Abstract
In our previous work, we found that perfusion of the rat cerebral cortex with hypoosmotic medium triggers massive release of the excitatory amino acid L-glutamate but decreases extracellular levels of L-glutamine (R.E. Haskew-Layton et al., PLoS ONE, 3: e3543). The release of glutamate was linked to activation of volume-regulated anion channels (VRAC), while mechanism(s) responsible for alterations in extracellular glutamine remained unclear. When mannitol was added to the hypoosmotic medium in order to reverse reductions in osmolarity, changes in microdialysate levels of glutamine were prevented, indicating an involvement of cellular swelling. Since the main source of brain glutamine is astrocytic synthesis and export, we explored the impact of hypoosmotic medium on glutamine synthesis and transport in rat primary astrocyte cultures. In astrocytes, a 40% reduction in medium osmolarity moderately stimulated the release of L-[3H]glutamine by ~2-fold and produced no changes in L-[3H]glutamine uptake. In comparison, hypoosmotic medium stimulated the release of glutamate (traced with D[3H]aspartate) by more than 20-fold. In whole-cell enzymatic assays, we discovered that hypoosmotic medium caused a 20% inhibition of astrocytic conversion of L[3H]glutamate into L-[3H]glutamine by glutamine synthetase. Using an HPLC assay we further found a 35% reduction in intracellular levels of endogenous glutamine. Overall, our findings suggest that cellular swelling (1) inhibits astrocytic glutamine synthetase activity, and (2) reduces substrate availability for this enzyme due to the activation of VRAC. These combined effects likely lead to reductions in astrocytic glutamine export in vivo and may partially explain occurrence of hyperexcitability and seizures in human hyponatremia.
Introduction
L-Glutamate and L-glutamine are two major amino acids which are involved in body nitrogen metabolism (Newsholme et al. 2003). Both glutamate and glutamine are present at high levels in the blood plasma, interstitial liquids, and inside all animal cells. In brain tissue, glutamate, in addition to its roles in metabolism and protein synthesis, serves as the main excitatory neurotransmitter. Accordingly, extracellular levels of glutamate in the central nervous system are tightly regulated and kept low due to the work of the glutamate-glutamine cycle, as originally proposed by Balazs et al. (1970) and van den Berg and Garfinkel (1971), and reviewed by Broer and Brookes (2001), Albrecht et al. (2007), and McKenna (2007). The precise control of extracellular glutamate is possible because brain tissue is separated from the circulation by the endothelial blood-brain barrier (BBB). The BBB strongly limits blood-to-brain transfer of glutamate and glutamine and isolates brain tissue from systemic fluctuations in amino acid concentrations (Smith 2000). Therefore, the bulk of CNS glutamate and glutamine are recycled rather than obtained from the circulatory system. Neuronal activity serves as the major source of extracellular glutamate. Upon release from synaptic vesicles and following activation of postsynaptic glutamate receptors, glutamate diffuses to the extrasynaptic space, where it is removed largely via the work of astrocytic glutamate transporters GLT-1 and GLAST (reviewed in Danbolt 2001). In astrocytes, glutamate is converted to glutamine by the ATP-dependent enzyme, glutamine synthetase or metabolized in mitochondria (Martinez-Hernandez et al. 1977; McKenna 2007). Under normal physiological conditions ~90% of synaptically released glutamate is recycled via conversion to glutamine by astrocytic glutamine synthetase (Kanamori et al. 2002). Astrocytic glutamine is then released by astrocytic glutamine transporters, SNAT3 and SNAT2, and taken into neuronal cells, predominantly by the SNAT1 system (Broer and Brookes 2001; Deitmer et al. 2003). In neurons, glutamine is hydrolyzed back to glutamate by the phosphate-activated glutaminase (Roberg et al. 1995; Laake et al. 1999). The resulting glutamate is re-packaged into synaptic vesicles, hence completing the glutamate-glutamine cycle, or diverted to biosynthetic and metabolic reactions (Danbolt 2001; McKenna 2007).
Numerous brain pathologies disrupt glutamate-glutamine homeostasis and commonly lead to pathological elevations in extracellular glutamate levels. The mechanisms of such disruptions are pathology-specific but frequently have a common denominator – pathological cell swelling (Mongin and Kimelberg 2005). For example, in ischemia, cell swelling occurs due to the anoxic opening of multiple ion permeability pathways and osmotic accumulation of inorganic ions, mainly Na+ and Cl− (Mongin and Kimelberg 2005; Mongin 2007). In hyponatremia, a systemic drop in blood plasma [NaCl], causes an osmotic shift of water from plasma to brain tissue, and triggers the osmotic swelling of brain cells (Gullans and Verbalis 1993; Fraser and Arieff 1997). Regardless, of the initial causes, increases in cell volume lead to the opening of several volume-sensitive transport pathways, one of which—the volume regulated anion channel (VRAC)—is permeable to glutamate and several other small organic molecules (Kimelberg et al. 1990; Banderali and Roy 1992; Abdullaev et al. 2006). Release of glutamate from the cytosol to the extracellular space contributes to excessive activation of neuronal glutamate receptors, particularly the Ca2+-permeable NMDA receptor, causing excitotoxic cell damage and death (Choi 1988; Arundine and Tymianski 2004).
The majority of previous studies addressing the effects of cell swelling focused on changes in extracellular glutamate levels due to the obvious connection of this amino acid to neural toxicity. We and others found that perfusion of cortical brain tissue with hypoosmotic medium causes the massive release of glutamate, which is sensitive to a number of anion channel blockers (Lehmann 1989; Estevez et al. 1999; Haskew-Layton et al. 2008). Such release has a clear in vitro correlate – activation of the anion channel VRAC in astrocytes (Abdullaev et al. 2006). In contrast, changes in extracellular glutamine levels have received little attention. In our microdialysis work, we established that hypoosmotic medium induced gradual but robust (~70% over one hour) reductions in extracellular glutamine levels (Haskew-Layton et al. 2008). Similar findings were reported in a microdialysis study of (Taylor et al. 1995). Furthermore, chronic hyponatremia, i.e. a systemic reduction in blood NaCl levels, is associated with strong (~50–60%) decreases in whole-brain glutamine levels (Thurston et al. 1987; Verbalis and Gullans 1991; Lien et al. 1991). The mechanisms responsible for these phenomena have remained completely unknown. In the present study we explored whether cell swelling alters glutamine transport and metabolism in primary cultures of rat astrocytes. Findings of our in vitro experiments were compared to results of the in vivo microdialysis studies measuring extracellular glutamate and glutamine in the rat cortex.
Materials and methods
Materials
L-Glutamate, L-glutamine, 6-diazo-5-oxo-L-norleucine (DON), and L-methionine sulfoximine (MSO) were purchased from Sigma-Aldrich (St. Louis, MO). 4-[(2-Butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]butanoic acid (DCPIB) was obtained from Tocris Cookson (Ellisville, MO). D-[3H]aspartate, L-[3H]glutamate and L-[3H]glutamine were acquired from PerkinElmer-New England Nuclear (Waltham, MA). All cell culture media and sera were obtained from Invitrogen (Carlsbad, CA). All salts, buffers, solvents, and other reagents, were from Sigma-Aldrich, unless otherwise specified.
Primary astrocyte cultures
Confluent primary astrocyte cultures were prepared from the cerebral cortex of newborn Sprague-Dawley rats. These and other animal procedures were approved by the Albany Medical Center Institutional Animal Use and Care Committee and adhered to the NIH guidelines for care and use of laboratory animals. One-day old rat pups were euthanized by rapid decapitation. The cerebral cortices were separated from the meninges, hippocampi, and basal ganglia. Cortical tissue combined from 4 animals was minced and transferred into a solution of the recombinant protease TrypLE (Invitrogen), which was diluted 1:1 (v:v) with OptiMEM (Invitrogen). Cells were extracted using three 10-min incubations with TrypLE additionally supplemented with bovine pancreatic DNase I (Sigma). The first extraction was discarded, while the second and the third extractions were combined with Minimal Essential Medium (MEM) containing 10% heat inactivated horse serum (HIHS) and 50 U/ml penicillin plus 50 μg/ml streptomycin (Pen/Strep). Cells were sedimented by a brief centrifugation (1,000 g × 1.5 min) and then resuspended in MEM/HIHS. Dissociated cells were seeded on poly-L-lysine coated T75 culture flasks (Techno Plastic Products, TPP, Trasadingen, Switzerland) at the density of 250,000 cells/flask. Cultures were grown for two-three weeks in MEM/HIHS +Pen/Strep at 37°C in a humidified atmosphere of 5% CO2/95% air. The culture medium was changed twice a week. Culture purity was routinely confirmed by staining with antibody recognizing specific astrocytic marker, glial fibrillary acidic protein, (Sigma); ≥98% of cells were GFAP-positive. Confluent cells were re-plated as necessary on 6- or 12-well tissue culture plates (TPP), or 18-mm square coverslips (Carolina Biological, Burlington, NC).
Animal surgery and microdialysis procedures
Microdialysis procedures were performed in male Sprague-Dawley rats (Taconic Farms), weighing between 325 and 425 g. Rats were given atropine sulfate (0.05 mg/kg, i.m.) to reduce fluid secretion in the respiratory tract and initially anesthetized with 5% isoflurane. Animals were then intubated and mechanically ventilated with a gas mixture of 2.25% isoflurane in 30% O2/balance N2. Saline drip (0.9% NaCl) was administered intraperitoneally throughout the procedure to prevent dehydration. Body temperature was monitored throughout the experiment with a rectal probe and was maintained between 36°C and 36.5°C with a heating pad. Animals were placed in a stereotaxic frame and microdialysis probes (2 mm tip, 20 kD cutoff, CMA Microdialysis, North Chelmsford, MA) were slowly lowered through burr holes into the frontoparietal cortex (from bregma, 1 mm anterior; ±4 mm lateral; 2.6 mm down from the dura). Cortical tissue was perfused via the microdialysis probes with the artificial cerebral spinal fluid (aSCF) at a rate of 2 μL/min. aCSF contained (in mM): 120 NaCl, 25 NaHCO3, 2.7 KCl, 1 MgSO4, 1.2 CaCl2, and 0.05 ascorbic acid. The medium was bubbled with 5% CO2 to maintain pH=7.3. After two hours of probe stabilization, at least two 20-minute perfusate samples were collected by a CMA-170 refrigerated fraction collector (CMA Microdialysis) to determine baseline amino acid levels before the application of drug or hypoosmotic medium. Hypoosmotic aCSF contained (in mM): 25 NaCl, 25 NaHCO3, 2.7 KCl, 1 MgSO4, 1.2 CaCl2, and 0.05 ascorbic acid (pH=7.3). Hypoosmotic medium was perfused at the same rate (2 μL/min) for one hour but perfusate samples were collected more frequently: every 5 minutes. Each rat was implanted with two microdialysis probes placed bilaterally in the cortex, with one probe serving as a control (hypoosmotic solution only) and the probe on the other side (chosen at random) serving as the experimental condition (hypoosmotic solution plus drug).
Determination of the intracellular amino acid content in primary astrocyte cultures
Confluent astrocyte cultures grown in 6 well plates were preincubated for 40 min in basal HEPES-buffered medium containing (in mM): 135 NaCl, 3.8 KCl, 1.2 MgSO4, 1.3 CaCl2, 1.2 KH2PO4, 10 D-glucose, 10 HEPES (pH=7.4). Basal medium was then aspirated and replaced with fresh basal, or hypoosmotic, or isoosmotic low [NaCl] HEPES-buffered media. In hypoosmotic medium (HYPO), [NaCl] was reduced to 77 mM. In isoosmotic low [NaCl] medium (Low-Na): [NaCl] was also reduced to 77 mM but osmolarity was corrected by adding 116 mM mannitol. Cells were incubated in the experimental media for 30 min at 37°C. After completion of incubation, experimental media were aspirated and 1 mL of solution containing 5 mM Hepes and 1 mM EDTA was added to each well. Cells were scraped and sonicated for 4 minutes at room temperature. 100 μL aliquots of cell lysates were taken for protein assays, and the remaining lysates were clarified by rapid centrifugation (4 min × 12,100 g, MiniSpin centrifuge, Eppendorf, Hauppauge, NY). Supernatants were taken for HPLC analysis of amino acid content.
HPLC analysis of amino acid content in microdialysate samples and lysed cells
Amino acid levels in microdialysate samples and cell lysates were determined by a reverse-phase high performance liquid chromatography (HPLC) using an Agilent 1200 HPLC setup and Eclipse XDB-C18 column (4.6×150 mm, 5 μm particle diameter). Pre-column derivatization of amino acids was performed with freshly prepared mix of o-phthaldialdehyde and 2-mercaptoethanol in 0.4 M sodium tetraborate buffer (pH=9.5). The amino acid derivatives were eluted with solvent containing 30 mM NaH2PO4, 1% tetrahydrofuran, 30 mM sodium acetate, 0.05% sodium azide, and increasing concentration of the HPLC grade methanol (10–30%). Fluorescence signals were registered using a programmable 1200 series fluorescence detector (Agilent). Amino acid standards (L-aspartate, L-glutamate, L-glutamine, taurine, L-alanine), which were processed in the same fashion, were used to locate amino acid peaks and calculate concentrations of individual amino acids in the samples.
L-[3H]glutamine and L-glutamate (D-[3H]aspartate) release assay
To measure L-glutamine release, cells grown on 18-mm square glass coverslips were initially preincubated for 40 minutes with the glutaminase inhibitor 1 mM DON (Willis and Seegmiller 1977). This was necessary to prevent conversion of radiolabeled L-glutamine into L-glutamate by glutaminase. DON was washed out by transferring cells into basal medium, and cells were preloaded for 30 min with 4 μCi/mL of L[3H]glutamine plus 2 μM of unlabeled glutamine at 37°C. Coverslips were washed in 2 mL of basal medium to remove excess L-[3H]glutamine and transferred into a Lucite perfusion chamber. This chamber had a depression precisely cut in the bottom to accommodate the coverslip and a Teflon screw top leaving space above the cells of around 150–200 μm in height. Cells were superfused at a flow rate of 1.2 mL/min in an incubator set at 37°C with Basal, Low-Na, or HYPO media as indicated in figures (for media composition, see above). One-min perfusate fractions were collected and analyzed for [3H] content in a TriCarb 1900TR Liquid Scintillation Analyzer (PerkinElmer, Boston, MA) after adding 4 mLs of Ecoscint A scintillation cocktail (National Diagnostics, Atlanta, GA). At the end of each experiment, astrocytes on coverslips were lysed with 1 mL of 2% sodium dodecyl sulfate (SDS) plus 8 mM EDTA to calculate remaining isotope content. Fractional isotope release for each time point was calculated by dividing radioactivity released in each one min interval by the radioactivity left in the cells.
To determine the L-glutamate release rate we used its nonmetabolizable analogue D-[3H]aspartate, which is taken inside the cell by L-glutamate transporters and can be used as a tracer of L-glutamate fluxes. Cells were loaded for 30 min with 2 μCi/mL of D[3H]aspartate plus 2 μM of unlabeled L-glutamate at 37°C. The remaining procedures were identical to those described above.
L-[3H]glutamine uptake assay
Cells grown in 12-well plates were washed from serum-containing media with HEPES buffered Basal medium and pre incubated for 40 minutes in Basal medium with or without the irreversible glutaminase inhibitor 1 mM DON. They were then washed twice with 1 mL of Basal medium and incubated for 5–40 minutes at 37°C with either Basal, Low-Na or HYPO media containing additionally 4 μCi/mL of L-[3H]glutamine and 2 μM of unlabeled L-glutamine. Amino acid uptake was terminated by 3 washes with 1 mL of ice-cold Basal medium. Cells were then lysed in 1 mL of 2% SDS plus 8 mM EDTA. Lysates was transferred into scintillation vials and [3H] content was determined as described above.
Enzymatic assays of glutamine synthetase and glutaminase activity
We measured glutamine synthetase and glutaminase activity inside intact cells by quantifying enzymatic conversions of L-[3H]glutamate and L-[3H]glutamine after the separation of radiolabeled amino acids on anion exchange columns as originally proposed by Prusiner and Milner (1970).
For glutaminase activity assays, astrocytes grown in 6-well plates were preincubated for 40 minutes with the irreversible glutamine synthetase inhibitor 1 mM MSO (Ronzio and Meister 1968). Cells were washed twice with 2 mL of Basal solution and incubated for 30 minutes in Basal, Low-Na, or HYPO media containing 4 μCi/mL of L-[3H]glutamine plus 2 μM of unlabeled glutamine and 20 μM of DCPIB. The concentrations of labeled and unlabeled substrates were selected empirically to achieve high intracellular labeling levels. DCIPB was used to prevent loss of L-[3H]glutamate via volume-regulated anion channel (VRAC) in swollen cells (Abdullaev et al. 2006). After the completion of this 30-min reaction, the extracellular isotope was removed by 3 consecutive washes with 2 mL of ice-cold Basal solution. One mL of milliQ H2O was added to each well to lyse astrocytes; cells were scraped and sonicated for 4 min. Lysates were clarified by rapid centrifugation (4 min × 12,100 g). Each cell lysate (1 mL) was added onto AG 1-X8 Polyprep column (BioRad, Hercules, CA) for anion exchange separation of L-[3H]glutamate and L-[3H]glutamine. Column content was eluted with three 2-mL volumes of milliQ H2O, followed by three volumes of 0.1 M HCl. Water elution removes uncharged L-[3H]glutamine, while subsequent acid elution extracts negatively charged L-[3H]glutamate. Eluent fractions were transferred into scintillation vials and 4 mL of Ecoscint scintillation cocktail (National Diagnostics, Atlanta, GA) were added to each vial. [3H] content was calculated in a Tri-Carb 1900TR Liquid Scintillation Analyzer. The glutaminase activity was calculated as % conversion of L[3H]glutamine to L-[3H]glutamate normalized to the total [3H] recovered from each sample (L-[3H]glutamate plus L-[3H]glutamine).
Intracellular activity of glutamine synthetase was assayed in a similar fashion but in this case astrocytes we preincubated for 40 min with the irreversible glutaminase inhibitor 1 mM DON (Willis and Seegmiller 1977). Cells were washed twice with 2 mL of Basal solution and then incubated for 30 minutes in Basal, Low-Na or HYPO media containing 2 μCi/mL of L-[3H]glutamate plus 2 μM of unlabeled glutamate and 20 μM of ammonium sulfate to provide sufficient levels of NH4+ for the GS reaction. 20 μM DCPIB was also added to prevent L-[3H]glutamate loss via VRAC in swollen cells. L[3H]glutamine and L-[3H]glutamate were then separated using AG 1-X8 columns as described above. The glutamine synthetase activity was calculated as a percent conversion of L-[3H]glutamate to L-[3H]glutamine normalized to the total [3H] content recovered from each sample (L-[3H]glutamine plus L-[3H]glutamate).
Statistical analyses
The majority of data in this study are presented as mean values ±SE. Statistical differences between groups were determined using one-way ANOVA or repeated measures ANOVA and post hoc Tukey test for multiple comparisons. For enzymatic assays, the experimental data were normalized to controls in each experiment. In these latter experiments statistical differences were determined using one population t-test with post hoc Bonferroni correction if multiple comparisons were made. Origin 8.1 (Origin Labs, Northampton, MA) and Prism 5 (GraphPad Software, San Diego, CA) were used for statistical analyses.
Results
Effect of hypoosmotic medium on extracellular glutamine and glutamate levels in the cerebral cortex in vivo
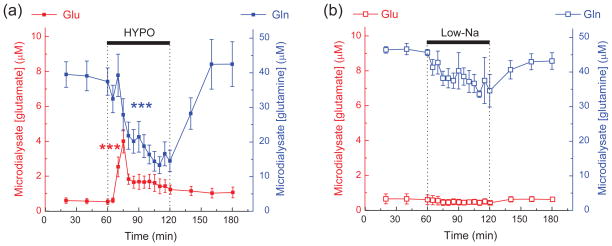
Our previous microdialysis study (Haskew-Layton et al. 2008), investigated the impact of hypoosmotic medium on extracellular amino acid levels in the cerebral cortex in vivo. In addition to finding transient increases in extracellular glutamate, aspartate and taurine levels, we unexpectedly discovered a strong decrease in extracellular glutamine levels. We now present in Fig. 1 the differential impact of hypoosmotic and isoosmotic low [NaCl] media on extracellular glutamine levels in the rat cortex. As seen in Fig. 1a, perfusion via a microdialysis probe of hypoosmotic medium in which [NaCl] was reduced from 120 to 25 mM (65% reduction in total osmolarity, total [Na+]o=50 mM, for complete medium composition see Methods) caused a gradual decrease in the extracellular glutamine by as much as ~70%. When [NaCl] was reduced ioosmotically by replacing it with mannitol, the reductions in the extracellular glutamine content were much less dramatic (~20%, Fig. 1b). For comparative purposes we also show the dynamics of extracellular glutamate in the same microdialysate samples. As previously reported, perfusion with hypoosmotic medium led to a substantial (~6.5-fold) increase in the extracellular concentrations of glutamate (Fig. 1a). Isoosmotic replacement of NaCl with mannitol had no effect on extracellular glutamate levels (Fig. 1b). Thus, extracellular levels of L-glutamine and L-glutamate are modulated via a mechanism that involves cellular swelling. Furthermore, peak increases in extracellular glutamate appear to precede changes in extracellular glutamine.
Fig. 1. Effects of hypoosmotic (a) and isoosmotic low [NaCl] (b) media on the extracellular levels of L-glutamate and L-glutamine in the rat brain cortex.
Brain tissue was perfused in anesthetized animals with hypoosmotic (HYPO) or isoosmotic low [NaCl] (Low-Na) media via two microdialysis probes placed bilaterally in the frontoparietal cortex. Extracellular levels of glutamate (red symbols) and glutamine (blue symbols) were measured in the microdialysate fractions using an off-line HLPC assay. The results are mean values ±SE from 5 animals. ***p<0.001, vs. basal release, repeated measures ANOVA. Note that the glutamine concentration scale is 5 times larger than the glutamate concentration scale. Data in (a) modified with permission from Haskew-Layton et al. (2008).
Effect of hypoosmotic medium on glutamate (D-[3H]aspartate) and L[3H]glutamine release and uptake in cultured rat astrocytes
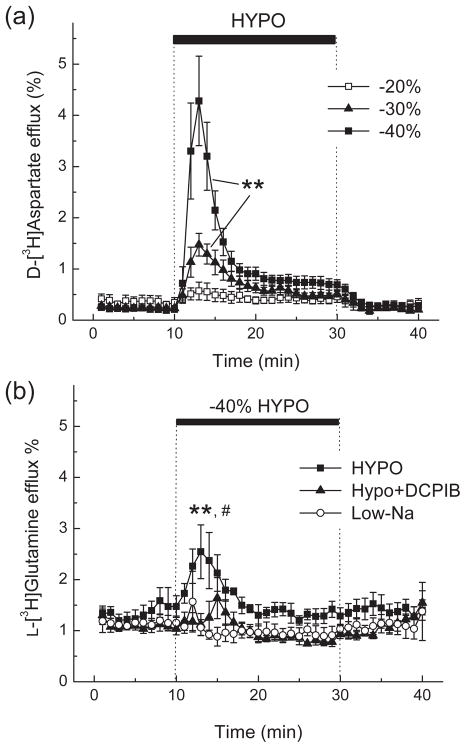
Since astrocytic synthesis and export is the main determinant of extracellular glutamine levels in vivo (Kanamori et al. 2002; McKenna 2007), we modeled the effects of hypoosmotic medium on L-glutamine transport in primary astrocyte cultures prepared from rat cortical tissue. L-Glutamate transport was first measured for comparative purposes. We used D-[3H]aspartate rather than L-[3H]glutamate to trace L-glutamate fluxes because it is a good substrate for the majority of glutamate transporters but, unlike L-glutamate, is not metabolized. In the first series of in vitro experiments, we quantified the dependence of D-[3H]aspartate release on medium osmolarity. Reductions in medium osmolarity in the range of 20–40% caused osmolarity-dependent increases in maximal L-glutamate (D-[3H]aspartate) release rates (Fig. 2a). For all the subsequent L[3H]glutamine transport experiments we used hypoosmotic medium in which osmolarity was reduced by 40%.
Fig. 2. Hypoosmotic medium strongly stimulates glutamate (D-[3H]aspartate) release and increases the release of L-[3H]glutamine in primary rat astrocytes.
(a) Dose dependence of osmolarity effects on the release of D-[3H]aspartate from astrocyte cultures. Cells were preloaded for 30 min with D-[3H]aspartate and then perfused with isoosmotic (basal) or hypoosmotic media (HYPO) in which osmolarity was reduced by 20, 30, or 40%. Data are the mean values ±SE of 4–5 experiments. **p<0.01 HYPO vs. basal release, repeated measures ANOVA. (b) Effect of 40% HYPO, isoosmotic low [NaCl] (Low-Na) medium, and the VRAC blocker DCPIB on L-[3H]glutamine release from primary astrocytes. For each experiment, cells were preincubated with the glutaminase inhibitor 1 mM DON and then preloaded for 30 min with L-[3H]glutamine. Data are the means ±SE of 4–5 experiments. **p<0.01 HYPO vs. Low-Na, #p<0.05, HYPO vs. HYPO+DCPIB.
In astrocyte cultures, the basal release rate for L-[3H]glutamine was 5–6-fold higher than that of D-[3H]aspartate (~1.25% vs ~0.2%, respectively, compare Fig. 2a and b). High glutamine release capacity in astrocytic cultures has been reported previously (Deitmer et al. 2003), and fits well with the idea of astrocytic glutamine export. Under our experimental conditions, intracellular L-glutamine is likely depleted below the KM values for astrocytic glutamine transporters, therefore the basal L-glutamine release rates presented here may be underestimated. When astrocytes were perfused with HYPO, we observed a transient increase in L-[3H]glutamine release by ~2-fold (Fig. 2b). The degree of stimulation was much smaller than a ~20-fold increase in the maximal rate of D[3H]aspartate release. Stimulation of L-[3H]glutamine release was due to cell swelling because we observed no changes in glutamine export in astrocytes perfused with Low-Na isoosmotic medium (Fig. 2b). Furthermore, such release was likely due to the opening of a VRAC permeability pathway because it was completely suppressed by the VRAC inhibitor DCPIB (Fig. 2b).
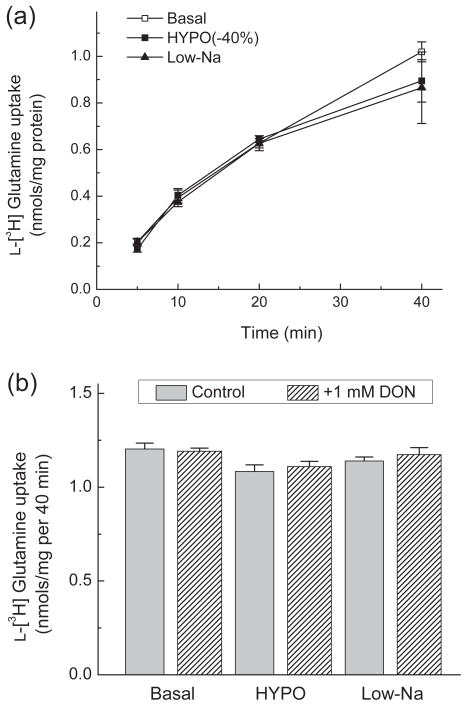
Swelling-activated increases in L-[3H]glutamine export in cultured astrocytes cannot explain why reductions in extracellular L-glutamine levels are seen in vivo in response to hypoosmotic medium (see Fig. 1a). An alternative explanation may involve the hypoosmotic stimulation of L-[3H]glutamine uptake that was reported in rat adipocytes (Ritchie et al. 2001). Therefore, we tested the effects of HYPO and Low-Na media on L-[3H]glutamine uptake in cultured astrocytes. In our experiments, astrocytic glutamine uptake was not significantly affected by Low-Na or HYPO media, even when glutamine catabolism was inhibited by pretreatment of cells with the irreversible glutaminase inhibitor DON (Fig. 3). These data in conjunction with the results of L[3H]glutamine release assays suggest that transmembrane glutamine transport in astrocytes is unlikely responsible for the changes in extracellular L-glutamine levels observed in vivo.
Fig. 3. L-[3H]glutamine uptake in astrocytes is not modified by hypoosmotic media or by the glutaminase inhibitor DON.
(a) For measurements of L-glutamine uptake, astrocytes were incubated with L-[3H]glutamine and 2 μM of unlabeled L-glutamine in Basal, −40% Hypo; or Low-Na media for 5–40 minutes. Data are the mean values ±SE of 6 independent measurements. (b) To exclude the contribution of the enzymatic conversion of L-[3H]glutamine to L-[3H]glutamate, astrocytes were treated with or without the glutaminase inhibitor DON (1 mM) for 40 min before transport measurements. L-[3H]glutamine uptake was measured for 40 min in the presence of 2 μM of unlabeled L-glutamine. Results are the means ± SE of 3 experiments.
Effect of hypoosmotic medium on intracellular amino acid levels
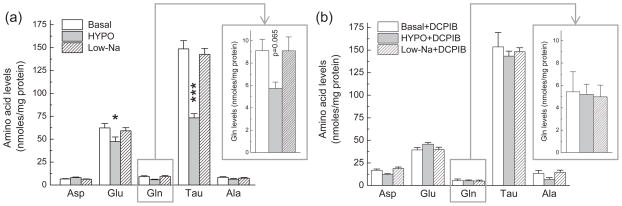
We next used HPLC analysis to quantify changes in the intracellular amino acid content. 30-min incubation in hypoosmotic medium resulted in a ~25% and ~35% decrease in the intracellular levels of L-glutamate and L-glutamine, respectively (Fig. 4a). Consistent with the previous literature reports (Sanchez-Olea et al. 1993, Olson 1999), we also found prominent reductions in intracellular levels of the sulfonic acid taurine, which is highly abundant in the cytosol of cultured astrocytes (~50% decrease, Fig. 4a). To confirm that changes in the intracellular amino acid levels were due to cell swelling, in parallel experiments astrocyte cultures were exposed to Low-Na medium, in which NaCl was isoosmotically replaced with mannitol. No changes in glutamate, glutamine, or taurine levels were found under isoosmotic low [NaCl] conditions (Fig. 4a). When the VRAC blocker DCPIB was added to the incubation media, it suppressed changes in glutamate, glutamine, and taurine levels under hypoosmotic conditions, as compared to basal and Low-Na controls (Fig. 4b), further suggesting that these amino acids are lost due to the activation of VRAC. Paradoxically, hypoosmotic medium did not decrease the intracellular levels of L-aspartate, another VRAC-permeable amino acid (23% increase, Fig. 4A). These findings are counterintuitive because aspartate has substantial VRAC permeability as reported in the past (Abdullaev et al. 2006) and also demonstrated in our present D-[3H]aspartate efflux experiments (Fig. 2a). Changes in the intracellular aspartate levels may be due to enhanced production from the intermediates of the TCA cycle and enhanced accumulation in the mitochondrial matrix (McKenna 2007).
Fig. 4. Hypoosmotic cell swelling reduces intracellular content of L-glutamate, L-glutamine, and taurine via a mechanism sensitive to the VRAC blocker DCPIB.
(a) Primary rat astrocytes were incubated in Basal, HYPO, or isoosmotic Low-Na media for 30 min. Levels of 5 intracellular amino acids were analyzed in cell lysates using an HPLC assay and normalized to protein content. Data are the mean values ± SE of 7–8 experiments. *p<0.05, ***p<0.001, vs. basal. Inset shows changes in glutamine levels on an expanded scale. (b) Effect of hypoosmotic medium on L-glutamate, L-glutamine, and taurine content was prevented by the VRAC inhibitor 20 μM DCPIB. Results are the means of 4 experiments. Inset shows changes in glutamine levels on an expanded scale.
Although DCPIB potently inhibited the effects of hypoosmotic medium on L-glutamate (D-[3H]aspartate) release (Abdullaev et al., 2006) and intracellular amino acid levels (present findings), we also observed that in the absence of cell swelling this compound on its own decreased intracellular levels of endogenous L-glutamate by ~30% and strongly increased intracellular L-aspartate (see Supplemental Fig. 1). This finding, however, should not affect interpretation of hypoosmotic experiments, or subsequent enzymatic assays, which were done with DCPIB present in all media (see below).
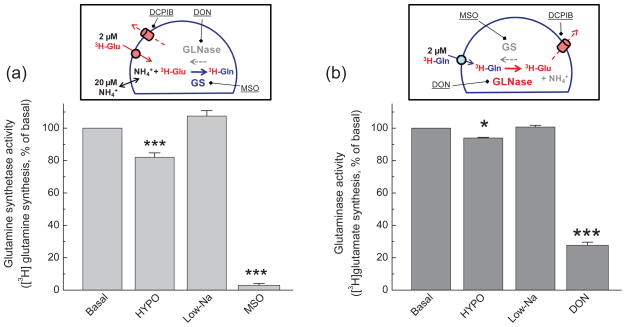
Impact of hypoosmotic medium on the activity of astrocytic glutamine synthetase and glutaminase
Since the results of our in vitro glutamine transport assays provided no mechanistic explanation for the in vivo observations of changes in extracellular glutamine levels, we turned our attention to potential modifications in astrocytic glutamine and glutamate metabolism. In the brain, up to 90% of synaptically released glutamate is recycled to glutamine via the activity of glutamine synthetase, an enzyme that is predominantly localized in astrocytes (Martinez-Hernandez et al. 1977; Kanamori et al. 2002). We measured the activity of glutamine synthetase in cultured astrocytes by monitoring the enzymatic conversion of L-[3H]glutamate to L-[3H]glutamine, using the method of (Prusiner and Milner 1970) that was adapted by us for assays in intact cells. Three important modifications to this assay were introduced to increase the sensitivity and specificity of the method. (1) To preserve newly synthesized L-[3H]glutamine, glutaminase activity was suppressed by preincubation with the irreversible glutaminase inhibitor DON. (2) To prevent the loss of L-[3H]glutamate and L-[3H]glutamine from swollen cells, VRAC activity was suppressed using the selective VRAC blocker DCPIB (Abdullaev et al. 2006). (3) To compensate for the loss of intracellular NH4+ during prolong incubation in chemically defined media, we added extracellular NH4+. With these modifications, we found that HYPO but not isoosmotic Low-Na medium inhibited glutamine synthetase activity by ~20% (Fig. 5a). To assure that our assay is selective towards glutamine synthetase, we carried out pharmacological controls. 40-min preincubation of cells with the irreversible glutamine synthetase blocker 1 mM MSO inhibited L-[3H]glutamate-to-L-[3H]glutamine conversion by 97% (Fig. 5a). Because in the preceding experiments we established that DCPIB reduces intracellular glutamate levels (see Supplemental Fig. 1), we additionally tested if DCPIB per se modulates the activity of glutamine synthetase. 20 μM DCPIB had no effect on the activity of this enzyme when tested under basal conditions (101.1 ±12.1%, n=6, see Supplemental Fig. 2).
Fig. 5. Hypoosmotic cell swelling suppresses enzymatic activity of glutamine synthetase (GS) and glutaminase (GLNase) in intact cultured astrocytes.
(a) The activity of GS was measured as enzymatic production of L-[3H]glutamine from L[3H]glutamate in intact astrocytes incubated under basal, HYPO and Low-Na conditions. To prevent enzymatic hydrolysis of L-[3H]glutamine by GLN and loss of L[3H]glutamate via VRAC, cells were preincubated with 1 mM DON, and 20 μM DCPIB was additionally added to all reaction media. Intracellular L-[3H]glutamine and L[3H]glutamate were extracted, separated, and quantified as described in Materials and methods. Pretreatment of cells with 1 mM MSO was used as a positive control for GS activity. Data are the mean values ± SE of 6–9 experiments. ***p<0.001 vs. basal. (b) The activity of glutaminase was analyzed in similar fashion but cells were preincubated with the irreversible inhibitor of GS 1 mM MSO. 20 μM DCPIB was added to all reaction media. Pretreatment of cells with 1 mM DON was used as a positive control for GLNase activity. Data are the mean values ± SE of 5–6 experiments. *p<0.05, ***p<0.001 vs. basal. Inset in each figure briefly summarizes experimental design.
Although the hypoosmotic medium-induced inhibition of glutamine synthetase that was observed in our experiments may partially explain drop in extracellular glutamine levels in vivo, such inhibition was rather small to fully account for the 40–45% reductions measured in vivo over a similar 30-min time interval. Therefore, we investigated whether other mechanisms may also contribute to in vivo effects. Specifically, we tested if cell swelling can stimulate the activity of the glutamine-hydrolyzing enzyme, glutaminase. Glutaminase activity was quantified by monitoring the enzymatic conversion of L-[3H]glutamine to L-[3H]glutamate. These assays were performed in cells that were pretreated with the glutamine synthetase inhibitor MSO to prevent reverse conversion of glutamate to glutamine, and in the presence of the VRAC blocker DCPIB to eliminate the loss of amino acids via a swelling-activated anion channel. With these modifications we found a 7% inhibition of glutaminase activity under hypoosmotic conditions but not in isoosmotic Low-Na medium (Fig. 5b). Thus, it is unlikely that enhanced hydrolysis in astrocytes is the cause for reductions in glutamine levels seen in microdialysis experiments.
To make sure that we selectively measure glutaminase activity, we tested the effects of the irreversible glutaminase inhibitor DON on L-[3H]glutamine-to-L[3H]glutamate conversion. 40-min preincubation of cells with 1 mM DON inhibited enzymatic conversion by ~75% (Fig. 5b). This number likely underestimates contribution of glutaminase to L-[3H]glutamine-to-L-[3H]glutamate reaction because Willis and Seegmiller (1977) reported that DON requires 2.5-hr preincubation to exert complete inhibitory action in cellular assays. Such prolonged preincubation was impractical for our experiments because it could lead to strong depletion of intracellular amino acids, especially L-glutamine. We further tested if DCPIB, which was added to prevent the loss of amino acids via VRAC in all enzymatic assays, has an effect on glutaminase activity. In control experiments performed under basal conditions, 20 μM DCPIB produced an apparent stimulation of glutaminase activity by 19% (n=3, p<0.05, Supplemental Fig. 2B) and reduced total isotope accumulation (“L-[3H]glutamine plus L-[3H]glutamate”) by ~40% (data not shown) that likely points to the effects of DCPIB on glutamine uptake.
Keeping these limitations in mind, it is important to stress that we found no evidence for the activation of astrocytic glutaminase in swollen cells, which could elucidate decreased extracellular glutamine levels in vivo. A possible explanation for quantitative differences between in vitro and in vivo results is provided in Discussion.
Discussion
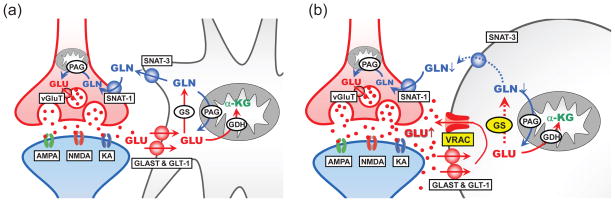
The major finding of this work is the suppression of glutamine synthetase activity in swollen cells under hypoosmotic conditions. This effect, in conjunction with loss of glial glutamate via swelling-activated channel VRAC (see schematic diagram in Fig. 6), may partially explain enigmatic reductions in extracellular glutamine levels seen in our microdialysis experiments employing hypoosmotic media. These latter results reveal strong changes in glutamate-glutamine metabolism in hyponatremic brain.
Fig. 6. Hypothetical scheme summarizing the effects of pathological cell swelling on glutamate-glutamine cycle in the brain tissue.
(a) Under physiological conditions extracellular glutamate (GLU) is recycled via the concerted work of glutamate uptake via GLAST-1 and GLT-1 transporters, conversion to glutamine (GLN) by glutamine synthetase (GS), and glutamine release and uptake by astrocytic SNAT-3 and neuronal SNAT-1. In neurons, glutamine is hydrolyzed to glutamate by phosphate-activated glutaminase (PAG). (b) Astrocytic cell swelling opens the glutamate-permeable channel VRAC and strongly inhibits activity of GS, resulting in lower levels of glutamine production and export. Other abbreviations: AMPA, NMDA, KA, ionotropic glutamate receptors; α-KG, α-ketoglutarate; GDH, glutamate dehydrogenase; vGluT, vesicular glutamate transporters. Note that for simplicity this diagram omits a number of alternative glutamine transporters and important metabolic enzymes.
Hypoosmotic cell swelling modifies glutamate-glutamine cycle via opening of VRAC channels and inhibition of glutamine synthetase activity
Glutamate is the major amino acid neurotransmitter in the CNS, which is responsible for 80–90% of excitatory synaptic signaling. As discussed in the introduction, the tight control of extracellular glutamate levels in brain tissue is achieved by the glutamate-glutamine cycle. An in vivo NMR study by (Kanamori et al. 2002) estimated that up to 90% of glutamate released during synaptic transmission is normally recycled via astrocytic conversion to glutamine. Therefore, sustained glutamatergic transmission strongly relies on glutamine export by astrocytes (reviewed in Broer and Brookes 2001; Hertz and Zielke 2004). We were surprised to find in microdialysis experiments that perfusion of hypoosmotic medium in the rat cortex caused a ~70% reduction in extracellular glutamine levels within 1 hour (Haskew-Layton et al. 2008) and the present study). This effect was dependent on cellular swelling because isoosmotic replacement of NaCl with mannitol had a very weak effect on extracellular glutamine concentrations. Similar to our work, Taylor et al. (1995) reported strong osmolarity-dependent reductions in extracellular glutamine in brains perfused with hypoosmotic medium. We hypothesized that changes in extracellular glutamine were due to inhibition of its export and/or synthesis in glial cells. In order to address this hypothesis, we analyzed the effects of hypoosmotic cell swelling on glutamine transport and metabolism in cultured astrocytes.
In our in vitro experiments, we found no evidence for volume-dependent changes in astrocytic glutamine uptake. Glutamine release in swollen cells was transiently stimulated by ~2-fold, which is opposite to what would be expected based on our in vivo observations. Lack of transient hypoosmotic stimulation of glutamine release in vivo may be due to a lower transmembrane gradient for this amino acid. Continuous astrocytic export results in high interstitial glutamine levels that are calculated to be 350–600 μM, while estimated cytosol concentrations are around 6–8 mM (Xu et al. 1998; Chaudhry et al. 2002). Thus, hypoosmotic stimulation of glutamine release in vivo is driven by ~10–20-fold glutamine gradient, as compared to the in vitro experiments, in which the absence of extracellular glutamine provides a stronger driving force for the amino acid efflux. Within the first 10 minutes of perfusion with hypoosmotic medium in astrocyte cultures, glutamine release levels returned to their baseline values. This time coincided with the beginning of a gradual decrease in microdialysate glutamine values in vivo (compare Figs. 1a and 2b). This subsequent drop in extracellular glutamine can at least partially be explained by decreased astrocytic synthesis. Our whole-cell enzymatic assays revealed partial inhibition of the glutamine-producing enzyme, glutamine synthetase, in hypoosmotic but not isoosmotic low-NaCl media. In comparison, hypoosmotic medium had a much smaller effect on the activity of the glutamine-hydrolyzing enzyme glutaminase.
The measurements of enzymatic activities were somewhat complicated by the fact that the VRAC blocker DCPIB, which was added to prevent amino acid loss from swollen cells, modified intracellular amino acid levels and glutamine uptake. Nevertheless, the effects of hypoosmotic medium on glutamine synthetase activity are valid, because all the assays were done under identical conditions with DCPIB, and because this compound did not have a direct effect on glutamine synthetase.
The 20% suppression of the glutamine synthetase activity measured after 30-min incubation in hypoosmotic medium in vitro, was accompanied by the 35% reduction in intracellular levels of glutamine quantified using an HPLC assay. However, the degree of glutamine synthetase inhibition seemed insufficient to fully account for the dramatic changes in the extracellular glutamine in vivo (~45% reduction over 30-min period, and ~70% over 1 hr). Several factors may contribute to quantitative differences between in vitro and in vivo results. (1) The conditions employed in our in vitro assays likely underestimate inhibition of glutamine synthetase. Particularly, treatment with DCPIB preventes the shunting glutamate from the cytosol to the extracellular space via VRAC in astrocyte cultures; and such shunting may decrease glutamate accessibility to glutamine synthetase in vivo (see diagram in Fig. 6). This idea is consistent with the finding that DCPIB prevented hypoosmotic changes in intracellular levels of both L-glutamate and L-glutamine (Fig. 4A). (2) Our in vitro experiments do not model potential changes in glutamine uptake and metabolism by neuronal cells. Although, astrocytic glutamine uptake and glutaminase activity were not affected by cell swelling, neuronal transport and metabolism may behave differently. (3) In vivo regulation of the glutamate-glutamine cycle may involve neuron-astrocyte interactions that are lacking in vitro.
Glutamine synthetase is one of the most heavily regulated metabolic enzymes. Besides modulation by intracellular levels of the substrates—glutamate, ammonia and ATP—glutamine synthetase is a subject to cumulative feedback inhibition by more than 40 biosynthetic products of nucleotide synthesis, and by adenylation (Stadtman 2001). As suggested above, inhibition of the enzyme in conjunction with limitation of glutamate accessibility due to amino acid loss via VRAC are likely causes for the reductions in glutamine synthesis. The exact mechanisms responsible for hypoosmotic inhibition of the enzymatic activity are not known but may involve decreases in intracellular ATP, hydrolytic increases in intracellular AMP, protein adenylation, or a number of other intracellular signaling reactions, which are associated with cellular swelling. It is known that hypoosmotic cell swelling triggers elevations in intracellular [Ca2+], activation of phospholipase A2 and numerous protein kinases, including PKC, members of the MAPK cascade, as well as tyrosine kinases of the src family (Mongin and Orlov 2001; Hoffmann et al. 2009). PKC has previously been implicated in the inhibition of glutamine synthetase activity and reduction of gene expression, albeit on a slower time scale that that employed in our experiments (Mangoura et al. 1995; Brodie et al. 1998).
A somewhat unexpected finding of this study was identification of swelling-activated glutamine release that was sensitive to the selective VRAC blocker DCPIB. VRAC, also termed in the literature ‘volume-sensitive anion/organic osmolyte channel’ (VSOAC), or ‘volume-sensitive outward rectifying’ channel (VSOR) (Strange et al. 1996; Okada 1997; Nilius et al. 1997) is permeable to negatively charged and neutral organic osmolytes, including glutamate, aspartate, taurine, and possibly myo-inositiol. L-Glutamine is generally considered to be excluded from this permeability pathway based on the size of the molecule (Nilius and Droogmans 2003). Our results point to limited VRAC permeability to L-glutamine. Similar conclusions were reached in previous studies based on the direct electrophysiological measurements of glutamine currents in human astrocytoma cells (Roy 1995), as well as indirect evidence involving inhibition of cell volume regulation by high levels of extracellular glutamine in cultured rat astrocytes (Pasantes-Morales et al. 1994).
Pathophysiological implications of cell volume-dependent regulation of glutamine synthetase activity
Although cellular swelling was found in numerous neuropathologies, our results are directly relevant to hyponatremia, the clinical syndrome caused by reductions in the blood plasma concentrations of Na+ and Cl−. This condition develops in ~1% of elective surgery patients, as well as in variety of metabolic disorders, in the syndromes of inappropriate secretion of antidiuretic hormone and the cerebral salt wasting, and as a result of psychogenic polydipsia (Fraser and Arieff 1997; Pasantes-Morales et al. 2002; Mongin and Kimelberg 2005). Symptomatic acute hyponatremia is associated with high mortality rates, reaching in some patient cohorts as much as 50% or higher (Arieff et al. 1976; Fraser and Arieff 1997). Although NaCl levels are reduced systemically, the morbidity and mortality in hyponatremic patients are due to deficits in the CNS. Following decreases in plasma osmolarity, water moves into the brain, enters its extracellular and intracellular compartments, triggering brain edema, compression of blood vessels, and brain herniation (Gullans and Verbalis 1993; Fraser and Arieff 1997). In addition to the critical impact on intracranial pressure, hyponatremia frequently triggers seizures and dramatically enhances susceptibility to generalized seizures if underlying conditions already exist (Andrew 1991). Both the previously described activation of glutamate release via VRAC (Kimelberg et al. 1990; Abdullaev et al. 2006; Haskew-Layton et al. 2008), and inhibition of glutamate recycling by astrocytic glutamine synthetase identified in the present work may contribute to the clinically observed hyperexcitability and seizures. Astrocytes are generally considered to be more sensitive to the effects of hypoosmotic swelling, as compared to neurons, perhaps due to low water permeability of neuronal cell membranes (Andrew et al. 2007). However, direct effects of hypoosmotic medium on neuronal excitability have also been reported (see for example Paoletti and Ascher 1994; Huang et al. 1997; Somjen 1999).
It remains to be explored if inhibition of glutamine synthetase by hypoosmotic medium is a long-lasting phenomenon. Glutamine belongs among the top five most abundant organic osmolytes, along with glutamate, creatine, taurine, and myo-inositol (Thurston et al. 1987; Verbalis and Gullans 1991; Gullans and Verbalis 1993; Pasantes-Morales et al. 2002). In chronic hyponatremia, total brain levels of glutamate and glutamine are persistently reduced by as much as 40–50 and 50–70%, respectively (Thurston et al. 1987; Verbalis and Gullans 1991; Massieu et al. 2004). Acute hyponatremia was reported to induce 20–40% reductions in the whole brain glutamate levels with no significant impact on the whole-brain glutamine (Thurston et al. 1975; Sterns et al. 1993; Silver et al. 1999). In cerebellum of water-intoxicated animals, Nagelhus et al. (1996) found ~40% increases in the total tissue glutamine levels and elevation in the intracellular glutamine-like immunoreactivity. These results differ from strong reductions in the extracellular glutamine measured by us and by Taylor et al (1995) using perfusion of hypoosmotic medium and microdialysis approach. These differences may suggest that in the hyponatremic brain changes in the extracellular glutamine levels precede drop in the whole tissue glutamine concentrations.
Besides hyponatremia, our findings may be relevant to other neuropathologies—such as stroke, traumatic brain injury, or epilepsy—that have also been associated with astrocytic swelling (reviewed in Mongin and Kimelberg 2005). Two-photon laser scanning microscopy done in vivo as well as post-mortem ultrastructural studies indicate that astrocyte swelling develops as rapidly as within 6–12 minutes after onset of global ischemia, and may be reliably demonstrated within 30–120 min after the initiation of focal ischemia, traumatic brain injury, or experimental epileptic seizures ((Jenkins et al. 1979; Barron et al. 1988; Fabene et al. 2006; Broberg et al. 2008; Risher et al. 2009). All these pathologies are accompanied by disruptions in glutamate-glutamine homeostasis and increased glutamate toxicity. Both VRAC activation and inhibition of glutamine synthetase activity may contribute to observed acute or delayed neurotoxicity (Kimelberg and Mongin 1998; Mongin 2007). At least some forms of human epilepsy and animal epilepsy models are associated with reductions in glutamine synthetase protein levels and normalized enzymatic activity (Eid et al. 2004; Bjornsen et al. 2007; Swamy et al. 2011). Lehmann (1987) and, more recently, Kanamori and Ross (2011) found rapid (within tens of minutes) and persistent elevations in hippocampal extracellular glutamate and strong reductions in extracellular glutamine levels during acute folate-induced seizures in rabbits and kainate-induced chronic seizures in rat. Such rapid changes cannot be explained by loss of protein but they are remarkably similar to our own observations in rat cortex perfused with hypoosmotic medium (Haskew-Layton et al. 2008). It should be noted, however, that the connection between cell swelling and amino acid levels in epilepsy has not been firmly established. Furthermore, a number of studies reported no changes in extracellular glutamate or glutamine levels (see for example Vezzani et al. 1985) or found seizure-associated elevations in both extracellular glutamate and glutamine (Kaura et al. 1995). Therefore, the mechanisms of pathological dysregulation of the glutamate-glutamine cycle are likely pathology- and animal model-specific and any generalizations should be treated with caution.
In conclusion, we propose a unified mechanism via which hypoosmotic swelling impacts brain glutamate-glutamine cycle in the brain tissue (summarized in Fig. 6). Our findings highlight the previously unknown inhibition of glutamine synthetase and a strong relationship between cell swelling, extracellular glutamate and glutamine levels, and intracellular glutamate metabolism.
Supplementary Material
Acknowledgments
We thank Dr. Alena Rudkouskaya for contribution to the pilot experiments leading to this study and Drs. Paul J. Feustel and Iskandar F. Abdullaev for helpful discussions and comments on the manuscript. This work was supported in part by grant from the National Institutes of Neurological Disorders and Stroke R01 NS061953. The authors have no conflict or financial interest to disclose.
References
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in rat cultured astrocytes. J Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci. 2007;12:332–343. doi: 10.2741/2067. [DOI] [PubMed] [Google Scholar]
- Andrew RD. Seizure and acute osmotic change: clinical and neurophysiological aspects. J Neurol Sci. 1991;101:7–18. doi: 10.1016/0022-510x(91)90013-w. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Labron MW, Boehnke SE, Carnduff L, Kirov SA. Physiological evidence that pyramidal neurons lack functional water channels. Cereb Cortex. 2007;17:787–802. doi: 10.1093/cercor/bhk032. [DOI] [PubMed] [Google Scholar]
- Arieff AI, Llach F, Massry SG. Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes. Medicine (Baltimore) 1976;55:121–129. doi: 10.1097/00005792-197603000-00002. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs R, Machiyama Y, Hammond BJ, Julian T, Richter D. The operation of the gamma-aminobutyrate bypath of the tricarboxylic acid cycle in brain tissue in vitro. Biochem J. 1970;116:445–461. doi: 10.1042/bj1160445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banderali U, Roy G. Anion channels for amino-acids in Mdck cells. Am J Physiol. 1992;263:C1200–C1207. doi: 10.1152/ajpcell.1992.263.6.C1200. [DOI] [PubMed] [Google Scholar]
- Barron KD, Dentinger MP, Kimelberg HK, Nelson LR, Bourke RS, Keegan S, Mankes R, Cragoe EJ., Jr Ultrastructural features of a brain injury model in cat. I. Vascular and neuroglial changes and the prevention of astroglial swelling by a fluorenyl (aryloxy) alkanoic acid derivative (L-644,711) Acta Neuropathol (Berl) 1988;75:295–307. doi: 10.1007/BF00690538. [DOI] [PubMed] [Google Scholar]
- Bjornsen LP, Eid T, Holmseth S, Danbolt NC, Spencer DD, de Lanerolle NC. Changes in glial glutamate transporters in human epileptogenic hippocampus: inadequate explanation for high extracellular glutamate during seizures. Neurobiol Dis. 2007;25:319–330. doi: 10.1016/j.nbd.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Broberg M, Pope KJ, Lewis T, Olsson T, Nilsson M, Willoughby JO. Cell swelling precedes seizures induced by inhibition of astrocytic metabolism. Epilepsy Res. 2008;80:132–141. doi: 10.1016/j.eplepsyres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Brodie C, Bogi K, Acs P, Lorenzo PS, Baskin L, Blumberg PM. Protein kinase C delta (PKCdelta) inhibits the expression of glutamine synthetase in glial cells via the PKCdelta regulatory domain and its tyrosine phosphorylation. J Biol Chem. 1998;273:30713–30718. doi: 10.1074/jbc.273.46.30713. [DOI] [PubMed] [Google Scholar]
- Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77:705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J Cell Biol. 2002;157:349–355. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Broer A, Broer S. Glutamine efflux from astrocytes is mediated by multiple pathways. J Neurochem. 2003;87:127–135. doi: 10.1046/j.1471-4159.2003.01981.x. [DOI] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Estevez AY, O’Regan MH, Song D, Phillis JW. Effects of anion channel blockers on hyposmotically induced amino acid release from the in vivo rat cerebral cortex. Neurochem Res. 1999;24:447–452. doi: 10.1023/a:1020902104056. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Weiczner R, Marzola P, Nicolato E, Calderan L, Andrioli A, Farkas E, Sule Z, Mihaly A, Sbarbati A. Structural and functional MRI following 4-aminopyridine-induced seizures: a comparative imaging and anatomical study. Neurobiol Dis. 2006;21:80–89. doi: 10.1016/j.nbd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med. 1997;102:67–77. doi: 10.1016/s0002-9343(96)00274-4. [DOI] [PubMed] [Google Scholar]
- Gullans SR, Verbalis JG. Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu Rev Med. 1993;44:289–301. doi: 10.1146/annurev.me.44.020193.001445. [DOI] [PubMed] [Google Scholar]
- Haskew-Layton RE, Rudkouskaya A, Jin Y, Feustel PJ, Kimelberg HK, Mongin AA. Two distinct modes of hypoosmotic medium-induced release of excitatory amino acids and taurine in the rat brain in vivo. PLoS ONE. 2008;3:e3543. doi: 10.1371/journal.pone.0003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- Huang R, Bossut DF, Somjen GG. Enhancement of whole cell synaptic currents by low osmolarity and by low [NaCl] in rat hippocampal slices. J Neurophysiol. 1997;77:2349–2359. doi: 10.1152/jn.1997.77.5.2349. [DOI] [PubMed] [Google Scholar]
- Jenkins LW, Povlishock JT, Becker DP, Miller JD, Sullivan HG. Complete cerebral ischemia. An ultrastructural study. Acta Neuropathol (Berl) 1979;48:113–125. doi: 10.1007/BF00691152. [DOI] [PubMed] [Google Scholar]
- Kanamori K, Ross BD. Chronic electrographic seizure reduces glutamine and elevates glutamate in the extracellular fluid of rat brain. Brain Res. 2011;1371:180–191. doi: 10.1016/j.brainres.2010.11.064. [DOI] [PubMed] [Google Scholar]
- Kanamori K, Ross BD, Kondrat RW. Glial uptake of neurotransmitter glutamate from the extracellular fluid studied in vivo by microdialysis and (13)CNMR. J Neurochem. 2002;83:682–695. doi: 10.1046/j.1471-4159.2002.01161.x. [DOI] [PubMed] [Google Scholar]
- Kaura S, Bradford HF, Young AM, Croucher MJ, Hughes PD. Effect of amygdaloid kindling on the content and release of amino acids from the amygdaloid complex: in vivo and in vitro studies. J Neurochem. 1995;65:1240–1249. doi: 10.1046/j.1471-4159.1995.65031240.x. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Mongin AA. Swelling-activated release of excitatory amino acids in the brain: Relevance for pathophysiology. Contrib Nephrol. 1998;123:240–257. doi: 10.1159/000059916. [DOI] [PubMed] [Google Scholar]
- Laake JH, Takumi Y, Eidet J, Torgner IA, Roberg B, Kvamme E, Ottersen OP. Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate activated glutaminase in rat cerebellum. Neuroscience. 1999;88:1137–1151. doi: 10.1016/s0306-4522(98)00298-x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. Alterations in hippocampal extracellular amino acids and purine catabolites during limbic seizures induced by folate injections into the rabbit amygdala. Neuroscience. 1987;22:573–578. doi: 10.1016/0306-4522(87)90354-x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. Effects of microdialysis-perfusion with anisoosmotic media on extracellular amino acids in the rat hippocampus and skeletal muscle. J Neurochem. 1989;53:525–535. doi: 10.1111/j.1471-4159.1989.tb07365.x. [DOI] [PubMed] [Google Scholar]
- Lien YH, Shapiro JI, Chan L. Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. Implications for the pathogenesis of central pontine myelinolysis. J Clin Invest. 1991;88:303–309. doi: 10.1172/JCI115292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoura D, Sogos V, Dawson G. Phorbol esters and PKC signaling regulate proliferation, vimentin cytoskeleton assembly and glutamine synthetase activity of chick embryo cerebrum astrocytes in culture. Brain Res Dev Brain Res. 1995;87:1–11. doi: 10.1016/0165-3806(95)00046-g. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Mongin AA. Disruption of ionic and cell volume homeostasis in cerebral ischemia: The perfect storm. Pathophysiology. 2007;14:183–193. doi: 10.1016/j.pathophys.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. Astrocytic swelling in neuropathology. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford University Press; Oxford/New York: 2005. pp. 550–562. [Google Scholar]
- Mongin AA, Orlov SN. Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology. 2001;8:77–88. doi: 10.1016/s0928-4680(01)00074-8. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Lehmann A, Ottersen OP. Neuronal and glial handling of glutamate and glutamine during hypoosmotic stress: a biochemical and quantitative immunocytochemical analysis using the rat cerebellum as a model. Neuroscience. 1996;72:743–755. doi: 10.1016/0306-4522(96)00003-6. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron. 1994;13:645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Franco R, Ordaz B, Ochoa LD. Mechanisms counteracting swelling in brain cells during hyponatremia. Arch Med Res. 2002;33:237–244. doi: 10.1016/s0188-4409(02)00353-3. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Murray RA, Sanchez-Olea R, Moran J. Regulatory volume decrease in cultured astrocytes. II. Permeability pathway to amino acids and polyols. Am J Physiol. 1994;266:C172–C178. doi: 10.1152/ajpcell.1994.266.1.C172. [DOI] [PubMed] [Google Scholar]
- Prusiner S, Milner L. A rapid radioactive assay for glutamine synthetase, glutaminase, asparagine synthetase, and asparaginase. Anal Biochem. 1970;37:429–438. doi: 10.1016/0003-2697(70)90069-2. [DOI] [PubMed] [Google Scholar]
- Risher WC, Andrew RD, Kirov SA. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia. 2009;57:207–221. doi: 10.1002/glia.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JW, Baird FE, Christie GR, Stewart A, Low SY, Hundal HS, Taylor PM. Mechanisms of glutamine transport in rat adipocytes and acute regulation by cell swelling. Cell Physiol Biochem. 2001;11:259–270. doi: 10.1159/000047812. [DOI] [PubMed] [Google Scholar]
- Roberg B, Torgner IA, Kvamme E. The orientation of phosphate activated glutaminase in the inner mitochondrial membrane of synaptic and non-synaptic rat brain mitochondria. Neurochem Int. 1995;27:367–376. doi: 10.1016/0197-0186(95)00018-4. [DOI] [PubMed] [Google Scholar]
- Ronzio RA, Meister A. Phosphorylation of methionine sulfoximine by glutamine synthetase. Proc Natl Acad Sci U S A. 1968;59:164–170. doi: 10.1073/pnas.59.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy G. Amino-acid current through anion channels in cultured human glial-cells. J Membr Biol. 1995;147:35–44. doi: 10.1007/BF00235396. [DOI] [PubMed] [Google Scholar]
- Silver SM, Schroeder BM, Bernstein P, Sterns RH. Brain adaptation to acute hyponatremia in young rats. Am J Physiol. 1999;276:R1595–R1599. doi: 10.1152/ajpregu.1999.276.6.r1595. [DOI] [PubMed] [Google Scholar]
- Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr. 2000;130:1016S–1022S. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Low external NaCl concentration and low osmolarity enhance voltage-gated Ca currents but depress K currents in freshly isolated rat hippocampal neurons. Brain Res. 1999;851:189–197. doi: 10.1016/s0006-8993(99)02185-x. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. The story of glutamine synthetase regulation. J Biol Chem. 2001;276:44357–44364. doi: 10.1074/jbc.R100055200. [DOI] [PubMed] [Google Scholar]
- Sterns RH, Baer J, Ebersol S, Thomas D, Lohr JW, Kamm DE. Organic osmolytes in acute hyponatremia. Am J Physiol. 1993;264:F833–F836. doi: 10.1152/ajprenal.1993.264.5.F833. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Swamy M, Yusof WR, Sirajudeen KN, Mustapha Z, Govindasamy C. Decreased glutamine synthetase, increased citrulline-nitric oxide cycle activities, and oxidative stress in different regions of brain in epilepsy rat model. J Physiol Biochem. 2011;67:105–113. doi: 10.1007/s13105-010-0054-2. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Davies SE, Obrenovitch TP, Doheny MH, Patsalos PN, Clark JB, Symon L. Investigation into the role of N-acetylaspartate in cerebral osmoregulation. J Neurochem. 1995;65:275–281. doi: 10.1046/j.1471-4159.1995.65010275.x. [DOI] [PubMed] [Google Scholar]
- Thurston JH, Hauhart RE, Jones EM, Ater JL. Effects of salt and water loading on carbohydrate and energy metabolism and levels of selected amino acids in the brains of young mice. J Neurochem. 1975;24:953–957. doi: 10.1111/j.1471-4159.1975.tb03661.x. [DOI] [PubMed] [Google Scholar]
- Thurston JH, Hauhart RE, Nelson JS. Adaptive decreases in amino acids (taurine in particular), creatine, and electrolytes prevent cerebral edema in chronically hyponatremic mice: rapid correction (experimental model of central pontine myelinolysis) causes dehydration and shrinkage of brain. Metab Brain Dis. 1987;2:223–241. doi: 10.1007/BF00999694. [DOI] [PubMed] [Google Scholar]
- van den Berg CJ, Garfinkel D. A stimulation study of brain compartments. Metabolism of glutamate and related substances in mouse brain. Biochem J. 1971;123:211–218. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbalis JG, Gullans SR. Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res. 1991;567:274–282. doi: 10.1016/0006-8993(91)90806-7. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Ungerstedt U, French ED, Schwarcz R. In vivo brain dialysis of amino acids and simultaneous EEG measurements following intrahippocampal quinolinic acid injection: evidence for a dissociation between neurochemical changes and seizures. J Neurochem. 1985;45:335–344. doi: 10.1111/j.1471-4159.1985.tb03993.x. [DOI] [PubMed] [Google Scholar]
- Willis RC, Seegmiller JE. The inhibition by 6-diazo-5-oxo-l-norleucine of glutamine catabolism of the cultured human lymphoblast. J Cell Physiol. 1977;93:375–382. doi: 10.1002/jcp.1040930308. [DOI] [PubMed] [Google Scholar]
- Xu GY, McAdoo DJ, Hughes MG, Robak G, de CR., Jr Considerations in the determination by microdialysis of resting extracellular amino acid concentrations and release upon spinal cord injury. Neuroscience. 1998;86:1011–1021. doi: 10.1016/s0306-4522(98)00063-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.