Abstract
Infection with HIV-1 frequently results in the loss of specific cellular immune responses and an associated lack of antibodies. Recombinant growth hormone (rGH) administration reconstitutes thymic tissue and boosts the levels of peripheral T cells, so rGH therapy may be an effective adjuvant through promoting the recovery of lost cellular and T-cell-dependent humoral immune responses in immunosuppressed individuals. To test this concept, we administered rGH to a clinically defined group of HIV-1-infected subjects with defective cellular and serological immune responses to at least one of three commonly employed vaccines (hepatitis A, hepatitis B or tetanus toxoid). Of the original 278 HIV-1-infected patients entering the trial, only 20 conformed to these immunological criteria and were randomized into three groups: Group A (n = 8) receiving rGH and challenged with the same vaccine to which they were unresponsive and Groups B (n = 5) and C (n = 7) who received either rGH or vaccination alone, respectively. Of the eight subjects in Group A, five recovered CD4 cellular responses to vaccine antigen and four of these produced the corresponding antibodies. In the controls, three of the five in group B recovered cellular responses with two producing antibodies, whereas three of the seven in Group C recovered CD4 responses, with only two producing antibodies. Significantly, whereas seven of ten patients receiving rGH treatment in Group A (six patients) and B (one patient) recovered T-cell responses to HIVp24, only two of six in Group C responded similarly. In conclusion, reconstitution of the thymus in immunosuppressed adults through rGH hormone treatment restored both specific antibody and CD4 T-cell responses.
Keywords: adjuvant, cellular responses, growth hormone, HIV, humoral responses, thymus
Introduction
Infection with HIV-1 frequently results in the loss of CD4 cellular and humoral specific immune responses to HIV, to other pathogens and to vaccines in general.1–3 Highly active antiretroviral therapy (HAART) typically results in virus clearance and an apparent recovery of the patients’ peripheral T-cell populations. Normal levels of peripheral T cells in such patients, however, do not necessarily reflect a fully functional immune system.4 A restricted T-cell repertoire is also a feature of adults with severe immunosuppression following chemotherapy for bone marrow transplantation,3 whereas children with a similar clinical history exhibit an essentially normal T-cell repertoire.1 As CD4 T cells are produced in the thymus, several strategies have been investigated for reconstituting thymic function; these includes the administration of recombinant human pituitary growth hormone (rGH) or somatotrophin,5,6 to both children and adults with growth hormone deficiency. More recently, the administration of rGH to HIV-1-infected subjects with lipodystrophy or persistently low CD4 T-cell counts (enrolled in the AIDS Clinical Trials Group phase 2 clinical trial) was reported to increase the thymic volume, the number of recent thymic emigrants, expand the CD4 T-cell pool, and increase the number of HIV-specific CD4 T cells.7–11 Finally, rGH therapy was also shown to stimulate the generation of bone marrow haematopoietic cells, including T-cell and B-cell precursors.12
Our primary aim was to determine whether growth hormone-mediated thymic restoration in adulthood can restore antigen-specific cellular and humoral responses to severly immunodepressed HIV-1 patients in vivo. As a proof of concept, therefore, we designed a clinical trial with three important differences from previous studies.5–9 First, and most importantly, the inclusion criteria for the HIV-positive patients were more stringent than in previous studies; specifically, they had to be seronegative for at least one of three commonly employed vaccines (hepatitis A, hepatitis B or tetanus toxoid), and to remain seronegative when revaccinated with the same vaccine(s) to which they were unresponsive. An additional requirement was that their peripheral CD4 cells failed to proliferate when cultured with the same challenge vaccines. Second, and as a strategy to amplify the number of newly formed specific T cells, the patients received 2 months of rGH treatment before their revaccination (with hepatitis A, hepatitis B or tetanus toxoid). Finally, and in addition to determining the recovery of serological responses to the vaccine, as in previous studies, we also investigated the impact of the rGH therapy on CD4 and CD8 T-cell responses to the vaccines and to HIV-1 gag and env antigens. Our results confirm and extend the potential utility of growth hormone therapy for the restoration of immune competence to severely immunodepressed HIV-1-positive patients and also, perhaps, to other clinical conditions involving an impaired immune system.
Materials and methods
The VIHCREC01 study was a pilot, investigator-initiated, randomized, parallel assignment clinical, open label multi-center study conducted in two Spanish hospitals, the Hospital Germans Trias i Pujol in Badalona and the Hospital Clinic in Barcelona. The design of this study is outlined in Fig. 1.
Figure 1.
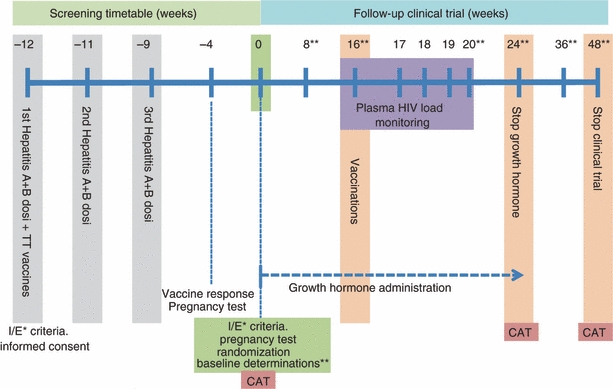
Study design. I/E* inclusion/exclusion crtieria; ** haematological, biochemical, virological and immunological assays and clinical examiniation; CAT, computed axial tomography of the thyroid.
We routinely tested patients attending the HIV-1 clinic for their serological status for hepatitis A, hepatitis B and tetanus toxoid. Patients who were negative for at least one of these antigens were selected for vaccination with these recall antigens; only those who remained antibody-negative were eligible for the study. Patients who complied with the following criteria were invited to participate in the intervention study after written informed consent had been obtained: HIV-1-positive patients, ≥ 18 years of age, undergoing HAART for at least 1 year before the beginning of the trial, Karnofsky performance status scale ≥ 80%, an undetectable HIV-1 plasma load (< 50 copies/ml) and > 50 CD4+ cells/mm3, alkaline phosphatase and alanine aminotransferase serum values no more than five times the normal superior limit, bilirubin in serum no more than twice the normal superior limit, granulocyte counts ≥ 1000 cells/mm3, haemoglobin ≥ 9·5 g/dl and platelets ≥ 75 000 per mm3. Exclusion criteria were HIV-1-wasting or current AIDS-defining diseases, serious chronic diseases (e.g. cancer, diabetes mellitus), allergy or hyper-reactivity to rGH or vaccines, drug or alcohol abuse, therapy with systemic glucocorticoids, and pregnancy. This study was approved by the Ethics Committee of Clinical Investigation of the Hospital Germans Trias i Pujol and the Hospital Clinic of Barcelona, and the Spanish Medicines Agency. The trial was registered at http://www.clinicaltrials.gov (ID protocol VIHCREC01, NCT 00287677).
All visits by HIV-1-positive patients took place at the Hospital Germans Trias i Pujol of Barcelona and the Hospital Clinic of Barcelona, were performed in parallel, and followed the same sample collection data sheet and case report form protocol. Healthy control volunteers were visited and followed up at the Hospital Germans Trias i Pujol; they partook in baseline investigations and participated in the standardized vaccination protocol but did not receive rGH intervention.
Eligible HIV-1-infected patients were randomized into three different groups: Group A, who received rGH for 6 months and were vaccinated with hepatitis A + B (Twinrix Adultos®; Glaxo SmithKline, Tres Cantos, Spain) and tetanus toxoid (Ditanrix Adulto®; SmithKline Beecham, Tres Cantos, Spain) at week 16; Group B, who received rGH but no vaccines; and Group C (HIV-1 control group), who received vaccines at week 16 but no rGH. The study medication was kindly provided by Pfizer Company, Barcelona, Spain (GENOTONORM® Kapiben 12 mg) via the hospital pharmacy. The rGH was injected intramuscularly into the quadriceps three times a week, preferentially in the morning, for 6 months. Seven healthy control volunteers participated in the baseline investigations and in the vaccination protocol as the vaccination control group (Fig. 1).
Growth hormone dosage
Although the dose of growth hormone recommended in the trial was 3 mg three times a week. Recombinant growth hormone induces secretion of the insulin-like growth factor-1 (IGF-1) which is closely associated with the levels of rGH and regulates the thymic homing of T-cell precursors5 but is also responsible for adverse effects such as carpal tunnel syndrome, acromegaly and myalgia. For this reason, we carefully monitored the levels of IGF-1 monthly during the trial and, as necessary, we reduced the doses of growth hormone until the levels of IGF-1 were within the normal range (< 500 U/ml).
Chest computed tomography
Chest computed tomography (CT) was performed in the Radiology Department of the Hospital Clinic and images were used to identify thymus tissue, as previously described.13
Measurement of T-cell receptor excision circles and proviral DNA
Peripheral blood mononuclear cells (PBMC) were stored frozen as pellets at −80° until DNA extraction. Genomic DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, Spain) according to the manufacturer's instructions. The number of copies of T-cell receptor excision circles (TREC), proviral DNA and copies of CCR5 were measured using real-time quantitative PCR performed in a spectrofluorometric thermal cycler (ABI PRISM 7000; Applied Biosystems, Foster City, CA) as previously described.14,15 The number of TREC and proviral DNA was related to the number of CCR5 copies in the same DNA samples. Two copies of CCR5 were present in each cell.
Immunophenotyping
Subpopulations of T cells were detected in fresh whole blood by a direct immunofluorescence antibody staining method.16 In summary, EDTA-treated peripheral blood was labelled with different combinations of antibodies against CD3, CD19, CD14 and CD56 for the detection of T and B cells, monocytes and natural killer cells; CD4, CD45RA and CD31 for the detection of recent thymic emigrants; CD62 ligand (CD62L), CD45RA and CD45RO for naive and memory populations; CXCR5 and CD25 for the identification of helper follicular cells and T regulatory cells; CXCR4 and CCR5 for the HIV-1 receptors; and CD8, CD45RO, CD38 and DR (Becton Dickinson Biosciences, Oxford, UK) for the levels of activated and terminal cells. The stained blood samples were depleted of red blood cells by treatment with Facs Lysing buffer (Becton Dickinson) and analysed with a FACscalibur cytometer (Becton Dickinson).
Human interferon-γ and interleukin-2 ELISPOTs
Human interferon-γ (IFN-γ) and interleukin-2 (IL-2) ELISPOTS were detected as previously described.17 Briefly, freshly isolated PBMC were either stimulated non-specifically with phytohaemagglutinin (5 μg/ml) (Sigma, Barcelona, Spain), or specifically with HIV-1 p24 (2·5 μg/ml) and gp160 antigens (5 μg/ml; Protein Science, Meriden, CT), cytomegalovirus virion (10 μg/ml; Serion, Walkersville, MD), recombinant hepatitis A virus proteins (2 μg/ml, Biodesign, Wurzburg, Alemania), serum antigen for hepatitis B virus (5 μg/ml; Prospec, Rehovot, Israel) or tetanus toxoid (10 μg/ml; Sigma). Cultures were prepared in 96 PVDF-bottomed-well plates coated with anti-IFN-γ or anti-IL-2 (Diaclone, Besancon, France), and incubated for 48 hr. The IFN-γ and IL-2 ELISPOTs were detected by a biotinylated anti-IFN-γ or IL-2 antibody followed by a streptavidin–alkaline phosphatase conjugate (Amersham Biosciences, Uppsala, Sweden), and development with a solution of 4-nitro-blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate, as recommended by the manufacturer (Sigma). A positive response was defined by an assay meeting the following criteria: strong response in the phytohaemagglutinin-stimulated positive control wells and the detection of at least 50 spot-forming units/106 PBMC after subtraction of the values obtained for the non-stimulated cells.17,18
Detection of HIV-1-specific CD8+ T-cell responses
An epitope-specific ELISPOT assay was used to measure antigen-induced IFN-γ release from CD8 T cells. All analyses were performed on cryopreserved PBMC. A mean of 16 (range 3–27) different HLA class I-restricted synthetic peptides from Gag, Pol, Env and Nef proteins were tested on cells from each individual. Different pools of overlapping HIV-1-1 Gag 15-mer peptides derived from the sequence of strain HXB2 were also tested in parallel. Ninety-six-well microtitre plates (Millipore, Barcelona, Spain) were coated overnight with a monoclonal antibody specific for human IFN-γ (monoclonal antibody 1-D1K; Mabtech, Stockholm, Sweden). The PBMC resuspended in RPMI-1640 plus 10% fetal calf serum were plated in the presence of different peptides at 4 μm and incubated overnight at 37° in 5% CO2. The plates were developed using biotinylated anti-human IFN-γ, the streptavidin–alkaline phosphatase conjugate, and a chromogenic substrate (BioRad, Barcelona Spain). Spot-forming cells were counted using an AID ELISPOT reader (Autoimmune Diagnostica GmHb, Germany). After subtracting the background counts obtained for the control PBMC cultured in medium alone, the results were normalized to spot-forming PBMC. A positive response was considered when the counts were greater than 40 spot-forming cells/106.18
Lymphoproliferation assay
The PBMC were resuspended at 2 × 106/ml and cultured in the presence or absence of pokeweed mitogen (10 μg/ml; Sigma), recombinant HIV-1 proteins gp160 and p24 (5 μg/ml; Protein Science), phytohaemagglutinin (5 μg/ml; Sigma), HIV-1 p24 or gp160 antigens (2·5 and 5 μg/ml, respectively; Protein Science), a live cytomegalovirus strain (1 : 800 v/v) (ref-AD-169 grown in human embryonic fibroblasts; Bio-Whittaker, Walkersville, MD), recombinant hepatitis A virus proteins (2 μg/ml; Biodesign), (10 μg/ml; Virion Serion, Würzburg, Germany), serum antigen for hepatitis B virus (5 μg/ml; Prospec), or tetanus toxoid (10 μg/ml; Sigma). Tritium-labelled thymidine (GE Healthcare, Barcelona, Spain) was added during the last 18 hr of the 7-day culture. After incubation, the cells were harvested and the incorporation of thymidine was evaluated using a betaplate reader (LKB, Wallac, Finland). The results were expressed as mean counts per minute (c.p.m.). The stimulation index was calculated for each sample as: c.p.m. for cells minus the c.p.m. of the spontaneous proliferation in the absence of any stimulus.19
Levels of IL-7 in plasma
The levels of IL-7 in plasma were measured using a commercial ELISA according to the manufacturer's instructions (Diaclone).
Neutralization assay
Pseudo viruses were generated by co-transfecting 293T cells with envelope (NL4-3 or AC10) expression plasmids and a Δenv HIV-1-1 backbone vector (pSG3ΔEnv); culture supernatants were harvested 24 hr after transfection, filtered (0·45 μm), and stored at −80°. The median tissue culture infective dose (TCID50) of the pseudo viruses was determined in TZM-bl cells, as described previously.20 Briefly, serial fivefold dilutions of pseudo virus were made in quadruplicate wells in a 96-well plate; 10 000 TZM-bl cells (containing 37·5 μg DEAE-dextran/ml) were added to each well. After 48 hr, the cultures were analysed using a luminometer (Labsystems, Waltham, MA) with the Britelite Reagent (Britelite Luminescence Reporter Gene Assay System; Perkin Elmer Life Sciences, Madrid, Spain), following the manufacturer's instructions.
Neutralizing antibodies against HIV-1, simian immunodeficiency virus and simian human immunodeficient virus type 1 were assessed in luciferase reporter gene assays.21 Specifically, neutralizing antibodies were measured as reductions in luciferase reporter gene expression after a single round of infection in TZM-bl cells as described previously20. Briefly, 200 TCID50 of pseudovirus was pre-incubated in 96-well plates with various dilutions of heat-inactivated sera (starting dilution 1/50, fivefold stepwise) in duplicate for 1 hr at 37°, 5% CO2. The TZM-bl cells were added at 10 000 cells/well, containing 37·5 μg DEAE-dextran/ml. Two days after infection, the cultures were analysed using luminometry (Labsystems, Buenos Aires, Argentina) using the Britelite Reagent (Britelite Luminescence Reporter Gene Assay System; Perkin Elmer Life Sciences), following the manufacturer's instructions. Then, neutralizing antibody titres (IC50), defined as the serum dilution required to reduce virus control Relative Light Units by 50%, were calculated (GraphPad Prism; GraphPad Software, La Jolla, CA).
Serology
The detection of antibodies against hepatitis A, hepatitis B and tetanus toxoid was performed in the routine assay by the Department of Microbiology at the Hospital Clinic, Barcelona, Spain.
Statistical analysis
The data were analysed using the graphpad statistical package. If the data were not normally distributed or there were low numbers of samples, they were expressed as a median and range. Data which were normally distributed were expressed as a mean and standard error of the mean. The Mann–Whitney U-test was used to compare groups. Spearman's correlation test was used to check the relationship between two sets of data. All tests of significance were two tailed. We used contingency tables to compare groups with categorical variables. Contingency tables were validated using Fisher's exact test.
Results
Patients
The patients attending our clinics were routinely tested for hepatitis A, hepatitis B and tetanus toxoid. As shown in Fig. 2 (patients chart), 278 patients tested negative for at least one of these antigens. Sixty-three patients volunteered to be revaccinated with hepatitis A, hepatitis B and tetanus toxoid, and 28 remained seronegative and agreed to participate in the trial. From these, six patients were excluded (Fig. 2), and 22 were randomized in the three groups: eight patients were treated with rGH and revaccinated with the three environmental antigens (Group A), six subjects received rGH only (Group B) and eight subjects were only vaccinated (Group C). One patient from Group B and one from Group C withdrew from the trial at 24 and 36 weeks, respectively. Finally, 20 patients completed the trial (eight, five and seven from Groups A, B and C, respectively) in whom all the analyses were performed.
Figure 2.
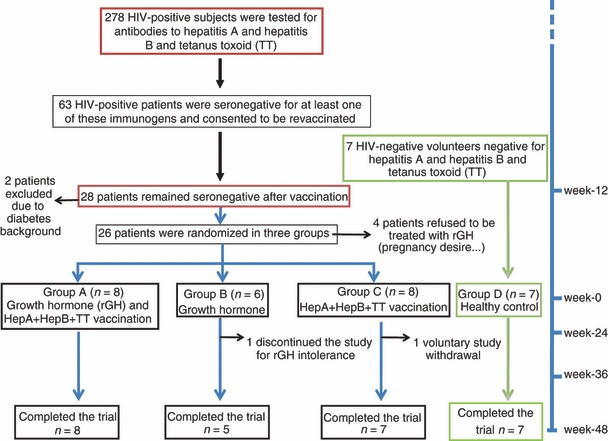
Patients chart flow.
As a positive control group (Group D), seven HIV-1-negative volunteers who were also seronegative for hepatitis A, hepatitis B and tetanus toxoid were vaccinated with these three antigens; all became seropositive with high titres of antibodies. The clinical characteristics of the patients are shown in Table 1.
Table 1.
Baseline characteristics of patients enrolled in the trial according to the experimental groups
| Characteristics | Group A | Group B | Group C |
|---|---|---|---|
| Intervention | rGH + vaccination | rGH | Vaccination |
| HIV status | Positive | Positive | Positive |
| Age (years) | 43 ± 2·3 | 37±4·2 | 41±3·6 |
| CD4 nadir1 (cells/mm3) | 175 ± 57·8 | 159 ± 72·0 | 226 ± 45·6 |
| HIV plasma load (copies/ml) | < 50 | < 50 | < 50 |
| Interleukin-7 (ng/ml) | 22 ± 4·57 | 17 ± 7·22 | 47 ± 4·3 |
| IGF-1 (U/ml) | 247± 41 | 219 ± 56 | 204 ± 36 |
| CD4 (cells/mm3) | 414 ± 64·18 | 528 ± 122·4 | 609 ± 115·6 |
| CD8 (cells/mm3) | 729 ± 105·3 | 845 ± 89·25 | 886 ± 134·6 |
| Neutralizing antibodies (IC50) | 294 ± 111 | 498 ± 104 | 1110 ± 452 |
CD4 nadir, the lowest CD4 cell value reached throughout patient's lifetime.
IGF-1, insulin-like growth factor-1; rGH, recombinant growth hormone.
The data are expressed as mean ± SE of mean.
Side-effects
During the trial, from the patients that received GH, five suffered from arthralgia, two from hand oedema, one from myalgia, one from peripheral neuropathy, one from carpal tunnel syndrome, one from asthenia and one from acute respiratory infections. In general, the adverse events were mild, but two patients had interrupted rGH therapy: one developed carpal tunnel syndrome and a second developed hand oedema. One patient required hospitalization because of acute respiratory infection.
Growth hormone doses
Nine patients required a dose reduction of rGH. Reasons for dose reduction were increased values of IGF-1 (five cases), arthralgia or myalgia (three cases), peripheral oedema (one case) and peripheral neuropathy (one case). Four patients needed at least two dose reductions to achieve either acceptable values of IGF-1 or an improvement of adverse events. In general, doses < 1 mg were well tolerated in all cases.
The administration of rGH increased thymic volume, levels of recent thymic emigrants, and absolute CD4 and CD8 T-cell counts
To assess whether the rGH had an effect on the volume of thymic tissue, computed axial tomography was performed at months 0, 6 and 12 after the initiation of the trial. Changes in volume, density and thymic index (TI) are reported in Fig. 3. From the 20 HIV-1-positive patients assessed, seven of the 12 who received rGH (five from Group A and two from Group B) showed a significant twofold increase in the volume of the thymus at the end of the 6 months of treatment, whereas no significant changes were observed in the untreated or control patients (Fig. 3a, Table 2). Six months later, although the average volume of the thymus had decreased in the rGH-treated subjects, their mean volume was still significantly larger than before the initiation of the trial (Fig. 3a). The capacity of rGH to restore the thymic volume was not affected by any of the following parameters: dosage of the hormone, age of the patients, CD4 nadir, CD4 at baseline, levels of IL-7, time between the CD4 nadir and the initiation of the trial, or the levels of IL-7 or IGF-1 (see supplementary material, Table S1). Similarly, there was no significant correlation between any of the above parameters and the thymic volume changes (see supplementary material, Table S1).
Figure 3.
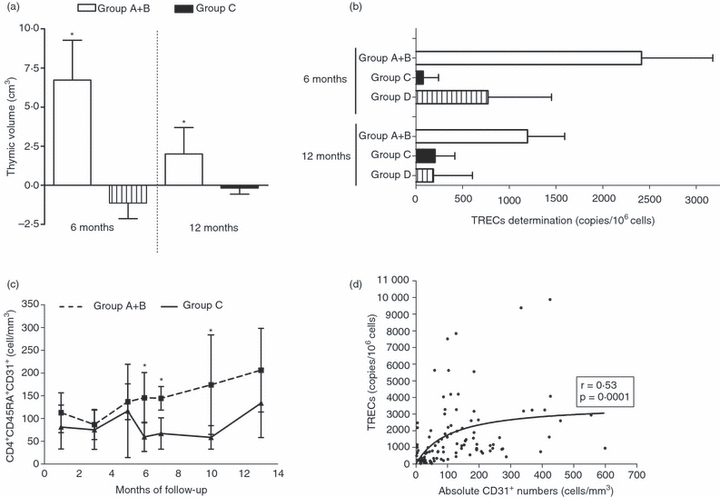
Effect of growth hormone therapy on the reconstitution of the thymus. Changes in thymic volume (cm3) at months 6 and 12 compared with baseline levels. Mean changes in the thymic volume of 12 HIV-1-infected patients who received recombinant growth hormone (Groups A + B) versus that of the seven control patients (Group C). The thymic tissue receded after GH treatment was stopped, but it remained larger than the pre-treatment size (a). Comparison of T-cell receptor excision cycles (TREC) in patients who received the recombinant growth hormone (Groups A + B; n = 12) compared with those who had only received vaccination (Group C; n = 7, and Group D; n = 7) at 6 and 12 months after the initiation of the trial. The results are expressed as copies of TRECs/106 cells (b).The absolute numbers of CD4+ CD45+ CD31+ recent thymic emigrants measured at baseline and during the next 12 months. Broken line: patients who received growth hormone. Continuous line: control group. There was a statistically significant difference between the two groups (P < 0·01) (c). A statistically significant correlation between the levels of TREC and the absolute numbers of CD4+ CD45RA+ CD31+ T cells (d).
Table 2.
Patients that reconstituted their cellular and humoral immune responses
| CD4 T-cell specific responses | ||||
|---|---|---|---|---|
| Patients (n) | Thymic volume increase | Recall antigens1 | HIV-p24 | Antibody production |
| Group A | ||||
| 1 | − | − | + | − |
| 2 | − | + | + | + |
| 3 | + | + | + | + |
| 4 | + | − | + | − |
| 5 | + | + | + | + |
| 6 | − | − | − | − |
| 7 | + | + | + | + |
| 8 | + | + | − | − |
| Group B | ||||
| 9 | + | + | + | + |
| 10 | + | + | NA | − |
| 11 | − | + | − | − |
| 12 | NA | − | NA | + |
| 13 | − | − | NA | − |
| Group C | ||||
| 14 | − | + | − | − |
| 15 | − | + | − | − |
| 16 | − | + | + | + |
| 17 | − | − | + | − |
| 18 | − | − | − | − |
| 19 | − | − | − | − |
| 20 | − | − | NA | + |
The recall antigens were hepatitis A, hepatitis B and tetanus toxoid.
NA, not available.
To assess whether additional thymic cells were migrated into the periphery, we measured changes in the levels of TREC to detect recent thymic emigrants (Fig. 3b) and changes in the absolute numbers of CD4+ CD45RA+ CD31+ T cells (Fig. 3c).14 Because of limited sample size, the number of TREC was assessed on whole PBMC. A strong correlation was detected between the level of TREC and the number of the CD4+ CD45RA+ CD31+ T cells (Fig. 3d). The TREC levels and CD4+ CD45RA+ CD31+ T cells were significantly increased in the periphery of patients who received rGH compared with those of hormonally untreated subjects [TREC 783 (119–1953) versus 128 (50–583) copies 106 cells (Fig. 3b); CD4+ CD45RA+ CD31+ T cells 136 (56–193) versus 45 (7–86) cells mm3 (P < 0·02), expressed as medians and the 25% quartile range]. Subgroup analysis revealed greater differences in thymic size between responders to rGH and those who did not respond [TREC 1051 (53–2205) versus 783 (387–1953); CD4+ CD45RA+ CD31+ T cells 154 (97–315) versus 25 (5–107) cells (Fig. 3c)].
Furthermore, the administration of rGH also increased the absolute numbers of CD4 T cells (Fig. 4a) and CD8 T cells (Fig. 4b); and this was more pronounced in the subjects showing an increase in the volume of thymic tissue. In contrast, the levels of CD4 and CD8 T cells from the patients who were only vaccinated (Group C) remained unchanged (Fig. 4a,b). The proportions of the different lymphoid subpopulations determined at months 2, 4, 5, 6, 9 and 12 remained stable in all groups during the trial. For example, no statistically significant changes were detected in the proportions of natural killer, B and T cells, the percentage of naive and memory cells, the levels of regulatory T cells or the T helper follicular cells (CXCR5+ CD57). Similarly, rGH treatment did not affect the percentage of cells expressing the HIV-1 co-receptors CXCR4 and CCR5, or the CD8 T-cell subsets, as measured by the expression of (CD8, CD28, CD45RA and CD45RO, CD57). Moreover, the rGH-treated patients showed no evidence of lymphocyte activation, as examined by the expression of CD38, class II, CD69, or CD62L in either of the two groups. Finally, the proportions of the above lymphocyte populations were similar in the rGH-treated patients regardless of the effect on thymic size.
Figure 4.
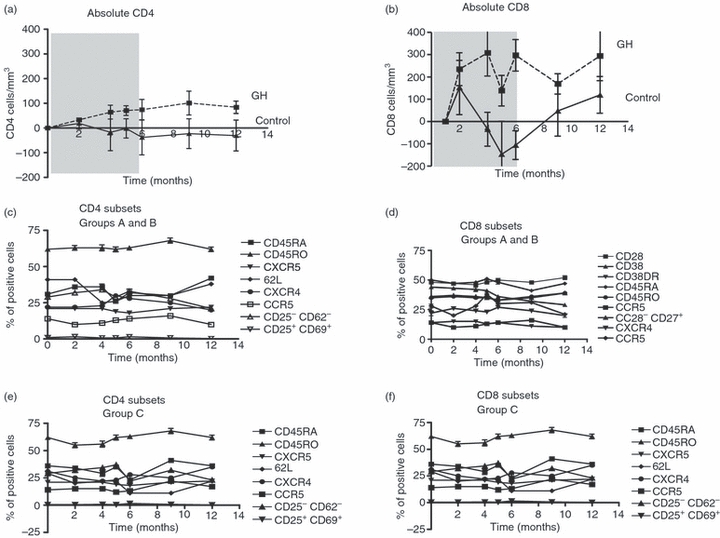
Effect of growth hormone treatment on the CD4 and CD8 subsets. The absolute numbers of CD4 (a) and CD8 (b) levels and the percentages of the CD4 and CD8 subsets were measured at months 0 (baseline), 2, 4, 5, 6, 9 and 12, as described in the Materials and methods. From the 20 HIV-1-positive individuals included in the trial, 13 were treated with recombinant growth hormone (rGH) for 6 months (indicated by grey shading). The mean and the standard error of the absolute CD4 (a) and CD8 (b) T-cell values from patients who received rGH (continuous line) (Groups A and B) and those who did not (discontinuous line, Group C). (c) to (f) show the percentages of CD4 and CD8 subpopulations from patients who received growth hormone (Group A and B; c, d) and those who did not (Group C; e, f).
The administration of rGH followed by vaccination induced the recovery of the CD4-specific responses to hepatitis A, hepatitis B, tetanus toxoid and HIV-1 p24 in subjects who were previously unresponsive
If the administration of rGH restores thymic function, then the predicted random generation of new T cells should result in an increase in CD4 T cells specific for hepatitis A, hepatitis B and tetanus toxoid. At baseline, our results showed that CD4 T cells from all the HIV-1-positive subjects failed to secrete IFN-γin vitro to any of the antigens tested by the ELISPOT assays. However, after rGH treatment, five out of eight Group A participants, three out of five Group B and three out of seven Group C subjects were positive for at least one of the hepatitis A, hepatitis B and tetanus toxoid antigens (Table 2).
When we investigated whether the reconstitution of the thymus increased the T-cell responses to HIV-1 p24 as measured by IFN-γ (ELISPOTs) any patient responded to HIV-1 p24 at baseline. However, after rGH treatment, six out of seven patients in Group A, one out of two in Group B and two out of six in Group C showed T cells producing IFN-γ to this HIV antigen. As expected, none of the HIV-1-negative patients responded to HIV-1 p24.
To check that the absence of CD4 responses was not the result of a general immune suppression, we measured responses to cytomegalovirus because the majority of HIV-1-infected patients are positive for this antigen. Seventeen out of 20 patients responded to cytomegalovirus before the initiation and at the end of the trial.
In contrast to published data,10 we did not see a statistically significant increase in the levels of proliferation of the mononuclear cells stimulated with hepatitis A, B or tetanus toxoid, as measured by the incorporation of titrated thymidine (see supplementary material, Table S2).
Restoration of the CD4 cellular responses by rGH therapy also restored serological responses to previously seronegative patients
As rGH treatment followed by vaccination induced CD4-specific responses, and CD4 T cells are necessary for the induction of humoral responses, we investigated whether the subjects who were previously seronegative to these antigens seroconverted after hormonal treatment. Significantly, four out of eight patients in Group A seroconverted to at least one antigen, whereas only two out of five of the subjects in Group B and two out of seven of Group C showed positive responses (Table 2). Interestingly, these patients who produced antibodies also recovered CD4-specific responses in vivo, consistent with the requirement of CD4 T-helper responses for the production of antibodies.
We then measured whether the rGH therapy and the expansion of CD4 T cells increased the titres of neutralizing antibodies to HIV-1. Twenty patients were tested before and after the trial and six of them showed antibody titres above 1/500 (see supplementary material, Table S2). Nevertheless, none of the 14 remaining patients had elevated their levels of neutralizing antibodies; the small changes detected were not statistically significant (P = 0·84; Groups A and B, 294 ± 111; 302 ± 141 at months 0 and 12, respectively; Group C, 498 ± 104 at month 0, 1110 ± 452 at month 12). As a negative control, we measured the level of neutralizing antibodies in the HIV-1 negative subjects and, as expected, none had detectable levels of antibodies. Furthermore, we could not detect any changes or an increase in the secretion of IFN-γ CD8 T cells in response to HIV-1 peptides (cytotoxic T-lymphocyte responses to HIV-1 peptides) (see supplementary material, Fig. S1).
The administration of GH did not increase proviral HIV-1 DNA and it did not increase the plasma viral load
Because HIV-1 can infect the cells of the thymus, it was essential to determine whether the stimulation of the thymus with growth hormone increased virally infected cells. To prevent viral replication and the infection and destruction of the newly formed cells, the patients were treated with HAART during the trial. Nevertheless, we feared that the arrival of ‘recent thymic emigrants’ to the periphery could subsequently lead to an increased plasma viral load. However, the viral load of all subjects remained undetectable throughout the study; the levels of proviral DNA did not change, except for a small non-significant peak of proviral viral load at month 2, at the time of vaccination (see supplementary material, Fig. S2b).
Discussion
One of the unresolved issues in immunology is the role of the thymus in the restoration and production of new antigen-specific cellular and humoral immune responses during adulthood, both in healthy and in diseased subjects. Although a functional thymus is present in normal healthy adults, after 60 years of age22 it is typically involuted. The thymus is sensitive to stress and is severely damaged in immunosuppressed individuals. Indeed, one of the challenges in producing a therapeutic vaccine for HIV-infected patients is their low CD4 T-cell numbers and related lack of CD4 responses to vaccines and to viral and bacterial pathogens.23 Because T-cell responses are crucial for antibody production, the exhaustion of these in HIV-1-positive patients seriously reduces the potential for efficient humoral immunity, not only for an HIV therapeutic vaccine, but also for other vaccines.
Several groups have shown that the administration of rGH to HIV-1-infected individuals induces an increase in thymic tissue with a concomitant increase in absolute CD4 T-cell counts, levels of TREC, recent thymic emigrants, and increased proliferative responses to HIV-1 proteins, the latter observation being consistent with previous reports.5,9,11,24,25 However, it is still controversial whether these T cells are indeed fully functional, and whether they are generated in the thymus or in the periphery. Therefore, we designed a clinical trial with highly restricted inclusion criteria to confirm and extend our understanding of the role of the thymus in the restoration of cellular and humoral responses in vivo.
Importantly, and in contrast to previous trials,11,24,25 our patients were HIV-1-infected individuals who were demonstrably immunosuppressed, having failed to make serological and CD4 cell immune responses to vaccination with at least one of the three selected antigens (hepatitis A, hepatitis B and tetanus toxoid). As a result of our stringent selection, only 28 of the original study group of 278 patients examined conformed to these criteria, possibly because the majority had been treated with anti-retrovirals for a long period and had never shown a large decrease in CD4 T cells. Finally, 20 subjects completed the trial; so although our trial is low in the number of subjects, its virtue is the clinical homogeneity of the groups studied. These experiences in selecting a clinically homogeneous group of subjects emphasize the diverse clinical characteristics of HIV-infected patients, a reality that considerably complicates the design of any experimental clinical intervention in the disease.
Having selected a group of patients with manifest immunoincompetence, both serological and cellular, our objective was to subject them to rGH therapy as a means to restore immune competence.
Because the T-cell repertoire is generated in the thymus by random mutation we feared that the number of newly formed cells with a defined specificity might be too low to be detected initially. Hence, a group of patients first received hormone and then were vaccinated to expand the newly formed cells (Group A). The administration of rGH induced a twofold increase in thymic tissue in seven of 13 subjects and five of these patients generated CD4-specific responses from whom four seroconverted for at least one of the three tested antigens, interestingly these four subjects belonged to Group A. Although we cannot exclude the possibility that the recovered responses resulted from the expansion of residual peripheral clones8,26,27 (two of seven subjects that were not treated also seroconverted), the observed increase in thymic tissue and the increase in absolute numbers after rGH administration strongly suggests that this treatment must have contributed to the recovery of the immune system, a critical requirement for the development of therapeutic vaccines for HIV-1. Specifically, the administration of rGH induced a higher increase in the absolute numbers of recent thymic emigrants (CD4+ CD45RA+ CD31+ T cells)14,27 compared with subjects who were only vaccinated or who were in the HIV-1-negative group. Although the size of the thymus slowly decreased after cessation of rGH treatment, even 6 months after treatment, it was still larger than the pre-treatment size. More significantly, responses to the challenge vaccine (hepatitis A, hepatitis B or tetanus toxoid) were still detectable at 9 and 12 months, suggesting that the restoration of immunocompetence might be long-lived and independent of rGH for its maintenance.
Significantly, the administration of rGH also increased the CD4 cellular responses to the HIV-1 antigen p24, which were absent before the trial in all subjects. The appearance of T cells recognizing HIV-1 p24, even though these subjects did not have detectable viral loads during the study, also suggests that the recovery of the thymic volume was also accompanied by the generation and emigration of new T cells. Residual viraemia may contribute to these new responses. Once again, the presence or recovery of CD4 cellular responses was correlated with production of antibodies. The increased numbers of CD4 and CD8 T cells associated with rGH-treated patients was also reflected by a proportionally similar increase in minor T-cell subpopulations. Hence, the relative proportions of follicular helper cells (CD4, CD45RO, CXCR5), regulatory T cells (CD4, CD25+)28 and Effector memory RA + T cells (T [EMRA cells])29 were maintained at normal levels during the trial. Similarly, the relative proportions of cells expressing the receptors CXCR4 and CCR5 were unchanged during the trial. In contrast to previous work suggesting that rGH induced an increase in natural killer cells30 and activation markers,31 in our study, the administration of rGH did not activate T cells, as assessed by the levels of DR, CD38 and CD69.32
The exact mechanisms of action of the rGH are not known. The hormone triggers a dose-dependent production of IGF-1, which may play a direct role in the thymic reconstitution.5–9 The lack of correlation between the increase in thymic volume and the IGF-1 levels that we observed is in agreement with other data showing that even much lower doses of rGH (0·7 mg/day) were still accompanied by an increase in IGF-1 levels.25 Furthermore, in our study, the increase in thymic tissue was not related to the rGH dose administered to the subjects. Similarly, there was no correlation between other factors that have been suggested to influence the recovery of the thymus in previous studies, including IL-7 levels, the CD4 nadir, numbers of CD4 cells or the age of the patients before the initiation of the trial. Finally, although the thymus can be infected with HIV-1, we did not detect differences in the levels of proviral HIV-1 or an elevated viral load, similar to previous studies,10 suggesting that the administration of HAART during the trial was sufficient to control viral load, the infection and destruction of newly formed T cells.
In conclusion, the main aim of our work was proof of concept to provide evidence that the restoration of the thymus in heavily immunosuppressed patients can reconstitute a fully functional and numerical recovery of the critically important CD4 T cells in a significant number of the treated individuals who responded to both vaccine and HIV antigens. Despite the relatively small sample size, we clearly demonstrated that the administration of rGH to HIV-infected patients before vaccination was associated with a significant recovery of cellular immunity. Hence, restoration of thymic function through rGH might improve the efficacy of therapies and other vaccines, not only in HIV-infected patients but also in other examples of immunosuppression, such as, bone marrow or solid organ transplantation where the loss of cellular immunity leads to infections and premature death.33
Acknowledgments
This project has been partly supported by the grants FIS PI 04/0503, FIS PI 07/0291 (M.P.) L.R. FIS (1081308) M.B.: FIS (PI051897), HIVACAT. M.P. is supported by the Instituto de Salud Carlos III and the Health Department of the Catalan Government (Generalitat de Catalunya). A.L. received a Research and Teaching Initiation Grant, University of Barcelona. This work was funded in part by the Fundació irsiCaixa and Red Temática Cooperativa de Investigación en SIDA (RD06/0006), FIS 1081308. The authors thank the patients who volunteered for this study, and the nursing staff who assisted at the site of the study. Growth hormone was kindly provided by Pfizer. We are also grateful to Red de Investigación en Sida (RIS): specifically, E. Jiménez, R. Peña, (Immunology: Badalona, Spain); L. Muñoz, M. J. Maleno, R. Escrig (Immunology: Barcelona, Spain); I. Bravo (Clinical: Badalona, Spain); R. Bellido, I. Erkizia (Virology: Badalona, Spain).
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Effect of growth hormone treatment on cytotoxic T-lymphocyte responses to HIV-1.
Figure S2. Correlation between the levels of insulin-like growth factor 1 (IGF-1) and CD4 counts.
Table S1. Characteristic of the patients who showed an increase in thymic tissue.
Table S2. Proliferation assays.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Sereti I, Anthony KB, Martinez-Wilson H, et al. IL-2-induced CD4+ T-cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood. 2004;104:775–80. doi: 10.1182/blood-2003-12-4355. [DOI] [PubMed] [Google Scholar]
- 2.Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. Aids. 2001;15:1749–56. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 3.Douek DC. The contribution of the thymus to immune reconstitution after hematopoietic stem-cell transplantation. Cytotherapy. 2002;4:425–6. doi: 10.1080/146532402320776035. [DOI] [PubMed] [Google Scholar]
- 4.Lederman HM, Williams PL, Wu JW, et al. Incomplete immune reconstitution after initiation of highly active antiretroviral therapy in human immunodeficiency virus-infected patients with severe CD4+ cell depletion. J Infect Dis. 2003;188:1794–803. doi: 10.1086/379900. [DOI] [PubMed] [Google Scholar]
- 5.Morrhaye G, Kermani H, Legros JJ, et al. Impact of growth hormone (GH) deficiency and GH replacement upon thymus function in adult patients. PLoS ONE. 2009;4:e5668. doi: 10.1371/journal.pone.0005668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berzins SP, Uldrich AP, Sutherland JS, Gill J, Miller JF, Godfrey DI, Boyd RL. Thymic regeneration: teaching an old immune system new tricks. Trends Mol Med. 2002;10:469–76. doi: 10.1016/s1471-4914(02)02415-2. [DOI] [PubMed] [Google Scholar]
- 7.Napolitano LA, Lo JC, Gotway MB, et al. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. Aids. 2002;16:1103–11. doi: 10.1097/00002030-200205240-00003. [DOI] [PubMed] [Google Scholar]
- 8.Al-Harthi L, Landay A. Immune recovery in HIV disease: role of the thymus and T cell expansion in immune reconstitution strategies. J Hematother Stem Cell Res. 2002;11:777–86. doi: 10.1089/152581602760404586. [DOI] [PubMed] [Google Scholar]
- 9.Pires A, Pido-Lopez J, Moyle G, Gazzard B, Gotch F, Imami N. Enhanced T-cell maturation, differentiation and function in HIV-1-infected individuals after growth hormone and highly active antiretroviral therapy. Antivir Ther. 2004;9:67–75. [PubMed] [Google Scholar]
- 10.Herasimtschuk AA, Westrop SJ, Moyle GJ, Downey JS, Imami N. Effects of recombinant human growth hormone on HIV-1-specific T-cell responses, thymic output and proviral DNA in patients on HAART: 48-week follow-up. J Immune Based Ther Vaccines. 2008;6:7. doi: 10.1186/1476-8518-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napolitano LA, Schmidt D, Gotway MB, et al. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest. 2008;118:1085–98. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French RA, Broussard SR, Meier WA, Minshall C, Arkins S, Zachary JF, Dantzer R, Kelley KW. Age-associated loss of bone marrow hematopoietic cells is reversed by GH and accompanies thymic reconstitution. Endocrinology. 2002;143:690–9. doi: 10.1210/endo.143.2.8612. [DOI] [PubMed] [Google Scholar]
- 13.Franco JM, Rubio A, Martinez-Moya M, Leal M, Merchante E, Sanchez-Quijano A, Lissen E. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002;99:3702–6. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Hernandez R, Jou A, Cabrera C, et al. Distribution of CD31 on CD4 T cells from cord blood, peripheral blood and tonsil at different stages of differentiation. Open Immunol J. 2010;3:19–26. [Google Scholar]
- 15.Massanella M, Puigdomenech I, Cabrera C, et al. Antigp41 antibodies fail to block early events of virological synapses but inhibit HIV spread between T cells. AIDS. 2009;23:183–8. doi: 10.1097/QAD.0b013e32831ef1a3. [DOI] [PubMed] [Google Scholar]
- 16.Bofill M, Lipman M, McLaughlin JE, Johnson MA, Poulter LW. Changes in lung lymphocyte populations reflect those seen in peripheral blood in HIV-1 positive individuals. Eur Respir J. 1998;11:548–53. [PubMed] [Google Scholar]
- 17.Darwich L, Cabrera C, Romeu J, et al. The magnitude of interferon-γ responses to human cytomegalovirus is predictive for HIV-1 disease progression. J Acquir Immune Defic Syndr. 2008;49:507–12. doi: 10.1097/QAI.0b013e318189a7af. [DOI] [PubMed] [Google Scholar]
- 18.De Rosa SC, Lu FX, Yu J, et al. Vaccination in humans generates broad T cell cytokine responses. J Immunol. 2004;173:5372–80. doi: 10.4049/jimmunol.173.9.5372. [DOI] [PubMed] [Google Scholar]
- 19.Plana M, Garcia F, Gallart T, Miro JM, Gatell JM. Lack of T-cell proliferative response to HIV-1 antigens after 1 year of highly active antiretroviral treatment in early HIV-1 disease. Immunology study group of Spanish EARTH-1 Study. Lancet. 1998;352:1194–5. doi: 10.1016/s0140-6736(05)60532-6. [DOI] [PubMed] [Google Scholar]
- 20.Montefiori DC, Metch B, McElrath MJ, Self S, Weinhold KJ, Corey L. Demographic factors that influence the neutralizing antibody response in recipients of recombinant HIV-1 gp120 vaccines. J Infect Dis. 2004;11:1962–9. doi: 10.1086/425518. [DOI] [PubMed] [Google Scholar]
- 21.Coligan JE. Commonly used detergents. Curr Protoc Protein Sci. 2001 doi: 10.1002/0471140864.psa01bs11. A1B1:A1B3 (online) [DOI] [PubMed] [Google Scholar]
- 22.Haynes BF, Hale LP, Weinhold KJ, et al. Analysis of the adult thymus in reconstitution of T lymphocytes in HIV-1 infection. J Clin Invest. 1999;103:453–60. doi: 10.1172/JCI5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shearer GM, Clerici M. Early T-helper cell defects in HIV infection. Aids. 1991;5:245–53. doi: 10.1097/00002030-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Lee JC, Boechat MI, Belzer M, et al. Thymic volume, T-cell populations, and parameters of thymopoiesis in adolescent and adult survivors of HIV infection acquired in infancy. AIDS. 2006;20:667–74. doi: 10.1097/01.aids.0000216366.46195.81. [DOI] [PubMed] [Google Scholar]
- 25.Hansen BR, Kolte L, Haugaard SB, et al. Improved thymic index, density and output in HIV-infected patients following low-dose growth hormone therapy: a placebo controlled study. AIDS. 2009;23:2123–31. doi: 10.1097/QAD.0b013e3283303307. [DOI] [PubMed] [Google Scholar]
- 26.Castro P, Plana M, Gonzalez R, et al. Influence of a vaccination schedule on viral load rebound and immune responses in successfully treated HIV-infected patients. AIDS Res Hum Retroviruses. 2009;25:1249–59. doi: 10.1089/aid.2009.0015. [DOI] [PubMed] [Google Scholar]
- 27.Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, Thiel A. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–94. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taams LS, Vukmanovic-Stejic M, Smith J, et al. Antigen-specific T cell suppression by human CD4+ CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Akondy RS, Miller JD, Doho G, et al. Molecular signature of human virus specific effector CD8+ T cells. J Immunol. 2009;183:7919–30. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodier MR, Imami N, Moyle G, Gazzard B, Gotch F. Loss of the CD56hi CD16– NK cell subset and NK cell interferon-γ production during antiretroviral therapy for HIV-1: partial recovery by human growth hormone. Clin Exp Immunol. 2003;134:470–6. doi: 10.1111/j.1365-2249.2003.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–9. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douek DC. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 2003;5:172–7. [PubMed] [Google Scholar]
- 33.Bofill M, Parkhouse RM. The increased CD38 expressed by lymphocytes infected with HIV-1 is a fully active NADase. Eur J Immunol. 1999;29:3583–7. doi: 10.1002/(SICI)1521-4141(199911)29:11<3583::AID-IMMU3583>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


