Abstract
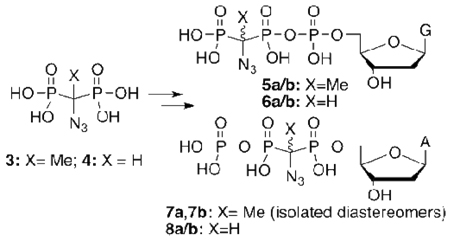
The first examples of α-azido bisphosphonates [(RO)2P(O)]2CX(N3) (1, R = i-Pr, X = Me; 2, R = i-Pr, X = H; 3, R = H, X = Me; 4, R = H, X = H) and corresponding β,γ-CX(N3) dGTP (5–6) and α,β-CX(N3) dATP (7–8) analogues are described. The individual diastereomers of 7 (7a, 7b) were obtained by HPLC separation of the dADP synthetic precursor (14a/b).
Triphosphate analogues in which a bridging oxygen is replaced by a functionalized carbon atom have been of interest as enzymatic probes since the early 1980s.1,2 Recently, we described the preparation of a series of highly purified and well characterized β,γ-CXY3,4 and α,β-CXY5 dNTP derivatives and applied them as probes of structure, function, and fidelity in DNA polymerase β (pol β),3–5 a base excision repair (BER) enzyme that is overexpressed in some cancers. By appropriate substitution on the CXY carbon (X, Y= H, Me, F, Cl, Br) a range of stereoelectronic properties is made available to probe binding and catalytic interactions with the enzyme. Linear free-energy relationship (LFER) plots of DNA pol β-catalyzed dGMP incorporation rates into nascent DNA from β,γ-CXY dGTPs have revealed a base match- dependent leaving group effect,4 and X-ray crystallographic data have provided evidence for stereospecific binding of analogues containing a CXF group.3,6
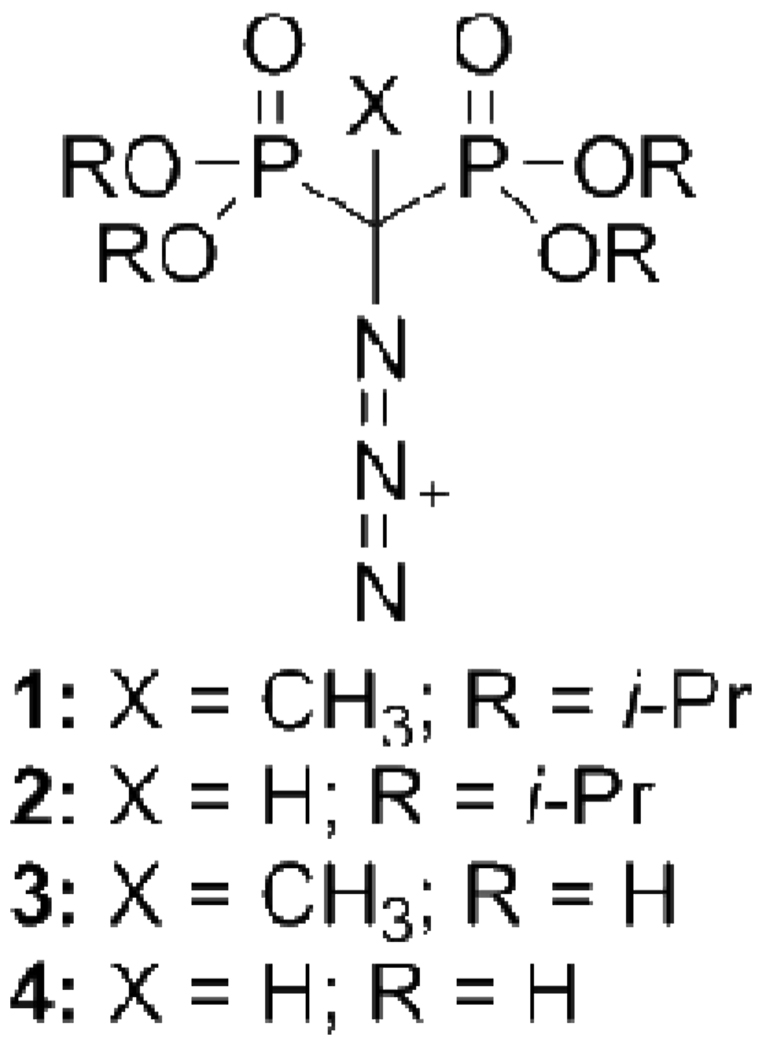
To extend these studies, we wished to construct β,γ (5, 6) and α,β (7, 8) dNTPs that incorporate a bridging CX(N3) group. As a pseudohalide7 the azido functionality could usefully extend the tunability of the analogue series, providing more information on molecular interactions at the active site arising from the stereochemistry at the bridging carbon. Introduction of the azido group might also facilitate separation of individual CXY diastereomers, which has not been accomplished with the various X,Y = methyl and halide derivatives prepared thus far.3,5 These considerations, combined with the prospect of developing a new and potentially useful bisphosphonate synthon, motivated an investigation of α-azido bisphosphonic esters and acids, a previously unknown class of compounds (Figure 1).
FIGURE 1.
Structures of α-azido bisphosphonate esters and acids.
Electrophilic azido transfer was explored as a methodology offering a direct route to 1 and 2 from readily available starting materials. In this approach, a sulfonyl azide reacts with a carbanion species to generate a triazene intermediate that can fragment into either diazo or azido products.8 Various factors influence the fragmentation pathway, but the nature of the sulfonyl azide is particularly important. Sulfonyl azides with sterically bulky and electron-donating substituents, such as 2,4,6-triisopropylbenzenesulfonyl azide (trisyl azide; TrN3), favor azido transfer8,9 while those containing strongly electron-withdrawing moieties, e.g. trifluoromethylsulfonyl azide (triflyl azide; TfN3), favor diazo transfer.9,10
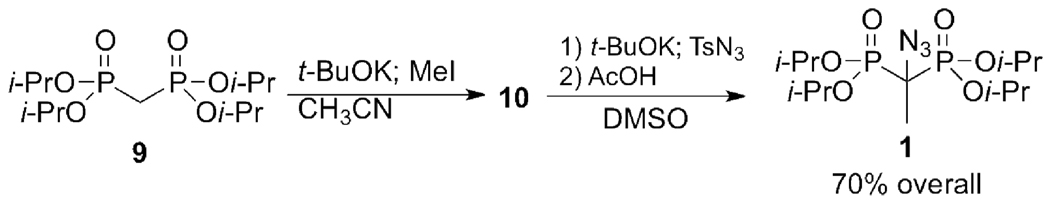
Although the methylene group of a bisphosphonate ester such as tetraisopropyl methylenebis(phosphonate) 9 is exposed in principle to either pathway, replacement of one α-H by a methyl group, as in tetraisopropyl ethylidenebis(phosphonate) 10, will enforce azido transfer. In the synthesis of 1 from 10, methylation of 9 with potassium tert-butoxide (t-BuOK) followed by methyl iodide gave a mixture of 78% 10 and 20% α,α-dimethyl side product. Fortunately, this crude 10 preparation could be directly treated with t-BuOK and then p-toluenesulfonyl azide (tosyl azide; TsN3) in DMSO at room temperature to give the azido ester 1 in 70% yield after purification by column chromatography (Scheme 1).
SCHEME 1.
Synthesis of 1.
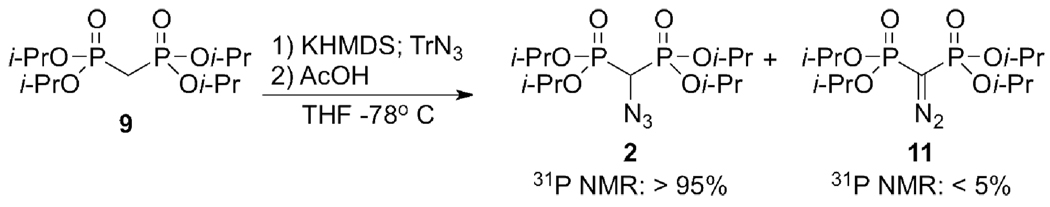
Attempts to synthesize 2 from 9 using tosyl azide led to the unwanted diazo transfer product. Reaction of a carbanion derived from an activated methylene group with a sulfonyl azide is a known route to tetraalkyl diazomethylenebis(phosphonates) and trialkyl α-diazo phosphonoacetates.11 However, use of trisyl azide with careful optimization of conditions (THF, −78° C, potassium counterion, fivefold azide reagent excess, immediate quenching by AcOH) generated 2 from the carbanion of 9 in greater than 95% yield by 31P NMR (Scheme 2). Despite the efficient conversion, purification of 2 proved to be problematic initially due to difficulty in removing the large excess of trisyl azide and a minor side product, tetraisopropyl diazomethylenebis(phosphonate) (11), by preparative chromatography on silica gel. Pure 2 (50% yield) was eventually obtained by crystallization of remaining trisyl azide, filtration, and selective oxidation of 11 with t-butyl hypochlorite12 in wet ethyl acetate13 prior to chromatography.
SCHEME 2.
Synthesis of 2.
Care must be taken during work-up of 2, which retains a relatively acidic α-H, because it decomposes slowly on silica gel, more quickly in aqueous solution at pH 6, and rapidly at pH 8. This contrasts with the reported stability of the malonate derivative14 and presumably reflects the resonance stabilization available in the enolate form of the latter compound.
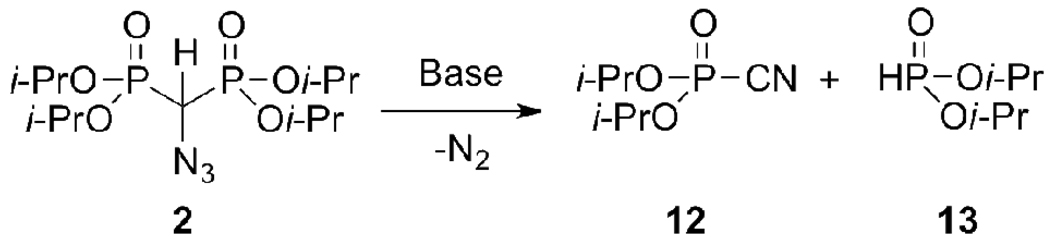
Exposure of 2 to bases such as BuLi, NaH, KHMDS, and t-BuOK results in the release of N2 with formation of diisopropyl cyanophosphate (12)15 and diispropyl phosphite (13) (Scheme 3). The base-induced elimination of nitrogen from α-azido esters,16 malonates,14 and sulfones17 has been previously reported and transition metal-catalyzed versions of this reaction have found synthetic utility.18,19 In contrast to diethyl azidomalonate, in which the carbanion was further derivatized at −15 °C without the loss of N2,14 2 loses N2 rapidly upon exposure to a variety of bases in organic solvents at −78 °C and does not give appreciable amounts of adducts in the presence of a large excess of N-chlorosuccinimide.
SCHEME 3.
Base-induced decomposition of 2.
The structures of 1–4 are supported by their suite of IR, NMR, and MS data. Esters 1 and 2 are both colorless oils with strong IR absorbances in the azido region (1: 2123, 2086 cm−1; 2: 2104, sh. at 2134 cm−1). The 1H NMR spectrum of 1 includes a CH3 triplet at δ 1.60 ppm (t, JHP 15 Hz) and that of 2 has an α-H triplet at δ 3.53 ppm (JHP 21 Hz). The 13C NMR spectrum of 1 has resonances at δ 17.6 (CH3) and δ 59.8 ppm (Cα t, JCP 150 Hz). The Cα of 2 resonates at δ 55.0 ppm (t, JCP 145 Hz). The 31P chemical shifts of 1 (δ 17.0 ppm) and 2 (δ 13.3 ppm) are consistent with a previously observed 3–4 ppm downfield effect of α-methylation in other bisphosphonate esters.20,21
Reaction of 1 and 2 with bromotrimethylsilane (BTMS)22 in acetonitrile at room temperature quantitatively gave the tetrakis(trimethylsilyl) esters, which display the expected upfield 31P shifts, Δδ +16–17 ppm. Desilylation with aqueous ethanol produced the acids 3 and 4 (> 98% overall) as hygroscopic white solids. These acids are stable in dilute aqueous solution pH 2–12 for at least several days at room temperature. They have IR bands at 2126, 2085 (sh) cm−1 (3) and 2113 cm−1 (4) attributed to the azido group. The 1H NMR spectra include resonances at δ 1.43 ppm (C(CH3)N3, t, JHP 13 Hz) for 3 and δ 3.26 ppm (CHN3, t, JHP 17 Hz) for 4 (CHN3). The 13C NMR displays resonances at δ 23.5 (CH3) and δ 68.4 ppm (Cα, t, JCP 129 Hz) for 3, and at δ 65.1 ppm (t, JCP 124 Hz) (Cα) for 4. The 31P chemical shifts are in the expected regions, δ 18.3 ppm (3) and δ 13.5 ppm (4). The HRMS m/z values for the [M−H]− anion of 3 and 4 match calculated values within error, confirming the assigned structures.
The present study and our previous interest in diazo transfer chemistry23,24 prompted us to reinvestigate a reported azido transfer to triethyl phosphonoacetate using triflyl azide in the presence of triethyl amine.25 Triflyl azide is known to be a powerful diazo transfer reagent9 and attempts by other authors to extend the procedure to benzocyclic β-keto esters were unsuccessful;8 nevertheless, the original reaction has been cited as a viable azido transfer reaction in recent reviews.26,27 In our hands, the procedure of Hakimelahi and Just25 produced triethyl diazophosphonoacetate, characterized by 1H, 13C, 31P NMR and MS (ESI and APCI) and generated no detectable amounts of azido transfer product. In light of these results and analysis of the literature,28 we propose that the reaction of triflyl azide with triethylphosphonoacetate is consistent with known diazo transfer chemistry and is not an anomalous case of azido transfer.
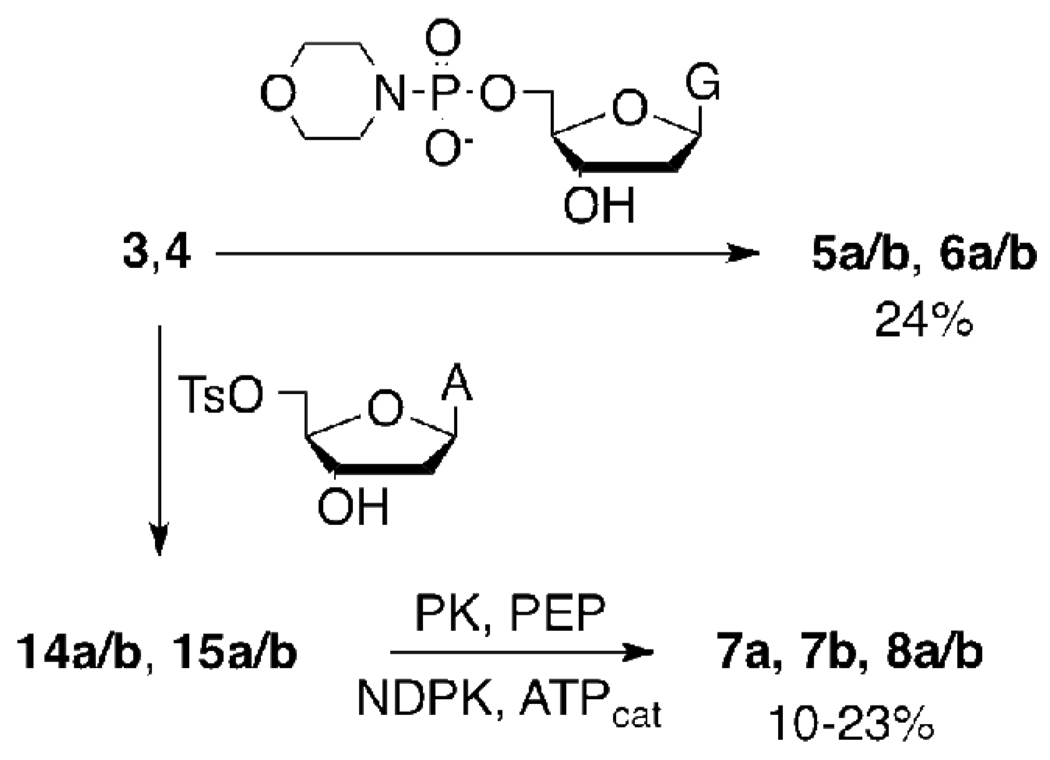
With acids 3 and 4 in hand, we then proceeded to the synthesis of β,γ (5, 6) and α,β (7, 8) azido methylene nucleotide triphosphate analogues. The β,γ-CXN3 dGTP analogues 5 and 6 were prepared by coupling3,4 the Bu3NH+ salts of 3 and 4 with the dGMP-morpholidate.29 α,β-CXN3 analogues of dATP (7, 8) were synthesized by reaction of the Bu4N+ salt of 3 or 4 with dA-5’-tosylate followed by enzymatic phosphorylation of the resulting α,β-CX(N3) dADP intermediates (14, 15) by ATP-phosphoenolpyruvate (PEP) catalyzed by pyruvate kinase (PK) and nucleoside diphosphate kinase (NDPK) in HEPES buffer, pH 7.55 (Scheme 4). The final compounds were purified by dual-pass (strong-anion exchange ‘SAX’ followed by reverse phase ‘RP’) HPLC to ≥ 97% as determined by 31P NMR and analytical HPLC, and were characterized by NMR (1H, 31P) and HRMS.
SCHEME 4.
Synthesis of nucleotide azidomethylene analogues.
Nucleotide triphosphate α,β-CXY or β,γ-bisphosphonate analogues with an asymmetrically substituted bridging carbon (X ≠ Y) are obtained as mixtures of diastereomers by existing synthetic procedures starting from a prochiral bisphosphonate precursors, and thus far none have been separated chromatographically.3, 5, 6 Diastereomeric β,γ-fluoromethylene dNTPs can be individually observed in the synthetic mixture by 19F NMR.3,6 In addition to the expected nucleoside resonances for dGuo, the 1H NMR of 5a/b shows a pair of doublets centered at δ 1.58–1.59 ppm (C(CH3)N3) and that of 6a/b an apparent triplet at δ 3.40 ppm (CHN3). However, although the proton-decoupled Pα and Pγ 31P NMR signals of 5a/b at 202 MHz are doublets at δ −9.45 and 14.5 ppm respectively, two resolvable (Δδ 0.03 ppm) diastereomeric pairs of doublets (J = 34, 19 Hz) are observed for Pβ. The δ vs. J assignments were confirmed by comparing 31P spectral data obtained at 162 and 202 MHz.
A similar pattern is seen with 6a/6b except that resolvable diastereomeric multiplets are observed for both Pα (δ −9.78, −9.81 ppm) and Pβ (δ 9.04, 9.06 ppm), but not for Pγ (10.4 ppm). This difference between 5a,b and 6a,b with respect to the sensitivity of the Pα chemical shift to the Pβγ CXN3 chiral center invites further investigation (we speculate that it might implicate an H-bonding effect involving the relatively acidic CHN3 hydrogen, or alternatively reflects a more asymmetric shielding effect of the CHN3 group on the Pα nucleus).
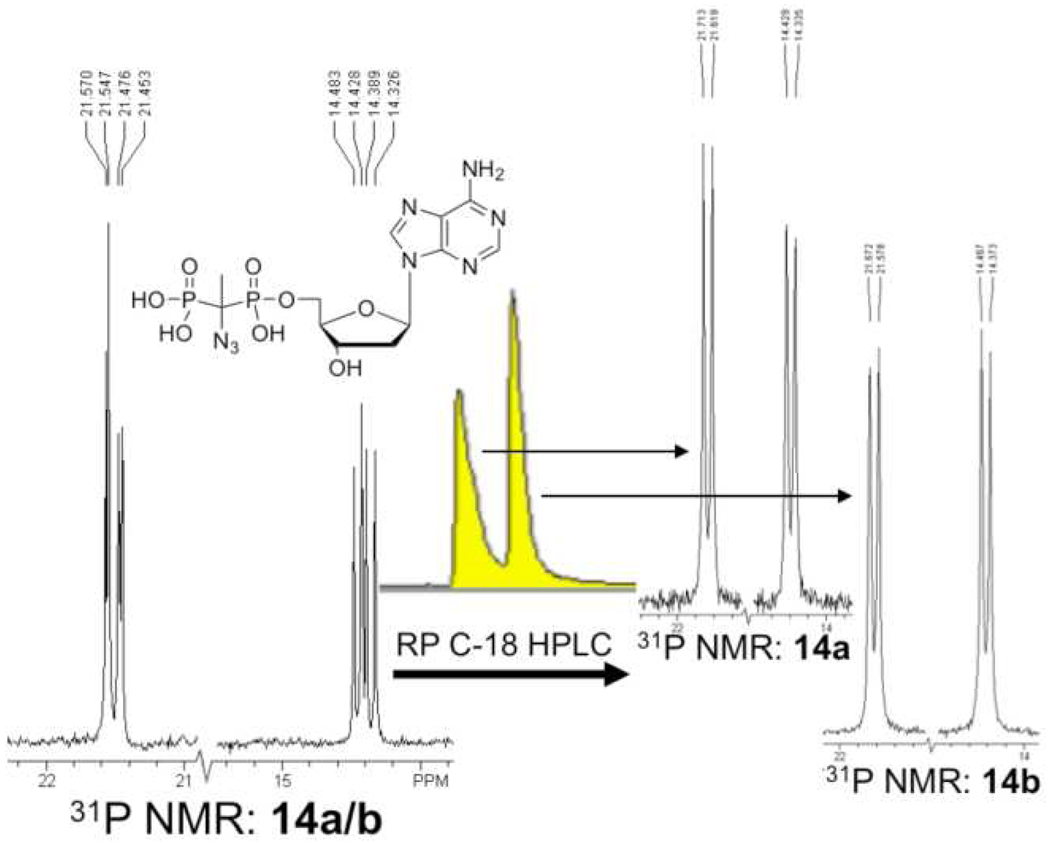
In the preparation of the α,β-C(CH3)N3 ATP analogues 7a and 7b, we found that the corresponding diphosphate intermediates (14a/b) could be isolated individually by isocratic RP HPLC (Figure 2). Subsequent enzymatic phosphorylation as described above then generated the individual diastereomers 7a and 7b. CD spectra measured for 7a and 7b showed prominent features of opposite polarity centered near 260 nm (7a, (−); 7b (+)). Curiously, the α,β-CHN3 dADP intermediate (15a/b) did not separate under similar conditions and its phosphorylation yielded the triphosphates 8a/b as a mixture of diastereomers which could not be distinguished by 31P NMR.
FIGURE 2.
Separation of 14 diastereomers by RP preparative HPLC. Left: 31P NMR spectrum of 14a/b as synthesized displays two pairs of doublets for both Pα and Pβ. Right: 31P NMR (14a and 14b) spectra of separated diastereomers show single pairs of doublets for Pα and Pβ.
In conclusion, we have described the first examples of α-azido bisphosphonate esters and acids. The latter compounds can be used to synthesize novel nucleotide analogues containing a CHN3 or CMeN3 at either the α,β or β,γ bridging position. The ability to obtain for the first time the individual diastereomers of the α,β-CH3N3 dADP (14a, 14b) and corresponding dATP analogues (5a, 5b) provides more refined probes for stereochemical interactions of these compounds with appropriate enzymes. A further use of these new bisphosphonates may be to prepare derivatives made more conveniently or uniquely accessible by the presence of the synthetically versatile azido function.
Experimental
General Experimental Methods
The nucleotide triphosphate analogues were prepared by adapting our previously published methods for β,γ-CXY3,4,6 and α,β-CXY5 dNTP analogues. Analytical HPLC analysis was conducted on a Varian PureGel SAX 10 mm × 100 mm 7 µL column eluted with A: H2O B: 0.5 M (0–50%) LiCl gradient over 30 min at a 4 mL/min flow rate. Products were detected at 259 nm for adenosine derivatives and at 253 nm for guanosine derivatives. All dNTP derivatives were prepared as triethylammonium salts.3–6 CD spectra were obtained on a JASCO J-815 spectropolarimeter.
Tetraisopropyl (1-azidoethane-1,1-diyl)bis(phosphonate), 1
Tetraisopropyl methylenebis(phosphonate) (1.75 g, 5 mmol) was treated with 1.2 equiv. t-BuOK and 1.2 equiv. MeI in anhydrous acetonitrile overnight at rt. Volatiles were removed at reduced pressure and the residue was dissolved in water, extracted into DCM and dried over MgSO4. Evaporation of the solvent left an oil (1.84 g) that contained ~20% dimethylated product, ~2% unmethylated material, and 78% monomethylated product. This mixture (~4 mmol monomethylated product) was dissolved in 20 mL DMSO and stirred with 0.689 g (6 mmol) t-BuOK. TsN3 1.05 g (5 mmol) was added dropwise. The reaction mixture was stirred for 30 s, quenched with 1.5 mL of AcOH, and then allowed to stir for several hours at rt. The reaction mixture was extracted with hexanes and the organic phase was dried over MgSO4. Following concentration by evaporation, the product was purified on silica gel using ACE:DCM (1:3), Rf = 0.69, giving 1 as a colorless oil. Yield: 1.45 g (70% overall). IR: ν (film) 2123 cm−1, 2086 (N3). δH (399.8 MHz; CDCl3): 1.33-1.37 (24 H, m, 4 × OCH(CH3)2), 1.60 (3H, t, JHP 15.2 Hz, PCCH3N3P), 4.83 (4 H, m, 4 × OCH(CH3)2); δC (100.5 MHz; CDCl3): 17.6 (t, JCP 3.9 Hz, PCCH3N3P), 23.7-23.8, 24.3-24.4 (OCH(CH3)3, 59.8 (t, JCP 150.3 Hz, PCP), 72.6-72.8 ((OCH(CH3)3; δP (161.9 MHz; CDCl3; 85% H3PO4): 17.0 ({1H} s; coupled JPH 15.2 Hz (qm), JPC 150 Hz (satellites)).
Tetraisopropyl (azidomethanediyl)bis(phosphonate), 2
In a 500 mL RB flask under N2, ~6 g (19 mmol) of TrN3 were dissolved in 30 mL of anhydrous THF and stirred vigorously at −78 °C. A solution of 1.517 g (4.41 mmol) of tetraisopropyl methylenebis(phosphonate) and 4.41 mL 1M KHMDS in 10 mL THF was mixed at rt, cooled to −78 °C and added at once to the TrN3 solution by syringe. The solution was stirred for 10 s and then quenched with 2.5 mL AcOH. The flask was moved to a −20 °C freezer and allowed to stand for 48 h, or until the triazene had decomposed as determined by 31P NMR. The precipitate was filtered and volatiles removed at reduced pressure. The reaction mixture was then seeded with solid TrN3 and allowed to sit overnight at 0 °C. The resulting precipitate was washed with H2O and filtered off. After removing water with rotovap, the residue was dissolved in 20 mL wet EtOAc. Ten drops of t-BuOCl were added and stirred at rt until evolution of N2 ceased. EtOAc was washed with a saturated aqueous NH4Cl solution, dried and concentrated. The residue (95% by 31P NMR) was purified on silica gel eluted with ACE:DCM (1:9), Rf = 0.36. Contaminating phosphite was removed by rotary evaporation (oil pump) with gentle warming to afford 0.83 g of 2 as a colorless oil (yield: 50% overall). IR: ν (film) 2134 (m), 2104 cm−1 (N3). NMR: δH (399.8 MHz; CDCl3): 1.31-1.34 (24 H, m, 4 × OCH(CH3)2), 3.53 (1H, t, JHP 20.8 Hz, PCHN3P), 4.79 (4 H, m, 4 × OCH(CH3)2); δC (100.5 MHz; CDCl3): 23.7-23.8, 24.1-24.2 **(OCH(CH3)3, 55.0 (t, JCP 144.8 Hz, PCP), 72.7, 72.9 (2t, JCP 3.5 Hz OCH(CH3)3); δP (161.9 MHz; CDCl3; 85% H3PO4): 13.3 ({1H} coupled, s; JPH 20.5 Hz (dm), JPC 145 Hz).
Base-induced decomposition of 2
5 mg (13 µmol) of 2 was dissolved in 1.5 mL anhydrous THF and cooled to −78 °C. 1.5 equiv. t-BuOK was added at once, causing rapid evolution of gas. Identical results were obtained with BuLi and KHMDS. NMR: δP (202.5 MHz; CDCl3; 85% H3PO4): 22.29 (diisopropyl cyanophosphate),15 4.8 (diispropyl phosphite) with integration 1:1.
(1-Azidoethane-1,1-diyl)bis(phosphonic acid), 3 and (azidomethanediyl)bis(phosphonic acid), 4
The appropriate bisphosphonate ester was dissolved in 2 mL anhydrous acetonitrile and 6 equiv. of BTMS were added by syringe. Stirring overnight gave the tetrakis(trimethylsilyl) esters (100% by 31P NMR). From 1: δP (202.5 MHz; CHCN, ext. 85% H3PO4): 0.9; from 2: δP −3.2 (s). Hydrolysis with ~1 mL of EtOH:H2O (1:1) and removal of volatiles by prolonged evaporation at low pressure provided the acids as hygroscopic white solids (>98% overall yield). Acid is further dried under vacuum in desiccator containing P2O5.
3: white solid, IR: ν (KBr): 2085 (m), 2126 cm−1 (N3). NMR: δH (399.8 MHz; D2O; pH 10.88): 1.43 (t, JHP 13.2 Hz, PCCH3N3P); δC (100.5 MHz; D2O; ext. pH 10.88): 23.5 (s, PCCH3P), 68.4 (t, JCP 129, PCP); δP (161.9 MHz; D2O; 85% ext. H3PO4; pH 10.88): 18.3 ({1H} s; coupled, JPH 13.2 Hz (q); JPC 129 Hz (satellites)). HRMS (ESI/APCi) [M−H]−: calcd for C2H6N3O6P2: 229.9737. Found: 229.9734.
4: IR: ν (KBr): 2113 cm−1 (N3). δH (399.8 MHz; D2O; pH 10.88): 3.26 (t, JHP 16.8 Hz, PCHN3P); δC (100.5 MHz; D2O; pH 10.88): 65.1 (t, JCP 124 Hz, PCP); δP (161.9 MHz; D2O; 85% ext. H3PO4; pH 10.88): 13.5 ({1H} s; coupled, JPH 16.5 Hz (d); JPC 122 Hz (satellites)); HRMS (ESI/APCi) [M−H]−: calcd for CH4N3O6P2, 215.9581. Found: 215.9584.
Ethyl diazo(diethoxyphosphoryl)acetate
from trifyl azide and ethyl(diethylphosphoryl)acetate. According to the procedure of Hakimelahi and Just,25 72 mg NaN3 (1.1 mmol) was suspended in 20 mL anhydrous DMF and 106 µL (1.0 mmol) CF3SO2Cl was added under N2. After several min, a solution of 222 mg (1.0 mmol) of ethyl(diethylphosphoryl)acetate and 101 mg (1.0 mmol) Et3N in 5 mL anhydrous DMF was added dropwise. After 40 min, a mixture of compound was detected by 31P NMR with δp (DMF) 20.8, 18.3, 10.4 in a ratio of 5:4:1. Gentle heating for an additional 30 min gave a binary mixture of the compounds at 10.4 (40%) and 20.8 (60%). Ether was added and the reaction mixture was washed 5 × with water. The organic layer was dried over MgSO4 and evaporated. A portion of the residue was purified by preparative TLC on silica gel eluted with ACE:DCM (1:9) giving a single mobile band (UV), Rf = 0.65 which was identified as ethyl diazo(diethoxyphosphoryl)acetate. NMR: δH (400.2 MHz; CDCl3): 1.32 (3H, t, JHH 7 Hz), 1.38 (6H, dt, JHH 7 Hz, JHP 0.8 Hz), 4.22 (4H, m), 4.29 (2H, q, JHH 7 Hz); δP (202.5 MHz, CDCl3; 85% H3PO4) 10.3 (s). MS (APCi, m/z), 251 (M+1)+; an MS/MS analysis of the m/z 251 species, gave a major fragment at m/z 223.11
2’-Deoxyguanosine 5’-triphosphate β,γ CCH3N3, 5a/b
Compound 3 (2 equiv. as the 1.5 tributylammonium salt) was coupled to 100 mg (0.242 mmol) dGuo-morpholidate in anhydrous DMSO to yield a white solid, 32.5 mg (23.8%) after dual pass (SAX then RP) HPLC purification.4 Analytical HPLC: retention time = 9.6 min; purity = 98%. NMR: δH (399.8 MHz; D2O; pH 10.88): 1.58 (dd, PCCH3N3P) 2.48-2.54, 2.72-2.79, 4.11-4.21, 4.12, 6.32 8.05. δP (161.9 MHz; D2O; 85% H3PO4; pH 10.88): −9.5 (d, Jαβ 33.7 Hz, Pα), 13.28 (dd, Jαβ 33.7 Hz, Jβγ 19.4 Hz, Pβ), 13.31 (dd, Jαβ 33.7 Hz, Jβγ 19.4 Hz, Pβ) 14.5 (d, Jβγ 19.4 Hz, Pγ). HRMS (ESI/APCi) [M−H]−: calcd for C12H18N8O12P3, 559.0263. Found: 559.0266.
2’-Deoxyguanosine 5’-triphosphate β,γ CHN3, 6a/b
Compound 4 (2 equiv. as the 1.5 tributylammonium salt) was coupled to 100 mg (0.242 mmol) dGuo-morpholidate in anhydrous DMSO to yield a white solid, 31.1 mg (23.5%) 6a/b after dual pass (SAX then RP) HPLC purification.4 Analytical HPLC: retention time = 10.3 min, purity = 98%. NMR: δH (499.8 MHz; D2O; pH 10.88): 2.48-2.53, 2.71-2.76, 3.53 (ddd, PCHN3P), 4.10-4.20, 4.25, 6.31 (t, J 6.0 Hz) 8.03, 8.03; δP (161.9 MHz; D2O; 85% H3PO4; pH 10.88): −9.78 (d, Jαβ 27.8 Hz, Pα), −9.81 (d, J 27.8 hz Pα), 9.04 (dd, Jαβ 27.8 Hz, Jβγ 12.9 Hz, Pβ), 9.06 (dd, Jαβ 27.8 Hz, Jβγ 12.9 Hz, Pβ) 10.414 (d, Jβγ 12.9 Hz, Pγ). HRMS (ESI/APCi) [M−H]−: calcd for C11H16N8O12P3, 545.0106. Found: 545.0121.
2’-Deoxyguanosine 5’-diphosphate, α,β C(CH3)N3, 14a/b
The tris(tetrabutylammonium) salt from 60 mg (0.260 mmol) of 3 was allowed to react with 115 mg (~1.1 equiv.) dA-5’-Ts in anhydrous acetonitrile5 to yield 65.1 mg of the nucleoside diphosphonate diasteromers in a ratio of 2:3; (54% crude conversion), which could be separated by RP HPLC. 14a: NMR: δH (399.8 MHz; D2O; pH 10.88): 1.50 (dd, JHP 12.4, JHP 15.2 Hz, PCCH3N3P), 2.57-2.63, 2.82-2.91, 4.10-4.16, 4.22-4.27, 6.48 (t, 6.4 Hz), 8.25, 8.52; δP (161.9 MHz; D2O; 85% H3PO4; pH 10.88) 14.5 (d, J 15 Hz, Pβ); 21.7 (d, J 15 Hz, Pα). 14b: NMR: δH (399.8 MHz; D2O; pH 10.88): 1.50 (dd, JHP JHP 12.8, 15.2 Hz, PCCH3N3P), 2.57-2.63, 2.82-2.88, 4.10-4.16, 4.22-4.27, 6.47 (t, 6.4 Hz), 8.22, 8.50; δP (161.9 MHz; D2O; 85% H3PO4; pH 10.88) 14.4 (d, J 15 Hz, Pβ); 21.6 (d, J 15 Hz, Pα).
2’-Deoxyadenosine 5’-triphosphate α,β C(CH3)N3, 7a/b
Enzymatic phosphorylation5 of 14a and 14b followed by HPLC purification of each product gave 17.3 mg (yield: 12.2% overall from starting acid) 7a and 14.9 mg (10.5%) 7b. 7a: Analytical HPLC: retention time = 9.5 min; purity = 98%. NMR: δH (499.8 MHz; D2O; pH 10.88): 1.58 (t, JHP 15.0 Hz, PCCH3N3P), 2.58-2.63, 2.83-2.89, 4.21-4.29, 6.50 (t, 6.5 Hz), 8.25, 8.54; δP (202.5 MHz; D2O; 85% H3PO4; pH 10.88) −4.5 (d, Jβγ 31.9 Hz, Pγ); 7.0 (dd, Pβ); 18.51 (d, Jαβ 19.0 Hz, Pα). HRMS (ESI/APCi) [M−H]−: calcd for C12H18N8O11P3, 543.0313. Found: 543.0333. 7b: Analytical HPLC: retention time = 9.5 min; purity = 96%. NMR: δH (499.8 MHz; D2O; pH 10.88): 1.59 (t, JHP 15 Hz, PCCH3N3P), 2.58-2.63, 2.85-2.90, 4.17-4.22, 6.51 (t, 6.5 Hz), 8.26, 8.56; δP (202.3 MHz; D2O; 85% H3PO4; pH 10.88) −4.4 (d, Jβγ 30.1 Hz, Pγ); 8.0 (dd, Pβ); 18.47 (d, Jαβ 18.2 Hz, Pα). HRMS (ESI/APCi) [M−H]−: calcd for C12H18N8O11P3, 543.0315. Found: 543.0315.
2’-Deoxyguanosine 5’-diphosphate, α,β CHN3, 15a/b
The tris(tetrabutylammonium) salt was prepared from 60 mg (0.276 mmol) of 4 and allowed to react with 120 mg (~1.1 equiv.) dAdo-5’-Ts in anhydrous acetonitrile5 to yield a white solid, 32 mg (26%). NMR: δp (242.8; D2O; pH 10.88): 9.9 (s, Pβ); 17.2 (s, Pα).
2’-deoxyadenosine 5’-triphosphate α,β CHN3, 8a/b
Enzymatic phosphorylation5 followed by HPLC purification gave 33.2 mg (22.7%) 8a/b. Analytical HPLC: retention time = 10.4 min; purity = 98%. NMR: δH (400.2 MHz; D2O; pH 10.88): 2.57-2.63, 2.82-2.90, 3.86 (dt, PCHN3P), 4.11-4.25, 4.29, 6.50 (t, J 6.5 Hz), 8.24, 8.528, 8.532; δP (202.3 MHz; D2O; 85% H3PO4; pH 10.88) −5.1 (d, Jβγ 25.1 Hz, Pγ); 2.8 (dd, Pβ); 14.3 (d, Jαβ 13.8 Hz, Pα). HRMS (ESI/APCi) [M−H]−: calcd for C11H16N8O11P3, 529.0157. Found: 529.0147.
Supplementary Material
Acknowledgment
This research was supported by NIH grant CA105010. We thank Ms. Inah Kang for assistance in preparing the manuscript and Dr. Ron New (UC Riverside Mass Spec Facility) for obtaining the HRMS data.
Footnotes
Supporting Information Available: IR (1–4), 1H NMR (1–8, 14, 15), gCOSY (1, 2), 13C NMR (1–4), 31P NMR (1–8, 14, 15), HRMS (3–8), UV/VIS and CD spectra (7a, 7b), HPLC data for 5–8, 31P NMR spectrum for conversion of 2 to 12 and 13, 31P NMR of 5a/b at two field strengths, 1H and 31P NMR and MS data for product from reaction of phosphonoacetate carbanion with trifyl azide. Available free of charge via the Internet at http://pubs.acs.org.
References
- 1.McKenna CE, Shen P-D. J. Org. Chem. 1981;46:4573. [Google Scholar]
- 2.Blackburn GM, Kent DE, Kolkmann F. J. Chem. Soc. Chem. Commun. 1981:1188. [Google Scholar]
- 3.Batra VK, Pedersen LC, Beard WA, Wilson SH, Kashemirov BA, Upton TG, Goodman MF, McKenna CE. J. Am. Chem. Soc. 2010;132:7617. doi: 10.1021/ja909370k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sucato CA, Upton TG, Kashemirov BA, Osuna J, Oertell K, Beard WA, Wilson SH, Florian J, Warshel A, McKenna CE, Goodman MF. Biochemistry. 2008;47:870. doi: 10.1021/bi7014162. [DOI] [PubMed] [Google Scholar]
- 5.Upton TG, Kashemirov BA, McKenna CE, Goodman MF, Prakash GKS, Kultyshev R, Batra VK, Shock DD, Pedersen LC, Beard WA, Wilson SH. Org. Lett. 2009;11:1883. doi: 10.1021/ol701755k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenna CE, Kashemirov BA, Upton TG, Batra VK, Goodman MF, Pedersen LC, Beard WA, Wilson SH. J. Am. Chem. Soc. 2007;129:15412. doi: 10.1021/ja072127v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis GP, Luscombe DK. Progress in Medicinal Chemistry. Vol. 31. Amsterdam: Elsevier; 1994. p. 125. [Google Scholar]
- 8.Evans DA, Britton TC, Ellman JA, Dorow RL. J. Am. Chem. Soc. 1990;112:4011. [Google Scholar]
- 9.Benati L, Nanni D, Spagnolo P. J. Org. Chem. 1999;64:5132. doi: 10.1021/jo9901541. [DOI] [PubMed] [Google Scholar]
- 10.Wurz RP, Lin W, Charette AB. Tetrahedron Lett. 2003;44:8845. [Google Scholar]
- 11.Jaszay ZM, Pham TS, Gonczi K, Petnehazy I, Toke L. Synth. Commun. 2010;40:1574. [Google Scholar]
- 12.Mintz MJ, Walling C. Org. Synth. 1969;49:9. [Google Scholar]
- 13.McKenna CE, Kashemirov BA, Li Z-M. Phosphorus Sulfur Silicon Relat. Elem. 1999;144–146:313. [Google Scholar]
- 14.Delacotte JM, Galons H. J. Chem. Res. Synopses. 1991:64. [Google Scholar]
- 15.Shi E, Xiao J, Pei C. Phosphorus Sulfur Silicon Relat. Elem. 2005;180:2155. doi: 10.1080/10426500590907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manis PA, Rathke MW. J. Org. Chem. 1980;45:4952. [Google Scholar]
- 17.Jarvis BB, Nicholas PE. J. Org. Chem. 1980;45:2265. [Google Scholar]
- 18.Chiba S, Zhang L, Ang GY, Hui BW-Q. Org. Lett. 2010;12:2052. doi: 10.1021/ol100522z. [DOI] [PubMed] [Google Scholar]
- 19.Ciez D. Org. Lett. 2009;11:4282. doi: 10.1021/ol901637w. [DOI] [PubMed] [Google Scholar]
- 20.McKenna CE, Khawli LA, Ahmad WY, Pham P, Bongartz JP. Phosphorus Sulfur Relat. Elem. 1988;37:1. [Google Scholar]
- 21.Hutchinson DW, Semple G. J. Organomet. Chem. 1985;291:145. [Google Scholar]
- 22.(a) McKenna CE, Higa MT, Cheung NH, McKenna MC. Tetrahedron Lett. 1977:155. [Google Scholar]; (b) McKenna CE, Schmidhauser J. J. Chem. Soc., Chem. Commun. 1979:739. [Google Scholar]
- 23.McKenna CE, Levy JN. J. Chem. Soc. Chem. Commun. 1989:246. [Google Scholar]
- 24.Khare AB, McKenna CE. Synthesis. 1991:405. [Google Scholar]
- 25.Hakimelahi GH, Just G. Synth. Commun. 1980;10:429. [Google Scholar]
- 26.Gajda A, Gajda T. Curr. Org. Chem. 2007;11:1652. [Google Scholar]
- 27.Sobhani S, Tashrifi Z. Tetrahedron. 2010;66:1429. [Google Scholar]
- 28.The IR band at 2100 cm−1 for the putative azido product25 is within the range of literature values30, 31 for triethyl diazophosphonoacetate including a sample prepared by a methodology that could not generate an azido product.32 The 1H NMR data do not positively identify a PC(HX)C hydrogen. The α-proton is distinguishable in analogous H-F,33 H-Cl, and H-Br34 triethyl phosphonoacetates and should be apparent in authentic trialkyl azidophosphonoacetate. The C.I. MS data in the original report identifies a m/z 223 peak which was assigned to a [M−N3]+ species. However, this peak also corresponds to a prominent fragment which we observe in the MS/MS of the triethyl diazophosphonoacetate parent peake [M+1]+ at m/z 251. Finally, the authors supported their structure by reducing their product to the aminophosphonoacetate with H2 Pd/C in EtOH. t-Butyl diazo(diethoxyphosphoryl)acetate is converted to a cognate PC(HNH2)C product under these conditions,35 demonstrating that the reduction would not necessarily distinguish the azido compound from triethyl diazophosphonoacetate.
- 29.Moffatt JG, Khorana HG. J. Am. Chem. Soc. 1961;83:649. [Google Scholar]
- 30.Takano S, Tomita S, Takahashi M, Ogasawara K. Synth. Commun. 1987:116. [Google Scholar]
- 31.Regitz M, Anshütz, Liedhegener A. Chem. Ber. 1968;101:3734. [Google Scholar]
- 32.Khokhlov PS, Kashemirov BA, Mikityuk AD, Strepikheev YA, Chimishkyan AL. Zh. Obshch. Khim. 1984;54:2785. [Google Scholar]
- 33.Marma MS, Khawli LA, Harutunian V, Kashemirov BA, McKenna CE. J. Fluor. Chem. 2005;126:1467. [Google Scholar]
- 34.McKenna CE, Khawli LA. J. Org. Chem. 1986;51:5467. [Google Scholar]
- 35.Shiraki C, Saito H, Takahashi K, Urakawa C, Hirata T. Synthesis. 1988:399. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.