Abstract
The non-genomic transmission of maternal behavior from one generation to the next illustrates the pervasive influence of maternal care on offspring development and the high degree of plasticity within the developing maternal brain. Investigations of the mechanisms through which these maternal effects are achieved have demonstrated environmentally-induced changes in gene expression associated with epigenetic modifications within the promoter region of target genes. These findings raise challenging questions regarding the pathways linking experience to behavioral variation and the broader ecological/evolutionary implications of the dynamic changes in neuroendocrine function that emerge. This review will highlight studies in laboratory rodents which demonstrate plasticity in the maternal brain and the role of maternally-induced changes in DNA methylation in establishing the link between variations in maternal care and consequent developmental outcomes. The persistence of maternal effects across generations and the trade-offs in reproduction that are evident in female offspring who experience high vs. low levels of maternal care contribute to our understanding of the divergent strategies that are triggered by the quality of early-life experiences. Evolving concepts of inheritance and the interplay between genes and the environment may advance our understanding of the origins of individual differences in phenotype.
Keywords: maternal, environment, transgenerational, epigenetic, ERα, reproductive strategy
The transmission of behavioral variation from parents to offspring can be considered at both a proximate/mechanistic level of analysis and within an ecological/evolutionary framework. Though traditional approaches to understanding the mechanisms of inheritance of traits has focused on the relationship between genetic variation and individual differences in phenotype, there is emerging evidence for the role of non-genomic factors in creating a transgenerational continuity in behavior. In many species, the emergence of developmental trajectories which lead to individual differences can be linked to early life experiences. In mammals, the quality of the environment experienced during perinatal development is shaped primarily by the interactions between mothers and offspring. Though these interactions can take on many different forms, depending on the particular species, a common feature of early development is the altered physiological, neuroendocrine, and behavioral patterns that emerge in the absence or disruption to these mother-infant interactions. However, even natural variations in the quality or quantity of maternal care can have a long-term impact on offspring brain and behavior. Interestingly, one of the consequences of variation in the experience of maternal care is in the development of neuroendocrine circuits which will shape the subsequent maternal and reproductive behavior of female offspring. Through this process, a transmission of individual differences in maternal behavior can be achieved. This finding leads us back to consideration of both the proximate/mechanistic and ecological/evolutionary pathways and implications of the inheritance of behavioral variation.
In this review, I will highlight studies which explore the environmental regulation of mother-infant interactions and the neurobiological substrates of individual differences in maternal care, with a particular focus on approaches using laboratory rodents. An emerging theme in these studies is the significant change in gene expression within the brain which can arise in response to environmental experiences occurring during the early stages of development. Evidence for environmentally-induced changes in transcriptional activity has triggered an evolution in our understanding of the interplay between genes and the environment. Rather than being constrained by the nature-nurture debate, we are rapidly moving forward and developing hypotheses that consider the common biological pathways through which genes and the environment operate. This common pathway may involve variation in the epigenetic landscape of the genome. Though the definition of epigenetic continues to be a topic of lively debate, a common theme in all definitions involves the factors that modify gene activity, through altered transcription, without altering the underlying gene sequence. Epigenetic factors may form a critical link between early life experiences and behavioral variation through dynamic yet stable changes in gene expression. Interestingly, the consequences of these environmentally-induced effects may persist across subsequent generations and thus illustrates another key feature of the definition of epigenetic: heritable effects. Here, I will discuss the evolving literature on the epigenetic mechanisms of maternal effects and the transmission of maternal behavior across generations.
While molecular and neurobiological investigation of the impact of early experiences have certainly inspired novel approaches to the study of the origins of behavioral variation which are contributing to the burgeoning field of Behavioral Epigenetics, these studies also raise important questions regarding the ecological/evolutionary implications of this gene-environment interplay. For example, if early life adversity in the form of reduced maternal care leads to impaired maternal behavior in subsequent generations, can this be considered adaptive? What advantages are conferred by dynamic yet potentially stable changes in gene expression, neurobiology, and behavior? To address these questions, I will discuss evidence for trade-offs in reproduction in the context of maternally induced behavioral variation and the conceptual advantage of including multiple mechanisms of inheritance in our thinking about maternal effects and the origins of variation.
Shaping the Maternal Brain
Genetic studies of maternal behavior suggest that, indeed, there are genes whose absence or variation can have a significant impact on parturition, lactation, and the quality of mother-infant interactions. For example, gene knock-out studies in laboratory mice indicate that deletion of estrogen receptor α (ERα) (Ogawa et al., 1998), fosB (Brown et al., 1996), prolactin receptor (Lucas et al., 1998), and the paternally expressed genes Peg1 (Lefebvre et al., 1998) and Peg3 (Champagne et al., 2009; Li et al., 1999) can alter various indices of postpartum behavior, including lactation/nursing, motivation to retrieve pups, nestbuilding, and pup licking/grooming (LG) [reviewed in (Leckman and Herman, 2002)]. The functional loss of these genes is associated with changes in the developing hypothalamus which may lead to decreased sensitivity to the priming effects on brain and behavior of circulating hormones during the gestational period or disruptions to the formation of circuits within the brain that are essential for maintaining offspring directed behavior.
Though genes certainly provide a biological substrate for the emergence of variations in maternal behavior, there is evidence that stable individual differences in the quality and quantity of mother-infant interactions can occur even in the absence of genetic variation. These effects occur in response to environmental experiences. Prenatal exposure to stress is associated with reduced LG in adult female offspring (Champagne and Meaney, 2006) whereas exposure to low doses of the endocrine disruptor bisphenol-A (BPA) is associated with increased postnatal LG but decreased overall contact with pups (Palanza et al., 2002). Postnatal disruption to the quality of mother-infant interactions likewise has long-term effects on later-life maternal behavior. Artificial rearing of rat pups, involving a complete absence of postnatal contact with mothers, has been found to significantly disrupt neurodevelopment leading to attention deficits, hyperactivity, and impaired social behavior (Levy et al., 2003; Lovic and Fleming, 2004). Females reared under these conditions are observed to provide reduced LG toward their own offspring (Gonzalez et al., 2001; Melo et al., 2006) and investigations of the mechanism of this effect suggest that artificially-reared females are less responsive to hormonally induced increases in maternal behavior (Novakov and Fleming, 2005). Reduced LG is also observed in females who experienced prolonged maternal separation during infancy (Boccia and Pedersen, 2001) or who were reared by females engaging in low levels of LG (Champagne et al., 2003a; Francis et al., 1999). Thus, the maternal brain can be shaped by the quality of the early life environment, leading to stable variations in the frequency of mother-infant interactions.
The study of natural variations in maternal care in laboratory rodents has provided critical insights into the origins of individual differences in behavior (Meaney, 2001) and the effect of environmental experiences on the neural circuits involved in mother-infant interactions (Champagne and Meaney, 2006; Champagne and Meaney, 2007). The frequency of postnatal LG behavior in a cohort of females is typically normally distributed, such that females can be selected based on observed “high LG” or “low LG” relative to the cohort mean. Females reared by high LG dams engage in higher frequencies of this form of maternal care compared to offspring reared by low LG dams (Champagne et al., 2003a; Francis et al., 1999). Importantly, cross-fostering studies have been used to illustrate that this effect is due to the quality of the rearing environment rather than the phenotype or genotype of the dam to which offspring are born (Champagne et al., 2003a). The neuroendocrine basis of this maternal effect appears to involve sensitivity to hormone induced up-regulation of hypothalamic oxytocin receptors (OTR). Post-parturient offspring reared by high LG dams have elevated levels of oxytocin receptors in numerous brain regions, including the medial preoptic area (MPOA) of the hypothalamus (Champagne et al., 2001; Francis et al., 2000). Amongst ovariectomized females, the experience of high LG during postnatal development predicts increasing levels of OTR and cfos in the MPOA in response to increasing levels of estradiol whereas amongst offspring reared by low LG dams, there is insensitivity to these estrogen-mediated effects (Champagne et al., 2001; Champagne et al., 2003b). Estrogen-insensivity and reduced up-regulation of OTR is a phenotype also observed amongst ERα knockout mice (Young et al., 1998), which may account for the impairments in maternal behavior observed in this transgenic model (Ogawa et al., 1998). The genomic effects of estradiol are dependent on estrogen-ER interactions (Klinge, 2001) and thus reduction or deletion of ER can lead to estrogen insensitivity. Amongst adult female offspring reared by low LG compared to high LG dams, there are reductions in ERα gene expression within the MPOA and these rearing effects emerge during the first week postpartum (Champagne et al., 2003b; Champagne et al., 2006). Thus, both genetic and environmental influences on the neuroendocrine pathways involved in responsivity to gonadal hormones can achieve similar effects on the developing maternal brain.
Early life experiences can certainly set the stage for subsequent maternal behavior, however, continued plasticity in the maternal brain may be observed in response to juvenile and adult experiences. Prolonged contact between mother and offspring during the weaning period can alter the quality of pre-weaning contact between female offspring and their own pups (Curley et al., 2009b). Social isolation of females during the post-weaning period has been demonstrated to reduce levels of ERα in the MPOA of prairie voles (Ruscio et al., 2009) and decrease OTR in female offspring of high LG dams (Champagne and Meaney, 2007). Conversely, the experience of post-weaning social and physical environmental enrichment can increase OTR in the female offspring of low LG dams. These post-weaning experiences are effective in shifting the transmission of maternal care from mother to offspring such that females reared by low LG dams who are housed in enrichment conditions exhibit increased LG and female offspring of high LG dams who are housed in isolation exhibit low LG (Champagne and Meaney, 2007). This plasticity in brain and behavior continues into adulthood to include experiences occurring during gestation and parturition/lactation. Stress during gestation leads to reduced levels of post-partum mother-infant interactions (Champagne and Meaney, 2006; Moore and Power, 1986), even when females are rearing fostered non-stressed pups. Amongst females who have previously been characterized as exhibiting high LG toward their pups, the experience of chronic gestational stress suppresses the frequency of postpartum LG associated with stress-induced reductions in OTR in the MPOA and amygdala (Champagne and Meaney, 2006). Reduction in LG persists when these females are re-mated, suggesting a long-term down-regulation of the neuroendocrine circuits underlying maternal behavior.
Maternal behavior can also be enhanced through changes in the social context of the postpartum environment. Though standard laboratory rearing of non-biparental rodents consists of a single lactating dam and litter, a more frequently observed rearing strategy in the wild or under semi-naturalistic conditions, consists of multiple lactating females caring for a communal nest of pups (Crowcroft and Rowe, 1963; Mennella et al., 1990; Schultz and Lore, 1993). Communal nursing has been demonstrated to alter cognition, exploratory behavior, and social interactions of offspring (Branchi, 2009; Curley et al., 2009a). Amongst lactating females who are rearing pups in a communal nest, there is an increased frequency of nursing and LG exhibited by each individual female (Curley et al., 2009a), suggesting that shifts in maternal behavior can be induced very rapidly through changes in the postpartum environment. Though the persistence of these effects and the underlying neuroendocrine changes associated with this behavioral variation have yet to be explored, it is apparent that maternal behavior exhibits an astonishing degree of plasticity in response to the quality of the environment with consequences for the neurodevelopment and behavior of offspring.
Epigenetics: How mothers leave their mark
An emerging theme in studies of the impact of mother-infant interactions on offspring development is the persistent and region-specific changes in gene expression that can be observed in adult offspring with varying early-life experiences. In the context of research on the developing maternal brain, up-regulation of the transcriptional activity of ERα in the MPOA in response to high levels of LG is observed during the early postpartum period and is stably maintained in adulthood with implications for maternal sensitivity (Champagne et al., 2003b; Champagne et al., 2006). Thus, to understand the transmission of maternal behavior from mother to offspring, it is necessary to explore the dynamic changes in gene expression that link experiences to behavior. Transcriptional regulation of the genome is elegant in its complexity and consists of dynamic interactions between a highly plastic molecular environment and a stable gene sequence. The process of transcription is dependent on the transition of chromatin from a densely packed heterochromatin to a more accessible euchomatin in which nucleotide sequences are able to interact with transcription factors and RNA polymerase. There are multiple mechanisms through which this accessibility can be altered, including post-translational modifications to histone proteins and DNA methylation (Peterson and Laniel, 2004; Razin, 1998). Histone proteins form the core of the nucleosome complex and are thus well positioned, both literally and figuratively, to interact with DNA. Modification to the N-terminal tails of the histone proteins through process such as acetylation, ubiquitination, and methylation can change the nature of the interaction between DNA and the surrounding nucleosome core. DNA methylation is a covalent modification to the DNA molecule whereby a methyl group is added to cytosine nucleotides within the gene sequence, typically at CpG sites. DNA methyltransferases (DNMT1 & DNMT3a/b) mediate this chemical modification which also attracts protein complexes, including methyl binding proteins, which add further “congestion” around the gene (Fan and Hutnick, 2005; Feng et al., 2007; Turner, 2001). The overall impact of these molecular changes for gene transcription will depend on the location and nature of the modification, however, DNA methylation is typically associated with decreased transcriptional activity. DNA methylation is one of the few mechanisms of gene regulation considered “epigenetic” as it is a modification of the DNA rather the surrounding proteins and patterns of DNA methylation within the genome are potentially heritable. DNA methylation serves a critical role in stably maintaining cell-specific gene expression patterns during cellular differentiation and these epigenetic marks are transmitted to daughter cells during the process of mitosis (Fukuda and Taga, 2005; Jones and Taylor, 1980). Without the capacity to induce this epigenetic modification, development would not proceed beyond the early fetal/postnatal stages.
Our understanding of the molecular biology of DNA methylation and the interactions between methylated DNA and transcriptional regulators within the cell nucleus is advancing rapidly and continues to highlight the elegance and complexity of this process. The question that has emerged in light of these advances is whether DNA methylation can be directed by the experiences of the organism and account for the long-term changes in gene expression that occur in response to those experiences. In particular, can the quality of mother-infant interactions during development lead to alterations in these epigenetic marks with consequence for individual differences in, amongst other behaviors, the maternal behavior of female offspring? Can this modification account for the differential expression of ERα in the MPOA of females reared by high vs. low LG dams? Analysis of the 1b promoter region of the ERα gene indicates that there are several CpG sites within this DNA sequence which could potentially become methylated (Freyschuss and Grandien, 1996; Schibler and Sierra, 1987) leading to reduced ERα mRNA. Using sodium bisulfite mapping combined with sequencing of treated DNA samples, the DNA methylation patterns within the ERα promoter region in MPOA tissue from high LG and low LG offspring have been mapped. Analysis of these findings suggest the presence of elevated levels of DNA methylation within the ERα gene promoter of female offspring reared by low LG dams (Champagne et al., 2006). Moreover, the direction of this epigenetic effect is consistent with the changes in gene expression that are observed between high and low LG offspring (Champagne et al., 2003b). Though these findings raise many questions regarding the pathways through which the quality of the environment can lead to shifts in these molecular imprints, these findings have stimulated novel perspectives on the dynamic yet stable epigenetic changes that may confer the high degree of developmental plasticity observed in the maternal brain.
Within the rapidly advancing field of Behavioral Epigenetics, which is highlighted in a recent special issue of Hormones & Behavior (in press), there continues to be exploration of the role of DNA methylation in linking variations in the quality of the maternal environment and long-term neurobehavioral outcomes. Gestational stress, variation in maternal caloric/nutrient intake, and various toxicological exposures during fetal development have been associated with altered DNA methylation within the brain and placenta leading to changes in gene expression of numerous target genes including: corticosterone releasing hormone (CRH), glucocorticoid receptor (GR), DNMT1, brain derived neurotropic factor (BDNF), and peroxisome proliferator-activated receptor α (PPARα) (Kovacheva et al., 2007; Lillycrop et al., 2005; Mueller and Bale, 2008; Novikova et al., 2008; Onishchenko et al., 2008). Postnatally, effects of prolonged maternal separation on vasopressin (AVP) mRNA in the paraventricular nucleus have been associated with separation-induced reductions in DNA methylation within the AVP gene that can be observed at 6 weeks postnatal and persist into adulthood (Murgatroyd et al., 2009). Daily exposure to abusive mother-interactions leads to reduced expression of BDNF in the prefrontal cortex in adulthood associated with increased DNA methylation within the BDNF IV promotor region (Roth et al., 2009). Natural variations in postnatal maternal LG in the rat are associated with changes in numerous receptor pathways, with effects on hippocampal GR being implicated in the high levels of HPA reactivity observed amongst offspring of low LG dams (Liu et al., 1997). Analysis of the GR 17 promotor region suggests that low levels of LG are associated with increased methylation within the GR promotor, decreased GR expression and an increased HPA response to stress (Weaver et al., 2004). Time course analysis has indicated that these maternally induced epigenetic profiles emerge during the postnatal period and are sustained into adulthood. Maternal LG also affects γ-aminobutyric acid (GABA) circuits and receptor subunit composition (Caldji et al., 2000; Caldji et al., 2003), and in a recent study, reduced hippocampal levels of glutamic acid decarboxylase (GAD1), the rate-limiting enzyme in GABA synthesis, were found in the male offspring of low LG dams associated with increased DNA methylation within the GAD1 promotor (Zhang et al., 2010). Though these studies incorporate a candidate gene approach, taken together, it is evident that maternal epigenetic effects may lead to changes in the transcriptional activity of multiple genes within a broad range of brain regions which are established in development and lead to long-term variation in gene expression and behavior.
Beyond Development: Transgenerational Impact of Maternal care
Across species, there is evidence for the transmission of individual differences in maternal behavior across multiple generations. In humans, this can be demonstrated for measures of parental bonding (Miller et al., 1997), attachment (Benoit and Parker, 1994), and abuse (Chapman and Scott, 2001; Egeland et al., 1987), in primates on indices of mother-infant contact and maternal rejection (Fairbanks, 1989; Maestripieri et al., 1997; Maestripieri, 2005), and in laboratory rodents on observed frequency of maternal LG and abusive interactions with pups (Champagne and Meaney, 2007; Francis et al., 1999; Roth et al., 2009). Moreover, when cross-fostering is used within these studies, it becomes clear that the inheritance of maternal behavior is not dependent on the transmission of genetic variation from mothers (F0) to daughters (F1) and grand-daughters (F2). Our increasing understanding of early-life influences on the developing maternal brain may provide insights into the mechanism of this transgenerational effect. As noted in the previous sections, variation in the experience of maternal care can shape the neuroendocrine pathways which will affect the maternal care exhibited by female offspring. In the case of natural variations in LG, the changes in DNA methylation associated with these experiences suggest that there is an epigenetic basis of the transmission of maternal behavior from mother to daughter (Champagne, 2008). A similar transmission of behavior is observed in response to postnatal abuse. Female rat pups exposed to abusive care-giving in infancy engage in abusive care-giving toward their own offspring and F2 offspring of these F1 females have elevated levels of methylation within the BDNF promotor in the cortex and hippocampus (Roth et al., 2009). Under stable environmental conditions, the persistence of altered gene expression within the maternal brain and the consequent variation in hormonal and neurobiological priming of maternal behavior that emerges can lead to a stable inheritance of maternal phenotype. Unlike genetic inheritance, this transmission of behavior is experience-dependent and may be altered by the conditions of postnatal, juvenile, and adult environment. For example, in Long-Evans rats reared post-weaning in standard juvenile housing conditions, there is a reliable transmission of LG phenotype that can be observed in grand-offspring (Champagne and Meaney, 2007). However, if offspring of low LG dams are reared under conditions of post-weaning social enrichment, they are observed to engage in high levels of LG and it is the high LG phenotype which is transmitted to F2 offspring of these females. Likewise, if offspring of high LG dams are reared under conditions of juvenile social isolation, they are observed to engage in low levels of LG and this altered LG phenotype is transmitted to F2 female offspring. A similar transmission of maternal phenotype can be observed in response to communal vs. standard rearing. Females rearing offspring in a communal nest are induced to display elevated levels of nursing and LG and when rearing their own offspring (under standard conditions) both F1 and F2 communal females exhibit increased maternal behavior (Curley et al., 2009a). Experience-dependent inheritance may also be an important consideration in the transmission of genetically induced variations in maternal behavior. The deficits in maternal behavior induced by mutation of Peg3 can be observed in F1 and F2 female offspring (Curley et al., 2009a) despite the epigenetic silencing of the mutant Peg3 allele in these females (a characteristic of paternally expressed genes which are inherited from mothers) . In this case, it is hypothesized that although genetic factors are critical in inducing the initial behavioral variation, the persistence of these effects in F1 and F2 offspring is mediated by the transgenerational influence of maternal care.
The implications of experience-dependent inheritance of variations in maternal behavior span beyond understanding of the origins of individual differences in maternal care. If maternal behavior is passed from generation to generation, so too are the neurobiological and behavioral consequences of variations in maternal care. In the case of low levels of maternal LG, this may include the heightened stress responsivity associated with increased DNA methylation and decreased expression of hippocampal GR and GAD1, reduced hippocampal plasticity associated with impaired learning and memory, and altered social behavior. Importantly, when considering the origins of these behavior phenotypes we must look to previous generations. Transgenerational perspectives are being increasingly incorporated in research designs and have provided new insights into the mechanisms of experience-dependent change (Skinner et al., 2010). An emerging theme in these studies is the potential of epigenetic marks within the germline in facilitating this intergenerational transmission. Recent studies of perinatal exposures indicate that changes in DNA methylation may be observed within the gametes of exposed individuals as well as their descendants (Anway et al., 2005; Franklin et al., 2010). Interestingly, this type of germline inheritance may be more evident in the patriline and account for the persistence of environmentally-induced effects in the absence of continued exposure to the trigger of the epigenetic modification. Overall, these studies suggest that our concept of inheritance and our understanding of the molecular and cellular pathways linking experiences in one generation to neurobehavioral phenotypes in the next may be rapidly evolving.
Trade-offs in Reproduction
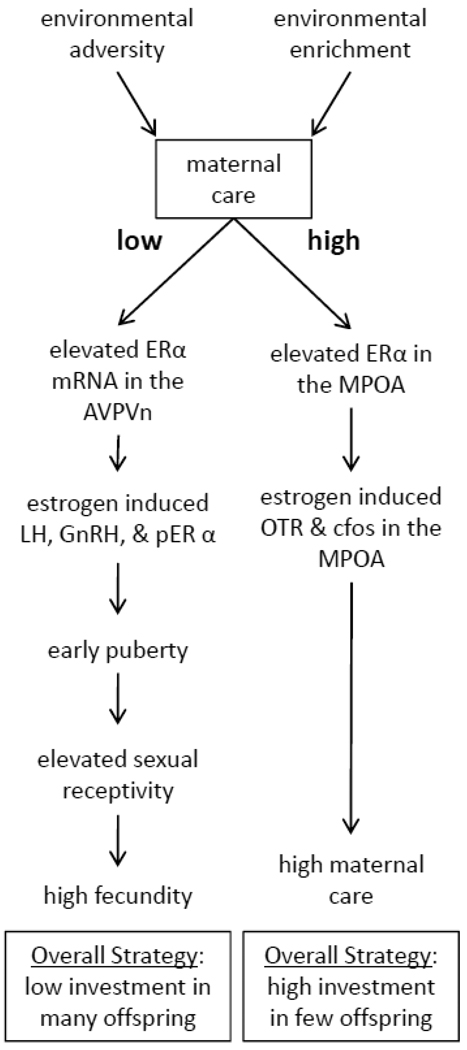
The profound effects of natural variations in maternal behavior within and across generations and the mechanisms involved in experience-dependent molecular and neurobiological outcomes continue to be explored. However, the occurrence of maternal imprints on offspring development also raises broader questions within ecological and evolutionary perspectives regarding the adaptive advantages of variation in the rearing environment. For example, if high LG confers advantages to offspring by increasing maternal care in females, reducing stress responsivity, and enhancing cognitive ability, what are the costs? Conversely, what are the benefits associated with the experience of low LG? Though costs/benefits are difficult to operationally define within the constraints of a laboratory environment, it would appear that there is a trade-off in reproduction that can be observed as a function of maternal care. Though offspring of high LG dams do provide high levels of maternal LG toward their own offspring, when compared to the offspring of low LG dams, they are far less sexually receptive (Cameron et al., 2008a; Cameron et al., 2008b; Uriarte et al., 2007). Despite exhibiting enhanced estrogen sensitivity within maternal circuits, female offspring of High LG dams are less responsive to estrogen induced lutenizing hormone (LH) levels, hypothalamic gonadotrophin-releasing hormone (GnRH), and phosphorylated ERα within the anteroventral paraventricular nucleus of the hypothalamus (AVPVn) (Cameron et al., 2008a). In contrast to the MPOA, in which there are elevated levels of ERα mRNA, offspring of high LG dams have reduced ERα mRNA in the AVPVn compared to offspring of low LG dams. Reduced ovulation and reproductive success following paced mating have also been observed in the offspring of high LG compared to low LG dams (Cameron et al., 2008a; Uriarte et al., 2007). These data suggest that the cost to enhanced maternal LG may be evident in the decreased sexual behavior of high LG offspring and there appears to be accelerated sexual maturation in the offspring who receive low LG. Puberty onset is earlier amongst offspring of low LG dams (Cameron et al., 2008a). Thus there is a trade-off between maternal and sexual behavior (see Figure 1) that is influenced by the quality of mother-infant interactions experienced during perinatal development.
Figure 1.
Illustration of the reproductive strategy that is associated with low vs. high levels of maternal licking/grooming (LG). Adverse environmental conditions experienced throughout development can lead to reduced maternal LG and a cascade of neuroendocrine changes in female offspring that induce early puberty, high sexual receptivity, and enhanced reproductive success following mating. In contrast, under conditions which promote high levels of LG, female offspring develop estrogen sensitivity within maternal circuits and inherit the high LG phenotype.
Reproductive strategies refer to the relative investment in different aspects of reproduction that leads to the generation of offspring. At a species level of comparison, these strategies have classically been divided into two main categories, described as r- and K-selection (Stearns, 1976). In r-selection, females are characterized as having a rapid maturation, increased sexual drive, an early age of reproduction, and reduced investment in the care of offspring. In contrast, K-selection females tend to delay sexual maturity, have reduced sexual drive, and invest high levels of energetic resources in the care of offspring. The parallels between these species-level strategies and the observed reproductive trade-offs in response to maternal LG in rodents are quite astonishing and suggest that the experience of variations in LG can set in motion the development of divergent within-species reproductive strategies. The relevance of these parallels becomes even more evident when we consider the environmental conditions that can shift maternal LG. As described in the previous sections, maternal deprivation during infancy, social isolation during juvenile development, and gestational stress can lead to reduced maternal LG (Boccia and Pedersen, 2001; Champagne et al., 2003a; Champagne and Meaney, 2006; Gonzalez et al., 2001), whereas social enrichment during juvenile development and communal rearing can lead to increased levels of maternal LG (Champagne and Meaney, 2007; Curley et al., 2009a). Life-history theories of reproduction would predict that adverse and unpredictable environments would favor an r-selection strategy, such that females are able to reproduce early in development and produce many offspring rather than conserving resources. Since the likelihood of survival is reduced, investment in growth and prenatal/postnatal investment in offspring would not be an optimal strategy for ensuring that sufficient numbers of offspring survive and reproduce. In contrast, more predictable and stable environments, in which resource availability is high and threat is low, would favor an K-selection strategy in which few offspring are produced and more offspring survive, promoting the benefits of increased maternal investment in the care of offspring. There is increasing empirical support for the predictions of these evolutionary theories, and in particular for the role of the early family environment in shaping reproductive strategies (Belsky et al., 1991; Ellis and Essex, 2007). Though r/K-selection has been formulated as a species level distinction, within-species variations in reproduction and rearing strategy have been observed (Krebs et al., 1973; McShea and Madison, 1984), particularly under conditions of threat/adversity (Sheriff et al., 2010), and may provide a framework for understanding the causes and consequences individual differences in behavior. Laboratory studies in rodents examining the effects of maternal care and the epigenetic mechanisms through which maternal influences shape the developing brain may serve as a tool for bridging the mechanistic and evolutionary levels of analysis in the study of behavioral variation.
Perspectives and Future Directions in the Study of Maternal Imprints
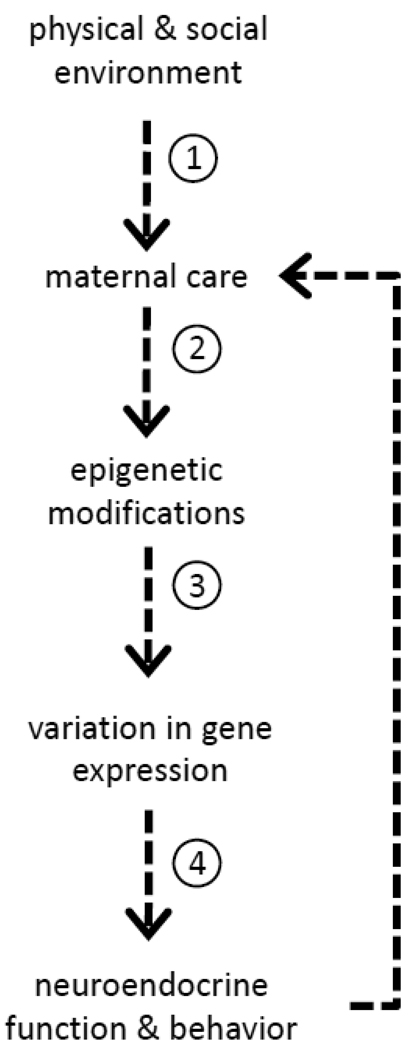
Mothers can certainly leave their mark. This is not to say that paternal influences are any less persistent and there is increasing evidence that both maternal and paternal environmental experiences may lead to altered offspring development (Curley et al., 2010). However, in all mammalian species, there is an intense period of mother-infant contact that is necessary for survival and thus maternal care represents a unique way in which mothers can influence the developmental trajectories of their offspring. In considering the topics discussed in this review - the plasticity of the maternal brain and behavior in response to environmental cues, emerging evidence for the influence of maternal care on DNA methylation, gene expression, and neuroendocrine function in offspring, the transmission of maternal care across generations, and the adaptive consequences of both high and low levels of maternal care within an evolutionary framework – it is perhaps evident that though we have made significant advances in understanding the origins of individual differences in behavior, there are still many questions remaining. Within the framework outlined in Figure 2, there are critical mechanistic issues that need further exploration:
Figure 2.
Illustration of the proposed pathways linking environmental experiences to the inheritance of behavioral variation via maternal care. Within this framework, there are many questions to be addressed, including (1) How do experiences occurring beyond the perinatal period lead to altered maternal care?, (2) How does the experience of maternal care induce epigenetic changes?, (3) How do we interpret the epigenetic code?, and (4) How do environmentally induced changes in gene expression shape the developing brain?
(1) How do experiences occurring beyond the perinatal period lead to altered maternal care?
Though plasticity in the maternal brain is evident, it is unclear whether early and later experiences target the same molecular mechanisms. This issue is particularly relevant in studies where there is an apparent reversal of the effects of early life experiences. Is this reversal or compensation? Studies which have explored this issue suggest that though amelioration of behavioral deficits induced by early life adversity can be achieved, the neural mechanisms underlying the deficit may remain unaltered (Bredy et al., 2003; Francis et al., 2002). For example, Francis et al. (2002) found that environmental enrichment of Long Evans rat pups during the post-weaning period was sufficient to attenuate the corticosterone response to stress amongst offspring who had experienced neonatal maternal separation. However, despite this physiological change, maternally separated pups reared in enriched environments maintained elevated levels of CRH mRNA in the hypothalamus. Thus, the juvenile environment was effective in inducing changes in behavioral/physiological outcomes without shifting the underlying molecular pathway targeted by early adverse rearing experiences. Similarly, plasticity in the maternal brain in response to the quality of the environment may be achieved through different mechanistic pathways in early compared to later developmental time-points.
(2) How does the experience of maternal care induce epigenetic changes?
Maternal care consists of a complex array of tactile, physiological, and behavioral interactions with offspring that occurs in the context of feeding/lactation. In the case of maternal LG, there is increasing theoretical and empirical support for the importance of the tactile component of this nurturing response. However, the relationship between tactile stimulation and the associated molecular changes will need to be elucidated. In the case of the reduced DNA methylation observed in the promoter regions of GR, GAD1, and ERα, it is hypothesized that LG-induced up-regulation of transcription factors (such as nerve growth factor-inducible factor A (NGFI-A) and signal transducer and activator of transcription 5a (Stat5a)) may promote transcriptional activation and reduced DNA methylation (Champagne et al., 2006; Weaver et al., 2007; Zhang et al., 2010). The tactile stimulation offspring experience during early development may also induce acute changes in growth hormone, oxytocin, and corticosterone (Pauk et al., 1986; Schanberg et al., 1984), which could also be explored as potential “triggers” of epigenetic modification. Establishing the signaling pathways through which these epigenetic effects occur in response to the broad range of social experiences which shape development may provide critical insights into the biological encoding of the quality of the environment.
(3) How do we interpret the epigenetic code?
The consequences of epigenetic change within the genome for gene expression are not always straightforward. Though elevated levels of DNA methylation are typically associated with gene silencing, the degree of methylation and location within the genome of the methylated DNA will certainly moderate the relationship between this epigenetic modification and transcriptional activity. This will be an important consideration when examining and interpreting group differences in DNA methylation profiles. To complicate matters further, it is necessary to consider the surrounding histones, methyl binding factors, methyltransferases, and co-factors which provide context to the genome and epigenome. A combination of in vitro and in vivo approaches to this daunting mechanistic issue may be essential to uncovering the meaning of epigenetic patterns.
(4) How do environmentally induced changes in gene expression shape the developing brain?
Though the pathways linking the experience of variations in maternal care and altered gene activity is certainly complex, this complexity continues when we consider the pathways linking differential transcription levels to individual differences in brain and behavior. Advances in the study of the relationship between target gene expression and synaptic plasticity, neuronal activation, and refinement in neural circuits are emerging (Fagiolini et al., 2009), and this may provide insights into the behavioral transmission of maternal effects. There are multiple cellular and molecular pathways through which early life experiences may induce long-term neurobiological changes, including variation in cell proliferation/survival, morphological changes in neurons and glia, and altered connectivity between brain regions (Holtmaat and Svoboda, 2009; Nithianantharajah and Hannan, 2006). The effect of environmental experiences may shape epigenetic changes in gene activity which then has consequences for these downstream pathways. Conversely, changes in cellular activity may have consequences for epigenetic marks leading to altered gene expression. Time-course studies will be essential to address this issue, where both the acute and long-term changes in epigenetic marks, gene expression, and neurobiology can be explored.
The observed pathways linking experiences to behavioral variation, both within and across generations, raises broader questions regarding adaptation, evolutionary significance, and expanding concepts of the mechanisms of inheritance. Within the framework of these broader issues, it is hypothesized that plasticity within the maternal brain leading to increased or decreased maternal care allows cues regarding the quality of the environment to shape offspring development in such a way that they are better prepared to survive and reproduce in that environment. In the case of variations in LG, it would appear that alternative reproductive strategies are induced by the quality of maternal care received. These findings also suggest that the effects of low LG would be adaptive under conditions of threat and unpredictability in the environment. Amongst offspring of high vs. low LG dams, elevated levels of plasma corticosterone lead to enhanced hippocampal plasticity in the offspring of low LG dams (Champagne et al., 2008), providing further support for the hypothesis that these offspring are optimally adapted to high threat conditions. Moreover, when adverse experiences lead to the intergenerational transmission of reduced maternal care, subsequent generations of offspring will not have to experience the threat themselves in order to be adapted to the high risk environment. These non-genomic effects have raised questions regarding the role of epigenetics and parental effects in understanding the process of evolution and have revived interest in Lamarckian inheritance of acquired characteristics (Jablonka and Lamb, 2002). Adopting a broader concept of inheritance, which has been described in the literature as “inclusive heritability” (Danchin and Wagner, 2010), may promote a more integrated perspective on the dynamic interactions between the environment, epigenetics, and the genome. The insights gained from such a perspective may advance our understanding of the role of maternal influences on the divergent phenotypes that arise in future generations.
Acknowledgments
Funding: This research was supported by Grant Number DP2OD001674 from the Office of the Director, National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Benoit D, Parker KC. Stability and transmission of attachment across three generations. Child Dev. 1994;65:1444–1456. doi: 10.1111/j.1467-8624.1994.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS One. 2008a;3:e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm Behav. 2008b;54:178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003a;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003b;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP, Swaney WT, Hasen NS, Keverne EB. Paternal influence on female behavior: the role of Peg3 in exploration, olfaction, and neuroendocrine regulation of maternal behavior of female mice. Behav Neurosci. 2009;123:469–480. doi: 10.1037/a0015060. [DOI] [PubMed] [Google Scholar]
- Chapman D, Scott K. The impact of maternal intergenerational risk factors on adverse developmental outcomes. Developmental Review. 2001;21:305–325. [Google Scholar]
- Crowcroft P, Rowe FP. Social organization and territorial behavior in the wild house mice (Mus musculus L.) Proc Zool Soc London. 1963;140:517–531. [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009a;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, Champagne FA. The meaning of weaning: influence of the weaning period on behavioral development in mice. Dev Neurosci. 2009b;31:318–331. doi: 10.1159/000216543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin E, Wagner RH. Inclusive heritability: combining genetic and non-genetic information to study animal behavior and culture. Oikos. 2010;119:210–218. [Google Scholar]
- Egeland B, Jacobvitz D, Papatola K. Child Abuse and Neglect: Biosocial Dimensions. Vol. New York: Aldine; 1987. [Google Scholar]
- Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks LA. Early experience and cross-generational continuity of mother-infant contact in vervet monkeys. Dev Psychobiol. 1989;22:669–681. doi: 10.1002/dev.420220703. [DOI] [PubMed] [Google Scholar]
- Fan G, Hutnick L. Methyl-CpG binding proteins in the nervous system. Cell Res. 2005;15:255–261. doi: 10.1038/sj.cr.7290294. [DOI] [PubMed] [Google Scholar]
- Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res. 2007;61:58R–63R. doi: 10.1203/pdr.0b013e3180457635. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Freyschuss B, Grandien K. The 5' flank of the rat estrogen receptor gene: structural characterization and evidence for tissue- and species-specific promoter utilization. J Mol Endocrinol. 1996;17:197–206. doi: 10.1677/jme.0.0170197. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Taga T. Cell fate determination regulated by a transcriptional signal network in the developing mouse brain. Anat Sci Int. 2005;80:12–18. doi: 10.1111/j.1447-073x.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Lovic V, Ward GR, Wainwright PE, Fleming AS. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Dev Psychobiol. 2001;38:11–32. doi: 10.1002/1098-2302(2001)38:1<11::aid-dev2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Jablonka E, Lamb MJ. The changing concept of epigenetics. Ann N Y Acad Sci. 2002;981:82–96. doi: 10.1111/j.1749-6632.2002.tb04913.x. [DOI] [PubMed] [Google Scholar]
- Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK. Gestational choline deficiency causes global- and Igf2 gene- DNA hypermethylation by upregulation of Dnmt1 expression. J Biol Chem. 2007 doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- Krebs CJ, Gaines MS, Keller BL, Myers JH, Tamarin RH. Population cycles in small rodents. Science. 1973;179:35–41. doi: 10.1126/science.179.4068.35. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Herman AE. Maternal behavior and developmental psychopathology. Biol Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- Lefebvre L, Viville S, Barton SC, Ishino F, Keverne EB, Surani MA. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet. 1998;20:163–169. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- Levy F, Melo AI, Galef BG, Jr, Madden M, Fleming AS. Complete maternal deprivation affects social, but not spatial, learning in adult rats. Dev Psychobiol. 2003;43:177–191. doi: 10.1002/dev.10131. [DOI] [PubMed] [Google Scholar]
- Li L, Keverne EB, Aparicio SA, Ishino F, Barton SC, Surani MA. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science. 1999;284:330–333. doi: 10.1126/science.284.5412.330. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task--reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–4107. doi: 10.1210/endo.139.10.6243. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Wallen K, Carroll KA. Infant abuse runs in families of group-living pigtail macaques. Child Abuse Negl. 1997;21:465–471. doi: 10.1016/s0145-2134(97)00006-9. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc Natl Acad Sci U S A. 2005;102:9726–9729. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShea WJ, Madison DM. Communal nesting between reproductively active females in a spring population of Microtus pennsylvanicus. Can J Zool. 1984;63:344–346. [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Melo AI, Lovic V, Gonzalez A, Madden M, Sinopoli K, Fleming AS. Maternal and littermate deprivation disrupts maternal behavior and social-learning of food preference in adulthood: tactile stimulation, nest odor, and social rearing prevent these effects. Dev Psychobiol. 2006;48:209–219. doi: 10.1002/dev.20130. [DOI] [PubMed] [Google Scholar]
- Mennella AM, Blumberg MS, McCiintock MK, Moltz H. Inter-litter competition and communal nursing among Norway rats: advantages of birth synchrony. Behavioral Ecology and Sociobiology. 1990;27:183–190. [Google Scholar]
- Miller L, Kramer R, Warner V, Wickramaratne P, Weissman M. Intergenerational transmission of parental bonding among women. J Am Acad Child Adolesc Psychiatry. 1997;36:1134–1139. doi: 10.1097/00004583-199708000-00022. [DOI] [PubMed] [Google Scholar]
- Moore CL, Power KL. Prenatal stress affects mother-infant interaction in Norway rats. Dev Psychobiol. 1986;19:235–245. doi: 10.1002/dev.420190309. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Novakov M, Fleming AS. The effects of early rearing environment on the hormonal induction of maternal behavior in virgin rats. Horm Behav. 2005;48:528–536. doi: 10.1016/j.yhbeh.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Novikova SI, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3:e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castren E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J Neurochem. 2008;106:1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110 Suppl 3:415–422. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauk J, Kuhn CM, Field TM, Schanberg SM. Positive effects of tactile versus kinesthetic or vestibular stimulation on neuroendocrine and ODC activity in maternally-deprived rat pups. Life Sci. 1986;39:2081–2087. doi: 10.1016/0024-3205(86)90359-0. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–R551. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. Embo J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny TD, Gomez A, Parker K, Carter CS. Social environment alters central distribution of estrogen receptor alpha in juvenile prairie voles. Physiol Behav. 2009;98:296–301. doi: 10.1016/j.physbeh.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanberg SM, Evoniuk G, Kuhn CM. Tactile and nutritional aspects of maternal care: specific regulators of neuroendocrine function and cellular development. Proc Soc Exp Biol Med. 1984;175:135–146. doi: 10.3181/00379727-175-41779. [DOI] [PubMed] [Google Scholar]
- Schibler U, Sierra F. Alternative promoters in developmental gene expression. Annu Rev Genet. 1987;21:237–257. doi: 10.1146/annurev.ge.21.120187.001321. [DOI] [PubMed] [Google Scholar]
- Schultz LA, Lore RK. Communal reproductive success in rats (Rattus norvegicus): effects of group composition and prior social experience. J Comp Psychol. 1993;107:216–222. doi: 10.1037/0735-7036.107.2.216. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology. 2010;91:2983–2994. doi: 10.1890/09-1108.1. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. Life-history tactics: a review of the ideas. Q Rev Biol. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- Turner B. Chromatin and Gene Regulation. Vol. Oxford: Blackwell Science Ltd; 2001. [Google Scholar]
- Uriarte N, Breigeiron MK, Benetti F, Rosa XF, Lucion AB. Effects of maternal care on the development, emotionality, and reproductive functions in male and female rats. Dev Psychobiol. 2007;49:451–462. doi: 10.1002/dev.20241. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, D'Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Donaldson R, Rissman EF. Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. Neuroreport. 1998;9:933–936. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci. 2010;30:13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]