Abstract
Circumcision significantly reduces female-to-male transmission of HIV infection, but changes in behavior may influence the overall impact on transmission. We sought to explore these effects, particularly for societies where women have less power to negotiate safe sex. We developed a compartmental epidemic model to simulate the population-level impact of various circumcision programs on heterosexual HIV transmission in Soweto. We incorporated gender-specific negotiation of condom use in sexual partnerships and explored post-circumcision changes in condom use. A 5-year prevention program in which only an additional 10% of uncircumcised males undergo circumcision each year, for example, would prevent 13% of the expected new HIV infections over 20 years. Outcomes were sensitive to potential changes in behavior and differed by gender. For Southern Africa, even modest programs offering circumcision would result in significant benefits. Because decreases in male condom use could diminish these benefits, particularly for women, circumcision programs should emphasize risk-reduction counseling.
Keywords: Male circumcision, Mathematical models, HIV prevention, Africa, Sexual behavior
Introduction
Male circumcision has recently been identified as a novel potential HIV prevention strategy that could be implemented immediately. Three large randomized controlled trials to evaluate the impact of circumcision on female-to-male (FTM) HIV transmission in Kenya, Uganda, and South Africa have proven the efficacy of adult male circumcision [1–3]. In addition, circumcision has been shown to be safe, affordable, and culturally acceptable to men in many African countries [4]. Yet the success of circumcision programs is not guaranteed.
As with any partially effective HIV prevention technology, it is possible that sexual risk behavior might change in response to the intervention, thus increasing or decreasing the potential benefits of that program [5]. Although there was no evidence of increased risk-taking behavior in the Kenyan and Ugandan circumcision trials [1, 2, 6], there was a moderate increase during the South African trial [3] and we are unable to predict what might occur outside of clinical trial conditions in an expanded circumcision program [7]. In another clinical trial to examine whether circumcision reduced male-to-female (MTF) transmission in Uganda, female partners of circumcised HIV-positive men did not report significant changes in risky sexual behaviors [8]. However, as with the three trials to assess the impact of circumcision on FTM transmission, this clinical trial involved regular and intensive risk reduction counseling. One prospective cohort study in Kenya found no risk compensation for the first year in men who underwent voluntary circumcision [9], but this is the only study of behavior following circumcision outside of a clinical trial and whether this represents permanent and widely applicable behavior patterns remains to be seen. In particular, the scale-up of circumcision is now being attempted in an environment of widespread knowledge regarding the effectiveness of circumcision for HIV prevention [10]. Additionally, the scale-up of circumcision may not include adequate risk reduction counseling because circumcision is seen as a single surgical event and because adequate resources have historically not been made available for behavior prevention programs [11].
Of further concern is the potential for gender-specific inequalities in the impact of risk compensation during the scale-up of male circumcision programs [12], particularly for risk compensation in the form of decreases in condom use. Questions have been raised regarding the ability of male circumcision programs to provide protection to women if they are unable to negotiate condom use or other forms of safer sex, if they experience a resulting increase in sexual violence, and if men do not receive the intensive behavioral counseling seen in clinical trial conditions [13–15]. An intervention given to men, who in turn may increase their risk-taking behavior, could potentially place women at greater risk for infection in societies where they have less power to negotiate safe sex practices.
Governments and international health policy makers are now considering or already recommending the addition of large-scale expanded adult male circumcision programs to their current arsenal of HIV prevention packages. Recent modeling studies of male circumcision programs have shown the enormous impact that this intervention could have in reducing HIV transmission in Southern Africa, particularly at high coverage levels [16–24]. While many of these studies have examined the potential for risk compensation in the form of increased sex partners to reduce the benefits of these programs, they have not addressed risk compensation in the form of widespread changes in condom use behavior by HIV-positive men in settings where women have a decreased ability to negotiate safe sex. Additionally, an expert panel recently concluded that current models have not identified potential harms to women at the population level resulting from an increase in risky behavior by circumcised HIV-positive men [25]. We believe that one reason potential harms to women have not been identified is that gender-specific power to negotiate condom use has not yet been examined. We therefore developed a mathematical model to examine the interaction between reductions in HIV transmission and changes in risk behavior following circumcision in the African setting, specifically allowing for gender differences in average condom use behavior as well as the ability to negotiate condom use. We used the model to estimate the potential impact of expanded adult male circumcision programs in Africa—particularly those with very modest coverage goals, which are of immediate interest as scale-up of male circumcision begins. We selected input parameters to simulate the late-stage, high prevalence HIV epidemic in the township of Soweto, South Africa as an example.
Methods
Model Description
We formulated a dynamic, compartmental model for HIV transmission and disease progression to examine the influence of male circumcision on heterosexual transmission of HIV (see Appendix). Individuals in the model progress through four disease stages: uninfected (HIV-negative), infected (HIV-positive) asymptomatic disease, infected (HIV-positive) symptomatic disease, and AIDS. Asymptomatic and symptomatic disease roughly corresponds to the World Health Organization (WHO) clinical stages 1/2 and 3, respectively, while AIDS corresponds to WHO clinical stage 4. The design includes factors specific to modeling the HIV epidemic in Africa such as predominantly heterosexual HIV transmission, limited knowledge of HIV infection status, and gender differences in circumstances surrounding sexual risk, which apply to all HIV prevention programs and which we have previously described in a related model on the impact of HIV vaccines [26]. The course of the epidemic is modeled as the flow of individuals between ten various ‘compartments’ or ‘health states’. Transitions between these compartments are defined by a set of deterministic, differential equations.
Circumcision
We incorporated the biological protective effect of circumcision in reducing heterosexual transmission of HIV in sexual partnerships and modeled only a reduction in FTM transmission for circumcised HIV-negative males. Because we modeled HIV transmission on an annual per-partnership basis, this protective effect applies to the probability of HIV transmission over the entire course of a partnership between a circumcised HIV-negative male and an HIV-positive female, rather than to a per-sex act probability of transmission (see Appendix).
We initialized our model with a protective effect (61%) based on results from the circumcision trial in South Africa [3], as it was conducted in a similar population and in close proximity to Soweto, but also conducted sensitivity analyses incorporating circumcision-related reductions in FTM HIV transmission of 20, 40, 60, and 80%. We incorporated current levels of male circumcision in South Africa (35%) [27, 28] into the model. We modeled expanded circumcision programs which targeted asymptomatic uncircumcised males aged 17 or older without testing them for HIV, therefore both HIV-negative and asymptomatic HIV-positive males would be eligible for circumcision. We explored the impact of expanded circumcision programs that covered an additional 10–20% of uncircumcised males each year, with further sensitivity analyses on program coverage levels.
Gender Differences in Risk Behavior
In our analyses, we modeled the potential effect of circumcision on behavior by exploring changes in levels of average condom use, although other changes in behavior could also occur. Based on previous survey research in South Africa [29] and the surge in public demand for circumcision following the mass publicity surrounding the results of the circumcision trial in South Africa [30], we examined behavior change for all circumcised men, irrespective of whether they were circumcised in the expanded program or elsewhere. We modeled exclusive male-negotiation of condom use in heterosexual partnerships in Soweto, as studies have shown significant imbalances in the ability of South African women to negotiate condom use with men in sexual partnerships [31–34]. Therefore, condom use in heterosexual partnerships was determined by the risk behavior pattern of the male. By employing average condom usage levels to explore changes in risk behavior, rather than numbers or types of partners or sex acts, we were able to capture these potentially important gender differences in risk compensation.
Input Parameters and Assumptions
We used published data sources for the majority of input parameters incorporated into the model (Table 1). We assumed that the partial protection afforded by circumcision would be life long. In order to simplify the model and understand the impact of circumcision apart from antiretroviral (ARV) therapy use, we assumed an ARV-naïve population because provision of ARV therapy in the population covered by the public-sector health system in South Africa is low at present (estimated at less than 20%, see Appendix) [35], and thus did not incorporate a reduction in transmission or increase in length of disease stage for individuals receiving ARV therapy (see Appendix). We initialized our model with an average condom use of 50% for all partnerships, prior to risk compensation. This estimate was based on mean condom use in a Soweto cohort [36] and on national surveys in South Africa of condom use at last sex [28, 37–40].
Table 1.
Parameter values
| Parameter name | Symbol | Valuea | Source |
|---|---|---|---|
| Preventive circumcision program parameters | |||
| Percentage of uncircumcised uninfected or uncircumcised asymptomatic HIV + males circumcised annually |
κ(t) | 0.10–0.20, t ≤ 5 0, t >5 |
Assumption |
| Circumcision protective effect (percent reduction in risk of female- to-male transmission of HIV infection) |
ε | 0.61 (0–1.0) | [3] |
| Change in (male-negotiated) condom use following circumcision, for males circumcised as adults following circumcision program implementation, or for males previously circumcised in childhoodb |
Δ | 1.0 (0–2.0) | Assumption |
| HIV transmission parameters | |||
| Male infectivity (per-partner probability of transmission to a female) | |||
| Asymptomatic period of HIV infection | βM1,j | 0.0684c | [66] |
| Symptomatic period of HIV infection | βM2,j | 0.1657c | [66] |
| Female infectivity (per-partner probability of transmission to a male) |
|||
| Asymptomatic period of HIV infection | βW1 | 0.1112c | [66] |
| Symptomatic period of HIV infection | βW2 | 0.2697c | [66] |
| Contact rate (number of new partners per year) of males or females | |||
| Uninfected | ρ0 | 3 | [36] |
| HIV infected, asymptomatic period | ρ3 | 3 | [36] |
| HIV infected, symptomatic period | ρ2 | 1 | Assumption |
| HIV infected, AIDS | ρ3 | 0 | Assumption |
| Baseline (male-negotiated) condom use for all partnerships (without implementation of an adult male circumcision program) |
hi,,j | 0.5 | [28, 36–40] |
| Condom failure rate for all partnerships | f | 0.14 | [67] |
| HIV disease duration parameters | |||
| Asymptomatic HIV infection (years) | 1/µ1 | 6.8 | [68–70] (with assumptions) |
| Symptomatic HIV infection (years) | 1/µ2 | 2.6 | [68–70] (with assumptions) |
| AIDS (years) | 1/µ3 | 0.8 | [68] |
| Population parameters, heterosexual men/women >16 years | |||
| Mean age (years) | 25.1 | [71] | |
| Non-AIDS life expectancy (years) | 60.8 | [72] | |
| Non-AIDS-related annual mortality rate | µ | 0.028d | [71, 72] |
| Initial population size | 823,000 | [28, 38–40, 73] | |
| Initial HIV prevalence, male population (%) | 11.6 | [38] | |
| Initial HIV prevalence, female population (%) | 20.0 | [38] | |
| Initial circumcision rate, baseline male population (%) | 0.35 | [27] | |
| Arriving male population HIV prevalence (%) | 0.03 | [28, 38, 40, 74] | |
| Arriving female population HIV prevalence (%) | 0.10 | [28, 38, 40, 74] | |
| Arriving male population circumcision rate (%) | 0.35 | [28] |
Values in parentheses are ranges used in sensitivity analyses
E.g., for Δ = 1.0, no change in condom use, for Δ = 1.30, 30% increase in condom use, for Δ = 0.70, 30% decrease in condom use
See Appendix for further details
Calculated[26]
We considered only heterosexual transmission of HIV in this analysis, as it is the predominant mechanism for adult HIV transmission in sub-Saharan Africa [41]. We examined HIV transmission over the entire course of a sexual partnership on an annual basis (see Appendix). The infectivity parameters (probability of transmitting HIV) for asymptomatic HIV-positive individuals were approximately 40% of the infectivity parameters for symptomatic HIV-positive individuals, and the asymptomatic infectivity parameter captured the higher rates of transmission seen during the acute stage of HIV infection [42] (see Appendix). We did not explicitly consider scenarios in which circumcised men resumed sex prior to complete wound healing. Although early resumption of sex post-circumcision does not increase the likelihood of FTM transmission [43], it can increase MTF transmission [8] and thus would place women at increased risk of infection during that period (approximately 6 weeks [44]). However, Hallett and colleagues have shown that the impact of potentially increased transmission to women during this period appears to be minimal [21].
We varied the sexual risk behavior between population subgroups, or compartments, according to disease stage for the number of sex partners per year and according to gender and circumcision status for condom use, but did not incorporate further risk stratifications. Details on the rationale used for estimates of input parameters which define other population characteristics, sexual risk behavior, HIV transmission, and disease progression have been reported elsewhere [26].
Further details on model equations, parameters, and the rationale and assumptions underlying the model specifications are provided in the Appendix.
Simulations
We first simulated predicted trends for the adult HIV epidemic in Soweto considering the reduction in HIV transmission from current rates of male circumcision to establish the baseline impact of present circumcision practices. We then explored the additional impact of expanded programs, which produced an increase in circumcision over baseline levels. We conducted simulations for potential programs which were implemented for a 5-year period and evaluated their incremental effects by examining the total number of HIV infections averted, the percentage of cumulative infections prevented, and the change in population HIV prevalence over time, as compared to baseline. We reported most outcomes for a 20-year period, which shows the longer-range impact of modest 5-year programs and allows for comparison with other studies.
We also simulated scenarios in which risk behavior changed following circumcision program implementation by varying condom use in circumcised males—exploring all values (0–100%) of post-circumcision program male-negotiated average condom use. Further, we explored varying levels of program coverage (the proportion of uncircumcised HIV-negative and asymptomatic HIV-positive men who are circumcised each year in the program) and varying levels of circumcision efficacy to assess the tradeoff between the true impact on HIV transmission of male circumcision, the program coverage level achieved, and the potential for changes in risk behavior with implementation of circumcision programs.
We implemented the model using difference equations and performed all simulations using Excel® 2003 spreadsheet software (Microsoft Corporation, Redmond WA). We calculated transitions between various compartments in the model using a cycle time of one year. Despite the lack of population HIV prevalence data for Soweto, we validated the model using overall predicted trends for Gauteng province (which includes Soweto township) population HIV prevalence (see Appendix for further details).
Results
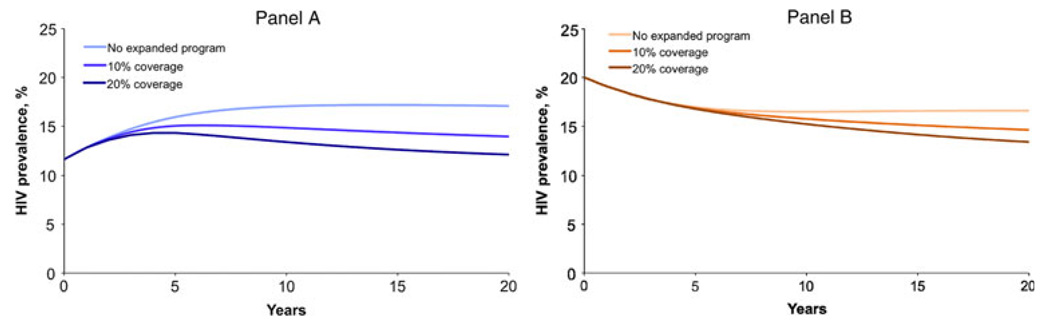
We first simulated the predicted trends in adult HIV prevalence in Soweto considering the reduction in HIV transmission from current rates of male circumcision. Assuming present levels of circumcision remain constant and a 61% reduction in FTM HIV transmission per partnership for circumcised males, male HIV prevalence would increase from 12 to 17% (Fig. 1a) and female HIV prevalence would decrease from 20 to 17% (Fig. 1b). Using a baseline population size of 823,000, the model predicted 142,000 male and 102,000 female infections would occur over the 20-year period.
Fig. 1.
Predicted trends in Soweto adult HIV prevalence over 20 years for male (panel A) and female (panel B) populations: model simulation considering the reduction in HIV transmission due to current rates of male circumcision, and following implementation of 5-year expanded circumcision HIV prevention programs targeting an additional 10 or 20% of uncircumcised adult males each year
We then considered the impact of various 5-year expanded adult circumcision programs, which covered an additional percentage of the uncircumcised male population each year (Figs. 1a,b). A program covering 10% of the uncircumcised adult male population each year for five years would result in a decrease in predicted HIV prevalence in 20 years from 17 to 14% for males and from 17 to 15% for females. If, instead, the program covered an additional 20% of the uncircumcised adult male population, the decrease in predicted HIV prevalence would be more substantial: predicted HIV prevalence would decrease to 12% for males and 13% for females. For both program coverage goals, approximately one-third of the total infections prevented over 20 years would be in females; and, looking at the long-term benefits of these short programs, the proportion of female infections prevented would increase to 40% of total infections prevented by these programs over 50 years.
We also simulated scenarios in which average condom use behavior changed for circumcised males following program implementation, for various levels of circumcision efficacy in a three-way sensitivity analysis (Table 2). We examined outcomes for varying circumcision efficacy (20, 40, 60, 80%), program coverage (10, 20%), and change in condom use behavior (+100%, +50%, +25%, 0%, −25%, −50%, −100%). While all circumcision programs in which condom use increased would be beneficial, many circumcision programs in which condom use decreased would still be beneficial—particularly for higher levels of circumcision protective effect. This was true regardless of whether the program coverage level was moderate or high (data not shown). Overall, for lower reductions in transmission due to circumcision (e.g., 20–40% efficacy), changes in condom use would play a larger role in determining the magnitude of program benefits, regardless of program coverage levels. For higher reductions in transmission due to circumcision (e.g., 60–80% efficacy), though, the impact of circumcision programs was significant and changes in condom use would have less influence; programs with lower coverage levels at high circumcision efficacy could tolerate greater decreases in condom use than those at lower circumcision efficacy while still providing a net benefit.
Table 2.
Sensitivity analysis on potential risk behavior change and protective circumcision effect: total infections prevented and percentage of cumulative infections prevented after 20 years by various 5-year expanded circumcision programsa
| Protective effect on HIV transmission due to circumcision | ||||
|---|---|---|---|---|
| Change in condom use post-circumcisionb | 20(%)c | 40(%)c | 60(%)c | 80(%)c |
| Programs with 10% coverage goals | ||||
| Condom use increases by 100% | 145,000 (49) | 126,000 (47) | 107,000 (43) | 87,000 (39) |
| Condom use increases by 50% | 87,000 (30) | 81,000 (30) | 74,000 (30) | 66,000 (30) |
| Condom use increases by 25% | 51,000 (17) | 53,000 (20) | 54,000 (22)d | 54,000 (25) |
| No change in condom use | 11,000 (4) | 21,000 (8) | 32,000 (13)d | 41,000 (19) |
| Condom use decreases by 25% | [34,000 (12)] | [14,000 (5)] | 7,000 (3)d | 27,000 (12) |
| Condom use decreases by 50% | [83,000 (28)] | [53,000 (20)] | [21,000 (8)] | 12,000 (5) |
| Condom use decreases by 100% | [186,000 (63)] | [139,000 (51)] | [84,000 (34)] | [23,000 (11)] |
| Programs with 20% coverage goals | ||||
| Condom use increases by 100% | 175,000 (59) | 158,000 (58) | 139,000 (57) | 119,000 (54) |
| Condom use increases by 50% | 109,000 (37) | 106,000 (39) | 102,000 (41) | 96,000 (44) |
| Condom use increases by 25% | 67,000 (23) | 73,000 (27) | 79,000 (32)d | 83,000 (38) |
| No change in condom use | 18,000 (6) | 36,000 (13) | 52,000 (21)d | 68,000 (31) |
| Condom use decreases by 25% | [37,000 (13)] | [7,000 (3)] | 23,000 (9)d | 51,000 (23) |
| Condom use decreases by 50% | [97,000 (33)] | [55,000 (20)] | [10,000 (4)] | 33,000 (15) |
| Condom use decreases by 100% | [222,000 (75)] | [161,000 (59)] | [87,000 (35)] | [9,000 (4)] |
Total infections prevented for male and female populations. Percentages reflect the proportion of expected infections prevented by each program scenario. Numbers in brackets represent negative values, which indicate the number and percentage of additional infections caused by a particular program
Considering an increase or decrease from baseline condom use levels of 50%. A 25% difference implies an absolute increase to 62.5% or decrease to 37.5% in condom use levels. A 50% difference implies an absolute increase to 75% or decrease to 25% in condom use levels. A 100% difference implies an absolute increase to 100% or decrease to 0% in condom use levels
Total expected infections over 20 years for various circumcision protective effects in the absence of expanded circumcision programs: 295,000 (20); 271,000 (40); 246,000 (60); and 220,000 (80%)
Outcomes for these simulations are examined in further detail in Fig. 2, which demonstrates infections prevented over time and stratified by gender for each of these scenarios
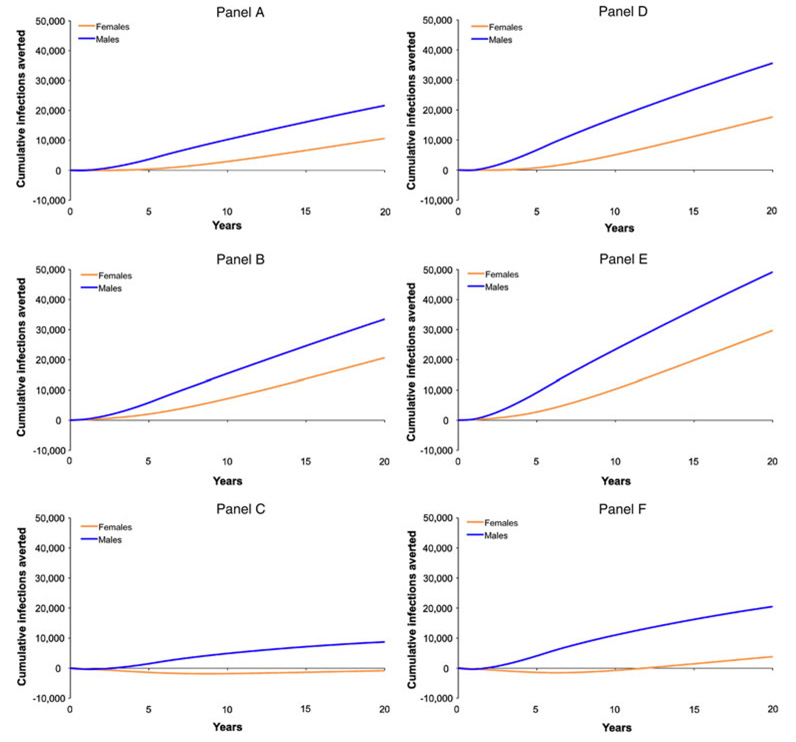
We then examined specific outcomes by gender in greater detail for several scenarios from Table 2 that indicated an overall positive effect in terms of infections prevented for the 60% circumcision efficacy assumption and a 25% increase or decrease in condom use (Fig. 2). This allowed for analysis of the quantitative differences in the number of infections prevented for men and women, as well as for variations in program outcomes over time. Panel 2A shows the baseline benefits of the 10% coverage program over 20 years by gender (10% of cumulative infections averted, or 11,000 infections prevented, in women; 15% of cumulative infections averted, or 22,000 infections prevented, in men). If average condom use increased by 25% (Panel 2B), the program would instead avert 20% (21,000) of the female and 24% (33,000) of the male infections whereas, if condom use decreased by 25% (Panel 2C), the program would avert 6% (9,000) of the male infections but result in a net additional 1,000 female infections. Panel 2D displays the baseline benefits of the 20% coverage program by gender (17% of cumulative infections averted, or 18,000 infections prevented, in women; 25% of cumulative infections averted, or 36,000 infections prevented, in men). If condom use increased by 25% (Panel 2E), the program would prevent 29% (30,000) of the female infections and 35% (49,000) of the male infections whereas, if condom use decreased by 25% (Panel 2F), the program would prevent 14% (20,000) of the male infections and 4% (4,000) of the net female infections, but with an initial period of increased infections in the female population. As shown in these simulations, analysis by gender reveals that even positive outcomes predicted at the population level can be deleterious for women in certain scenarios of increased risk behavior.
Fig. 2.
Cumulative HIV infections prevented over 20 years for male and female populations following implementation of 5-year expanded circumcision programs targeting: an additional 10% of uncircumcised adult males each year with no subsequent risk behavior change (panel A), with a 25% increase in condom use, corresponding to an absolute increase in condom use from 50 to 62.5% in circumcised males (panel B), or with a 25% decrease in condom use, corresponding to an absolute decrease in condom use from 50 to 37.5% in circumcised males (panel C); and similarly, an additional 20% of uncircumcised adult males each year with no subsequent risk behavior change (panel D), a 25% increase in condom use, corresponding to an absolute increase in condom use from 50 to 62.5% in circumcised males (panel E), or a 25% decrease in condom use, corresponding to an absolute decrease in condom use from 50 to 37.5% in circumcised males (panel F)
Discussion
We developed a model to estimate the potential impact of male circumcision programs for men and women in sub-Saharan Africa and examined the dynamic interaction between reduced susceptibility to HIV infection and subsequent behavioral disinhibition in the use of condoms by circumcised males. Overall, we found that circumcision programs could prevent a substantial number of new HIV infections in the African setting, and that moderate changes in condom use behavior do not significantly influence this outcome. The magnitude of the benefits from circumcision programs was sensitive to changes in sexual risk behavior, which for this study we approximated with changes in condom use to examine potential gender discrepancies: increased condom use would naturally lead to augmented program benefits but decreases in condom use would lead to decreased program benefits. All circumcision programs in which circumcision efficacy was approximately 60% or greater provided net benefits to the overall population in terms of infections prevented regardless of moderate changes in condom use behavior; only very large changes in condom use behavior (e.g., 50% or greater decrease in condom use) could significantly offset these benefits. In addition, we found that even relatively small circumcision programs would be beneficial and would continue to produce reductions in HIV transmission during the following decades. We estimated that conservative 5-year expanded circumcision programs targeting only 10–20% of uncircumcised men each year could produce substantial benefits over a wide range of values for circumcision protective effect, while tolerating moderate decreases in condom use.
We also found that a substantial number of infections would be prevented in women indirectly due to the decrease in male infections afforded by the circumcision programs and their associated potential for risk-reduction counseling to increase condom use—although the benefits to females were quantitatively less than the benefits to males. After twenty years, one-third of all infections prevented by these 5-year programs with 10 and 20% coverage goals would occur in the female population, but these gender differences in program benefits would gradually diminish over time. Additionally, transmission of HIV to females was still decreased for a wide variety of program simulations involving potential changes in risk behavior.
However, our model showed substantial gender disparities in program outcomes for some scenarios in which condom use decreased, which addresses concerns that an increase in risky sexual behavior by circumcised HIV-positive men might cause an increase in HIV prevalence in women, which could then increase the risk to men in a cyclical fashion [45]. Although the majority of potential program scenarios we estimated resulted in a positive net value for the number of infections prevented, women might experience a greater number of HIV infections over the 20-year period in certain simulations if condom use decreased following program implementation. This could occur despite a positive net value for the overall number of infections prevented because of the difference in magnitude between the outcomes of the program for men and women. And even if women did not experience a greater number of HIV infections over the 20-year period (e.g., a positive net value for the total infections prevented in women), some scenarios with decreased condom use still resulted in periods of increased HIV infections for women over several years of the program.
Due to gender disparities in the protective effect of this intervention, circumcised males receive a direct benefit due to the protective effect of circumcision, while females receive only an indirect benefit from reduced population HIV prevalence. Further, because we modeled condom use in sexual partnerships to be determined by males, changes in sexual risk behavior for men receiving the intervention of circumcision directly correlated with changes in sexual risk behavior that place women at risk for HIV infection. In addition to highlighting the differences between potential benefits for men and women, our analyses demonstrate that risk behavior changes in the form of increases or decreases in condom use may be more or less influential in programs where an intervention can provide a direct benefit to only a fraction of the population (such as the case with male circumcision) versus the entire population (such as the case with HIV vaccines [26]).
Our results have confirmed the findings of previous model-based studies on the impact of male circumcision programs [16–24], but have allowed for the examination of modest program coverage goals, post-circumcision changes in condom use behaviors, and gender interactions in heterosexual HIV transmission and program outcomes in greater detail—demonstrating the benefits of circumcision programs even when taking into account potentially offsetting factors such as minimal program coverage and moderate condom use behavior change in settings where the negotiation of condom use is gender-based. Although a recent expert panel on mathematical modeling of male circumcision programs concluded that increases in risk behavior would decrease the benefits of male circumcision programs overall [25], whether these programs could cause potential harm—rather than just a decrease in benefit—to women at the population level has not been examined. Our analyses show the potential for circumcision programs to place women at greater overall risk for HIV infection in certain settings, and further exploration of gender-specific outcomes of male circumcision interventions is needed.
While the inclusion of gender-specific condom use negotiation and risk compensation is a strength of our model, our assumption of exclusive male negotiation of condom use for these analyses is one of its limitations. Although there is an imbalance of power in the negotiation of safe sex in Southern Africa, it is not absolute, and the impact of variations in male and female negotiating power warrants further investigation. In addition, the focus of the present analysis was to explore the impact of gender differences in power to negotiate safe sex, which is primarily manifested in the ability to specify condom use during sexual partnerships, on circumcision program outcomes. Other behavioral manifestations of gender differences in power to negotiate safe sex, such as polygamy, should be explored in the future.
The reduction in FTM HIV transmission of 61% (95%CI: 34,77%) used in our base case analyses for Soweto was based on the randomized controlled trial of adult male circumcision in South Africa, which was a single site study conducted nearby in Orange Farm [3]. The other two trials in Kenya and Uganda reported efficacy results of 53% (95%CI: 22,72%) [2] and 51% (95%CI: 16,72%) [1], respectively, and preliminary long term follow-up data in Kenya indicates that circumcision efficacy is sustained and possibly strengthened to 64% (95%CI: 43,77%) at 42 months [46]. Additional long-term follow-up data on circumcision efficacy is not yet available and the effectiveness of circumcision in preventing HIV transmission might vary in other populations and non-trial conditions. For these reasons, we explored values for the reduction in FTM HIV transmission from 20 to 80%, which encompass the trial confidence intervals, in sensitivity analyses, although a number of ecological and cohort studies indicate that the protective effect is indeed likely to be very high [47–56]. We found that the model predictions were consistent over a wide variety of values and thus our results can likely be generalized to other populations with similar epidemic profiles and predominantly heterosexual spread of HIV, despite the initialization of the model with parameters specific to the African setting. However, the results cannot be extended to populations with major differences in route of HIV transmission or stage of the epidemic.
We examined only the impact of circumcising adult males, but modeling studies examining the impact of increased pediatric circumcision are certainly merited. Despite the immediate epidemiological benefits and reduced ethical issues surrounding the circumcision of sexually active adults able to provide informed consent, circumcision of infants and boys would eventually be more practical and provide greater public health benefits [57]. We also did not simulate the impact of circumcision on other sexually transmitted infections (STIs) or their effect on HIV transmission. While circumcision may reduce other STIs, which in turn contributes to a reduction in HIV transmission, this effect is likely to be minimal compared with the effect of circumcision on HIV transmission itself [58].
Additionally, our results do not take into account the fact that circumcision programs would involve a one-time intervention while programs to increase condom use require an intervention that must be practiced with every sex act over the life of the individual; therefore the best manner in which to allocate resources between different programs cannot be determined by our analyses. Our model also does not consider the impact of multiple, simultaneous prevention programs such as education on delaying intercourse, partner reduction, promotion of condom use, access to HIV counseling and testing, syringe exchange programs, STI treatment, treatment of HIV-positive individuals to reduce viral load, and eventually, chemoprophylaxis regimens, microbicides, and prophylactic or therapeutic vaccines [59–61]. Further work on assessing the impact of multiple partially effective prevention programs is merited to allow for relative comparisons between different intervention package combinations.
Male circumcision has been shown to be effective, safe, affordable, acceptable, and will likely confer lifelong benefits. However, implementation of even the modest circumcision programs described in our study will depend on the ability to scale up operations: many clinics in Africa are already struggling to cope with the increased demand for circumcision following coverage in the media [30] and a study in Soweto has shown limited capacity in public sector hospitals [62]. Further, programs must ensure that all male circumcision procedures are both safe and voluntary and must remain sensitive to the cultural practices of different populations—although even cultures and ethnic groups which do not currently encourage circumcision may find it acceptable, as was shown in the South African Zulu and Tswana populations [63, 64]. Circumcision programs will need to provide risk-reduction counseling, both to individuals as well as the community. This is particularly important in the South African context, where studies have revealed beliefs that circumcised men can increase their sexual risk behavior [29] and where risk behavior was already shown to increase slightly during the large circumcision trial conducted near Soweto [3]. Further, in an HIV vaccine trial in Soweto, men who elected to undergo male circumcision reported higher levels of risky sexual behavior at baseline [65]. Additionally, this risk-reduction counseling may need to be targeted in particular to men, given the reduced ability of women to negotiate condom use in the African setting and the fact that men will be receiving the intervention. Finally, programs should be evaluated on their effectiveness, with particular emphasis on whether changes in sexual risk behavior have occurred and the individual benefits to men and women.
Ackowledgments
The authors thank Edward Kaplan for his assistance with the model structure, and the anonymous reviewers who have provided valuable insights on the content of the paper. K. M. Andersson also thanks the Department of Epidemiology and Public Health and the MD/PhD Program at the Yale University School of Medicine for supporting the dissertation research from which portions of this article evolved.
This work was supported by the National Institute on Drug Abuse (RO1DA015612), the National Institute of General Medical Sciences Medical Scientist Training Program (GM07205), and the Agency for Health Research and Quality Training Program in Health Services Research (T32HS017589). The authors do not have any associations that may pose a potential conflict of interest.
Footnotes
Research undertaken in part during graduate studies in the MD/PhD program at Yale University and under the maiden name Kyeen Mesesan.
Electronic supplementary material The online version of this article (doi:10.1007/s10461-010-9784-y) contains supplementary material, which is available to authorized users.
Contributor Information
Kyeen M. Andersson, Email: kyeen@aya.yale.edu, Division of Health Policy & Administration, Department of Epidemiology & Public Health, Yale University School of Medicine, 60 College Street, New Haven, CT 06510, USA.
Douglas K. Owens, VA Palo Alto Health Care System, Palo Alto, CA, USA Center for Primary Care and Outcomes Research/Center for Health Policy, Stanford University, Stanford, CA, USA.
A. David Paltiel, Division of Health Policy & Administration, Department of Epidemiology & Public Health, Yale University School of Medicine, 60 College Street, New Haven, CT 06510, USA.
References
- 1.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):1112–1122. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westercamp N, Bailey RC. Acceptability of male circumcision for prevention of HIV/AIDS in sub-Saharan Africa: a review. AIDS Behav. 2007;11(3):341–355. doi: 10.1007/s10461-006-9169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassell MM, Halperin DT, Shelton JD, Stanton D. Risk compensation: the Achilles’ heel of innovations in HIV prevention? BMJ. 2006;332(7541):605–607. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattson CL, Campbell RT, Bailey RC, et al. Risk compensation is not associated with male circumcision in Kisumu, Kenya: a multi-faceted assessment of men enrolled in a randomized controlled trial. PLoS ONE. 2008;3(6):e2443. doi: 10.1371/journal.pone.0002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wegbreit J, Bertozzi S, DeMaria LM, Padian NS. Effectiveness of HIV prevention strategies in resource-poor countries: tailoring the intervention to the context. AIDS. 2006;20(9):1217–1235. doi: 10.1097/01.aids.0000232229.96134.56. [DOI] [PubMed] [Google Scholar]
- 8.Wawer MJ, Makumbi F, Kigozi G, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374(9685):229–237. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agot KE, Kiarie JN, Nguyen HQ, Odhiambo JO, Onyango TM, Weiss NS. Male circumcision in Siaya and Bondo Districts, Kenya: prospective cohort study to assess behavioral disinhibition following circumcision. J Acquir Immune Defic Syndr. 2007;44(1):66–70. doi: 10.1097/01.qai.0000242455.05274.20. [DOI] [PubMed] [Google Scholar]
- 10.Weiss HA, Halperin D, Bailey RC, Hayes RJ, Schmid G, Hankins CA. Male circumcision for HIV prevention: from evidence to action? AIDS. 2008;22(5):567–574. doi: 10.1097/QAD.0b013e3282f3f406. [DOI] [PubMed] [Google Scholar]
- 11.Eaton L, Kalichman SC. Behavioral aspects of male circumcision for the prevention of HIV infection. Curr HIV/AIDS Rep. 2009;6(4):187–193. doi: 10.1007/s11904-009-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berer M. Male circumcision for HIV prevention: what about protecting men’s partners? Reprod Health Matters. 2008;16(32):171–175. doi: 10.1016/S0968-8080(08)32418-5. [DOI] [PubMed] [Google Scholar]
- 13.Gruskin S. Male circumcision, in so many words. Reprod Health Matters. 2007;15(29):49–52. doi: 10.1016/S0968-8080(07)29310-3. [DOI] [PubMed] [Google Scholar]
- 14.Hankins C. Male circumcision: implications for women as sexual partners and parents. Reprod Health Matters. 2007;15(29):62–67. doi: 10.1016/S0968-8080(07)29311-5. [DOI] [PubMed] [Google Scholar]
- 15.Berer M. Male circumcision for HIV prevention: perspectives on gender and sexuality. Reprod Health Matters. 2007;15(29):45–48. doi: 10.1016/S0968-8080(07)29304-8. [DOI] [PubMed] [Google Scholar]
- 16.Gray RH, Li X, Kigozi G, et al. The impact of male circumcision on HIV incidence and cost per infection prevented: a stochastic simulation model from Rakai, Uganda. AIDS. 2007;21(7):845–850. doi: 10.1097/QAD.0b013e3280187544. [DOI] [PubMed] [Google Scholar]
- 17.Williams BG, Lloyd-Smith JO, Gouws E, et al. The potential impact of male circumcision on HIV in sub-Saharan Africa. PLoS Med. 2006;3(7):1032–1040. doi: 10.1371/journal.pmed.0030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagelkerke NJD, Moses S, de Vlas SJ, Bailey RC. Modelling the public health impact of male circumcision for HIV prevention in high prevalence areas in Africa. BMC Infect Dis. 2007;7:16–30. doi: 10.1186/1471-2334-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn JG, Marseille E, Auvert B. Cost-effectiveness of male circumcision for HIV prevention in a South African setting. PLoS Med. 2006;3(12):2349–2358. doi: 10.1371/journal.pmed.0030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podder CN, Sharomi O, Gumel AB, Moses S. To cut or not to cut: a modeling approach for assessing the role of male circumcision in HIV control. Bull Math Biol. 2007;69(8):2447–2466. doi: 10.1007/s11538-007-9226-9. [DOI] [PubMed] [Google Scholar]
- 21.Hallett TB, Singh K, Smith JA, et al. Understanding the impact of male circumcision interventions on the spread of HIV in southern Africa. PLoS ONE. 2008;3(5):e2212. doi: 10.1371/journal.pone.0002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White RG, Glynn JR, Orroth KK, et al. Male circumcision for HIV prevention in sub-Saharan Africa: who, what and when? AIDS. 2008;22(14):1841–1850. doi: 10.1097/QAD.0b013e32830e0137. [DOI] [PubMed] [Google Scholar]
- 23.Bollinger LA, Stover J, Musuka G, et al. The cost and impact of male circumcision on HIV/AIDS in Botswana. J Int AIDS Soc. 2009;12(1):7. doi: 10.1186/1758-2652-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Londish GJ, Murray JM. Significant reduction in HIV prevalence according to male circumcision intervention in sub-Saharan Africa. Int J Epid. 2008;37(6):1246–1253. doi: 10.1093/ije/dyn038. [DOI] [PubMed] [Google Scholar]
- 25.UNAIDS/WHO/SACEMA expert group on modelling the impact and cost of male circumcision for HIV prevention. Male circumcision for HIV prevention in high HIV prevalence settings: what can mathematical modelling contribute to informed decision making? PLoS Med. 2009;6(9):e1000109. doi: 10.1371/journal.pmed.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson KM, Owens DK, Vardas E, Gray GE, McIntyre JA, Paltiel AD. Predicting the impact of a partially effective HIV vaccine and subsequent risk behavior change on the heterosexual HIV epidemic in low- and middle-income countries: a South African example. J Acquir Immune Defic Syndr. 2007;46(1):78–90. doi: 10.1097/QAI.0b013e31812506fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shisana O, Simbayi L. Nelson Mandela/HSRC study of HIV/ AIDS: South African National HIV prevalence, behavioural risks and mass media: household survey 2002. Cape Town: Human Sciences Research Council Press; 2002. [PubMed] [Google Scholar]
- 28.Pettifor AE, Rees HV, Kleinschmidt I, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005;19(14):1525–1534. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 29.Lagarde E, Dirk T, Puren A, Reathe R-T, Auvert B. Acceptability of male circumcision as a tool for preventing HIV infection in a highly infected community in South Africa. AIDS. 2003;17(1):89–95. doi: 10.1097/00002030-200301030-00012. [DOI] [PubMed] [Google Scholar]
- 30.Wise J. Demand for male circumcision rises in a bid to prevent HIV. Bull World Health Organ. 2006;84(7):509–511. [PMC free article] [PubMed] [Google Scholar]
- 31.Simbayi L, Strebel A, Wilson T, et al. Sexually transmitted diseases in the south african public sector. Cape Town: National department of health’s directorate of health systems research and epidemiology in conjunction with the directorate of HIV/AIDS and STDs; 1999. Aug, [Google Scholar]
- 32.Pettifor AE, Measham DM, Rees HV, Padian NS. Sexual power and HIV risk, South Africa. Emerg Infect Dis. 2004;10(11):1996–2004. doi: 10.3201/eid1011.040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ackermann L, de Klerk GW. Social factors that make South African women vulnerable to HIV infection. Health Care Women Int. 2002;23(2):163–172. doi: 10.1080/073993302753429031. [DOI] [PubMed] [Google Scholar]
- 34.Dunkle KL, Jewkes RK, Brown HC, et al. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363(9419):1415–1421. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- 35.The World Health Organization (WHO), the joint United nations programme on HIV/AIDS (UNAIDS), and the United nations children’s fund (UNICEF) Toward universal access: scaling up priority HIV/AIDS interventions in the health sector: Progress Report 2008. Geneva: WHO; 2008. [Google Scholar]
- 36.Andersson KM, Van Niekerk RM, Niccolai LM, et al. Sexual risk behaviour of the first cohort undergoing screening for enrollment into Phase I/II HIV vaccine trials in South Africa. Int J STD AIDS. 2009;20(2):95–101. doi: 10.1258/ijsa.2008.008207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shisana O, Rehle T, Simbayi LC, et al. South African National HIV prevalence, incidence, behaviour and communication survey, 2008: a turning tide among teenagers? Cape Town: Human Sciences Research Council Press; 2009. [Google Scholar]
- 38.Shisana O, Rehle T, Simbayi LC, et al. South African National HIV prevalence, HIV incidence, behaviour and communication survey, 2005. Cape Town: Human Sciences Research Council Press; 2005. [Google Scholar]
- 39.Simbayi LC, Chauveau J, Shisana O. Behavioural responses of South African youth to the HIV/AIDS epidemic: a nationwide survey. AIDS Care. 2004;16(5):605–618. doi: 10.1080/09540120410001716405. [DOI] [PubMed] [Google Scholar]
- 40.Pettifor AE, Rees HV, Steffenson A, et al. HIV and sexual behavior among young South Africans: a national survey of 15–24 year olds. Johannesburg: reproductive health research unit, University of the Witwatersrand; 2004. Apr 6, [Google Scholar]
- 41.The Joint United Nations Programme on HIV/AIDS (UNAIDS) Report on the global AIDS epidemic. Geneva: UNAIDS; 2006. [Google Scholar]
- 42.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 43.Mehta SD, Gray RH, Auvert B, et al. Does sex in the early period after circumcision increase HIV-seroconversion risk? Pooled analysis of adult male circumcision clinical trials. AIDS. 2009;23(12):1557–1564. doi: 10.1097/QAD.0b013e32832afe95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kigozi G, Gray RH, Wawer MJ, et al. The safety of adult male circumcision in HIV-infected and uninfected men in Rakai, Uganda. PLoS Med. 2008;5(6):e116. doi: 10.1371/journal.pmed.0050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalichman S, Eaton L, Pinkerton S. Circumcision for HIV prevention: failure to fully account for behavioral risk compensation. PLoS Med. 2007;4(3):e138. doi: 10.1371/journal.pmed.0040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey R, Moses S, Parker C, et al. The protective effect of male circumcision is sustained for at least 42 months: results from the Kisumu, Kenya Trial. XVII international AIDS conference; Mexico city. 2008. [abstract THAC0501] [Google Scholar]
- 47.Simonsen JN, Cameron DW, Gakinya MN, et al. Human immunodeficiency virus infection among men with sexually transmitted diseases: experience from a center in Africa. N Engl J Med. 1988;319(5):274–278. doi: 10.1056/NEJM198808043190504. [DOI] [PubMed] [Google Scholar]
- 48.Weiss HA, Quigley MA, Hayes RJ. Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS. 2000;14(15):2361–2370. doi: 10.1097/00002030-200010200-00018. [DOI] [PubMed] [Google Scholar]
- 49.Siegfried N, Muller M, Deeks J, et al. HIV and male circumcision–a systematic review with assessment of the quality of studies. Lancet Infect Dis. 2005;5(3):165–173. doi: 10.1016/S1473-3099(05)01309-5. [DOI] [PubMed] [Google Scholar]
- 50.Baeten JM, Richardson BA, Lavreys L, et al. Female-to-male infectivity of HIV-1 among circumcised and uncircumcised Kenyan men. J Infect Dis. 2005;191(4):546–553. doi: 10.1086/427656. [DOI] [PubMed] [Google Scholar]
- 51.Gray RH, Kiwanuka N, Quinn TC, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. AIDS. 2000;14(15):2371–2381. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 52.Meier AS, Bukusi EA, Cohen CR, Holmes KK. Independent association of hygiene, socioeconomic status, and circumcision with reduced risk of HIV infection among Kenyan men. J Acquir Immune Defic Syndr. 2006;43(1):117–118. doi: 10.1097/01.qai.0000224973.60339.35. [DOI] [PubMed] [Google Scholar]
- 53.Gray R, Azire J, Serwadda D, et al. Male circumcision and the risk of sexually transmitted infections and HIV in Rakai, Uganda. AIDS. 2004;18(18):2428–2430. [PubMed] [Google Scholar]
- 54.Auvert B, Buve A, Ferry B, et al. Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub-Saharan Africa with different levels of HIV infection. AIDS. 2001;15 Suppl 4:S15–S30. doi: 10.1097/00002030-200108004-00003. [DOI] [PubMed] [Google Scholar]
- 55.Auvert B, Buve A, Lagarde E, et al. Male circumcision and HIV infection in four cities in sub-Saharan Africa. AIDS. 2001;15 Suppl 4:S31–S40. doi: 10.1097/00002030-200108004-00004. [DOI] [PubMed] [Google Scholar]
- 56.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 57.Rennie S, Muula AS, Westreich D. Male circumcision and HIV prevention: ethical, medical and public health tradeoffs in low-income countries. J Med Ethics. 2007;33(6):357–361. doi: 10.1136/jme.2006.019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desai K, Boily MC, Garnett GP, Masse BR, Moses S, Bailey RC. The role of sexually transmitted infections in male circumcision effectiveness against HIV—insights from clinical trial simulation. Emerg Themes Epidemiol. 2006;3:19. doi: 10.1186/1742-7622-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen J. Prevention cocktails: combining tools to stop HIV’s spread. Science. 2005;309(5737):1002–1005. doi: 10.1126/science.309.5737.1002. [DOI] [PubMed] [Google Scholar]
- 60.Potts M, Halperin DT, Kirby D, et al. Public health: reassessing HIV prevention. Science. 2008;320(5877):749–750. doi: 10.1126/science.1153843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coates TJ, Richter L, Caceres C, Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372(9639):669–684. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Bruyn G, Smith MD, Gray GE, et al. Circumcision for prevention against HIV: marked seasonal variation in demand and potential public sector readiness in Soweto, South Africa. Implement Sci. 2007;2:2. doi: 10.1186/1748-5908-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scott BE, Weiss HA, Viljoen JI. The acceptability of male circumcision as an HIV intervention among a rural Zulu population, Kwazulu-Natal, South Africa. AIDS Care. 2005;17(3):304–313. doi: 10.1080/09540120412331299744. [DOI] [PubMed] [Google Scholar]
- 64.Rain-Taljaard RC, Lagarde E, Taljaard DJ, et al. Potential for an intervention based on male circumcision in a South African town with high levels of HIV infection. AIDS Care. 2003;15(3):315–327. doi: 10.1080/0954012031000105379. [DOI] [PubMed] [Google Scholar]
- 65.de Bruyn G, Martinson NA, Nkala BD, et al. Uptake of male circumcision in an HIV vaccine efficacy trial. J Acquir Immune Defic Syndr. 2009;51(1):108–110. doi: 10.1097/QAI.0b013e3181a03622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 67.The National Institute of Allergy and Infectious Diseases (NIAID), the National Institutes of Health (NIH), and the Department of Health and Human Services (DHHS) Workshop Summary: Scientific evidence of condom effectiveness for sexually transmitted disease (STD) prevention. Herndon, Virginia: NIAID, NIH & DHHS; 2000. Jun, pp. 12–13. [Google Scholar]
- 68.Morgan D, Mahe C, Mayanja B, Okongo JM, Lubega R, Whitworth JA. HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries? AIDS. 2002;16(4):597–603. doi: 10.1097/00002030-200203080-00011. [DOI] [PubMed] [Google Scholar]
- 69.Owens DK, Edwards DM, Shachter RD. Population effects of preventive and therapeutic HIV vaccines in early- and late-stage epidemics. AIDS. 1998;12(9):1057–1066. [PubMed] [Google Scholar]
- 70.Edwards DM, Shachter RD, Owens DK. A dynamic HIV-transmission model for evaluating the costs and benefits of vaccine programs. Interfaces. 1998;28(3):144–166. [Google Scholar]
- 71.Statistics South Africa. Mid-year population estimates, south africa: 2004. Pretoria: statistics South Africa; 2004. Jul 27, [Google Scholar]
- 72.The Actuarial Society of South Africa. [Accessed 20 Oct 2007];ASSA2000 Model. Available at: http://www.actuarialsociety.org.za/. [PubMed]
- 73.Statistics South Africa. [Accessed 20 Oct 2007];South African census. 2001 Available at: www.statssa.gov.za.
- 74.Centre for AIDS development, research and evaluation (CADRE) [Accessed 11 Jan 2006];HIV prevalence, incidence, behavior and communication survey 2005. Available at: www.cadre.org.za.