Abstract
The Ras-ERK (extracellular signal-regulated kinase) and PI3K (phosphatidylinositol 3-kinase)-mTOR (mammalian target of rapamycin) signaling pathways are the cell’s chief mechanisms for controlling cell survival, differentiation, proliferation, metabolism, and motility in response to extracellular cues. Components of these pathways were among the first to be discovered when scientists began cloning proto-oncogenes and purifying cellular kinase activities in the 1980s. Ras-ERK and PI3K-mTOR were originally modeled as linear signaling conduits activated by different stimuli, yet even early experiments hinted that they might intersect to regulate each other and co-regulate downstream functions. The extent of this crosstalk and its significance in cancer therapeutics are now becoming clear.
Core components
The Ras-ERK pathway
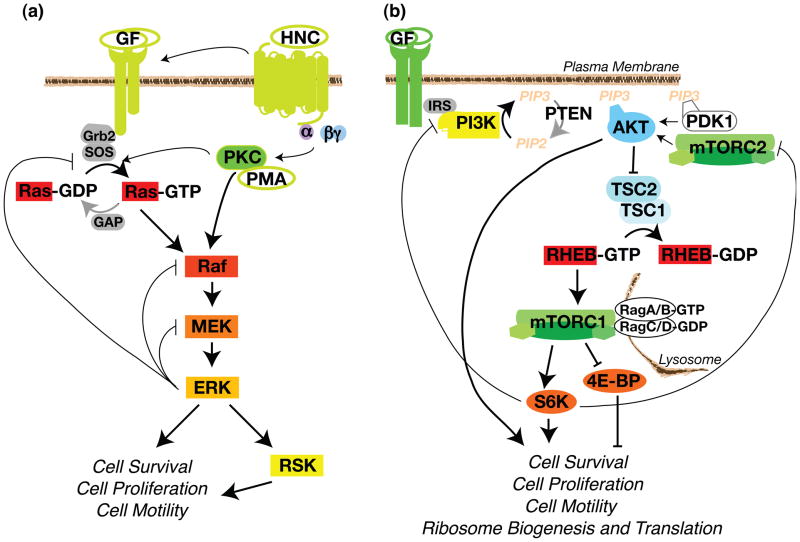
ERK (extracellular signal-regulated kinase) is a MAPK (mitogen-activated protein kinase) that functions as the major effector of the Ras oncoprotein. MAPK pathways consist of an initial GTPase (guanosine triphosphatase)-regulated kinase (MAPKKK), which phosphorylates and activates an intermediate kinase (MAPKK), which in turn phosphorylates and activates an effector kinase (MAPK, Box 1). In the ERK-MAPK pathway, these components are the Ras GTPase and the protein kinases Raf, MEK, and ERK (Figure 1a). Ras and the ERK-MAPK pathway are activated by growth factors, polypeptide hormones, neurotransmitters, chemokines, and phorbol esters, which signal through their cognate RTKs (receptor tyrosine kinases) and GPCRs (G protein-coupled receptors), or by direct activation of PKC (protein kinase C) (Figure 1a) [1, 2].
Box 1. Types of Ras-ERK and PI3K-mTOR signaling proteins.
GTPases
These proteins have the intrinsic ability to catalyze the conversion of GTP (guanine tri-phosphate) to GDP (guanine di-phosphate). GTPases are generally active when bound to GTP and inactive when bound to GDP. GEFs (guanine nucleotide exchange factors) activate GTPases by inducing GTPases to release their bound GDP and thereby facilitating GTP binding. GAPs (GTPase activating proteins) inactivate GTPases by inducing the GTPases to hydrolyze their bound GTP to GDP and become inactive.
Kinases
These proteins have the intrinsic ability to catalyze the transfer of the gamma-phosphate from an ATP molecule to another protein. Most kinases require phosphorylation of their activation loop of the catalytic kinase domain to be in an active conformation. Many kinases are additionally phosphorylated at several other residues, which can regulate recruitment of the activation loop kinase, kinase folding and stability, or kinase localization.
Phosphatases
These proteins have the intrinsic ability to catalyze the removal of a phosphate from another protein.
Adaptor proteins
These proteins physically bridge two signaling proteins through protein–protein interaction domains. Such interaction domains can bind peptide sequences, modified peptide sequences that are phosphorylated, methylated, acetylated, or ubiquitylated, or an identical interaction domain within other proteins.
Docking proteins
Similar to adaptor proteins, docking proteins are generally larger and contain a membrane-recruitment domain and multiple protein–protein interaction motifs.
Figure 1.
The Core Pathway Components. The RAS-MAPK and PI3K-mTOR pathways respond to extracellular and intracellular cues to control cell survival, proliferation, motility, and metabolism. (a) The Ras-ERK-MAPK Pathway. In quiescent cells, inactive Ras-GDP associates with the plasma membrane and inactive Raf, MEK, and ERK are largely cytoplasmic. GF (growth factor) binding activates RTK auto-phosphorylation, generating binding sites for the SHC and GRB2 adaptor molecules that recruit SOS, the RasGEF (GTPase exchange factor), to the membrane. SOS catalyzes Ras GTP exchange and Ras-GTP then recruits Raf to the membrane, where it gets activated [1]. HNC (polypeptide hormone, neurotransmitter, and chemokine) activation of GPCRs feed into the MAPK cascade by trans-activating upstream RTKs, thereby inducing SOS translocation, and/or Raf activation [2]. Cell-permeable phorbol esters such as PMA directly bind and activate PKC by mimicking the natural PKC ligand diacylglycerol. The mechanism by which PKC activates ERK is not resolved and could be through activation of SOS or Raf [2]. Raf activates MEK and MEK activates ERK via activation loop phosphorylation. ERK also feeds back to negatively regulate the pathway. (b) The PI3K-mTOR Pathway. In quiescent cells, the lipid phosphatase PTEN maintains low levels of PIP3, resulting in AKT inactivation. TSC2, in complex with TSC1, maintains RHEB in the GDP-bound state. Insulin and IGF1 bind their cognate RTKs, and subsequent receptor autophosphorylation creates binding sites that then recruit IRS, an adaptor protein for PI3K. Different RTKs activate PI3K through distinct docking proteins, such as FRS (FGF Receptor Substrate) or GAB (c-Met or EGFR), or via direct binding of PI3K (Platelet-derived Growth Factor Receptor) [11]. Activated PI3K phosphorylates PIP2 to generate membrane-bound PIP3. Pleckstrin homology (PH) domains in AKT and PDK1 recognize PIP3 and translocate to the membrane. PDK1 phosphorylates the activation loop and mTORC2 phosphorylates the hydrophobic motif of AKT, thus promoting AKT activation and phosphorylation of TSC2. This TSC2 phosphorylation inhibits TSC2 GAP activity. RHEB-GTP localizes to the lysosome and activates mTORC1 following its recruitment by the Rag GTPases [6].
Activated ERK phosphorylates cytoplasmic signaling proteins, including RSK (p90 ribosomal S6 kinase), and end-point effectors such as transcription factors. RSK similarly phosphorylates several cytoplasmic targets and transcriptional regulators. ERK nuclear targets include the TCF (Ternary Complex Factor) transcription factors, which play a major role in inducing IEG (Immediate Early Gene) expression. The IEG products, such as c-Fos and c-Myc, induce late-response genes that promote cell survival, cell division, and cell motility [3, 4]. Confoundingly, non-physiological hyper-activation of Ras-ERK signaling can induce the expression of CDK (cyclin-dependent kinase) inhibitors and trigger cell cycle arrest [5].
The target specificity of active ERK is controlled by substrate availability (cell type, cell cycle phase, and extracellular environment), by ERK scaffolding, and by subcellular localization. Scaffolding proteins tether MEK and ERK to specific substrates and subcellular locales and are required for ERK phosphorylation of the corresponding bound or local substrates [1].
The PI3K-mTOR pathway
The cell’s other key mechanism for controlling cell survival, division and metabolism is the PI3K (phosphatidylinositol 3-kinase)-mTOR (mammalian target of rapamycin) pathway. mTOR complex 1 (mTORC1) responds to growth factors, energy status, amino acid levels, and cellular stress. Growth factors activate the lipid kinase PI3K through either direct PI3K recruitment to the growth factor receptors or indirect recruitment involving the IRS (insulin receptor substrate) or GAB (GRB2-associated binder) docking proteins. PI3K generates PIP3 (phosphatidyl inositol 3,4,5 tri-phosphate), which recruits the protein kinase AKT to the plasma membrane where it is activated by 3-phosphoinositide-dependent kinase 1 (PDK1) and the second mTOR complex, mTORC2 (see below). AKT phosphorylates many survival, proliferation, and motility factors as well as the TSC2 (tuberous sclerosis complex 2) GAP (GTPase activating protein). AKT phosphorylation of TSC2 releases TSC inhibition of the GTPase RHEB (Ras homolog enriched in brain). RHEB-GTP directly activates mTORC1 ([6], Figure 1b).
mTORC1 consists of the Ser/Thr kinase mTOR, a scaffolding protein RAPTOR (regulatory-associated protein of mTOR) and mLST8 (mammalian lethal with Sec13 protein 8). This complex phosphorylates eukaryotic initiation factor 4E (eIF4E)-binding protein (4E-BP) and S6K (p70 ribosomal S6 Kinase), events which modulate ribosome biogenesis and the translation of proteins that promote cell growth and division, including c-Myc and cyclin D (Figure 1b). Notably, 4E-BP phosphorylation inhibits its ability to bind and sequester the eIF4E mRNA cap-binding protein, thereby permitting the assembly of the cap-binding complex and subsequent translation initiation. S6K, by contrast, is activated by mTORC1 phosphorylation. S6K acts on many substrates, including transcription factors, the ribosomal protein S6, RNA helicases and other protein substrates involved in translation initiation and elongation [6].
Amino acid availability, energy status, and oxygen levels feed into this core PI3K-mTORC1 pathway. Amino acids positively regulate mTORC1 through many mechanisms, one of which involves activation of the Rag GTPases, which recruit mTORC1 to lysosomes where it co-localizes with RHEB (Figure 1b). Glucose deprivation and hypoxia negatively regulate mTORC1 by increasing AMP:ATP ratios, resulting in AMPK (5’ AMP-activated protein kinase) activation. Activated AMPK then phosphorylates TSC2, priming it for additional activating phosphorylations by GSK3 (glycogen synthase kinase 3) α and β. Activation of the GAP function of TSC results in inhibition of RHEB-mTORC1 signaling. AMPK also suppresses mTORC1 signaling by directly phosphorylating RAPTOR [6].
mTOR also functions in a second protein complex called mTORC2, which contains the scaffolding protein RICTOR (rapamycin-insensitive companion of mTOR), and the regulatory proteins mLST8 and mSIN1 (mammalian stress-activated protein kinase interacting protein). Just as RAPTOR scaffolds mTOR to mTORC1 substrates, RICTOR directs mTOR to mTORC2 substrates [7]. Growth factors and PI3K signaling activate mTORC2; however, the mechanism is poorly understood. mTORC2 phosphorylates and activates AKT, SGK (serum glucocorticoid-induced kinase), and specific PKC isoforms (Figure 1b). mTORC2 activity regulates cytoskeleton organization, cell survival, and lipid metabolism, but the relative roles of AKT, SGK, and PKC in these phenotypes has not been thoroughly investigated [8].
Ras-ERK and PI3K-mTORC1 signaling dynamics
The intensity and duration of pathway activation are regulated by the strength of the stimulus and by feedback loops. Importantly, the agonists involved in Ras-ERK activation only partially overlap with those that signal to PI3K-mTORC1. PMA (phorbol 12-myristate 13-acetate) is generally a strong activator of the Ras-ERK pathway. By contrast, insulin, and IGF1 (insulin growth factor-1) are weaker Ras-ERK activators, but strong PI3K-mTORC1 activators [9, 10]. However, the degree of pathway activation by specific growth factors often depends on the amount of growth factor, the expression and cell surface localization of their cognate RTKs, and the expression of receptor family members and various docking proteins. [11]
Signaling dynamics are also often greatly influenced by positive feedforward and negative feedback loops, which function in both the Ras-ERK and PI3K-mTORC1 pathways. One positive loop involves the GAB docking proteins, which bind the GRB2–SOS complex on activated RTKs. GABs can bind RasGAP, SHP2, PI3K, and PIP3 (Src homology 2 domain-containing protein-tyrosine phosphatase). SHP2 dephosphorylates the RasGAP docking sites on GAB1 and its associated RTKs, thereby reducing Ras inactivation and augmenting Ras-ERK signaling [12]. GAB1 recruitment of PI3K generates local PIP3, which recruits additional GAB1 to the membrane and further increases PI3K signaling [11, 12].
By contrast, negative feedback loops within each pathway can dampen Ras-ERK and PI3K-mTORC1 signaling. For example, ERK phosphorylates and inhibits SOS, Raf, and MEK1, thereby reducing ERK activation (Figure 1a, [4]). ERK also induces the expression of genes encoding sprouty proteins which interfere with Raf-mediated MEK activation, as well as MAPK phosphatases, which inactivate ERK [4]. Examples of PI3K-mTORC1 negative feedback loops are S6K phosphorylation of IRS and RICTOR, which reduce AKT activity and mTORC1 signaling (Figure 1b, [6, 13–15]).
Mechanisms of pathway integration
One of the first hints of Ras-ERK and PI3K-mTORC1 pathway integration arose during the early 1990s when our lab described both PI3K-dependent and–independent inputs into p70S6K activation [16]. In the intervening years, many mechanisms and modes of crosstalk have been uncovered. These include cross-inhibition, cross-activation, and pathway convergence on substrates (box 2).
Box 2. Mechanisms of pathway crosstalk.
Negative feedback loop
In such loops, a member of one signaling pathway inhibits its own upstream activator.
Cross-inhibition
During cross-inhibition, a member of one pathway negatively regulates an upstream component of another pathway, thereby inhibiting the other pathway’s ability to signal.
Cross-activation
In cases of cross-activation, a member of one pathway positively regulates an upstream component of the another pathway, thereby increasing the activity of the other pathway.
Pathway convergence
This phrasing refers to instances in which two or more signaling pathways directly act on the same complex or protein. The converging signaling pathways both positively or both negatively regulate the complex or protein’s function.
Whereas some of the kinases (Raf, MEK, mTORC1) involved in these pathways have very narrow substrate specificity, others (ERK, RSK, AKT, S6K) phosphorylate several members of the core signaling pathways as well as numerous effector proteins. Accordingly, much of the pathway integration occurs via these latter kinases. ERK is a proline-directed kinase that phosphorylates PXS/TP motifs [17]. RSK, AKT, and S6K are all members of the AGC kinase family and preferentially phosphorylate RXRXXS/T motifs [18]. Interestingly, ERK and the AGC kinases often converge to phosphorylate the same substrate or complex (see below).
Cross-inhibition
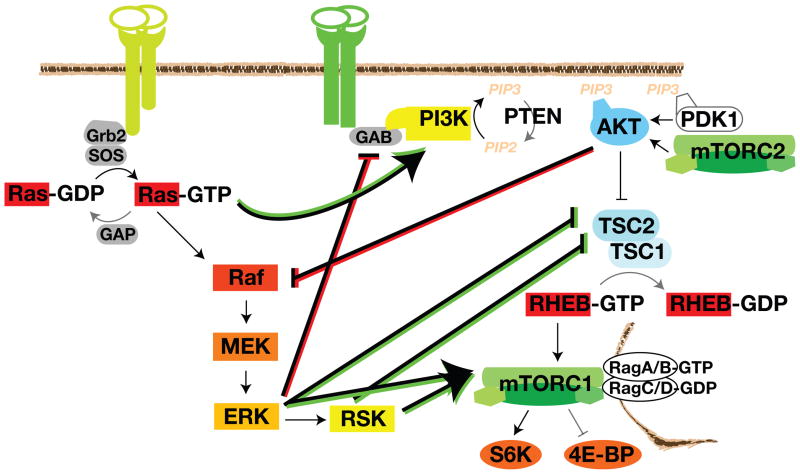
The Ras-ERK and PI3K-AKT pathways can negatively regulate each other’s activity. Such cross-inhibition is often revealed when one pathway is chemically blocked, thereby releasing the cross-inhibition and effectively activating the other pathway. For example, MEK inhibitors enhance epidermal growth factor (EGF)-induced AKT activation [19, 20]. This process might involve EGF-induced ERK phosphorylation of GAB1, which inhibits GAB1-mediated recruitment of PI3K to the EGF receptor (EGFR) (Figure 2). ERK phosphorylates four residues on GAB1, and substitution of these sites abrogates the ability of constitutively active MEK to decrease phospho-AKT levels [21]. These ERK phospho-sites could function to recruit SHP2, which not only dephosphorylates the RasGAP binding sites (see above), but also regulates PI3K binding [22].
Figure 2.
Pathway Crosstalk. The Ras-MAPK and PI3K-mTORC1 pathways regulate each other via cross-inhibition (red) and cross-activation (green). Each pathway has a mechanism to negatively feed onto the other: ERK phosphorylation of GAB and AKT phosphorylation of Raf. Components of the Ras-ERK pathway (Ras, Raf, ERK, and RSK) also positively regulate the PI3K-mTORC1 pathway. TSC2 and mTORC1 are key integration points that receive many inputs from both the Ras-ERK and PI3K signaling. Positive regulation of the substrate protein is shown as an arrow. Negative regulation of the substrate protein is depicted as a blunt-ended line.
An analogous cross-inhibition between AKT and Raf is induced by strong IGF1 stimulation [23]. AKT negatively regulates ERK activation by phosphorylating inhibitory sites in the Raf N-terminus (Figure 2, [24–27]). In response to cAMP agonists, PKA (protein kinase A), another AGC kinase, also phosphorylates the conserved 364/259 site [28, 29]. 14-3-3 dimers recognize the phospho-site and sequester the auto-inhibited Raf in the cytosol, away from Ras and MEK [29]. AKT’s inhibitory phosphorylations on Raf are removed by PP1 (protein phosphatase 1) and/or PP2A during mitogen-stimulated Raf activation [1]. Interestingly, this cross-inhibition is necessary for the progression of benign nevi or moles to melanoma. The benign lesions harbor activating mutations in RAS or RAF, which induce Ras-ERK signaling to such high and sustained levels that cell cycle arrest or senescence occurs. Secondary mutations that activate PI3K-AKT dampen RAS-ERK signaling to levels that cooperate with AKT to induce transformation [27].
Pathway cross-activation
The Ras-ERK pathway cross-activates PI3K-mTORC1 signaling by regulating PI3K, TSC2, and mTORC1. Ras-GTP can directly bind and allosterically activate PI3K [30–32]. Strong activation of the RAS-ERK pathway can also lead to mTORC1 activity by ERK and RSK signaling to the TSC complex, an archetype of signal integration (Figure 2). As discussed above, TSC2 is a heavily phosphorylated protein that senses a variety of growth factor and stress signals [6]. EGF, phorbol esters, and constitutively active Ras mutants can induce ERK and RSK-mediated phosphorylation of TSC2 [8, 33]. The ERK and RSK sites are different from those phosphorylated by AKT, but similarly function to inhibit TSC’s GAP function and promote mTORC1 activity and tumorigenesis [8]. Similar stimuli also induce phosphorylation of RAPTOR by ERK and RSK. RAPTOR phosphorylation promotes mTORC1 phosphorylation of 4E-BP [18, 34, 35]. In this way, phorbol esters and constitutively active Ras and Raf induce PI3K-independent mTORC1 phosphorylation of 4E-BP and tumorigenicity [8, 18, 34–36]. mTORC1 activity towards S6K was not assayed in these studies and the role of S6K-mediated phosphorylation of S6 in tumorigenesis in currently unclear.
MAPK scaffolds appear to regulate several steps of the mTORC1 signaling pathway. MP1 (MEK1 scaffolding protein), which scaffolds MEK and ERK at late endosomes and is required for sustained ERK activity, also scaffolds the Rag GTPases to the lysosome [1, 6, 37]. Thus, MP1 could provide a mechanism which co-localizes ERK and mTORC1 signaling, thereby promoting crosstalk. Recent reports indicate that the KSR (kinase suppressor of Ras) MAPK scaffold interacts with mTOR, RAPTOR, RICTOR, and the TSC2-activating kinases AMPK and GSK3 [38, 39]. In response to growth factors, KSRs translocate from the cytoplasm to the cell membrane where they direct the co-localization of RAF, MEK, and ERK needed for ERK activation [1]. A recent investigation in to the role of KSR interaction with AMPK found KSR2 positively regulates AMPK activation[39] and promotes glucose and fatty acid metabolism, a process also regulated by mTORC1 and mTORC2 [8, 39]. Future research will hopefully clarify any role for KSRs in balancing AMPK activation, which can inhibit mTORC1, with Ras-ERK activation.
Pathway convergence
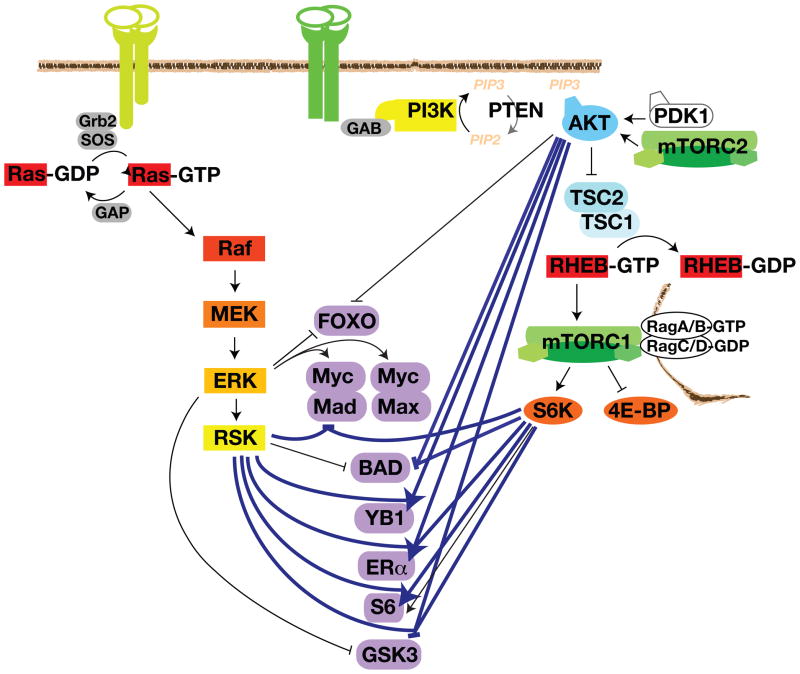
Once activated, ERK, RSK, AKT, and S6K often act on the same substrate, sometimes in concert, to promote cell survival, proliferation, metabolism, and motility. Examples include the FOXO (forkhead box O) and c-Myc transcription factors, BAD (BCL2-associated agonist of cell death), and GSK3. FOXOs regulate the expression of apoptotic proteins and cell cycle regulators to suppress cell survival and proliferation. ERK phosphorylates FOXO3A Ser294, Ser344, and Ser425, which increases FOXO3A interaction with the E3-ubiquitin ligase MDM2, thereby directing FOXO3A’s ubiquitin-proteasome-mediated degradation [40]. AKT and SGK also phosphorylate FOXO1 and FOXO3A at Thr32, Ser253, and Ser315 (FOXO3A numbering) (Figure 3, [41-44]). 14-3-3 proteins bind the phosphorylated sites and sequester FOXOs in the cytosol, thereby precluding their ability to enter the nucleus and activate quiescence and apoptotic gene expression programs [45].
Figure 3.
Pathway Convergence and AGC Kinase Promiscuity. ERK and the AGC kinases often regulate the same substrates to bring about the same phenotypic effects. Situations in which the same residue is phosphorylated by multiple AGC kinases are symbolized in bold blue. Representative substrates for different combination of ERK and AGC kinase inputs are depicted in purple. FOXO and GSK3 are examples of Ras-ERK and PI3K-mTORC1 convergence at different residues on the same substrate via ERK and AGC kinase inputs. The Myc–Mad1 and Myc–Max dimers are examples of the two pathways converging on different members of the same complex. BAD and S6 are examples of AGC kinases regulating different motifs in the same substrate. YB1 and ERα are examples of AGC kinase promiscuity, in which several AGC kinases phosphorylate the same residue. For simplicity, inputs from SGK and PKC are not included.
c-Myc functions as an obligate heterodimer with Max to positively regulate growth and survival transcriptional programs. The related transcription factor Mad1 competes with Max for c-Myc binding, and the Myc–Mad heterodimer represses gene transcription of growth and survival genes. ERK-mediated phosphorylation of Ser62 stabilizes c-Myc [46]. RSK and S6K feed into this pathway by phosphorylating Mad1 on Ser145 (Figure 3). This promotes Mad1 ubiquitylation and degradation, Myc–Max dimerization, and the induction of pro-survival and growth genes [47].
BAD is a pro-apoptotic BCL2 family BH3-only protein. Hypophosphorylated BAD interacts with and neutralizes the pro-survival BCL2 family proteins, which frees BAX and BAK to induce apoptosis at the mitochondria. PKA and RSK phosphorylate BAD at Ser112 [48–50], and AKT and S6K phosphorylate BAD on Ser136 (Figure 3, [41, 51]). In some cell types, the related kinases PKC can also phosphorylate BAD on these and an additional site (Ser155) [52, 53]. Both Ser 112 and Ser136 reside within RXRXXS consensus motifs that when phosphorylated, are recognized by 14-3-3 proteins. 14-3-3 binding promotes cell survival by sequestering BAD in the cytosol, away from the mitochondria and pro-survival BCL2 family members [54]. Inhibition of both MEK and PI3K is required to release BAD from 14-3-3 and induce apoptosis [55]. BIM, another pro-apoptotic BH3-only protein, is also regulated by ERK phosphorylation-induced proteasomal degradation and AKT-induced sequestration by 14-3-3 [56].
Wnt, insulin, and other growth factors inactivate the GSK3 α/β Ser/Thr kinases. This releases GSK3-mediated inhibition of pro-survival, proliferation, and motility proteins, such as adhesion proteins and the β-catenin transcription factor that drives the expression of cyclin D and MYC. Among many other substrates involved in these functions, GSK3 also phosphorylates TSC2, thereby activating TSC2 and inactivating mTORC1 [6]. ERK phosphorylates GSK3β at Thr43, which primes GSK3β for subsequent phosphorylation and inactivation by RSK (Ser9) (Figure 3, [57]). In different cell types and under different stimulatory conditions, AKT, PKA, PKC, and S6K can each phosphorylate this AGC kinase site and will be further discussed in the following section.
AGC kinase promiscuity
The Ras-ERK and PI3K-mTOR pathways additionally converge via AGC kinase promiscuity. RSK’s N-terminal kinase domain, AKT, S6K, SGK, and PKC are all AGC kinases that share a similar architecture and a requirement for PDK1 phosphorylation of their activation loop [18]. The AKT–PDK1 interaction requires PDK1 interaction with PIP3 at the plasma membrane. By contrast, RSK, S6K, SGK, and PKC interact with PDK1 in the cytosol via their phosphorylated hydrophobic motifs [3, 18]. When RSK’s C-terminal kinase domain’s activation loop is phosphorylated by ERK, the C-terminal kinase domain autophosphorylates the hydrophobic motif within the N-terminal kinase domain [3]. mTORC1 phosphorylates the hydrophobic motif on S6K and mTORC2 phosphorylates the hydrophobic motifs of AKT, PKC and SGK [18]. The data do not exclude contributions from other kinases to the phosphorylation of these AGC kinases’ hydrophobic motifs under specific conditions. In addition, AKT hydrophobic motif phosphorylation increases AKT kinase activity, but its role in mediating AKT activity towards specific substrates is unclear.
Owing to their propensity to phosphorylate RXRXXS/T motifs, RSK, S6K, AKT and SGK often, but not always, phosphorylate the same regulatory sites on the same substrates (Figure 3). AKT strictly requires an Arg in the -5 position, whereas RSK, S6K, and SGK phosphorylate motifs with either Arg or Lys in this position [41]. Some RSK, AKT, S6K, and SGK substrates are also targeted by conventional PKCs that recognize RKXS/TXR motifs [58]. A specific substrate can be phosphorylated by one or a combination of AGC kinases at a given time in any given cell type, depending on the stimulus type, strength, and duration and the individual AGC kinase’s expression level, subcellular location, and cell cycle-dependent activation status.
Common AGC kinase substrates: cell survival, proliferation, and motility
The AGC kinases co-regulate several proteins involved in ERK- and PI3K-mTOR-mediated cell survival, proliferation, and motility. As discussed above, the GSK3 α/β kinases regulate cell survival, proliferation, metabolism, and motility through their phosphorylation of transcription factors, and cytosolic, cytoskeleton, and adhesion proteins. AGC kinases phosphorylate GSK3 α/β on Ser21/Ser9, which creates pseudo-substrate sites that induce intra-molecular inhibition (Figure 3, [8, 41, 59]). RSK phosphorylates these sites in response to EGF and phorbol ester stimulation and HBX (hepatitis virus B protein X) expression [57, 60, 61]. AKT phosphorylates these sites in response to insulin and IGF stimulation [8, 41, 60]. However, when AKT is inhibited by an S6K-mediated negative feedback loop, S6K phosphorylates GSK3β Ser9 [18]. PKA, which is activated by cAMP, can also phosphorylate these sites [62]. Furthermore, conventional PKCs can phosphorylate GSK3β, but not GSK3α, in vitro [63]. HBX, insulin, and in vitro RSK and S6K-induced GSK3 phosphorylation inhibit GSK3 activity towards many substrates including β-catenin and glycogen synthase [55, 62, 63].
RSK, AKT, and S6K also co-regulate cell survival and proliferation by phosphorylating the Mad1, YB1 (Y-box binding protein 1), and ERα (estrogen receptor α) transcription factors. These transcription factors are widely expressed in human cancers, and they respectively bind E-boxes, Y-boxes, and EREs (estrogen-response elements) in the promoters of their unique and mutual target genes, thereby inducing pro-growth and motility genes and repressing cell death and cell cycle arrest genes. As mentioned above, RSK and S6K phosphorylate Mad1 on Ser145, inducing its proteasomal degradation and allowing Myc heterodimerization with Max (Figure 3, [47]). Myc–Max induces the expression of genes that promote cell division, protein biosynthesis, and metabolism and represses the expression of genes that induce cell adhesion and inhibit cell division [47].
AKT- and RSK phosphorylate YB1 on Ser102, which upregulates its transcriptional activity, thereby inducing proliferation and anchorage-independent growth (Figure 3, [66, 67]). YB1 also binds and represses the translation of cap-dependent pro-growth mRNAs; Ser102 phosphorylation inhibits this function [68]. Whereas YB1 can also promote the translation of specific mRNAs involved in EMT (epithelial-to-mesenchymal transition) and motility, this latter function is not known to be regulated by AGC kinase phosphorylation.
ERα also drives the expression of survival and proliferative genes, including cyclin D1, EGFR, FOS, and MYC. RSK, AKT, and S6K phosphorylate ERα on Ser167; this confers estrogen-independent transcriptional activity (Figure 3, [69–71]). ERα Ser167 exemplifies how RSK and S6K inputs on the same site can be temporally restricted: MEK inhibition eliminates the phosphorylation of ERα that occurs within the first 2–15 minutes of PMA stimulation, whereas mTORC1 inhibition eliminates the phosphorylation that occurs after 30–90 minutes of stimulation. The two time frames correlate with the activation status of RSK and S6K, respectively, suggesting that the kinases’ signaling specificity is achieved by their activation kinetics [69].
The AGC kinases additionally converge on p27kip1 and filamin A to regulate cell motility. p27kip1 is a cell cycle regulatory protein that also functions in the cytoplasm to bind and inhibit RhoA GTP-loading and Rho-associated, coiled-coil containing protein kinase (ROCK) activation [72, 73]. p27kip1 is compartmentalized into the cytoplasm by 14-3-3 binding to phosphorylated Thr198 [74, 75]. Depending on the cell type and stimulus condition, RSK, AKT, and/or SGK phosphorylate p27 on Thr198 [73, 75, 76]. AKT and SGK also phosphorylate Thr157 [76, 77]. Ser10 and Thr187 are also phosphorylated by AKT, although contributions from other AGC kinases cannot be excluded [74]. Interestingly, Ser10 and Thr157 phosphorylation regulates p27kip1 cytoplasmic localization via a 14-3-3-independent mechanism. Ser10 phosphorylation promotes nuclear export and Thr157 phosphorylation, which occurs in the cytoplasm during the G1 phase of the cell cycle. Thr157 phosphorylation interferes with p27kip binding to importin α, thereby preventing nuclear-re-entry [77].
Filamin A promotes cell motility by cross-linking and stabilizing actin filaments. RSK and the un-related PAK (p21-activated kinase) phosphorylate filamin A at Ser2152 [78, 79] and this phosphorylation is required for membrane ruffling. AKT or S6K might also phosphorylate this site: the phosphorylation increases with IGF1 stimulation and is sensitive to PI3K inhibition [80]. However, the data are not definitive given that PI3K activates GEFs for the Rac and CDC42 (cell division control 42) GTPases, which can activate S6K and PAK [81, 82]. Additional RSK and AKT substrates involved in cell motility, such as the Fra-1 transcription factor, girdin and palladin actin-binding proteins, and the endosomal sorting protein ACAP1, have not been thoroughly investigated for phosphorylation by other AGC kinases.
Common AGC kinase substrates: protein synthesis
The AGC kinases are key regulators of protein synthesis and folding. In addition to regulating unique substrates that influence protein synthesis, RSK, AKT, and S6K share the ability to phosphorylate and regulate eIF4B (eukaryotic initiation factor 4B), eEF2K (eukaryotic elongation factor 2 kinase), rpS6 (ribosomal protein S6), and the CCT (chaperonin containing TCP1). eIF4B is a co-factor for the eIF4A ATPase/helicase that unwinds the secondary structures of 5’ untranslated regions (UTRs) during translation initiation [6]. RSK, AKT, and S6K phosphorylate eIF4B on Ser422, which promotes eIF4B association with the pre-initiation complex [6, 83, 84]. RSK and S6K also phosphorylate eIF4B Ser406. Phosphorylation of either Ser422 or Ser406 is required for efficient translation [84]. RSK and S6K regulate translation elongation by phosphorylating eEF2K, an inhibitor of the translation elongation factor eEF2. eEF2, in turn, promotes translocation of growing polypeptide chains through the ribosome, and RSK and S6K inhibit eEF2K by phosphorylating Ser366, thereby eliminating eEF2 inhibition and activating translation elongation [18].
Originally identified as kinases that phosphorylate rpS6, RSK and S6K phosphorylate rpS6 Ser235/Ser236, and S6K additionally phosphorylates Ser240/244 (Figure 3). These phosphorylations positively regulate rpS6 binding to the 7-methylguanosine cap complex, subsequent cap-dependent translation, and cell size [85, 86]. RSK and S6K might also regulate protein synthesis at the level of folding. These two AGC kinases phosphorylate CCTβ on Ser260. Although it is not known if the phosphorylation affects CCT’s binding to or folding of substrates, the phosphorylation does promote cell proliferation [87].
Common AGC kinase substrates: other cellular processes
As the known repertoire of AGC kinase substrates grows, novel co-regulated substrates involved in various cellular functions continue to be discovered. For example, RSK and AKT phosphorylate RanBP3 on S58, which promotes nuclear import by maintaining the cytoplasmic to nuclear Ran gradient [88]. RSK, AKT, and SGK also phosphorylate the same site on the Rab GAP AS160 (AKT substrate of 160 kDa). In unstimulated 3T3-L1 adipocyctes, AS160 restricts GLUT4 (glucose transporter) localization to intracellular vesicles. AKT phosphorylates a series of residues on AS160 [18, 89, 90], of which Ser318, Ser588, Thr642, and Ser751 phosphorylation are required for insulin-induced GLUT4 translocation to the plasma membrane [18]. These sites are probably also targeted by RSK and SGK, given that the phosphorylations are sensitive to RSK inhibition and can occur in vitro with purified RSK and SGK [89].
Therapeutic inhibition of Ras-ERK and PI3K-mTORC1
As major regulators of cell survival, proliferation, metabolism, and motility, the Ras-ERK and PI3K-mTORC1 pathways are commonly activated during oncogenesis. Owing to cross-activation and pathway convergence, the resulting activation of Ras-ERK and PI3K-mTOR signaling could theoretically facilitate the development of resistance to therapeutics targeting only one pathway. Indeed, concurrent KRAS/BRAF and PI3K/PTEN mutations reduce the cytostatic response of cancer cell lines to AKT and mTOR inhibitors [8, 91]. Furthermore, [92, 93]chronic treatment of melanoma cells harboring activating BRAFV600E mutations can induce resistance via Raf isoform switching, upregulation of the MAPKKK COT (also called TPL2) and IGF1R-PI3K-AKT signaling pathways [94–96]. Tumor samples from phase I clinical trials with Raf inhibitors also exhibit NRAS mutations and PDGFR upregulation [97]. The Raf isoform switching, COT upregulation, and RAS mutation mechanisms still rely on ERK signaling and would thus be susceptible to MEK or ERK inhibition. However, RTK-mediated activation of alternative survival pathways requires co-inhibition of PI3K-mTORC1 signaling. Encouragingly, treatment with inhibitors to both pathways kills resistant melanoma lines and effectively inhibits tumor growth in prostate and lung cancer mouse models [27, 92–94].
Even in cases of “oncogene addiction” in which one signaling pathway appears to drive cancer progression, pathway cross-inhibition reduces the effectiveness of single agents. Basal-like breast cancers exhibit high levels of activated ERK and EGFR and RAS-like transcriptional profiles that predict a proliferation arrest to MEK inhibitors [20, 98]. However, treatment of basal-like cell lines with MEK inhibitors increases AKT activity and is cytostatic, rather than cytotoxic. Supplementing MEK inhibition with PI3K inhibition induced cell death in vitro and tumor regression in xenograft models [20, 98]. Conversely, analysis of tumor biopsies from phase I clinical trials with an mTORC1 inhibitor revealed an upregulation of ERK activity in metastatic breast cancer patients [97]. Studies in normal cells and cancer cell lines uncovered an undefined mechanism of cross-inhibition in which S6K signaling inhibits Ras-ERK activity [99].
Concluding remarks
The Ras-ERK and PI3K-mTORC1 pathways represent key mechanisms for cells to regulate cell survival, proliferation, and motility. In addition to their independent signaling programs that provide compensatory mechanisms, the pathways extensively cross-talk to both positively and negatively regulate each other. Encouragingly, co-inhibition of both pathways has been successful in reducing tumor growth in xenograft cancer models and importantly, also in genetically engineered mouse models [92, 93]. The utility of specific RTK inhibitors, Raf versus MEK, or PI3K versus mTOR versus dual PI3K-mTOR inhibitors must be assessed for each cancer subtype based on the known activation of RAS-MAPK and PI3K-mTOR signaling. Thus, the ability to uncover a patient’s signaling signature will play a major role in the future development of personalized therapies. Although more work is required to understand this specificity (Box 3), two decades of experimentation aimed at understanding the mechanisms of Ras-ERK and PI3K-mTORC1 signaling are beginning to pay off.
Box 3. Outstanding questions.
How is positive cross-talk balanced with negative cross-inhibition?
Do the regulatory loops cause signaling between the two pathways to oscillate, and would such oscillations contribute to a cells’ ability to continuously respond to sustained extracellular stimulation during growth, cell cycle progression, development, or motility?
Do MAPK scaffolds contribute to Ras-MAPK and mTORC1 cross-talk?
Can biomarkers for nodes in each pathway be used to determine the relative activity of cross-pathway signaling and predict which therapy combinations would best target cancers of a specific tissue type (personalized therapy)?
Could scaffolds or proteins involved in complex assembly and stability (such as heat shock protein 90; HSP90), be viable therapeutic targets in combination therapies with Ras-MAPK and PI3K-mTORC1 pathway inhibitors?
Acknowledgments
This work was funded by the Susan G. Komen (M.C.M.) and NCI R37CA46595 (J.B.). JB is an Established Investigator of the LAM Foundation. There are many primary research papers and excellent reviews that we were unable to reference due to space limitations. We apologize for this situation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26(22):3113–21. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 2.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213(3):589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 3.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9(10):747–58. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon AS, et al. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 5.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26(22):3227–39. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 6.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40(2):310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alessi DR, Pearce LR, Garcia-Martinez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2(67):pe27. doi: 10.1126/scisignal.267pe27. [DOI] [PubMed] [Google Scholar]
- 8.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerk A, et al. Peptide growth factors signal differentially through protein kinase C to extracellular signal-regulated kinases in neonatal cardiomyocytes. Cell Signal. 2006;18(2):225–35. doi: 10.1016/j.cellsig.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Weng LP, et al. PTEN inhibits insulin-stimulated MEK/MAPK activation and cell growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos complex formation in a breast cancer model. Hum Mol Genet. 2001;10(6):605–16. doi: 10.1093/hmg/10.6.605. [DOI] [PubMed] [Google Scholar]
- 11.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohrle FU, Daly RJ, Brummer T. Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun Signal. 2009;7:22. doi: 10.1186/1478-811X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29(21):5657–70. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julien LA, et al. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol. 2010;30(4):908–21. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treins C, et al. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 2010;29(7):1003–16. doi: 10.1038/onc.2009.401. [DOI] [PubMed] [Google Scholar]
- 16.Chung J, et al. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370(6484):71–5. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 17.Hutti JE, et al. A rapid method for determining protein kinase phosphorylation specificity. Nat Methods. 2004;1(1):27–9. doi: 10.1038/nmeth708. [DOI] [PubMed] [Google Scholar]
- 18.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11(1):9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 19.Yu CF, Liu ZX, Cantley LG. ERK negatively regulates the epidermal growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase. J Biol Chem. 2002;277(22):19382–8. doi: 10.1074/jbc.M200732200. [DOI] [PubMed] [Google Scholar]
- 20.Hoeflich KP, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15(14):4649–64. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 21.Lehr S, et al. Identification of major ERK-related phosphorylation sites in Gab1. Biochemistry. 2004;43(38):12133–40. doi: 10.1021/bi049753e. [DOI] [PubMed] [Google Scholar]
- 22.Zhang SQ, et al. Receptor-specific regulation of phosphatidylinositol 3'-kinase activation by the protein tyrosine phosphatase Shp2. Mol Cell Biol. 2002;22(12):4062–72. doi: 10.1128/MCB.22.12.4062-4072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moelling K, et al. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277(34):31099–106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286(5445):1741–4. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 25.Dhillon AS, et al. Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J. 2002;21(1-2):64–71. doi: 10.1093/emboj/21.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan KL, et al. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem. 2000;275(35):27354–9. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- 27.Cheung M, et al. Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68(9):3429–39. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhillon AS, et al. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol. 2002;22(10):3237–46. doi: 10.1128/MCB.22.10.3237-3246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumaz N, Marais R. Protein kinase A blocks Raf-1 activity by stimulating 14-3-3 binding and blocking Raf-1 interaction with Ras. J Biol Chem. 2003;278(32):29819–23. doi: 10.1074/jbc.C300182200. [DOI] [PubMed] [Google Scholar]
- 30.Kodaki T, et al. The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol. 1994;4(9):798–806. doi: 10.1016/s0960-9822(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Viciana P, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370(6490):527–32. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 32.Suire S, Hawkins P, Stephens L. Activation of phosphoinositide 3-kinase gamma by Ras. Curr Biol. 2002;12(13):1068–75. doi: 10.1016/s0960-9822(02)00933-8. [DOI] [PubMed] [Google Scholar]
- 33.Roux PP, et al. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101(37):13489–94. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carriere A, et al. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J Biol Chem. 2011;286(1):567–77. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster KG, et al. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J Biol Chem. 2010;285(1):80–94. doi: 10.1074/jbc.M109.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbert TP, Tee AR, Proud CG. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J Biol Chem. 2002;277(13):11591–6. doi: 10.1074/jbc.M110367200. [DOI] [PubMed] [Google Scholar]
- 37.Nada S, et al. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J. 2009;28(5):477–89. doi: 10.1038/emboj.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dougherty MK, et al. KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol Cell. 2009;34(6):652–62. doi: 10.1016/j.molcel.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costanzo-Garvey DL, et al. KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 2009;10(5):366–78. doi: 10.1016/j.cmet.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JY, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10(2):138–48. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biggs WH, 3rd, et al. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96(13):7421–6. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rena G, et al. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274(24):17179–83. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 44.Tang ED, et al. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274(24):16741–6. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 45.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 46.Sears R, et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci U S A. 2008;105(18):6584–9. doi: 10.1073/pnas.0802785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonni A, et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286(5443):1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 49.Shimamura A, et al. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr Biol. 2000;10(3):127–35. doi: 10.1016/s0960-9822(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 50.Tan Y, et al. p90(RSK) blocks bad-mediated cell death via a protein kinase C-dependent pathway. J Biol Chem. 1999;274(49):34859–67. doi: 10.1074/jbc.274.49.34859. [DOI] [PubMed] [Google Scholar]
- 51.Harada H, et al. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci U S A. 2001;98(17):9666–70. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harada H, et al. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3(4):413–22. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- 53.Thimmaiah KN, Easton JB, Houghton PJ. Protection from rapamycin-induced apoptosis by insulin-like growth factor-I is partially dependent on protein kinase C signaling. Cancer Res. 2010;70(5):2000–9. doi: 10.1158/0008-5472.CAN-09-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zha J, et al. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87(4):619–28. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 55.She QB, et al. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8(4):287–97. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281(2):813–23. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 57.Ding Q, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19(2):159–70. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Nishikawa K, et al. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272(2):952–60. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 59.Dajani R, et al. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105(6):721–32. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 60.Shaw M, Cohen P. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS Lett. 1999;461(1–2):120–4. doi: 10.1016/s0014-5793(99)01434-9. [DOI] [PubMed] [Google Scholar]
- 61.Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303(Pt 3):701–4. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang X, et al. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97(22):11960–5. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goode N, et al. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992;267(24):16878–82. [PubMed] [Google Scholar]
- 64.Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem. 2000;275(42):32475–81. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 65.Sutherland C, Cohen P. The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994;338(1):37–42. doi: 10.1016/0014-5793(94)80112-6. [DOI] [PubMed] [Google Scholar]
- 66.Sutherland BW, et al. Akt phosphorylates the Y-box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene. 2005;24(26):4281–92. doi: 10.1038/sj.onc.1208590. [DOI] [PubMed] [Google Scholar]
- 67.Stratford AL, et al. Y-box binding protein-1 serine 102 is a downstream target of p90 ribosomal S6 kinase in basal-like breast cancer cells. Breast Cancer Res. 2008;10(6):R99. doi: 10.1186/bcr2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evdokimova V, et al. Akt-mediated YB-1 phosphorylation activates translation of silent mRNA species. Mol Cell Biol. 2006;26(1):277–92. doi: 10.1128/MCB.26.1.277-292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 584(1):124–8. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun M, et al. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61(16):5985–91. [PubMed] [Google Scholar]
- 71.Joel PB, et al. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol Cell Biol. 1998;18(4):1978–84. doi: 10.1128/mcb.18.4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Besson A, et al. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18(8):862–76. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larrea MD, et al. RSK1 drives p27Kip1 phosphorylation at T198 to promote RhoA inhibition and increase cell motility. Proc Natl Acad Sci U S A. 2009;106(23):9268–73. doi: 10.1073/pnas.0805057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fujita N, et al. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2002;277(32):28706–13. doi: 10.1074/jbc.M203668200. [DOI] [PubMed] [Google Scholar]
- 75.Fujita N, Sato S, Tsuruo T. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2003;278(49):49254–60. doi: 10.1074/jbc.M306614200. [DOI] [PubMed] [Google Scholar]
- 76.Hong F, et al. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30(6):701–11. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 77.Shin I, et al. Phosphorylation of p27Kip1 at Thr-157 interferes with its association with importin alpha during G1 and prevents nuclear re-entry. J Biol Chem. 2005;280(7):6055–63. doi: 10.1074/jbc.M412367200. [DOI] [PubMed] [Google Scholar]
- 78.Woo MS, et al. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24(7):3025–35. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vadlamudi RK, et al. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4(9):681–90. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 80.Ravid D, et al. Filamin A is a novel caveolin-1-dependent target in IGF-I-stimulated cancer cell migration. Exp Cell Res. 2008;314(15):2762–73. doi: 10.1016/j.yexcr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 81.Chou MM, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85(4):573–83. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 82.Whale A, et al. Signalling to cancer cell invasion through PAK family kinases. Front Biosci. 2011;16:849–64. doi: 10.2741/3724. [DOI] [PubMed] [Google Scholar]
- 83.Holz MK, et al. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123(4):569–80. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 84.van Gorp AG, et al. AGC kinases regulate phosphorylation and activation of eukaryotic translation initiation factor 4B. Oncogene. 2009;28(1):95–106. doi: 10.1038/onc.2008.367. [DOI] [PubMed] [Google Scholar]
- 85.Roux PP, et al. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282(19):14056–64. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31(6):342–8. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Abe Y, et al. p90 ribosomal S6 kinase and p70 ribosomal S6 kinase link phosphorylation of the eukaryotic chaperonin containing TCP-1 to growth factor, insulin, and nutrient signaling. J Biol Chem. 2009;284(22):14939–48. doi: 10.1074/jbc.M900097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoon SO, et al. Ran-binding protein 3 phosphorylation links the Ras and PI3-kinase pathways to nucleocytoplasmic transport. Mol Cell. 2008;29(3):362–75. doi: 10.1016/j.molcel.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geraghty KM, et al. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J. 2007;407(2):231–41. doi: 10.1042/BJ20070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kane S, et al. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277(25):22115–8. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 91.Di Nicolantonio F, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 2010;120(8):2858–66. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kinkade CW, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118(9):3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14(12):1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flaherty KT, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Villanueva J, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18(6):683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johannessen CM, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–72. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nazarian R, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mirzoeva OK, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69(2):565–72. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carracedo A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118(9):3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]