini Mental State Examination. In: Lutz F, editor. Psychological Assessment Resources. 2004. [Google Scholar]
- 19.Wechsler D. In: Wechsler Adult Intelligence Scale-III. Corporation P, editor. San Antonio: 1987. [Google Scholar]
- 20.Brandt J, Benedict R. Proffesional Manual Psychological Assessment Resources. Lutz, Florida: 2001. Hopkins Verbal Learning Test, revised. [Google Scholar]
- 21.Kaplan E, Goodglass H, Weintraub S. In: The Boston naming test. Kaplan E, Goodglass H, editors. Boston, MA: 1978. [Google Scholar]
- 22.Benton A, Hamsher K, Sivan S. Multilingual aphasia examination. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 23.Lucas J, Ivnik RJ, Smith GE, Bohac DL, Tanglaos EG, Graff-Radford N, Petersen RC. Mayo’s older American normative studies: Category fluency norms. J Clin Exp Neuropsych. 1998;5:594–610. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- 24.Reitan R. Validity of the train making test as an indicator of organic brain damage. Perceptual Motor Skills. 1985;8:271–276. [Google Scholar]
- 25.Blair J, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. Clin Neuropsych. 1989;3:129–136. [Google Scholar]
- 26.Yesavage J, Brink T, Rose T. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 27.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 28.Smith S, Levante T, Meier B, Ernst R. Computer simulations in magnetic resonance. An object-oriented programming approach. J Magn Reson Series A. 1994;106(1):75–105. [Google Scholar]
- 29.Dumoulin M, Zimmerman E, Hurd R, Hancu I. Increased brain metabolite T2 relaxation times in patients with Alzheimer’s disease. Proc 13-th Meeting of the Int Soc Magn Reson Med; 2005; Miami. p. 1179. [Google Scholar]
- 30.Hancu I, Dumoulin M, Blezek D, Zimmerman E, Hurd R. Effects of T2 relaxation on 1H MRS data: to correct or not to correct for?. Proc 13-th Meeting of the Int Soc Magn Reson Med; 2005; Miami. p. 2765. [Google Scholar]
- 31.Bartha R. Effect of signal-to-noise ratio and spectral linewidth on metabolite quantification at 4 T. NMR Biomed. 2007;20(5):512–521. doi: 10.1002/nbm.1122. [DOI] [PubMed] [Google Scholar]
- 32.Macri MA, Garreffa G, Giove F, Guardati M, Ambrosini A, Colonnese C, Maraviglia B. In vivo quantitative 1H MRS of cerebellum and evaluation of quantitation reproducibility by simulation of different levels of noise and spectral resolution. Magn Reson Imaging. 2004;22(10):1385–1393. doi: 10.1016/j.mri.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Lee PL, Yiannoutsos CT, Ernst T, Chang L, Marra CM, Jarvik JG, Richards TL, Kwok EW, Kolson DL, Simpson D, Tang CY, Schifitto G, Ketonen LM, Meyerhoff DJ, Lenkinski RE, Gonzalez RG, Navia BA. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging. 2003;17(6):625–633. doi: 10.1002/jmri.10295. [DOI] [PubMed] [Google Scholar]
- 34.Hancu I. Optimized glutamate detection at 3T. J Magn Reson Imaging. 2009;30(5):1155–1162. doi: 10.1002/jmri.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heun R, Freymann K, Erb M, Leube DT, Jessen F, Kircher TT, Grodd W. Successful verbal retrieval in elderly subjects is related to concurrent hippocampal and posterior cingulate activation. Dement Geriatr Cogn Disord. 2006;22(2):165–172. doi: 10.1159/000094558. [DOI] [PubMed] [Google Scholar]
- 36.Machulda MM, Senjem ML, Weigand SD, Smith GE, Ivnik RJ, Boeve BF, Knopman DS, Petersen RC, Jack CR. Functional magnetic resonance imaging changes in amnestic and nonamnestic mild cognitive impairment during encoding and recognition tasks. J Int Neuropsychol Soc. 2009;15(3):372–382. doi: 10.1017/S1355617709090523. [DOI] [PMC free article] [PubMed] [Google Scholar]