Abstract
The identification of developmental stages in natural killer (NK) cells, especially in human NK cells, has lagged for decades. We characterize four novel populations defined by CD11b and CD27, which can represent the distinct stages of human NK cells from different tissues. Nearly all NK cells from peripheral blood are CD11b+ CD27− populations whereas NK cells from cord blood have CD11b+ CD27− and CD11b+ CD27+ populations. Interestingly, we have found large CD11b− CD27− populations of NK cells from deciduas. We also demonstrate that each population could be characterized by unique functional and phenotypic attributes. CD11b− CD27− NK cells display an immature phenotype and potential for differentiation. CD11b− CD27+ and CD11b+ CD27+ NK cells show the best ability to secrete cytokines. CD11b+ CD27− NK cells exhibit high cytolytic function. We demonstrate that human NK cells at different developmental stages have special functions and describe a new model of human NK cell differentiation.
Keywords: cell development, cellular immunology, decidual natural killer cells, human natural killer cells, innate immunity
Introduction
Natural killer (NK) cells are large granular lymphocytes of the innate immune system that recognize and kill aberrant cells and rapidly produce soluble factors such as interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α). Similar to T and B cells, human NK cells are thought to be derived from CD34+ haematopoietic stem cells through discrete stages of maturation.1–4 Elegant research has provided evidence that the secondary lymphoid tissue is an important site for human NK-cell maturation.5 In contrast to our copious knowledge about T and B cells, we still have very limited knowledge about the development of human NK cells.
Natural killer cells have been considered a heterogeneous population and may be divided into different subsets.6 In humans, evidence has suggested that NK-cell differentiation can be characterized by analysing the surface expressions of CD34, CD117 and CD94 cells.5 Other studies show that NK cells in stage 3 (CD34− CD117+ CD94−) are capable of producing interleukin-22 (IL-22), suggesting the heterogeneous characteristic of this population.7–11 Given that human NK cells have been usually defined as CD56+ CD3− and the CD56 marker is only highly expressed during stage 4 (CD34− CD117− CD94+),5,12 the heterogeneity of NK cells in stage 4 has garnered additional attention. Many efforts have been made to identify the different subsets of CD56+ CD3− NK cells. Two subsets have been identified based on their levels of CD56 expression. CD56dim NK cells are found predominantly in the peripheral blood and have powerful cytotoxic function. CD56bright NK cells are found mostly in the lymphoid organs and have poor cytolytic function.13–15 NK cell terminal differentiation has been identified to be based on a decrease in NKG2A and an acquisition of Killer-cell immunoglobulin-like receptors (KIRs).16 However, there has been a lack of evidence for the discrete stages that represent human NK subsets not only in maturation stages but also in functional divisions; in addition, there has also been a lack of the way to build a cross-link between human and mouse that can compare data from these two species.
Recent studies have reported that CD27 of the TNF receptor family is an important marker dividing NK-cell subsets.17,18 The surface density of CD27 and CD11b divides murine NK cells into four subsets and represents their level of maturation.19,20 To investigate whether the same subsets exist in humans and to explain the heterogeneous characteristics of CD56+ CD3− human NK cells, we selected three types of human NK cells from peripheral blood, cord blood and decidua tissues. Here, we provide evidence that the expression of CD11b and CD27 may reflect the distinct populations of human NK cells. Nearly all the NK cells from peripheral blood are CD11b+ CD27− (CD11b+SP) population whereas NK cells from cord blood have CD11b+ CD27−(CD11b+SP) and CD11b+ CD27+ (DP) populations. However, consistent with their immature developmental stage, NK cells from the decidua have large CD11b− CD27− (DN) populations. We further demonstrate that these stages may also be associated with NK-cell effector functions. The DN NK cells displayed an immature phenotype and a potential for differentiation. CD11b− CD27+ (CD27+SP) and DP NK cells had the best ability to secrete cytokines. Furthermore, CD11b+SP NK cells showed high cytolytic function. Such a variety of characteristics demonstrates that human NK cells in different developmental stages have special functions. On the basis of these results, we developed and functionally characterized a new model for the development of human NK cells.
Materials and methods
Human samples and isolation
Peripheral blood mononuclear cells were obtained from the Blood Centre of Anhui Province (Hefei, China). They were from buffy coats obtained from healthy donors and prepared by centrifugation through Ficoll. Fourteen decidual samples in the term trimester were obtained from placenta collected after full-term gestation. Eleven cord blood samples were donated after gestation. Forty-one decidual samples of normal pregnancies during the first trimester were collected from elective pregnancy terminations. All three types of samples were acquired from Anhui Provincial Hospital. Ethical approval to use these samples was obtained from the Ethics Committee of the University of Science & Technology of China. Informed consent was obtained from each donor before surgery. Decidual lymphocytes were isolated by digesting the tissue with 1% collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 50 μg/ml DNAse I (Shanghai Sangon, Shanghai, China) and by purifying using density gradient centrifugation (Percoll; GE Healthcare, Uppsala, Sweden) as described.
Flow cytometry
Lymphocyte suspensions were prepared and stained with the following human monoclonal antibodies: anti-CD3, anti-CD56, anti-CD16, anti-CD27, anti-CD2, anti-CD34, anti-CD117, anti-CD94, anti-NKG2A, anti-NKG2D, anti-NKG2C, anti-CD11b, anti-CD7 and anti-CD11c, which were all mouse antibodies from BD Bioscience (San Jose, CA). Mouse serum was used to block non-specific Fc-receptor binding, and homologous IgGs were used as negative control antibodies. FACS staining was performed according to the manufacturer's instructions. Samples were run on a FACSCalibur flow cytometer, a fluorescence-activated cell sorter (BD Biosciences), and analysed by WinMDI (http://www.methods.info/software/flow/winmdi.html) and Flowjo softwares (Tree Star, Inc. Ashland, OR). For CD107a analysis, lymphocytes from peripheral blood mononuclear cells, cord blood mononuclear cells and decidual mononuclear cells of the first trimester were cultured with K562 at the ratio of 1 : 1 with monensin (10 μg/ml; Sigma, St. Louis, MO) and phycoerythrin-conjugated anti-CD107a (BD Bioscience) for 4 hr. Then, the cells were washed, blocked and stained with surface antibodies for ordinary FACS staining.
Intracellular cytokine staining for IFN-γ and TNF-α
Intracellular staining was performed on the decidual cells of the first trimester after 4 hr of stimulation with PMA (50 ng/ml; Sigma) and monensin (10 μg/ml; Sigma) in the presence of ionomycin (1 μg/ml; Calbiochem, Darmstadt, Germany). Cells were collected, washed and blocked using mouse serum. The fluorochrome-conjugated monoclonal antibodies to the cell surface markers were first added as described above; next, the cells were washed in PBS, fixed and resuspended in permeabilization buffer. FITC-anti-IFN-γ, phycoerythrin-anti-TNF-α or an isotype-matched control antibody was added, and the cells were incubated for 30 min at 4°. After incubation, the cells were washed, resuspended and analysed by FACSCalibur (BD Bioscience).
Cell sorting and culture
For cell culture, decidual lymphocytes in the term trimester after full-term gestation were isolated from deciduas and cultured with human IL-15 (10 ng/ml; Peptech, Burlington, MA) or IL-2 (100 U/ml; Peptech) in RPMI-1640 with 10% fetal calf serum. Half of the medium was replaced every 3 days to replenish the cytokines. Cells were collected after 7 or 14 days of culturing and analysed by flow cytometry. For the DN NK-cell subset culturing, decidual cells of the term trimester were stained with anti-CD3, anti-CD56, anti-CD11b and anti-CD27, then sorted as gated CD56+ CD3− CD11b− CD27− DN NK cells by FACS Aria (BD Bioscience).The purity of DN NK cells after sorting was ≥ 95%.
Statistical analyses
We used two-tailed paired Student's t-tests (difference between two groups) or two-tailed unpaired Student's t-tests to determine the statistical significance.
Results
CD11b and CD27 expression reflect the different developmental stages of human NK cells
To confirm the maturation of these human NK cells, we noted that the maturation of mouse NK cells had been successfully defined by CD27 and CD11b into four developmental stages.20 We determined whether these markers may also be used to reflect the maturation of NK cells in humans. Interestingly, approximately 20% of NK cells from decidual mononuclear cells (dNK cells) and NK cells from cord blood mononuclear cells (cNK cells) were CD27+ NK cells, whereas less than 5% of NK cells from peripheral blood mononuclear cells (pNK cells) were CD27+ NK cells. Furthermore, the majority of cNK and pNK cells were CD11b+, but among the dNK cells, < 30% of the CD3− CD56+ NK cells expressed CD11b. As in mouse NK cells, here we found that in human NK cells, CD11b and CD27 staining distinguished four subsets: CD11b− CD27− (DN), CD11b− CD27+ (CD27+ SP), CD11b+ CD27+ (DP) and CD11b+ CD27− (CD11b+ SP) (Fig. 1a). More than 95% of the pNK cells were in the CD11b+ SP subset, suggesting their mature characteristics (Fig. 1d). The majority of cNK cells were CD11b+ SP, and approximately 20% of them exhibited the DP phenotype (Fig. 1c). Less than 5% of the pNK and cNK cells were categorized in the DN or CD27+ SP subsets. However, among the dNK cells, approximately 60% were in the DN subset and 10% in the CD27+ SP subset, confirming their immature phenotype (Fig. 1b,c). We also compared dNK cells from the first trimester with those from the term trimester. There was no significant difference between these two kinds of dNK cells in the distribution of these four subsets. Both of them had a high percentage of DN NK cells. Hence, the four subsets defined by CD11b and CD27 reflected the developmental stages of human NK cells and it is the first time that such a large DN NK subpopulation has been found in human decidua samples.
Figure 1.
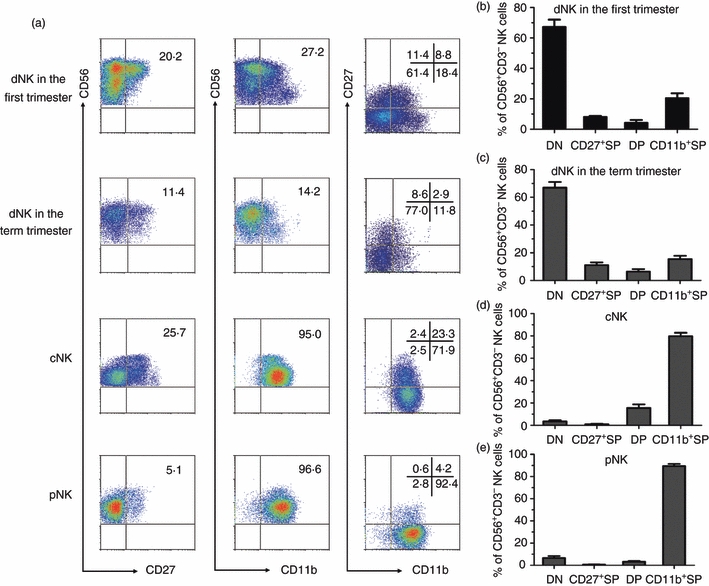
CD11b and CD27 reflect different developmental stages in natural killer cells from decidua (dNK), cord blood (cNK) and peripheral blood (pNK) mononuclear cells. (a) Representative flow cytometry analysis of the expression of CD27 and CD11b on gated CD56+ CD3− dNK of the first trimester, dNK of the term trimester, cNK and pNK. (b) The frequency of each subset in dNK cells of the first trimester (n = 10). (c) The frequency of each subset in dNK cells of the term trimester (n = 8). (d) The frequency of each subset in cNK cells (n = 9) (e). The frequency of each subset in pNK cells (n = 9).
Based on the expression of CD34, CD117 and CD94, four developmental stages of human NK cells have recently been described in secondary lymphoid tissue (SLT).5 Previous reports showed that CD117+ stage 3 cells were present in deciduas and peripheral blood.7 Here we confirmed that there were no stage 1 (CD34+ CD117− CD94−) and stage 2 (CD34+ CD117+ CD94−) cells in pNK, cNK and dNK cells but about 0·2–5·0% CD117+ NK cells (Fig. 2a). We also confirmed that all three types of NK cells had high CD94 expression and there was no significant difference in CD94 expression among the subsets (Fig. 2b). Hence, the three types of human CD56+ NK cell were mainly found in the most mature stage 4 (CD34− CD117− CD94+/−).
Figure 2.
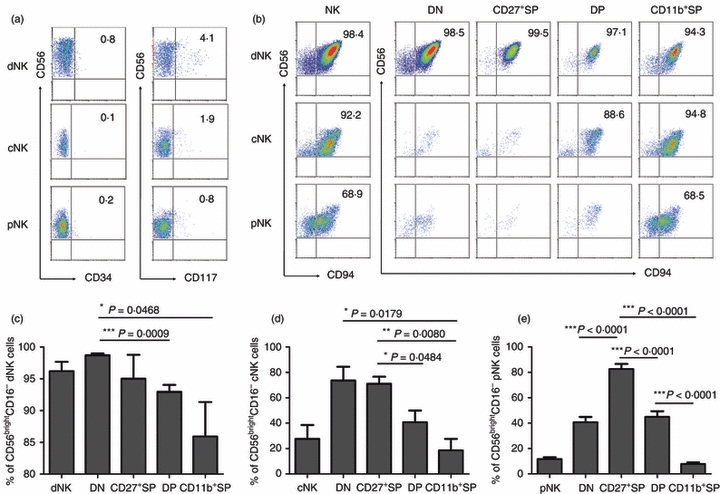
Each of the four subsets are mainly in a CD34− CD117− CD94+/− phenotype, among which double negative (DN) and CD27+ single-positive (SP) subsets are the majority of CD56bright CD16− NK cells. (a) Representative flow cytometry analysis of CD34 and CD117 expression on gated CD56+ CD3− decidual natural killer (dNK) cells of the first trimester, cord blood NK (cNK) cells and peripheral blood NK (pNK) cells. Results are representative of five experiments. (b) Representative flow cytometry analysis of CD94 expression on dNK, cNK, pNK and the four subsets of NK cells. Results are representative of five experiments. (c) Frequency of CD56bright CD16− NK cells in total cells and in each subset of dNK cells (n = 6). (d) Frequency of CD56bright CD16− NK cells in total cells and in each subset of cNK cells (n = 4). (e) Frequency of CD56bright CD16− NK cells in total cells and in each subset of pNK cells (n = 8).
Moreover, CD56+ CD3− human NK cells were identified as CD56bright and CD56dim subsets.13,14 Our results showed that dNK had about 95% CD56bright CD16− NK cells, cNK had > 20% CD56bright CD16− NK cells, compared with < 10% CD56bright CD16− NK cells in pNK cells. Among the four subsets defined by CD11b and CD27, DN and CD27+ SP cells were the majority of cells in the CD56bright CD16− subsets (Fig. 2c–e), confirming their immature phenotype. The majority of CD11b+ SP cells had the CD56dim CD16+ phenotype, displaying their mature phenotype. Collectively, the four-subset model defined by CD11b and CD27 reflects the developmental stages of human NK cells and is an important guideline for defining the stages of human NK cell development.
DN NK cells represent an immature phenotype
To verify whether maturation subsets defined by CD11b and CD27 are associated with progressive functions, we investigated each of the four subsets separately. First, we investigated whether dNK cells represented an immature phenotype. Representative surface molecules were tested in gated CD56+ CD3− dNK, cNK and pNK cells. NKG2A, reported to be highly expressed on cells in the immature developmental stage,16,21 was expressed > 80% in dNK, which was significantly higher compared with 60% in cNK and < 20% in pNK cells. In contrast, NKG2C and NKG2D were expressed at higher levels in pNK than in cNK and dNK cells. Expressions of CD11c, CD7 and CD2 cells have been reported to be an important phenotype in mature cells;5,7,22 which were expressed at significantly lower levels in dNK cells but much higher levels in cNK and pNK cells (Fig. 3a). These results confirmed that the developmental stages progressed in the following order: dNK < cNK < pNK.
Figure 3.
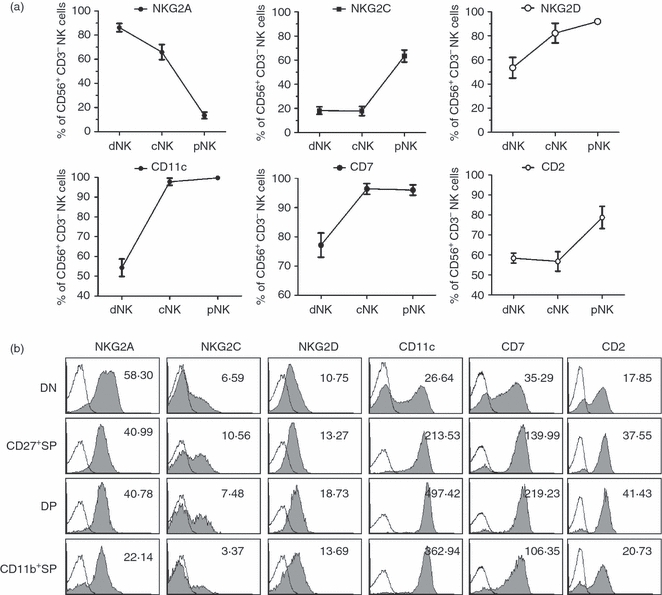
Double-negative natural killer (DN NK) cells show a more immature phenotype compard with the other NK-cell subsets. (a) The percentages of surface molecule expression using the gated CD56+ CD3− decidual NK (dNK) cells of the first trimester, cord blood NK (cNK) and peripheral blood NK (pNK) cells (n = 8) for each experiment. (b) Expression of various surface molecules on gated DN dNK cells versus the other three NK subsets. Results are representative of five experiments. Numbers showed the average fluorescence intensity of each surface molecule.
Second, we investigated the phenotypes in each NK subset defined by CD11b and CD27 expression. These results have been obtained using dNK cells, because cNK and pNK cells have too limited DN and CD27+ SP populations. As expected, we found that DN NK cells expressed significantly higher levels of NKG2A but much lower levels of NKG2C and NKG2D and even lower levels of CD11c, CD7 and CD2 cells than the other three subsets (Fig. 3b). These data suggest that the DN NK subset is in the immature state compared with the other three subsets. Therefore, including a large population of cells in the DN NK subset led to the designation of the immature phenotype for dNK cells.
DN NK cells have developmental potential
To further evaluate the potential developmental capability of DN NK cells, we cultured freshly isolated human decidual lymphocytes of the term trimester in different cytokine environments and evaluated their phenotypes at several time-points, for it is difficult to collect aseptic decidual samples of the first trimester from clinic. In medium supplemented with IL-2, DN NK cells decreased from 50·7% to 47·2% by day 7 of culturing and decreased further, to 39·0%, by day 14. In contrast, DP NK cells increased from 13·0% to 30·8% at day 14 (Fig. 4a,b). We also induced maturation in medium supplemented with IL-15 as well as in medium with both IL-2 and IL-15. The three different cytokine environments could induce maturation among the NK cells (Fig. 4d,f). However, IL-2 seemed to be more effective than IL-15 at inducing maturation.
Figure 4.
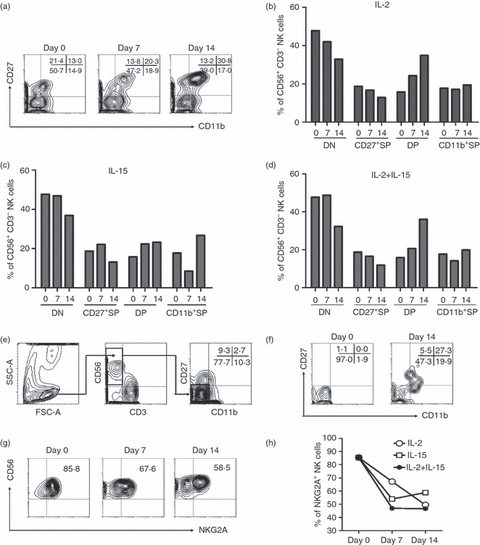
Double-negative natural killer (DN NK) cells have developmental potential. Decidual cells in the term trimester were cultured with interleukin-2 (IL-2; 100 U/ml) and/or IL-15 (10 ng/ml) for 7 or 14 days. (a) Representative flow cytometry analysis of the expression of CD27 and CD11b on IL-2-cultured decidual NK (dNK) cells at each time-point. Dot plots were gated on live NK cells using a lymphocyte gate using forward scatter versus side scatter and an NK-cell gate using CD56+ CD3− cells. (b) The frequency of each subset of NK cells at different time-points. Cells were cultured with IL-2. (c) The frequency of each subset of NK cells at different time-points. Cells were cultured with IL-15. (d) The frequency of each NK-cell subset at different time-point. Cells were cultured with IL-2 and IL-15. (e) Gating strategy of sorting DN NK cells. Dot plots were gated on live NK cells using a lymphocyte gate using forward versus side scatter and an NK-cell gate using CD56+ CD3− cells. Then DN NK cells were sorted by gating CD56+ CD3− CD11b− CD27−. The purity of DN NK cells after sorting was > 95%. (f) Representative flow cytometry analysis of the expression of CD27 and CD11b on IL-2-cultured sorted DN NK cells. (g) Representative flow cytometry analysis of the expression of NKG2A on gated IL-2-cultured dNK cells. (h) The frequency of NKG2A+NK cells at each time-point under different culture conditions in (b), (c) and (d). Each result is representative of three experiments.
To further verify the culturing effect of DN NK cells alone, we sorted the DN NK subset by decidual cells of the term trimester, which were stained with anti-CD3, anti-CD56, anti-CD11b and anti-CD27. The gating strategy has been shown in Fig. 4(e). We gated the lymphocytes first, then gated CD56+ CD3− NK cells, and sorted DN NK cells as CD56+ CD3− CD11b− CD27− cells by FACS Aria (BD Bioscience).The purity of DN NK cells after sorting was ≥ 95%. After 14 days culturing of IL-2 (100 U/ml), DN NK cells have developed into the other three subsets (Fig. 4f). To further verify this procession, we evaluated the changes in NK receptors during these cultures. NKG2A, which had been shown to be expressed at a higher level on immature NK cells (Fig. 3), decreased from 85·4% to 58·5% after 14 days of culturing (Fig. 4g,h), suggesting that maturation had progressed. Hence, the DN NK subset had an immature phenotype and developmental potential.
CD27+ SP and DP NK cells displayed the highest secretion of cytokines
The main functions of NK cells include killing aberrant cells and producing cytokines, which can be antimicrobial or may prime other cells of the immune system.13,14 We further questioned the sequence of effector function acquisition by NK cells in the four subsets defined by CD11b and CD27. Because cNK and pNK have very limited DN and CD27+ SP NK cells, typical cytokines have been analysed by human decidual NK cells of the first trimester. The DN NK cells produced the lowest levels of IFN-γ and TNF-α that were consistent with their immature developmental stages. CD27+ SP and DP NK cells had the highest cytokine expression, suggesting that NK cells had acquired the ability to produce cytokines during these two stages. Furthermore, CD11b+ SP NK cells secreted lower levels of cytokines compared with CD27+ SP and DP NK cells, but they still secreted more than DN NK cells (Fig. 5a–c). These data suggest that there is a division of labour between the four NK-cell subsets, which would account for the ability of CD27+ SP and DP NK cells to secrete cytokines.
Figure 5.
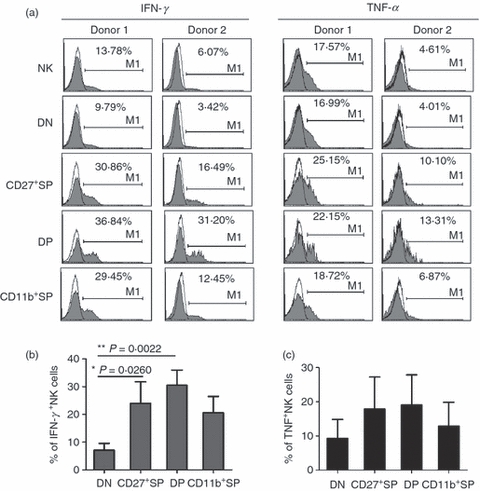
CD27+ single-positive (SP) and double-positive (DP) natural killer (NK) cells have greater cytokine secretion. Typical cytokines produced by human decidual NK cells of the first trimester have been analysed in the four subsets defined by CD27 and CD11b. (a) Representative flow cytometry analysis of interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) in different NK-cell subsets. Results are representative of seven experiments. (b) Frequency of IFN-γ-secreting NK cells in different NK-cell subsets (n = 7). (c) Frequency of TNF-α-secreting NK cells in different NK-cell subsets (n = 7).
CD11b+ SP NK cells have the strongest cytotoxic capacity
We further evaluated the cytotoxic capacity of the four NK-cell subsets. CD16 (Fcγ receptor III), which has been described as facilitating antibody-dependent cellular cytotoxicity and killing,23 was expressed on < 5% of the dNK cells, whereas the marker was found on 70% of cNK cells and > 90% of pNK cells (Fig. 6a). In each subset defined by CD27 and CD11b, the DN and CD27+ SP NK cells had very low levels of CD16 expression compared with the other two subsets. CD11b+ SP NK cells had the highest CD16 expression among all three types of human NK cells (Fig. 6b,c), suggesting that they have the strongest cytotoxic capacity.
Figure 6.
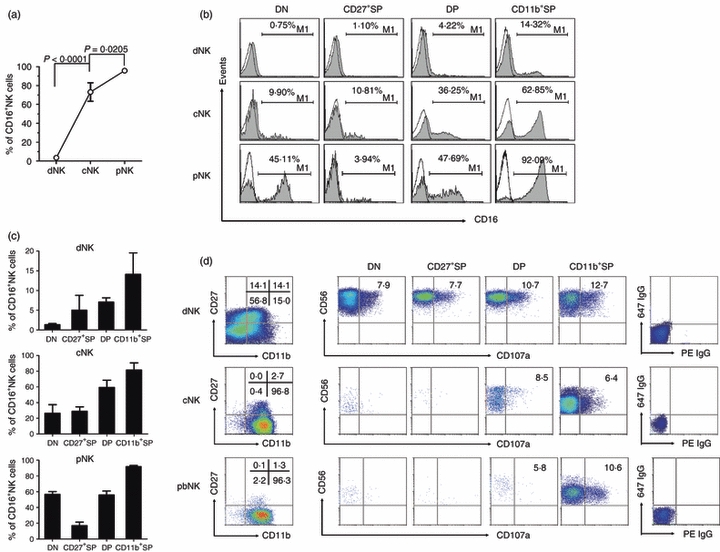
CD11b+ single-positive natural killer (SP NK) cells have the best ability for killing. (a) The frequency of CD16+ NK cells among decidual (dNK), cord blood (cNK) and peripheral blood (pNK) NK cells (n = 10) for each NK cell type. (b) Representative flow cytometry analysis of CD16 expression on cells from each subset of dNK, cNK and pNK cells. Results are representative of six experiments. (c) The frequency of CD16+ NK cells in different subsets of dNK, cNK and pNK cells (n = 6). (d) Representative flow cytometry analysis of CD27/CD11b expression on gated NK cells and CD107a expression in different NK subsets and in dNK, cNK and pNK cells. Results are representative of five experiments.
We then analysed the expression of CD107a, which is a functional marker for identifying NK cell-mediated lysis of target cells.24 Lymphocytes from peripheral blood, cord blood and decidual mononuclear cells were cultured with K562 at the ratio of 1 : 1 with monensin (10 μg/ml; Sigma) and phycoerythrin-conjugated anti-CD107a for 4 hr. Then, the cells were washed, blocked and stained with other surface antibodies for ordinary FACS staining. Each of the four subsets occupied > 10% of the total NK cells. The CD11b+ SP subset had higher CD107a expression than the DP subset and much higher expression than the DN and CD27+ SP subsets. Among the pNK and cNK cells, which had limited cell numbers in DN and CD27+ SP subsets, the CD11b+ SP subset was also the main source of CD107a (Fig. 6d), consistent with previous data that CD11b can mediate cytotoxic priming by beta-glucan.25 Hence, the CD11b+ SP subset was the main source of cytolysis in each type of human NK cell.
Discussion
The identification of the developmental stages of B and T cells has advanced for decades, whereas our knowledge regarding NK-cell development, especially human NK-cell development, remains in its infancy. Although the developmental stages of mouse NK cells were recently described,19,20 whether similar populations existed in humans had not yet been reported. In this study, we characterized four novel NK populations (DN, CD27+ SP, DP, and CD11b+ SP) that appeared to represent the developmental stages of human pNK, cNK and dNK cells. Furthermore, we demonstrated that each population could be characterized by unique functional and phenotypic attributes. Hence, our data provide evidence that human NK cells at different developmental stages have specific functional roles in tissues and may also reflect a novel model of human NK-cell differentiation.
One primary goal of our study was to identify surface markers that could be used to specifically identify the developmental stages of human NK cells in vivo. These markers would be important for determining the developmental states of NK cells in different tissues or in different pathologies. In this regard, CD27 and CD11b are useful markers because they provide a developmental continuity for human NK cells; we found that these markers can differentiate between the four functionally heterogeneous populations with the potential to identify additional subsets or clinical usage within each of these populations. In addition, these markers provide a bridge between human and mouse data, which will be useful for comparisons between the two species and provide benefit for future research.
Much of our data are consistent with previous mouse and human data on this subject. Specifically, we observed that CD27 distinguished between two NK-cell subsets in human and mouse peripheral blood mononculear cells.17,18 The two NK-cell subsets corresponded to the previously described CD56bright and CD56dim NK cells. The NK cells decreased their expression of NKG2A but acquired NKG2D, CD11c, CD2, CD7 and CD11b during development.5,16 Published data have identified that the human lymph nodes and tonsils, the location of immature NK cells, express a low level of the CD11b integrin.5 We further observed in human decidual NK cells that more than half were CD11b− NK cells. Hence, a low level of CD11b is characteristic of immature human NK cells. In addition, we observed that CD27 was expressed at a much higher level on human cNK and dNK cells. The developmental stages of human CD56+ NK cells were easily identified using CD11b and CD27. Our data also suggest that the CD56dim (major CD11b+ SP) population is more mature than the CD56bright (major DN and CD27+ SP) population, supporting Lanier et al.'s original proposal26 and consistent with many recent studies.13 Another novel finding was that although the majority of DN and CD27+ SP subsets had a CD56bright CD16− phenotype, the DN subset actually expressed few cytokines. These results suggest that the CD56bright CD16− population is still heterogeneous.
Decidual stage 3 NK cells have the capability to differentiate to stage 4 not only in SLT but also in uterine mucosa.5,7 Here we provided evidence that human stage 4 NK cells are heterogeneous and can be divided into DN, CD27+ SP, DP and CD11b+ SP. This is important for three reasons. First, we confirm and suggest decidua could represent a novel site of NK cell development. It has not previously been described that so many immature DN population existed in decidual NK cells. Second, we observe the sequence of effector function acquisition by NK cells in the four subsets is accompanied with their developmental stages. Third, it is interesting that there are DN and CD27+ SP populations in dNK cells but not in pNK and cNK cells. Do those immature NK cells develop in lymphoid tissue and recruit to the circulatory system? We suggest that this is the case because NK cells are the first lymphocytes appearing in patients recovering from haematopoietic stem cell transplantation and those cells are mainly CD56bright NK cells,27–30 which in our study have been proven to mainly include DN and CD27+ SP populations. Why do cNK cells maintain a DP population and why are they not as mature as pNK cells? Such questions will be interesting to investigate.
In light of the aforementioned data, it is also noteworthy that IL-15 alone was insufficient for promoting DN NK cells to the three other populations compared with IL-2. Published results have provided evidence that much IL-15 was found in the decidual tissues.31 As presented here and in previous reports, NK-cell subsets and developmental stages displayed tissue preference, implying that tissue-specific environments have an impact on NK-cell differentiation.5,13,32–36 Much IL-15 maintains the decidual NK subset that exists in an immature stage and acts with immunomodulatory potential.32,36–38 This process could possibly be regulated by dendritic cells because they have already been shown to control NK-cell proliferation and effector functions.39–41 However, many new questions have arisen from this research. For example, it is still unclear which specific factors allow for decidual NK cells to remain in an immature stage. Although we have shown that prolonged incubation by the cytokines IL-2 and IL-15 can induce DN NK cells to develop to other subsets, we still cannot exclude the possibility that this process may not be a physiological recapitulation of NK-cell development and maturation. DNK cells were distinct from pNK cells for less than 20% dNK cells were mature CD11b+SP NK cells. An unanswered question is why such NK cells occupy > 70% of the total lymphocytes at the human fetal–maternal interface. In future studies, it will be important to refine the specific functions of human NK cells at different developmental stages and in different tissues.
Acknowledgments
This work was supported by the Natural Science Foundation of China (#30730084, #30721002) and the Ministry of Science & Technology of China (973 Basic Science Project 2007CB512405,2009CB522403).
Glossary
Abbreviations
- CBMC
cord blood mononuclear cells
- CD11b+ SP
CD11b+ CD27− NK cells
- CD27+ SP
CD11b− CD27+NK cells
- cNK
NK cells from cord blood mononuclear cells
- DMC
decidual mononuclear cells
- DN
CD11b− CD27− NK cells (double-negative)
- dNK
NK cells from decidual mononuclear cells
- DP
CD11b+ CD27+ natural killer cells (double-positive)
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- pNK
NK cells from PBMC
Disclosures
All authors declare no competing financial interests.
References
- 1.Galy A, Travis M, Cen DZ, Chen B. Human T-cells, B-cells, natural-killer, and dendritic cells arise from a common bone-marrow progenitor-cell subset. Immunity. 1995;3:459–73. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 2.Miller JS, Alley KA, Mcglave P. Differentiation of natural-killer (Nk) cells from human primitive marrow progenitors in a stroma-based long-term culture system – identification of a Cd34+ 7+ Nk progenitor. Blood. 1994;83:2594–601. [PubMed] [Google Scholar]
- 3.Shibuya A, Kojima H, Shibuya K, Nagayoshi K, Nagasawa T, Nakauchi H. Enrichment of interleukin-2-responsive natural-killer progenitors in human bone-marrow. Blood. 1993;81:1819–26. [PubMed] [Google Scholar]
- 4.Freud AG, Becknell B, Roychowdhury S, et al. A novel human CD34+ subset that constitutively expresses the high affinity interleukin-2 receptor traffics to lymph nodes and differentiates into CD56Bright natural killer cells. Blood. 2004;104:93a–93a. [Google Scholar]
- 5.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–43. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huntington ND, Vosshenrich CAJ, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–14. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 7.Male V, Hughes T, McClory S, Colucci F, Caligiuri MA, Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. 2010;185:3913–8. doi: 10.4049/jimmunol.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes T, Becknell B, McClory S, et al. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113:4008–10. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+ IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207:281–90. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes T, Becknell B, Freud AG, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–14. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 13.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–25. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 15.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 16.Beziat V, Descours B, Parizot C, Debre P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS ONE. 2010;5:e11966. doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–24. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 18.Vossen MT, Matmati M, Hertoghs KM, et al. CD27 defines phenotypically and functionally different human NK cell subsets. J Immunol. 2008;180:3739–45. doi: 10.4049/jimmunol.180.6.3739. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 20.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–96. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 21.Le Garff-Tavernier M, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging cell. 2010;9:527–35. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 22.Allavena P, Bianchi G, Paganin C, Giardina G, Mantovani A. Regulation of adhesion and transendothelial migration of natural killer cells. Nat Immun. 1996;15:107–16. [PubMed] [Google Scholar]
- 23.Vivier E, Morin P, O'Brien C, Druker B, Schlossman SF, Anderson P. Tyrosine phosphorylation of the Fc gamma RIII(CD16): zeta complex in human natural killer cells. Induction by antibody-dependent cytotoxicity but not by natural killing. J Immunol. 1991;146:206–10. [PubMed] [Google Scholar]
- 24.Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Arico M, Moretta L, Pende D. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316–23. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 25.Xia Y, Borland G, Huang J, Mizukami IF, Petty HR, Todd RF, Ross GD. Function of the lectin domain of Mac-1/complement receptor type 3 (CD11b/CD18) in regulating neutrophil adhesion. J Immunol. 2002;169:6417–26. doi: 10.4049/jimmunol.169.11.6417. [DOI] [PubMed] [Google Scholar]
- 26.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–6. [PubMed] [Google Scholar]
- 27.Beziat V, Nguyen S, Lapusan S, et al. Fully functional NK cells after unrelated cord blood transplantation. Leukemia. 2009;23:721–8. doi: 10.1038/leu.2008.343. [DOI] [PubMed] [Google Scholar]
- 28.Dulphy N, Haas P, Busson M, et al. An unusual CD56bright CD16low NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation. J Immunol. 2008;181:2227–37. doi: 10.4049/jimmunol.181.3.2227. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen S, Dhedin N, Vernant JP, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105:4135–42. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- 30.Vago L, Forno B, Sormani MP, et al. Temporal, quantitative, and functional characteristics of single-KIR-positive alloreactive natural killer cell recovery account for impaired graft-versus-leukemia activity after haploidentical hematopoietic stem cell transplantation. Blood. 2008;112:3488–99. doi: 10.1182/blood-2007-07-103325. [DOI] [PubMed] [Google Scholar]
- 31.Verma S, Hiby SE, Loke YW, King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62:959–68. doi: 10.1095/biolreprod62.4.959. [DOI] [PubMed] [Google Scholar]
- 32.Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–12. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 34.Verma S, King A, Loke YW. Expression of killer cell inhibitory receptors on human uterine natural killer cells. Eur J Immunol. 1997;27:979–83. doi: 10.1002/eji.1830270426. [DOI] [PubMed] [Google Scholar]
- 35.Brodin P, Karre K, Hoglund P. NK cell education: not an on–off switch but a tunable rheostat. Trends Immunol. 2009;30:143–9. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Hanna J, Mandelboim O. When killers become helpers. Trends Immunol. 2007;28:201–6. doi: 10.1016/j.it.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Karimi K, Blois SM, Arck PC. The upside of natural killers. Nat Med. 2008;14:1184–5. doi: 10.1038/nm1108-1184. [DOI] [PubMed] [Google Scholar]
- 38.Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med. 2006;12:1065–74. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Hagihara M, Ando K, et al. Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells. J Immunol. 2001;166:1590–600. doi: 10.4049/jimmunol.166.3.1590. [DOI] [PubMed] [Google Scholar]
- 40.Borg C, Jalil A, Laderach D, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–75. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 41.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–17. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


