Abstract
Estradiol (E2) exerts an inhibitory effect on food intake in a variety of species. While compelling evidence indicates that central, rather than peripheral, estrogen receptors (ERs) mediate this effect, the exact brain regions involved have yet to be conclusively identified. In order to identify brain regions that are sufficient for E2's anorectic effect, food intake was monitored for 48 h following acute, unilateral, microinfusions of vehicle and two doses (0.25 and 2.5 μg) of a water-soluble form of E2 in multiple brain regions within the hypothalamus and midbrain of ovariectomized rats. Dose-related decreases in 24-h food intake were observed following E2 administration in the medial preoptic area (MPOA), arcuate nucleus (ARC), and dorsal raphe nucleus (DRN). Within the former two brain areas, the larger dose of E2 also decreased 4-h food intake. Food intake was not influenced, however, by similar E2 administration in the paraventricular nucleus, lateral hypothalamus, or ventromedial nucleus. These data suggest that E2-responsive neurons within the MPOA, ARC, and DRN participate in the estrogenic control of food intake and provide specific brain areas for future investigations of the cellular mechanism underlying estradiol's anorexigenic effect.
Keywords: ventromedial hypothalamus, lateral hypothalamus, paraventricular nucleus of the hypothalamus, satiety, female rat, estrogens
Introduction
Behavioral studies in rats reveal that estradiol's (E2's) anorexigenic effect occurs slowly, within hours to days following acute, peripheral E2 treatment (Geary and Asarian, 1999; Asarian and Geary, 2002). This has prompted investigations of the relative contributions of the two nuclear estrogen receptor (ER) subtypes, ERα and ERβ, to the estrogenic control of food intake. Studies involving ER null mice and pharmacological manipulations of ERα and ERβ suggest that activation of ER is both sufficient and necessary for E2's anorexigenic effect (Geary et al., 2001; Roesch, 2006; Santollo et al., 2007; Thammacharoen et al., 2009; Santollo et al., 2010). Less is known, however, regarding the sites of the critical ERs. While E2 is released by the ovaries, thereby gaining easy access to peripheral ERs, it also readily crosses the blood-brain barrier, thereby gaining access to central ERs. As such, E2's anorexigenic effect could be mediated by activation of peripheral and/or central ERs.
The relative contributions of peripheral and central ERs to E2's anorexigenic effect have been addressed in three studies involving ICI 182,780, an ER antagonist with limited ability to cross the blood-brain barrier. In the earlier studies, peripheral administration of ICI 182,780 failed to influence the anorexigenic effect of chronic E2 treatment in ovariectomized (OVX) rats and hamsters (Wade et al., 1993a; Wade et al., 1993b). Recently, we extending these reports by demonstrating that peripheral administration of ICI 182,780 fails to alter the anorexia following acute E2 treatment in OVX rats. Peripheral administration of ICI 182,780 did, however, block E2's proliferative effect on uterine tissue, thereby confirming extensive blockade of peripheral ERs (Rivera and Eckel, 2010). In comparison, central administration of ICI 182,780 was sufficient to block E2's acute, anorexigenic effect in OVX rats. Our observation that E2 continued to induce vaginal estrus in these same rats confirms that centrally administered ICI-182,780 did not leak into the periphery (Rivera and Eckel, 2010). Taken together, these studies demonstrate that activation of central, rather than peripheral, ERs is necessary for E2's anorexigenic effect.
It is likely that E2's anorexigenic effect is mediated by a distributed neuronal network since E2 influences the activity of a number of hypothalamic neuropeptide and neurotransmitter systems implicated in the control of food intake. For example, studies in rodents reveal that estradiol decreases neuropeptide Y (NPY) and melanin-concentrating hormone (MCH) mRNA expression in the arcuate nucleus (ARC) and the lateral hypothalamus (LH), respectively (Baskin et al., 1995; Murray et al., 2000; Pelletier et al., 2007). In addition, estradiol increases pro-opiomelanocortin (POMC) and corticotropin-releasing factor (CRF) mRNA expression in the ARC and paraventricular nucleus of the hypothalamus (PVN), respectively (Pelletier et al., 2007). Thus, it is likely that the critical, central ERs underlying E2's anorexigenic effect are located in multiple brain areas.
One approach to identify the locations of the critical ERs involves direct administration of E2 in specific brain areas. Of the hypothalamic nuclei, the PVN has received the most attention. Butera and colleagues were the first to report that administration of dilute, crystalline E2 in the PVN decreased food intake in OVX rats and guinea pigs (Butera and Czaja, 1984; Butera and Beikirch, 1989) and that subcutaneous administration of E2 failed to decrease food intake in OVX rats with bilateral lesions of the PVN (Butera et al., 1992). Because others have failed to replicate these findings (Dagnault and Richard, 1994; Hrupka et al., 2002), the role of the PVN in the estrogenic control of food intake remains unresolved. More recently, the involvement of additional hypothalamic and hindbrain sites have been examined. Similar to the PVN, there are conflicting reports regarding the medial preoptic area's (MPOA's) role in the estrogenic control of food intake. Richard and colleagues reported dose-related decreases in food intake following microinfusions of a water-soluble form of E2 in the MPOA (Dagnault and Richard, 1997), whereas Geary and colleagues were unable to replicate these findings when placing dilute crystalline E2 implants in the MPOA (Hrupka et al., 2002). One report exists that direct administration of E2 in the nucleus of the solitary tract is sufficient to decrease food intake in OVX rats (Thammacharoen et al., 2008). Finally, there has been agreement to date that the ventromedial hypothalamus (VMH) is not involved in the estrogenic control of food intake. Implants of both pure and dilute crystalline E2 in the VMH fail to reduce food intake (Palmer and Gray, 1986; Butera et al., 1989) and rats with VMH lesions display a reduction in food intake following E2 treatment (King and Cox, 1973).
Although the available literature suggests that E2 acts in the brain to decrease food intake, methodological differences among previous studies may have contributed to the equivocal findings regarding the sites of the critical ERs. Studies attempting to replicate previous findings often used different forms of E2 (i.e. crystalline or water-soluble) that may have resulted in differing concentrations of E2 in the brain. Other methodological inconsistencies across studies involve the duration of E2 treatment (acute versus chronic application), the type of E2 (E2 benzoate, 17-β E2, water-soluble E2), and differing rat strains (Long Evans, Wistar, Sprague Dawley). The present study was designed to address some of these methodological inconsistencies by examining food intake in a single strain of OVX rats receiving similar site-specific, unilateral microinfusions of a water-soluble form of E2 in various hypothalamic brain areas. The small volume and lipophobic properties of this form of E2 were chosen to minimize the spread of E2. In addition, acute hormone treatment was used in an attempt to more accurately model the cyclic rise in endogenous E2 secretion in the intact female rat and to prevent a sustained elevation in central E2 concentration.
The goal of this experiment was to target multiple brain areas using a unified approach involving acute E2 administration to identify nuclei sufficient for E2's anorexigenic effect. The lateral ventricle was included as a positive control and to establish an upper boundary of E2 doses to be administered in specific brain regions. The PVN and MPOA were chosen on the basis of at least one positive report that site-specific administration of E2 within these brain areas reduced food intake in OVX rats (Butera et al., 1989; Dagnault et al., 1997). The ARC, LH, and DRN were chosen because they each synthesize either a neuropeptide or a neurotransmitter (e.g., NPY, MCH, and serotonin respectively) that has been implicated in the estrogenic control of food intake (Rivera and Eckel, 2005; Eckel et al., 2005; Messina et al., 2006; Santollo and Eckel, 2008a; Santollo and Eckel, 2008b). The VMH was chosen as a negative control region because it is well established that E2 does not act in the VMH to decrease food intake (Palmer et al., 1986; Butera et al., 1989).
Materials and methods
Animals and housing
Female, Long-Evans rats (Charles River Breeding Laboratory, Raleigh, NC) weighing 225–250 g at study onset, were housed individually in custom-designed cages. Each cage was equipped with a feeding niche that provided access to a spill-resistant food cup. Rats were given free access to powdered rat chow (Purina 5001) and tap water, except where otherwise noted. The testing rooms were maintained at 20 ± 2°C under a reverse 12:12-h light–dark cycle (dark onset=1300 h). Animal usage and all procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
Surgery
Rats were anesthetized by intraperitoneal (i.p.) injections of a mixture of ketamine (50 mg/kg; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (4.5 mg/ml; Rompun, Mobay, Shawnee, KS) and then bilaterally OVX using an intra-abdominal approach. Immediately following ovariectomy surgery, rats were implanted with single, stainless-steel, guide cannulae (26-g (nucleus-specific) or 22-g (lateral ventricle), Plastics One, Roanoke, VA) targeting the MPOA (AP: −0.7 mm; ML −0.5 mm; DV: −8.0 mm; n = 12), ARC (AP: −3.5 mm; ML: −0.4 mm; DV: −9.3 mm; n = 10), DRN (AP: −7.8 mm; ML: −4.4 mm; DV: −7.2 mm; 35° angle; n = 13), PVN (AP: −1.8 mm; ML: −0.4 mm; DV: −7.4 mm; n = 10), LH (AP: −2.6 mm; ML: −1.1 mm; DV: −8.0 mm; n = 9), VMH (AP: −2.4 mm; ML: −0.7 mm; DV: −9.0 mm; n = 12), or lateral ventricle (AP: −0.6 mm; ML: −1.7 mm; DV: −3.5 mm; n = 18). The coordinates were chosen in an attempt to target the portion of each brain area containing the greatest density of ERα expression (Jacobs and Azmitia, 1992; Yokosuka et al., 1997; Sato et al., 2005; Muschamp and Hull, 2007). The depths of the cannulae were chosen to target the upper border of the brain area of interest in order to limit tissue damage within targeted brain areas. Following surgery, rats received i.p. injections of butorphanol (0.5 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and gentamicin (10 mg/ml; Pro Labs Ltd, St. Joseph, MO) to minimize post-surgical pain and the risk of infection, respectively. Behavioral testing did not commence until food intake and body weight returned to pre-surgical levels (~ 7 days).
Cannula verification
Ventricular cannulae were verified following post-operative recovery by monitoring light-phase water intake following intracerebroventricular (i.c.v.) microinfusion of 50 ng of angiotensin II (Sigma–Aldrich, St. Louis, MO), delivered in 5 μl of saline vehicle over a period of 1 min. Only those rats that consumed at least 5 ml of water in 20 min were included in the study. All but three rats passed this criterion (15 of 18 placements were correct; mean consumption = 8.8 ± 1.2 ml).
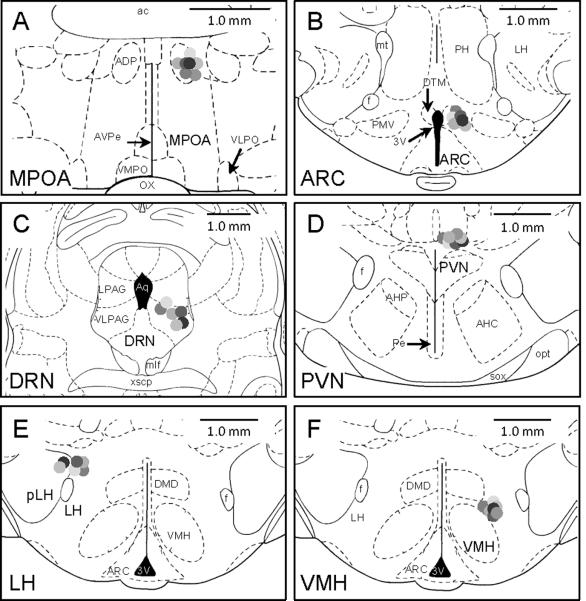
Nuclei-specific cannulae were verified using postmortem histology. At the end of the study, rats were anesthetized with 0.5 ml of sodium pentobarbital (50 mg/ml Henry Schein, Melville, NY) and, once unresponsive, decapitated. Their brains were removed and stored in 10% formalin (Sigma–Aldrich, St. Louis, MO) for 48 h and then stored in a 30% sucrose solution for cryoprotection. One week later, brains were sectioned on a cryostat (Ziess) at 40 μm intervals. Serial sections were obtained through the nuclei of interest and then stained with cresyl violet (Sigma–Aldrich). A rater, unaware of the behavioral data, assessed cannula placements in accordance with a rat stereotaxic atlas (Paxinos and Watson, 1998). Rats in which the tips of the cannulae were misplaced (greater than 300 μm beyond the targeted area in any direction, i.e. dorsal, ventral, rostral or caudal) were excluded from data analysis. This resulted in 39 rats being included in the data analysis; 7 out of 12 MPOA, 6 out of 10 ARC, 6 out of 13 DRN, 7 out of 10 PVN, 6 out of 9 LH, and 7 out of 12 VMH (Fig. 1).
Fig. 1.
Summary of cannula placements. The line drawings represent the cannula placements of rats included in the study. Rats with misplaced cannulae were excluded from data analysis. This resulted in 39 rats being included in the data analysis (MPOA, −0.4 bregma, n=7; ARC, −3.4 bregma, n=6; DRN, −7.8 bregma, n=6; PVN, −1.8 bregma, n=7; LH, −2.6 bregma, n=6; VMH, −2.5 bregma, n=7)
Drugs
The form of E2 used in the present study was synthesized to be water soluble by the addition of a carrier molecule, 2-Hydroxypropyl-b-cyclodextrin, which accounted for 95.3% of the drug dry weight. Drug solutions of varying concentrations were created by dissolving the β-E2/cyclodextrin carrier molecule (E4389; Sigma-Aldrich, St. Louis, MO) in artificial cerebral spinal fluid (aCSF) to yield concentrations of E2 ranging from 0.25 – 10 μg of E2 per microinfusion volume. Similarly, vehicle solutions were created by dissolving the cyclodextrin carrier molecule (C0926; Sigma-Aldrich) alone in aCSF to produce varying concentrations that matched the amount of carrier present in the E2 solutions.
Experiment 1: ventricular microinfusions of E2
Each day at 0900 h, food intake and body weight were recorded and water and food cups were refilled. On test days, food and water were removed from the rats' cages 1-h prior to dark onset. Thirty min prior to dark onset, dust caps were removed and replaced with an internal injector that extended 0.5 mm past the guide cannula and in the lateral ventricle. Using a within-subjects, counter-balanced design, rats received i.c.v. microinfusions of either 0 and 5 μg E2 in 2.5 μl vehicle (group 1, n = 7) or 0 and 10 μg E2 in 2.5 μl vehicle (group 2, n = 8) over a period of 1 min via a hand-pump syringe. The injector remained in the guide cannula for an additional 1 min to allow for the drug to diffuse away from the tip of the injector. At dark onset, food and water were returned to the cages and food intake was measured at 1, 2, 4, 24, and 48 h following treatment.
Experiment 2: site specific microinfusions of E2
The procedure for this second series of experiments was the same as Experiment 1 with the following exceptions. The internal injector extended 0.5 mm past the guide cannula with the exception of the following brain regions: MPOA = 1.0 mm projection and LH = 0.8 mm projection. Longer projections were necessary in these brain areas in order to target the regions containing the greatest density of ERα while still targeting the upper borders of the nuclei for the guide cannula depth. Using a within-subjects, counter-balanced design, rats received microinfusions of 0, 0.25 and 2.5 μg E2 in 200 nl of vehicle at 4-day intervals. All microinfusions were administered over a period of 3 min via a microinfusion pump (Harvard Apparatus). The injector remained in the guide cannula for an additional 3 min to allow for the drug to diffuse away from the tip of the injector. These drug doses were chosen based on the results from Experiment 1 and a previous study in which water-soluble E2 was administered in the MPOA (Dagnault et al., 1997).
Data Analysis
Food intake is presented as mean ± SEM throughout. Food intakes following drug treatment for each brain site were analyzed using repeated-measures ANOVAs (drug treatment X time). Newman Keuls post-hoc tests were used to investigate differences between groups following significant ANOVA effects (P < 0.05). To assess drug spread in animals with misplaced cannulae, 24-h food intake following vehicle and 2.5 μg E2 treatments were analyzed using paired t-tests.
Results
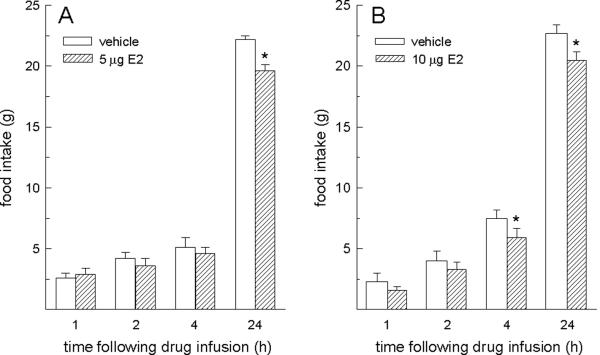
Food intake following ventricular microinfusions of both doses of E2 was influenced by interactive effects of hormone treatment and time, F-values = 4.36 and 6.31, Ps < 0.005 (Fig. 2). A reduction in 24-h food intake was detected in rats receiving the lower (5 μg) dose of E2, relative to vehicle-treated rats, P < 0.05 (Fig. 2a). Reductions in 4- and 24-h food intake were detected in rats receiving the higher (10 g) dose of E2, relative to vehicle-treated rats, Ps < 0.05 (Fig. 2b).
Fig. 2.
Food intake following ventricular (i.c.v.) microinfusions of E2. Microinfusions of E2 into the lateral ventricle decreased food intake in OVX rats. (A) The lower (5 μg) dose of E2 decreased 24-h food intake. (B) The higher (10 μg) dose of E2 decreased both 4- and 24-h food intake. *E2 < vehicle at corresponding time points, P < 0.05.
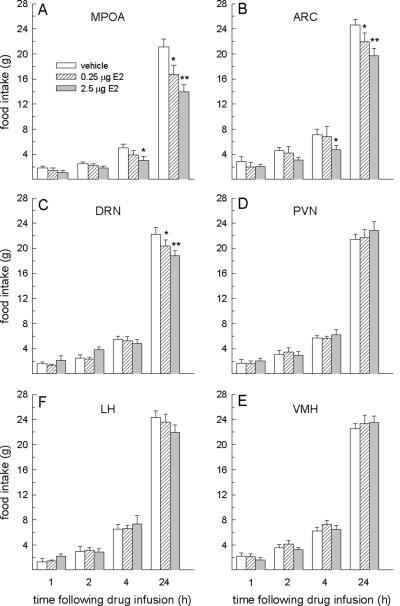
Food intake following site-specific microinfusions of E2 in the MPOA, ARC, and DRN was influenced by interactive effects of hormone treatment and time, F-values = 2.44 – 9.12, Ps < 0.05 – 0.001 (Fig. 3a–c). Dose-dependent decreases in 24-h food intake were observed in rats receiving microinfusions of E2 in each of these brain regions (Ps < 0.05). In addition, a decrease in 4-h food intake was also apparent, but this effect was limited to rats receiving the larger dose of E2 in the MPOA and ARC only. The anorexigenic effect of E2 in each of these three brain areas (MPOA, ARC, DRN) was limited to 24 h, since food intake in all rats had returned to basal levels by 48 h following E2 treatment (data not shown). Food intake following site-specific microinfusions of E2 in the PVN, LH and VMH was not influenced by either main or interactive effects of hormone treatment, F-values = 0.63–2.03, n.s., (Fig. 3d–f). Food intake at all time points was similar between E2- and vehicle-treated rats.
Fig. 3.
Food intake following site-specific microinfusions of E2. Microinfusions of E2 in the MPOA, ARC and DRN, but not in the PVN, LH, or VMH decreased food intake in OVX rats. In the MPOA and ARC (A and B, respectively), the larger dose of E2 decreased 4- and 24-h food intake, whereas the smaller dose of E2 decreased 24-h food intake only. In the DRN (C), dose-related decreases in 24-h food intake were observed following E2 treatment. Neither dose of estradiol affected food intake in the PVN (D), LH (E) or VMH (F). *E2 < vehicle at corresponding time points, P < 0.05. **2.5 μg dose of E2 < 0.25 μg dose of E2, P < 0.05.
As a measure of drug spread, we compared 24-h food intake measurements following microinfusions of vehicle and the larger dose of E2 that were obtained from the 26 rats with misplaced cannulae (one rat was excluded from this analysis as the misplaced cannula targeted the third ventricle and, as would be expected, decreased food intake). Analysis of the feeding data by individual brain areas and combined across all brain areas (to increase statistical power) failed to yield any significant effects of hormone treatment (Table 1).
Table 1.
Food intake following infusions of vehicle and 2.5 μg estradiol (E2) in rats with misplaced cannulae that failed to target the indicated brain areas.
| Brain area | Vehicle | 2.5 μg E2 | t-test results |
|---|---|---|---|
| MPOA | 19.5 ± 0.6 g | 20.4 ± 1.0 g | t(4) = 0.702, p = 0.52 |
| ARC | 23.1 ± 0.8 g | 22.7 ± 0.8 g | t(2*) = 1.245, p = 0.34 |
| DRN | 21.9± 1.2 g | 21.0 ± 1.6 g | t(6) = 1.073, p = 0.32 |
| PVN | 20.9 ± 0.6 g | 22.1 ± 2.1 g | t(2) = 0.831, p = 0.49 |
| LH | 20.1 ± 2.0 g | 22.0 ± 1.7 g | t(2) = 2.495, p = 0.12 |
| VMH | 22.9 ± 0.9 g | 21.8 ± 0.7 g | t(4) = 0.918, p = 0.41 |
| Combined Nuclei | 21.5 ± 0.5 g | 21.5 ± 0.6 g | t(25*) = 0.008, p = 0.99 |
Data are presented as mean ± SEM. Group differences were assessed via dependent t-tests, which are presented in the last column. Twenty-four h food intake was similar following microinfusions of vehicle or E2 in these rats with misplaced cannulae.
One rat with a misplaced cannula in the ARC was excluded from the ARC and combined nuclei analyses because the cannula extended into the third ventricle and, as expected, reduced 24-h food intake.
Abbreviations: MPOA = medial preoptic area; ARC = arcuate nucleus; DRN = dorsal raphe nucleus; PVN = paraventricular nucleus; LH = lateral hypothalamus; VMH = ventromedial hypothalamus.
Discussion
These findings clearly show that unilateral microinfusions of E2 in the MPOA, ARC and DRN are sufficient to produce dose-related decreases in 24-h food intake in OVX rats. In contrast, similar microinfusions of E2 in the PVN, LH and VMH do not influence food intake. Identification of the brain areas that mediate the anorexigenic effects of E2 is important for multiple reasons. First, knowing where E2 acts in the brain to reduce food intake is a prerequisite to understanding both the cellular mechanisms and the neuronal circuitry underlying the hormone's robust anorexigenic action. Second, identification of the critical brain areas will help to identify feeding-related peptides and neurotransmitter systems that may be modulated by E2, thereby contributing to sex differences in food intake.
E2 in the MPOA, ARC, and DRN
The present data, along with a previous report by Richard and colleagues (Dagnault et al., 1997), demonstrate that E2 in the MPOA is sufficient to decrease food intake. Richard's group tested the involvement of the MPOA in E2's anorexigenic effect in food-restricted, OVX rats trained to consume food during two, 2-h test meals separated by 9 h. Microinfusions of the two highest doses of water soluble E2 (0.25 and 2.5 μg) in the MPOA decreased food intake during the first, 2-h test meal, occurring 4 h after E2 treatment, but not during the second, 2-h test meal, occurring 15 h after E2 treatment (Dagnault et al., 1997). Our findings are in partial agreement with this report in that MPOA infusions of the higher (2.5 μg) dose of E2, but not the lower (0.25 μg) dose of E2, was sufficient to decrease 4-h food intake in OVX rats. However, we, unlike Richard's group, detected a dose-related decrease in 24-h, food intake in E2-treated rats. It is surprising that Richard's group did not report a reduction in food intake during the second, 2-h feeding test because the available literature suggests that E2 acts via a genomic mechanism to reduce feeding (e.g., Geary et al., 1999; Asarian et al., 2002). Although, a behavioral change within 4 h could still be the result of a genomic mechanism, our observation that the anorexigenic effect of the lower dose of E2 was limited to 24 h is clearly consistent with a genomic effect. The reduction in food intake observed at 4 h in both studies could suggest that the larger dose of E2, 2.5 μg, represents a pharmacological hormone dose.
Our findings, however, are in contrast with Geary and colleagues (Hrupka et al., 2002), who reported that dilute crystalline E2 implants in the MPOA failed to reduce food intake but produced reliable decreases in body weight. In addition, Butera et al. did not detect an anorexigenic effect of dilute crystalline E2 in the MPOA on 3-day food intake in OVX rats (Butera et al., 1989). However, these discrepancies could reflect methodological differences among studies, such as the use of different E2 preparations. We and Richard's group used acute microinfusion of water soluble E2, whereas the other reports used dilute crystalline implants. Also, the coordinates used to target the MPOA differed. We and Richard's group targeted the caudal MPOA, which has the greatest expression of ERα (Sato et al., 2005), the ER subtype that appears to mediate E2-induced decreases in food intake (Roesch, 2006; Santollo et al., 2007; Santollo et al., 2010). Therefore, the inefficacy of E2 treatment by others could result from insufficient activation of ERα within this brain region.
Our MPOA data suggest that CRF may be one mediator of E2's anorexigenic action in female rats. CRF is an anorexigenic peptide that is co-localized with ERs in the caudal MPOA (Dagnault et al., 1997) and it has previously been demonstrated that acute i.c.v. microinfusion of a CRF antagonist blocks E2's anorexigenic effect in OVX rats (Dagnault et al., 1993). An important next step will be to determine if other feeding-related peptides in the MPOA also contribute to E2's anorexigenic effect.
A novel finding from this study is that E2 microinfusions in the ARC produced dose-related decreases in 24-h food intake, which implicate the ARC as an important nucleus for E2's anorexigenic effect. Similar to our findings involving the MPOA, microinfusions of the larger dose of E2 in the ARC produced a significant decrease in 4-h food intake. Again, this could suggest that the larger dose of E2 is pharmacological because a behavioral change this rapidly is not normally observed following administration of a physiological dose of E2 (Geary et al., 1999; Asarian et al., 2002). Our finding, that microinfusion of E2 in the ARC is sufficient to decrease food intake in OVX rats, is consistent with previous studies demonstrating that E2 can influence a variety of peptides in this nucleus. For example, E2 increases the expression of the anorexigenic peptide POMC but it also decreases the expression of the orexigenic peptides NPY and AgRP (Crowley et al., 1985; Baskin et al., 1995; Clegg et al., 2007; Pelletier et al., 2007). It has also been demonstrated that both peripheral and central (i.c.v.) administration of E2 increases leptin's anorexigenic effect in female rats (Clegg et al., 2006). Because leptin receptors are abundantly expressed in the ARC, where they influence activity of both orexigenic and anorexigenic peptide populations, our data suggest that the interaction between E2 and leptin could occur locally in the ARC. Taken together, the available evidence suggests that E2 acts in the ARC to increase anorexigenic signaling and decrease orexigenic signaling, which leads to an overall reduction in food intake.
Our demonstration that E2 microinfusion in the DRN is sufficient to decrease food intake is novel. We hypothesized that E2 acts in the DRN to decreases food intake because it is the major locus of serotonin cell bodies and because we previously demonstrated that increased serotonin neurotransmission mediates, in part, E2's anorexigenic effect. Estrous females are more sensitive to the anorexigenic effect of the non-specific serotonin agonist fenfluramine than males or diestrous females (Rivera et al., 2005). Moreover, exogenous E2 increases the anorexigenic effect of fenfluramine in OVX rats (Eckel et al., 2005), suggesting that the aforementioned sex and estrous-related changes in fenfluramine's anorexigenic effect are mediated by endogenous E2. In addition, E2 increases expression of the Pet-1 and serotonin transporter genes, which have been implicated in the regulation of serotonin neurons in the DRN during the same time period that E2 decreases food intake and body weight in OVX rats (Rivera et al., 2009). In support of our hypothesis, similar to our findings in the MPOA and ARC, dose-related decreases in 24-h food intake were observed following E2 microinfusion in the DRN. Our data support the notion that E2 can act within the DRN to alter serotonin neuronal activity and, thereby, decrease food intake.
In summary, our findings confirm and extend a previous report that E2 acts in the MPOA to decrease food intake (Dagnault et al., 1997) and identify two additional brain areas, the ARC and DRN, in which E2 acts to decrease food intake. We are confident that none of the positive effects are due to leakage of E2 in surrounding brain regions. As a control, we examined the rats with cannulae that missed their targets in the MPOA, ARC, and DRN. In each case we found no effect of E2 microinfusion on food intake. We also examined the rats with cannulae that missed their targets in the PVN, VMH and LH and again we found no effect of E2 microinfusion on food intake. This further supports our claim that there was minimal drug spread because these three nuclei are in close proximity to areas where one would expect E2 to reduce food intake, such as the 3rd ventricle and ARC. Our work is also consistent with previous data demonstrating that E2 modulates the potency of feeding-related neuropeptides/neurotransmitters residing in each of these brain regions, including NPY, CRF, POMC and serotonin (Crowley et al., 1985; Baskin et al., 1995; Dagnault et al., 1997; Rivera et al., 2005; Eckel et al., 2005; Clegg et al., 2007; Pelletier et al., 2007; Rivera et al., 2009).
E2 in the PVN, LH, and VMH
Microinfusions of E2 in the PVN failed to decrease food intake in OVX rats. Our finding is consistent with some, but not all, previous studies investigating the involvement of the PVN in E2's anorexigenic effect. For example, implants of dilute crystalline E2 are reported to either decrease (Butera et al., 1989) or have no effect (Hrupka et al., 2002) on food intake in OVX rats. In addition, bilateral lesions of the PVN may either block (Butera et al., 1992) or have no effect (Dagnault et al., 1994) on E2's anorexigenic effect in OVX rats. It is difficult to reconcile these discrepant findings. In a previous report where E2 in the PVN had no effect on food intake, norepinephrine microinfusion in the PVN did decrease food intake. This suggests that misplaced cannulae and/or tissue damage did not contribute to the negative finding (Hrupka et al., 2002). It is possible that the positive results from previous reports resulted from drug spreading in the 3rd ventricle. However, this still cannot account for the positive (but unreplicated) finding obtained from the lesion study. This leaves open the possibility that had our study used bilateral, instead of unilateral, E2 microinfusions in the PVN a decrease in food intake may have been observed. However, additional data suggest otherwise. Since the early positive reports by Butera and colleagues (Butera et al., 1989; Butera et al., 1992), it is now well established that ERα is both sufficient and necessary for E2's anorexigenic effect (Roesch, 2006; Santollo et al., 2007; Thammacharoen et al., 2009; Santollo et al., 2010). Because of the negligible expression of ERα in the PVN (Shughrue et al., 1997), one would expect that E2 in the PVN would fail to influence food intake. Therefore, our data suggest that the critical ERs that mediate E2's anorexigenic effect reside outside of the PVN and that either the neuronal populations in the PVN do not contribute to E2's anorexigenic effect or if they are involved they are mediated by E2 indirectly from other upstream nuclei. For example, although CRF is abundantly expressed in the PVN (Liposits et al., 1987) and may be involved in mediating E2 anorexigenic effect (Dagnault et al., 1993), based on the data here it is likely that the site of this interaction is not in the PVN. Instead the interaction could be in the MPOA, based on our current findings and the work of Richard and colleagues (Dagnault et al., 1997), or mediated in nuclei upstream of the PVN.
A surprising result was that E2 in the LH did not decrease food intake because available evidence demonstrates that E2 interacts with two feeding related peptides located in the LH, MCH and orexin. For example, E2 decreases MCH gene expression in the LH of obese male rats (Morton et al., 2004) and E2 treatment decreases the orexigenic effect of MCH in OVX rats (Messina et al., 2006; Santollo et al., 2008b). In addition, orexin protein expression changes across the estrous cycle with a peak during proestrous (Porkka-Heiskanen et al., 2004). While these studies provide a sound rational for targeting the LH, our findings that microinfusions of E2 in the LH are not sufficient to decrease food intake fail to implicate the LH in mediating E2's anorexigenic effect. Although it is possible, based on the large rostral-caudal extent of the LH, that E2 microinfusions in a different part of the LH would be capable of decreasing food intake. However, we believe that this is unlikely because we chose our coordinates based on the location of the greatest ERα expression within this nucleus (Muschamp et al., 2007). This suggests that the neuronal populations in the LH are not direct targets of E2 that contribute to its anorexigenic actions. Instead, if neuropeptides such as MCH and orexin are involved in E2's anorexigenic effect, it is likely that the critical ERs mediating these interactions reside outside of the LH.
As expected, E2 in the VMH was not sufficient to reduce food intake. This finding is in agreement with other studies in which implants of both pure and diluted crystalline E2 in the VMH failed to affect food intake (Palmer et al., 1986; Butera et al., 1989). Additionally, rats with VMH lesions still show a reduction in food intake following E2 treatment (King et al., 1973). In a more recent study, OVX mice with viral-mediated knockdown of ERα in the VMH were as equally responsive to the anorexigenic effect of E2 as control mice (Musatov et al., 2007). In an attempt to reveal an inhibitory effect of E2 on food intake within the VMH, we chose coordinates that targeted the region containing the greatest density of ERα cell bodies (Yokosuka et al., 1997). However, E2 microinfusion still failed to decrease food intake. These observations, in combination with previous research, provide compelling evidence that the VMH is neither sufficient nor necessary for E2's anorexigenic effect. Thus, if any peptides in the VMH contribute to E2's anorexigenic effect, it is likely through an indirect pathway originating outside of the VMH.
Perspectives
Here we demonstrated that the MPOA, ARC and DRN are brain regions where E2 can act to decrease food intake, whereas the PVN, LH and VMH are not sufficient for this behavioral effect. Although the neural mechanisms by which E2 decreases food intake are poorly understood, these findings add to our knowledge of the brain circuitry involved in mediating E2's anorexigenic effect. Our data strengthen a previous report that the MPOA is involved in mediating E2's anorexigenic effect (Dagnault et al., 1997). In addition, we have provided the first evidence that E2 administration in the ARC and DRN are sufficient to reduce food intake in OVX rats. Each brain region identified as being sufficient for mediating the anorexigenic effect of E2 contains neurons that express various peptides and neurotransmitters that are implicated in the physiological control of food intake, in general, and the estrogenic control of food intake, in particular. For example, our data suggest that the peptides in the ARC such as NPY, AgRP and the POMC products, such as α-melanocyte stimulating hormone, are likely mediators of the estrogenic control of food intake. In addition, CRF in the MPOA and serotonin in the DRN are also likely to play a role. Geary and colleagues previously identified the nucleus of the solitary tract (NTS) as an additional site where E2 acts to decrease food intake (Thammacharoen et al., 2008). Because cholecystokinin, POMC and serotonergic neurons are all expressed in the NTS, and either their behavioral effects or gene expression are increased by estradiol (Geary et al., 1994; Eckel et al., 2002, Eckel et al., 2005; Rivera and Eckel, 2005; Rivera et al., 2009, Pelletier et al., 2007), it is likely that these signals from the NTS play a role in mediating the estrogenic inhibition of food intake. A major challenge for future studies will be to determine the relative contributions of the critical ERs in each of the brain areas identified in the present study and in the NTS along with understanding how these E2-sensitive sites communicate with one another. Such information is critical to further our understanding of the neural circuitry underlying E2's anorexigenic effect. At a more molecular level, it will be important to determine how the activation of ERs within these nuclei affects the transcription of genes implicated in the estrogenic control of food intake. Answers to these questions will be important to further our understanding of sex differences in food intake.
Acknowledgments
We thank Dr. Anne Etgen for helpful comments on the manuscript. This work was supported by grants from the NIH: DK073936 (LAE), NS062667 (JS) and DC000044 (A-MT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 2.Baskin DG, Norwood BJ, Schwartz MW, Koerker DJ. Estradiol inhibits the increase of hypothalamic neuropeptide Y messenger ribonucleic acid expression induced by weight loss in ovariectomized rats. Endocrinology. 1995;136:5547–5554. doi: 10.1210/endo.136.12.7588307. [DOI] [PubMed] [Google Scholar]
- 3.Butera PC, Beikirch RJ. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 4.Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- 5.Butera PC, Willard DM, Raymond SA. Effects of PVN lesions on the responsiveness of female rats to estradiol. Brain Res. 1992;576:304–310. doi: 10.1016/0006-8993(92)90694-5. [DOI] [PubMed] [Google Scholar]
- 6.Clegg DJ, Brown LM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decreases in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 7.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 8.Crowley WR, Tessel RE, O'Donohue TL, Adler BA, Kalra SP. Effects of ovarian hormones on the concentrations of immunoreactive neuropeptide Y in discrete brain regions of the female rat: Correlations with serum Luteinizing Hormone (LH) and median eminence LH-Releasing Hormone. Endocrinology. 1985;117:1151–1155. doi: 10.1210/endo-117-3-1151. [DOI] [PubMed] [Google Scholar]
- 9.Dagnault A, Ouerghi D, Richard D. Treatment with alpha-helical-CRF(9–41) prevents the anorectic effect of 17-beta-estradiol. Brain Res. Bull. 1993;32:689–692. doi: 10.1016/0361-9230(93)90175-b. [DOI] [PubMed] [Google Scholar]
- 10.Dagnault A, Richard D. Lesions of hypothalamic paraventricular nuclei do not prevent the effect of estradiol on energy and fat balance. Am. J. Physiol. (Endocrinology) 1994;267:E32–E38. doi: 10.1152/ajpendo.1994.267.1.E32. [DOI] [PubMed] [Google Scholar]
- 11.Dagnault A, Richard D. Involvement of the medial preoptic area in the anorectic action of estrogens. Am. J. Physiol. (Regulatory Integrative Comp. Physiol.) 1997;272:R311–R317. doi: 10.1152/ajpregu.1997.272.1.R311. [DOI] [PubMed] [Google Scholar]
- 12.Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brain of ovariectomized rats. Am. J. Physiol. (Regulatory Integrative Comp. Physiol. 2002;283:R1378–R1385. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- 13.Eckel LA, Rivera HM, Atchley DPD. The anorectic effect of fenfluramine is influenced by sex and stage of the estrous cycle in rats. Am. J. Physiol. (Regulatory Integrative Comp. Physiol. 2005;288:R1486–R1491. doi: 10.1152/ajpregu.00779.2004. [DOI] [PubMed] [Google Scholar]
- 14.Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol. Behav. 1999;67:141–147. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 15.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa N. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 16.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol. Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 17.Hrupka BJ, Smith GP, Geary N. Hypothalamic implants of dilute estradiol fail to reduce feeding in ovariectomized rats. Physiol. Behav. 2002;77:233–241. doi: 10.1016/s0031-9384(02)00857-0. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 19.King J, Cox VC. The effects of estrogens on food intake and body weight following ventromedial hypothalamic lesions. Physiol. Psychol. 1973;1:261–269. [Google Scholar]
- 20.Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- 21.Messina MM, Boersma G, Overton JM, Eckel LA. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol. Behav. 2006;88:523–528. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Morton GJ, Mystkowski P, Matsumoto AM, Schwartz MW. Increased hypothalamic melanin concentrating hormone gene expression during energy restriction involves a melanocortin-independent, estrogen sensitive mechanism. Peptides. 2004;25:667–674. doi: 10.1016/j.peptides.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Murray JF, Baker BI, Levy A, Wilson CA. The influence of gonadal steroids on pre-pro melanin-concentrating hormone mRNA in female rats. J Neuroendocrinol. 2000;12:53–59. doi: 10.1046/j.1365-2826.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- 24.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen recepor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muschamp JW, Hull EM. Melanin concentrating hormone and estrogen receptor-alpha are coexstensive but not coexpressed in cells of male rat hypothalamus. Neurosci. Lett. 2007;427:123–126. doi: 10.1016/j.neulet.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer K, Gray JM. Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol. Behav. 1986;37:187–189. doi: 10.1016/0031-9384(86)90404-x. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- 28.Pelletier G, Luu-The V, Labrie F. Oestrogenic regulation of proopiomelanocortin, neuropeptide Y and corticotropin-releasing hormone mRNAs in mouse hypothalamus. J. Neuroendocrinol. 2007;19:426–431. doi: 10.1111/j.1365-2826.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 29.Porkka-Heiskanen T, Kalinchuk A, Alanko L, Huhtaniemi I, Stenberg D. Orexin A and B levels in the hypothalamus of female rats: the effects of the estrous cycle and age. European J. Endocrinology. 2004;150:737–742. doi: 10.1530/eje.0.1500737. [DOI] [PubMed] [Google Scholar]
- 30.Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol. Behav. 2005;86:331–337. doi: 10.1016/j.physbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Rivera HM, Eckel LA. Activation of central, but not peripheral, estrogen receptors is necessary for estradiol's anorexigenic effect in ovariectomized rats. Endocrinology. 2010;151:5680–5688. doi: 10.1210/en.2010-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera HM, Lockwood DR, Kwon BS, Houpt TA, Eckel LA. Estradiol treatment increases Pet-1 and serotonin transporter (5HTT) gene expression in the OVX rat. Brain Res. 2009;1259:51–58. doi: 10.1016/j.brainres.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol. Behav. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav. Brain Res. 2008a;191:173–177. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santollo J, Eckel LA. The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiol. Behav. 2008b;93:842–850. doi: 10.1016/j.physbeh.2007.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Activation of ER alpha is necessary for the estradiol-induced reductions in food intake in female rats. Horm. Behav. 2010;85:872–877. doi: 10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am. J. Physiol. (Regulatory Integrative Comp. Physiol. 2007;293:R2194–R2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- 38.Sato S, Braham CS, Putnam SK, Hull EM. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: co-localization and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Res. 2005;1043:205–213. doi: 10.1016/j.brainres.2005.02.074. [DOI] [PubMed] [Google Scholar]
- 39.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-alpha agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Res. 2009;1268:88–96. doi: 10.1016/j.brainres.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 41.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wade GN, Blaustein JD, Gray JA, Meredith JM. ICI 182,780: a pure antiestrogen that affects behaviors and energy balance in rats without acting in the brain. Am. J. Physiol. (Regulatory Integrative Comp. Physiol. 1993a;34:R1392–R1398. doi: 10.1152/ajpregu.1993.265.6.R1392. [DOI] [PubMed] [Google Scholar]
- 43.Wade GN, Powers JB, Blaustein JD, Green DE. ICI 182,780 antagonizes the effects of estradiol on estrous behavior and energy balance in Syrian hamsters. Am. J. Physiol. (Regulatory Integrative Comp. Physiol.) 1993b;265:R1399–R1403. doi: 10.1152/ajpregu.1993.265.6.R1399. [DOI] [PubMed] [Google Scholar]
- 44.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala of the rat. J. Comp. Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]