Abstract
We investigated the influence of drug network characteristics including trust, size, and stability on HIV risk behaviors and HIV testing among injection drug users (IDUs) in St. Petersburg, Russia. Overall, male and female IDUs who reported having high levels of trust in their drug networks were significantly more likely to share syringes than those with lower levels of trust (OR [95% CI]) 2.87 [1.06, 7.81] and 4.89 [1.05, 21.94], respectively). Male and female IDUs in larger drug networks were more likely to share syringes than those in smaller networks (4.21 [1.54, 11.51] and 4.80 [1.20, 19.94], respectively). Characteristics that were significantly associated with not having been HIV tested included drug network instability among men and larger network size among women. High trust, large size, and instability were positively and significantly associated with syringe sharing and not having been HIV tested. Effectiveness of interventions in Russia to reduce the risk of HIV infection may be enhanced if network characteristics are addressed.
Keywords: Drug networks, HIV, Russia, IDU
Introduction
Since the dissolution of the Soviet Union, Russia has been experiencing one of the fastest growing HIV epidemics in the world [1]. According to government officials, as of October 2009, there were 516,167 registered cases of HIV, however estimates are believed to be much higher [2]. Economic and political upheaval in the early and mid-1990s allowed illicit drug markets, most notably that for heroin, to flourish and fuel dual epidemics of injection drug use and HIV. Consequently, the HIV epidemic in Russia is concentrated among injection drug users (IDUs), with more than 80% of all HIV cases attributed to injection of illicit substances [3]. In St. Petersburg, the second largest city in Russia, 1.81% of the 4.6 million inhabitants were estimated to be IDUs [4]. With HIV prevalence approaching 50% among IDUs in St. Petersburg [5–7] and an incidence rate estimated to be 14.1/100 person-years [8], a clearer understanding of the social and behavioral aspects of HIV transmission among IDUs is required.
The influence of social networks on behaviors, perceptions, and norms has been researched extensively. One’s social environment directly affects individual behavior and norms [9–11]. Within the context of injection drug use, drug network characteristics such as large size and close ties to others in the network have been implicated in high-risk injection practices [12–15].
Most of the research on drug network influences and HIV risk has been conducted in the United States, which is economically and culturally distinct from Russia. In the Russian Federation, high levels of distrust in the government exist, thus individuals often seek support and advice from within their social networks [16, 17]. Indeed, strong ties within injecting dyads in St. Petersburg were significantly associated with receptive and distributive syringe sharing [18]. The purpose of the present study was to extend research from injecting dyads to the broader issue of drug networks and those network characteristics that influence HIV-related behaviors. Specifically, we hypothesized roles for trust, size, and stability of drug networks and examined their influence on high-risk injection practices and HIV testing. We were particularly interested in these drug network characteristics because previous research has shown that trust [19], network size [13, 20], and stability [21, 22] are influential in HIV risk behavior in injecting drug environments. Furthermore, based on members of the research team experience working with this population, we believed that obtaining data regarding these network characteristics could provide new insight into the role of peer influence on HIV-risk and serve as essential foundations for larger-scale network interventions in Russia. It was also deemed feasible to collect these measures using a cross-sectional study design while maintaining anonymity for study participants.
The outcomes of interest in the present study are important because high-risk injection practices continue to propagate ongoing transmission, and knowing one’s HIV status is an important factor in risk reduction. In Russia, HIV testing coverage is suboptimal and primarily conducted in the context of a doctor or hospital visit for another reason or contact with an institution that conducts routine testing such as prisons and drug treatment centers [6]. Because testing coverage is low (approximately 80% of IDUs have ever been tested for HIV) and more often routine than voluntary, there is room for improvement in coverage via voluntary testing; thus, understanding factors associated with not having been tested is important. A thorough understanding of the network characteristics significantly associated with HIV testing could highlight new targets in future HIV testing strategies in order to increase coverage of IDU populations.
Methods
Sample Recruitment
We used two methods to recruit IDUs into the study. First, participants were recruited by convenience sampling from health care centers (addiction treatment clinics and public infectious disease hospitals) in St. Petersburg by social workers, from June 2009 to August 2009. The social workers, who were familiar with the target population through their work, offered IDUs the opportunity to enroll in the study and if the potential participant agreed, an appointment was arranged for an interview at a later time. Research staff, consisting of trained sociologists and the social workers involved with recruiting, administered the interviews in clinics at various locations throughout the city. The second recruitment strategy was peer referral using dual incentives to recruit members of hidden populations [23]. Participants who were recruited into the study were given three coupons to distribute to peers in their drug networks. All participants were reimbursed for participation and additional reimbursement was provided for successful recruitment of peers.
Eligibility criteria included being at least 18 years of age and reporting injection drug use within the past 30 days. All respondents initialed the consent form to maintain anonymity after the interviewer provided a detailed description of the nature of the study. Reimbursement for participation in the study included several gifts including tea, chocolate, hygiene products, and compensation for transportation costs (total value approximately 10$ US). After the conclusion of the interview, participants received information on HIV prevention and locations for HIV testing. Ethics approval was granted by Yale University Human Investigation Committee and the St. Petersburg State University Institutional Review Board.
Measures
The survey instrument included sections pertaining to sociodemographics, characteristics of drug networks including size and composition of networks and trust within the networks (as described below), drug and syringe/needle behaviors, and HIV testing and disclosure of HIV status to injection and sexual partners. Sociodemographic variables of interest included age, education, employment, marital status, type of drugs used, duration of drug use, and self-reported HIV status.
The independent variables of interest were levels of trust within the injecting networks, network size measured by the number of injecting partners in the past 30 days, and stability measured by having strangers or someone whom the participant did not know very well present when injecting with others. The trust items consisted of four questions related to trust in networks about injecting behaviors and communication pertaining to HIV testing and status. Specifically, the four trust questions were: (1) “If someone with whom you inject drugs gives you a sterile syringe, how sure can you be that this syringe is actually sterile?” (2) “If someone with whom you inject drugs informs you that he/she went to get tested for HIV, how sure are you that this is true?” (3) “If someone with whom you inject drugs tells you that he/she is HIV-negative, how sure are you that he/she is actually HIV-negative?” (4) “If someone with whom you inject drugs is infected with HIV, how sure would you be that this person would disclose his/her status to you?” Responses ranged from “absolutely not sure” to “absolutely sure” and were coded from 1 to 5. Cronbach’s alpha was highest for items 2, 3, and 4 (α = 0.84) so a composite score was based on these three items as a global measure of trust related to HIV risk. The scores from these three measures were summed into a new composite trust score that ranged from 3 to 15 (3 = lowest trust, 15 = highest trust). For statistical modeling, the composite trust variable was dichotomized at the median score of 8 into “low levels of trust” and “high levels of trust”.
We determined network size by asking, “In the past 30 days, how many additional people, excluding yourself, usually inject drugs in your group?” Drug network size was dichotomized at the median into small drug networks (<3 other injectors) and large drug networks (≥3 other injectors). Regarding network stability, we asked the respondents “How often, in the past 30 days, has a stranger (someone whom you did not know before injecting) used drugs in your group?” Because the scope and cross-sectional nature of this study precluded a full assessment of network members and networks’ changing composition over time, responses to the question about whether there was a stranger present in the network in the past 30 days (yes/no) was used as a marker of network instability. We used this measure as a marker of stability because of our a priori belief that a stranger reflects a relatively new member of the network, indicating turnover and therefore an unstable composition of network members. These measures were applicable only to respondents who reported having at least one injecting partner in the past 30 days.
The two outcomes of interest were high-risk injection practices and HIV testing behavior. A high-risk injection was defined as having shared, sold, or lent an already used syringe in the past 30 days. Sharing, defined in this question, was having either distributed or received a used syringe from another IDU. HIV testing behavior was defined as having ever been tested for HIV.
Statistical Analyses
The sample was described with summary statistics, including frequency distributions and means, for all variables of interest. A series of logistic regression models (total of 6) were then generated to assess the associations between the three predictors (network trust, size, and stability) and two outcomes of interest (syringe sharing and HIV testing). Predictors of interest were first entered into an unadjusted logistic regression model. An interaction term for each independent variable and sex was created to examine effect modification; these were also included in each model. Interaction terms were included to determine if there were differences in the associations for male and female IDUs, and to assess effects for male and female IDUs separately. Sociodemographic variables that were associated at the P < 0.2 level with the outcome of interest in the bivariate analysis were then included in a multivariate model. Final multivariate models were determined by performing stepwise backward elimination to remove covariates not significant at the P < 0.05 level. The adjusted odds ratios (OR) and 95% confidence intervals from these final models are reported. All statistical analyses were performed using SAS 9.2 [24].
Results
Sample Description
A total of 124 participants were recruited into the study. The majority of the sample was recruited by outreach workers (93%) while the remaining 7% was recruited by peers. Descriptive statistics and frequencies for variables of interest are found in Table 1. The majority of the sample was male (67%), lacked a permanent place of employment (85%), and was single or never married (84%). The average age of the respondents was 28.8 ± 5 years and 87% of the sample had less than a university level education. Ninety-three percent of the participants reported injecting heroin and a sizeable minority of respondents (20%) reported injecting methamphetamines in the past 30 days. The average length of time injecting drugs was 9.6 ± 4.7 years and 77% of respondents had injected in the company of others in the past 30 days. Of those who had injected with others, over half (62%) injected with fewer than three partners. Drug network size varied considerably between men and women. Less than a third (27%) of women reported injecting with 3 or more individuals while 44% of men had injected in larger groups. Approximately one-third of all respondents reported injecting in the presence of a stranger in the past 30 days; this was also greater among males (43%) than females (19%). Having a larger network was significantly associated with having a stranger present in the network (P < 0.01).
Table 1.
Descriptive statistics of study sample (n = 124) of IDU in St. Petersburg, Russia
| Total samplea (n = 124) | Males (n = 82) | Females (n = 42) | |
|---|---|---|---|
| Age (mean, SD) | 28.8, 5.0 | 29.7, 5.0 | 26.9, 4.8 |
| Highest level of education, n (%) | |||
| High school graduate or less | 50 (40) | 32 (39) | 18 (43) |
| Technical school | 10 (8) | 7 (8) | 3 (7) |
| Specialist education | 48 (39) | 35 (43) | 13 (31) |
| Any university education | 16 (13) | 8 (10) | 8 (19) |
| Permanent place of employment, n (%) | |||
| Yes | 105 (85) | 70 (85) | 35 (83) |
| No | 19 (15) | 12 (15) | 7 (17) |
| Marital status, n (%) | |||
| Married or living together | 20 (16) | 12 (15) | 8 (19) |
| Not married or separated | 104 (84) | 70 (85) | 34 (81) |
| Drug used: heroin, n (%) | |||
| Yes | 115 (93) | 77 (94) | 38 (90) |
| No | 9 (7) | 5 (6) | 4 (10) |
| Drug used: methamphetamines, n (%) | |||
| Yes | 24 (19) | 16 (20) | 8 (19) |
| No | 100 (81) | 66 (80) | 34 (81) |
| Duration of drug use (mean, SD) | 9.6, 4.7 | 10.2, 4.9 | 8.5, 4.2 |
| Self-reported HIV status, n (%) | |||
| Positive | 60 (48) | 39 (48) | 20 (48) |
| Negative/Do not know | 64 (52) | 43 (52) | 22 (52) |
| Trust in drug networks,b n (%) | |||
| High | 43 (35) | 23 (38) | 20 (50) |
| Low | 79 (65) | 59 (62) | 20 (50) |
| Network size, n (%) | |||
| < 3 | 74 (62) | 44 (56) | 30 (73) |
| ≥3 | 46 (38) | 35 (44) | 11 (27) |
| Stranger present in network,c n (%) | |||
| Yes | 32 (34) | 26 (43) | 6 (19) |
| No | 61 (66) | 35 (57) | 26 (81) |
| Shared, sold, or lent an already used syringe, n (%) | |||
| Yes | 50 (41) | 34 (43) | 16 (38) |
| No | 72 (59) | 46 (57) | 26 (62) |
| Ever tested for HIV, n (%) | |||
| Yes | 101 (81) | 68 (82) | 33 (79) |
| No | 23 (19) | 14 (17) | 9 (21) |
May not sum to 124 due to missing data
Sum of trust questions 2–4, dichotomized at midpoint (low trust corresponds to trust score <8 and high trust ≥ 8)
Only reported from IDU who injected with at least one other person in the past 30 days
Forty-one percent of the sample reported sharing, lending, or selling an already used syringe (defined as high-risk injection practices). Most of the respondents (81%) had been tested for HIV at least once and 48% of the sample reported being positive for HIV.
The distribution of the trust measures is shown in Fig. 1. In general, there was a range of responses across all trust measures, with 24–44% of respondents being absolutely not trusting across the four measures, and 9–24% being absolutely trusting across measures. The distribution of trust was higher for injection practices than for communication about HIV status and testing. Female IDUs tended to be more trusting than their male counterparts, with 50% reporting high levels of trust of the composite score measuring global trust compared to only 28% of male IDUs reporting high levels of trust. The P-values for interaction terms were not significant in any of the models, however we calculated odds ratios for men and women separately using parameter estimates from the models with interaction terms to assess differences in magnitudes of effect. In a few instances the differences between the calculated adjusted OR for men and women were markedly divergent. For example, the OR for women not having been tested for HIV in large networks was 6.34 (95% CI: 1.03, 39.03), while the estimate for men was much lower 1.92 (95% CI: 0.50, 7.33).
Fig. 1.
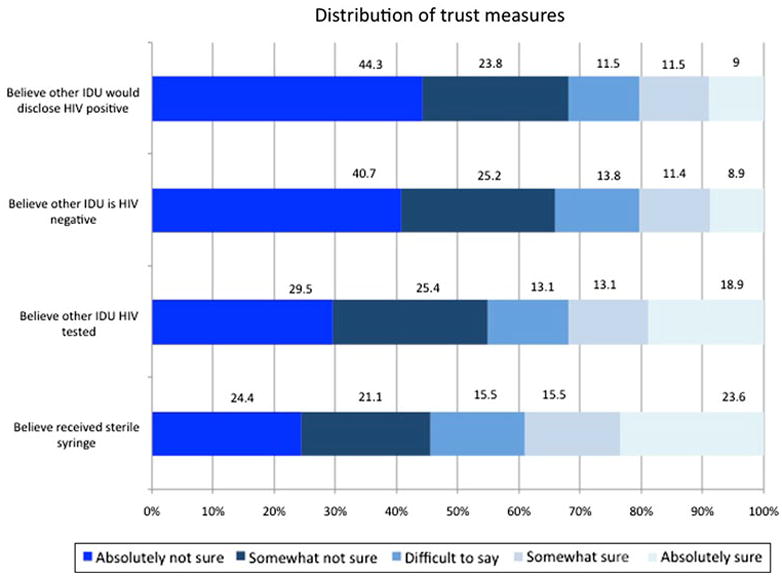
Distribution of trust measures
Association Between Trust and Outcomes
Results for all associations between network characteristics and high-risk HIV behaviors can be found in Table 2. In the models that examined the global trust composite as a correlate of risky injection practices and not having been tested for HIV, the associations were positive for both dependent variables, though not always statistically significant. For both male and female IDUs, we found statistically significant associations between high trust and risky injection practices (OR = 2.87, 95% CI: [1.06, 7.81] and OR = 4.89, 95% CI: [1.20, 19.94]), respectively.
Table 2.
Final multivariate logistic model estimates measuring associations between network characteristics and high-risk behaviors associated with HIV transmission for male and female IDU in St. Petersburg, Russia
| Network characteristic | High risk injection practices | Not tested for HIV |
|---|---|---|
| Males | ||
| Trust | ||
| High | 2.87 (1.06, 7.81)* | 1.39 (0.36, 5.41)a |
| Low | 1.0 | 1.0 |
| Size | ||
| ≥3 | 4.21 (1.54, 11.51)*b | 1.92 (0.50, 7.33)a |
| < 3 | 1.0 | 1.0 |
| Stranger present | ||
| Yes | 2.37 (0.80, 7.00)b | 16.67 (1.89, 147.12)*a |
| No | 1.0 | 1.0 |
| Females | ||
| Trust | ||
| High | 4.89 (1.20, 19.94)* | 1.75 (0.37, 9.66)a |
| Low | 1.0 | 1.0 |
| Size | ||
| ≥3 | 4.80 (1.05, 21.94)*b | 6.34 (1.03, 39.03)*a |
| < 3 | 1.0 | 1.0 |
| Stranger present | ||
| Yes | NMLc | 2.14 (0.15, 31.20)a |
| No | 1.0 | 1.0 |
Models adjusted for duration of drug use
Models adjusted for methamphetamine use
NML no maximum likelihood estimate calculated due to quasi-complete separation of data points (all females who had injected in the presence of a stranger had engaged in high-risk injection practices)
P < 0.05
Association Between Network Size and Outcomes
Larger network size was strongly and significantly associated with risky injection practices for both male and female IDUs (OR = 4.21, 95% CI [1.54–11.51] OR = 4.89, 95% CI [1.05, 21.94], respectively). For the outcome of not having been tested for HIV, the association was positive but not significant among male IDUs, and positive and significant among female IDUs (OR = 6.34 95% CI: [1.03, 39.03]) after adjusting for potential confounders.
Association Between Stranger Present in Injecting Network and Outcomes
In the models that examined having a stranger in the network as a marker of network instability, men and women were included in a combined model examining the association with high-risk injections because of quasi-complete separation in the calculation of the maximum likelihood estimate among women only. This was due to all women who reported a stranger in their network also reported unsafe injections (n = 6). In the combined model, men and women who reported a stranger in their network were more likely to report unsafe injections (OR = 2.37, 95% CI = [0.80, 7.00]). In the models examining the association with not having been tested for HIV, the association was strong and significant only for men (OR = 16.67 95% CI [1.89, 147.12]).
Discussion
In this study we found significant associations of drug network characteristics including more trust, larger network size, and presence of stranger with risky injection practices and not having been tested for HIV among IDUs in St. Petersburg, Russia. These are the first findings from Russia detailing the role of drug networks on IDUs’ risk behaviors, and they support previous research from other parts of the world [12, 25–27]. While some associations between the independent and dependent variables were not significant, all adjusted odds ratios among both men and women were greater than one indicating a strong pattern of increased odds of engaging in risky HIV-related behaviors associated with each examined network characteristic. The lack of significance, in many cases, may be due to limited statistical power resulting from the small sample size.
We hypothesized that being too trustful within one’s injecting drug network would lead to more risky behaviors through a mechanism in which trust might create a false sense of security. For example, expecting or assuming another IDU to disclose an HIV positive status to an injector in the same network may lead to risky injection practices in the absence of disclosure. It is also possible that asking another IDU directly about his/her HIV status could be seen as “insulting” and could strain a friendship. Conversely it may be an easier topic to broach with someone who is not considered a close friend. HIV testing behavior could also be influenced by a more trusting environment via a similar mechanism. IDUs may not feel compelled to be tested for HIV if they believe they are in insulated injecting environments and trust other IDUs to disclose their status to them. This might be particularly important for female IDUs, who are often introduced to injecting drugs after beginning a sexual relationship with male IDUs [28]. In such relationships trust may be high as it relates to intimacy and female IDUs may believe their sex partner will disclose his HIV positive serostatus. Indeed, we found strong and significantly higher odds of unsafe injections and not being tested for HIV among women with higher levels of trust, after adjusting for possible confounders. Women may be at more risk since they are more likely to engage in receptive syringe sharing when injecting with their sex partner [29–34]. While female networks tend to be small, leading to perhaps more secure immediate injecting environments, their male IDU partners tend to have larger networks, increasing their risk for encountering blood-borne viruses. Thus, trust may be seen as a risk factor for HIV transmission in IDU networks that include sexual partnerships due to trusting a partner to disclose infection status and susceptibility in engaging in risky behaviors, both injection and sexual [12, 33–35].
Similar to other published studies [13, 20], larger network sizes were associated with high-risk injection practices. The adjusted odds ratios for both male and female IDUs were statistically significant. Furthermore, IDUs in large networks were less likely to have ever been tested for HIV suggesting that large networks create environments that could facilitate HIV-transmission through lack of knowledge of serostatus of network members due to lack of HIV testing and communication about HIV status compounded by risky injection practices among IDUs.
Reported injecting drug networks in this sample were generally smaller than reported in urban areas in the United States [36]. Despite this, many of the networks in our St. Petersburg sample were not insulated from unfamiliar injectors. Not unexpectedly, these factors were associated in our sample. However, they were modeled separately as independent variables because their meanings could be distinct. IDUs with large and unstable networks had significantly greater odds of risky injection practices. This finding appears to be divergent from other studies suggesting strong ties are normally associated with syringe sharing [15], however this may be attributed to the differences between American and Russian IDUs that include age, experience with HIV testing, and access to IDU-targeted HIV prevention services. Thus, this research adds to a growing body of evidence that the injecting drug environment and associated health risks in Russia are culturally dissimilar to those in the United States.
Some limitations of this study include the small sample size and lack of exhaustive recruitment of IDUs deep within networks. The peer referral recruitment strategy did not accelerate (a stated requirement for obtaining a representative sample [37]). Possible explanations could be the relatively small network sizes, high levels of stigma experienced by IDUs in Russia [38], and inconvenience of study site locations for the participants due to long distances from neighborhoods with associated with drug using communities. Therefore, we relied on convenience sampling by outreach workers familiar with the target population, which might have led to a non-representative sample. However, our sample characteristics related to demographics and drug use patterns are consistent with characteristics of IDUs sampled by peer referral in recent studies conducted in St. Petersburg [5, 6]. Another limitation imposed by the relatively small sample size was the need to generate more statistical power by dichotomizing variables of interest. Finally, due to the cross-sectional and anonymous study design, we did not collect data from network members directly, thoroughly evaluate network stability over time, or use already established network stability indices [39]. Though previous research using ego data only has been shown to be a viable method [40, 41], we believe that true network studies are needed to further elucidate the effect of these characteristics on high-risk behaviors.
Despite these limitations, our findings indicate important injection and HIV testing risk behaviors associated with drug network characteristics among IDUs in St. Petersburg. We found differences in terms of high-risk behaviors associated with HIV-transmission between male and female IDUs, which warrants further investigation with larger sample sizes to explore possible mechanisms. Future studies should continue to measure differences between male and female IDU drug network environments, especially those that include both members of IDU sexual dyads. Furthermore, future work should assess network stability and corresponding injection behaviors over time to assess more deeply the potential influences on HIV transmission dynamics within injecting drug networks. Future interventions should focus on improving communication regarding HIV status and promoting safer injection practices within IDU networks. Additionally, peer counseling should be implemented to create safer injection environments within large networks and those with unstable or weak ties. Effectiveness of interventions to reduce the risk of HIV infection may be enhanced if network characteristics are addressed.
Acknowledgments
The work was supported by the Downs Fellowship and Committee on International Health at Yale University, an AIDS International Training and Research Program grant from the NIH Fogarty International Center (5D43TW001028), and by a grant from the Civilian Research and Development Foundation to NGO Stellit and Yale University School of Public Health (RUB- 7001-ST-08). The authors are tremendously thankful for all the assistance provided by the research team at NGO Stellit in St. Petersburg.
Contributor Information
Javier A. Cepeda, Email: jacepeda@gmail.com, Department of Epidemiology and Public Health, Yale School of Public Health, 60 College Street, New Haven CT 06520, USA
Veronika A. Odinokova, Saint-Petersburg Non-Governmental Organization of Social Projects “Stellit”, Saint-Petersburg, Russia
Robert Heimer, Department of Epidemiology and Public Health, Yale School of Public Health, 60 College Street, New Haven CT 06520, USA, Center for Interdisciplinary Research on AIDS, Yale School of Public Health, 135 College Street, New Haven, CT 06520, USA.
Lauretta E. Grau, Department of Epidemiology and Public Health, Yale School of Public Health, 60 College Street, New Haven CT 06520, USA, Center for Interdisciplinary Research on AIDS, Yale School of Public Health, 135 College Street, New Haven, CT 06520, USA
Alexandra Lyubimova, Saint-Petersburg Non-Governmental Organization of Social Projects “Stellit”, Saint-Petersburg, Russia.
Liliya Safiullina, Saint-Petersburg Non-Governmental Organization of Social Projects “Stellit”, Saint-Petersburg, Russia.
Olga S. Levina, Saint-Petersburg Non-Governmental Organization of Social Projects “Stellit”, Saint-Petersburg, Russia
Linda M. Niccolai, Department of Epidemiology and Public Health, Yale School of Public Health, 60 College Street, New Haven CT 06520, USA, Center for Interdisciplinary Research on AIDS, Yale School of Public Health, 135 College Street, New Haven, CT 06520, USA
References
- 1.World Health Organization. Russian Federation: summary country profile for HIV/AIDS treatment scale-up. 2005. [Google Scholar]
- 2.Cohen J. Late for the epidemic: HIV/AIDS in Eastern Europe. Science. 2010;329(5988):160, 162–164. doi: 10.1126/science.329.5988.160. [DOI] [PubMed] [Google Scholar]
- 3.Goliusov AT, Dementyeva LA, Ladnaya NN, et al. Country progress report of the Russian Federation on the implementation of the declaration of commitment on HIV/AIDS. [Accessed 13 May 2010];Ministry of Health and Social Development of the Russian Federation: Federal service for surveillance of consumer rights protection and human well-being of the Russian Federation. 2009 Available at http://data.unaids.org/pub/Report/2008/russia_2008_country_progress_report_en.pdf.
- 4.Heimer R, White E. Estimation of the number of injection drug users in St Petersburg, Russia. Drug Alcohol Depend. 2010;109(1–3):79–83. doi: 10.1016/j.drugalcdep.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niccolai LM, Toussova OV, Verevochkin SV, Barbour R, Heimer R, Kozlov AP. High HIV prevalence, suboptimal HIV testing, and low knowledge of HIV-positive serostatus among injection drug users in St Petersburg, Russia. AIDS Behav. 2010;14(4):932–41. doi: 10.1007/s10461-008-9469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyarmathy VA, Li N, Tobin KE, et al. Correlates of unsafe equipment sharing among injecting drug users in St. Petersburg, Russia. Eur Addict Res. 2009;15(3):163–70. doi: 10.1159/000220344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niccolai LM, Shcherbakova IS, Toussova OV, Kozlov AP, Heimer R. The potential for bridging of HIV transmission in the Russian Federation: sex risk behaviors and HIV prevalence among drug users (DUs) and their non-DU sex partners. J Urban Health. 2009;86(Suppl 1):131–43. doi: 10.1007/s11524-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niccolai LM, Verevochkin SV, Toussova OV, White E, Barbour R, Kozlov AP, Heimer R. Estimates of HIV incidence among drug users in St. Petersburg, Russia: continued growth of a rapidly expanding epidemic. Eur J Public Health. 2010 doi: 10.1093/eurpub/ckq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asch SE. Rules and values. In: Asch SE, editor. Social psychology. Upper Saddle River, NJ: Prentice Hall; 1952. pp. 350–63. [Google Scholar]
- 10.Sherif M. The psychology of social norms. Oxford: Harper Torchbooks; 1966. [Google Scholar]
- 11.Hyman HH. The psychology of status. Arch Psychol. 1942;269:5–91. [Google Scholar]
- 12.Gyarmathy VA, Neaigus A. The effect of personal network exposure on injecting equipment sharing among Hungarian IDUs. Connections. 2006;27(1):29–42. [PMC free article] [PubMed] [Google Scholar]
- 13.Latkin C, Mandell W, Vlahov D, Oziemkowska M, Celentano D. People and places: behavioral settings and personal network characteristics as correlates of needle sharing. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(3):273–80. doi: 10.1097/00042560-199611010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Latkin C, Mandell W, Oziemkowska M, Vlahov D, Celentano D. The relationships between sexual behavior alcohol use, and personal network characteristics among injecting drug users in Baltimore, Maryland. Sex Transm Dis. 1994;21(3):161–7. doi: 10.1097/00007435-199405000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Valente TW, Vlahov D. Selective risk taking among needle exchange participants: implications for supplemental interventions. Am J Public Health. 2001;91(3):406–11. doi: 10.2105/ajph.91.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amirkhanian YA, Kelly JA, Kirsanova AV, et al. HIV risk behaviour patterns, predictors, and sexually transmitted disease prevalence in the social networks of young men who have sex with men in St Petersburg, Russia. Int J STD AIDS. 2006;17(1):50–6. doi: 10.1258/095646206775220504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetherel C, Plakans A, Wellman B. Social networks, kinship, and community in Eastern Europe. J Interdiscipl Hist. 1994;24(4):639–63. [Google Scholar]
- 18.Gyarmathy VA, Li N, Tobin KE, et al. Injecting equipment sharing in Russian drug injecting dyads. AIDS Behav. 2009;14(1):141–51. doi: 10.1007/s10461-008-9518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes T, Davis M, Judd A. Hepatitis C and its risk management among drug injectors in London: renewing harm reduction in the context of uncertainty. Addiction. 2004;99:621–33. doi: 10.1111/j.1360-0443.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- 20.Mandell W, Kim J, Latkin C, Suh T. Depressive symptoms, drug network, and their synergistic effect on needle-sharing behavior among street injection drug users. Am J Drug Alcohol Abuse. 1999;25:117–27. doi: 10.1081/ada-100101849. [DOI] [PubMed] [Google Scholar]
- 21.Costenbader EC, Astone NM, Latkin CA. The dynamics of injection drug users’ personal networks and HIV risk behaviors. Addiction. 2006;101(7):1003–13. doi: 10.1111/j.1360-0443.2006.01431.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann JP, Su SS, Pach A. Changes in network characteristics and HIV risk behavior among injection drug users. Drug Alcohol Depend. 1997;46(1–2):41–51. doi: 10.1016/s0376-8716(97)00038-0. [DOI] [PubMed] [Google Scholar]
- 23.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Social Problems. 1997;44:174–99. [Google Scholar]
- 24.SAS Institute. SAS for Windows (Version 9.2) Cary, NC: 2010. [Google Scholar]
- 25.Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61(5):1026–44. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Williams ML, Zhou Z, Siegal HA, Robles RR, Trotter RT, Jones A. A comparison of drug use networks across three cities. Social networks, drug abuse, and HIV transmission. NIDA Research Monograph. 1995:109–30. [PubMed] [Google Scholar]
- 27.Latkin CA, Mandell W, Vlahov D, Oziemkowska M, Celentano DD. The long-term outcome of a personal network-oriented HIV prevention for injection drug users: the SAFE Study. Am J Community Psychol. 1996;24(3):341–64. doi: 10.1007/BF02512026. [DOI] [PubMed] [Google Scholar]
- 28.Sherman SG, Smith L, Laney G, Strathdee SA. Social influences on the transition to injection drug use among young heroin sniffers: a qualitative analysis. Int J Drug Policy. 2002;13(2):113–20. [Google Scholar]
- 29.De P, Cox J, Boivin J, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007;102(11):1730–9. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 30.Barnard M. Needle sharing in context: patterns of sharing among men and women injectors and HIV risks. Addiction. 1993;88(6):805–12. doi: 10.1111/j.1360-0443.1993.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 31.Gossop M, Griffiths P, Strang J. Sex differences in patterns of drug taking behavior. Br J Psychiatry. 1994;164(1):101–4. doi: 10.1192/bjp.164.1.101. [DOI] [PubMed] [Google Scholar]
- 32.Hunter GM, Donoghoe MC, Stimson GV, Rhodes T, Chalmers CP. Changes in the injecting risk behaviour of injecting drug users in London, 1990–1993. AIDS. 1995;9(5):493–501. [PubMed] [Google Scholar]
- 33.Neaigus A, Friedman SR, Goldstein MF, Ildefonso G, Curtis R, Jose B. Using dyadic data for a network analysis of HIV infection and risk behaviors among injecting drug users. NIDA Res Monogr. 1995;151:20–37. [PubMed] [Google Scholar]
- 34.Pivnick A, Jacobson A, Eric K, Doll L, Drucker E. AIDS, HIV infection, and illicit drug use within inner-city families and social networks. Am J Public Health. 1994;84(2):271–4. doi: 10.2105/ajph.84.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unger JB, Kipke MD, De Rosa CJ, Hyde J, Ritt-Olson A, Montgomery S. Needle-sharing among young IV drug users and their social network members: the influence of the injection partner’s characteristics on HIV risk behavior. Addict Behav. 2006;31(9):1607–18. doi: 10.1016/j.addbeh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Latkin C, Mandell W, Oziemkowska M, et al. Using social network analysis to study patterns of drug use among urban drug users at high risk for HIV/AIDS. Drug Alcohol Depend. 1995;38(1):1–9. doi: 10.1016/0376-8716(94)01082-v. [DOI] [PubMed] [Google Scholar]
- 37.Heckathorn D. Respondent driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Social Problems. 2002;49(1):11–34. [Google Scholar]
- 38.Bobrova N, Rhodes T, Power R, et al. Barriers to accessing drug treatment in Russia: a qualitative study among injecting drug users in two cities. Drug Alcohol Depend. 2006;82(Suppl 1):S57–63. doi: 10.1016/s0376-8716(06)80010-4. [DOI] [PubMed] [Google Scholar]
- 39.Morgan DL, Neal MB, Carder P. The stability of core and peripheral networks over time. Soc Networks. 1997;19(1):9–25. [Google Scholar]
- 40.Evans JL, Hahn JA, Page-Shafer K, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study) J Urban Health. 2003;80(1):137–46. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyarmathy VA, Li N, Tobin KE, et al. Correlates of unsafe equipment sharing among injecting drug users in St. Petersburg, Russia. Eur Addict Res. 2009;15(3):163–70. doi: 10.1159/000220344. [DOI] [PMC free article] [PubMed] [Google Scholar]


