Abstract
This paper describes an experimental model of neuroinflammation based on the production of interleukin-6 (IL-6) by neural glial cells infected with Theiler's murine encephalomyelitis virus (TMEV). Production of IL-6 mRNA in mock-infected and TMEV-infected SJL/J murine astrocytes was examined using the Affymetrix murine genome U74v2 DNA microarray. The IL-6 mRNA from infected cells showed an eightfold increase in hybridization to a sequence encoding IL-6 located on chromosome number 5. Quantitative real-time reverse transcription PCR (qPCR) was used to study the regulation of IL-6 expression. The presence of IL-6 in the supernatants of TMEV-infected astrocyte cultures was quantified by ELISA and found to be weaker than in cultures of infected macrophages. The IL-6 was induced by whole TMEV virions, but not by Ad.βGal adenovirus, purified TMEV capsid proteins, or UV-inactivated virus. Two recombinant inflammatory cytokines, IL-1α and tumour necrosis factor-α were also found to be potent inducers of IL-6. The secreted IL-6 was biologically active because it fully supported B9 hybridoma proliferation in a [3H]thymidine incorporation bioassay. The cerebrospinal fluid of infected mice contained IL-6 during the acute encephalitis phase, peaking at days 2–4 post-infection. Finally, this in vitro neuroinflammation model was fully inhibited, as demonstrated by ELISA and qPCR, by five selective oestrogen receptor modulators.
Keywords: astrocytes, encephalomyelitis virus, inflammation, interleukin-6, oestrogen receptor modulators
Introduction
Theiler's murine encephalomyelitis virus (TMEV) is a picornavirus that persistently infects the central nervous system (CNS) of mice.1 The intracerebral infection of the low-neurovirulence BeAn strain in demyelination-sensitive strains of mice (including the SJL/J strain) is currently used as a model for studying human demyelination processes such as multiple sclerosis. The demyelination that occurs in this model has been shown to be primarily mediated by the immune system.2–4
Retroviral infection stimulates the molecular machinery of cells within minutes of infection. Some early viral proteins act as transcriptional regulators that trigger the expression of inflammatory genes controlling the synthesis of defence proteins, including a large number of cytokines.5 Distinct subsets of cytokines are secreted by effector populations of immune cells (although mainly by CD4+ T cells) in response to pathogens such as viruses and other stimuli.6 The nervous system target cells of TMEV, the glial astrocytes, also produce cytokines and chemokines after infection as part of the pro-inflammatory response. This, however, finally induces myelin breakdown.3 The present work reports how, using DNA microarray analysis, the increased expression of a gene coding for interleukin-6 (IL-6) was detected in TMEV-infected SJL/J astrocytes. Astrocytes and the immune mediators they produce in response to TMEV infection regulate the ensuing adaptive immune response that eventually induces demyelination as a harmful side-effect. Cytokine/chemokine production by astrocytes following in vitro TMEV infection is likely to reflect the situation that would be generated in vivo.
Interleukin-6 is an important activator of inflammation and directs the transition from innate to acquired immunity.7 It was originally identified as a B-cell differentiation factor,8 but is today known to be a pleiotropic cytokine with important effects on cell growth, differentiation, migration and inflammation.9 Production of IL-6 in astrocytes can be induced in different model systems involving infection with mouse hepatitis virus 4, vesicular stomatitis virus or Newcastle disease virus,10–12 brain injury13 and even TMEV infection.14 Its presence is indicative of the infectious origin of some immune diseases. Hence, astrocytes from patients with Aicardi–Goutières syndrome produce the chemokine CXCL10 but not IL-6, suggesting that this neuroinflammatory disease may be caused by perturbations of the innate immune system rather than by a viral infection.15
In the present work, DNA hybridization and quantitative real-time PCR (qPCR) were used to examine the expression of the IL-6 gene in astrocytes in an in vitro TMEV-induced neuroinflammation model, and its inhibition by a number of selective oestrogen receptor modulators (SERMs). The main SERM, oestradiol (a regulator of reproduction), is also involved in the growth and differentiation of a number of tissues, including those of the CNS. It also has a neuroprotective effect on the CNS,16–18 partly through its reduction of inflammation.19–21 Oestrogens inhibit the release of cytokines from different cell types,22 suggesting that pro-inflammatory genes are the major signalling targets for oestrogen receptors α and β.23 Recent studies have indicated that oestrogen receptor ligands exert their anti-inflammatory effects by repressing genes that promote inflammation, such as those coding for cytokines and chemokines.24,25
Materials and methods
Astrocyte and macrophage cell cultures
Astrocyte cultures were prepared by mechanical dissociation of the cerebral cortex of newborn SJL/J Harlan mice26 purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were maintained on standard feed and water provided ad libitum at the Instituto Cajal. The cortex was isolated under a dissecting microscope and cleaned of the choroid plexus and meninges. Cell suspensions were filtered through a 135-μm pore mesh into Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS) and gentamicin (Gibco BRL, Paisley, UK). After centrifugation, cells were filtered through a 40-μm nylon cell strainer (Falcon-Becton Dickinson, Le Pont De Claix, France) and cultured in 75-cm2 tissue culture flasks (Costar, Cambridge, MA) at 37°. The medium was changed after 4 days of culture and subsequently twice per week over the entire culture period. Cultures were enriched for astrocytes by the removal of less adherent microglia and oligodendrocytes by shaking overnight at 250 r.p.m. (at 37°) in a table-top shaker (Thermo Forma, Marietta, OH). Cellular confluence was observed 10 days after plating, producing around 1 × 107 cells per flask; the cells showed a flat, polygonal morphology. A mean astrocyte content of 98% was confirmed by indirect immunofluorescence staining of methanol-fixed cultures using rabbit anti-glial fibrillar acidic protein antiserum (Dakopatts, Glostrup, Denmark). The lack of mature oligodendrocytes and microglia/macrophages was confirmed using a guinea-pig anti-myelin basic protein antiserum prepared as described elsewhere,27 and the monoclonal anti-Mac-1 antibody (Serotec, Oxford, UK). Secondary fluorescein-labelled antibodies were purchased from the Sigma Chemical Co. (St Louis, MO).
To prepare macrophage cultures, resident peritoneal exudate cells were harvested after injection of 4 ml DMEM into the peritoneum of SJL/J mice. After centrifugation in the cold, cells were resuspended in DMEM containing 10% FCS and gentamicin, and macrophages were allowed to adhere to the plastic flasks at 37° for 1 hr. Non-adherent cells were removed by vigorous washing. The remaining adherent macrophages were used for TMEV infection over the next 24 hr. We routinely checked the cultures for the absence of endotoxin using the Limulus amoebocyte lysate assay and for Mycoplasma contamination using the Mycoplasma PCR ELISA test (Boehringer Mannheim GmbH, Mannheim, Germany).
Viruses and infection
The BeAn 8386 strain of TMEV, isolated in 1957 from a feral mouse in Belem, Brazil, was used in all infections. Baby hamster kidney cells (BHK-21) were grown at 37° in DMEM containing 10% FCS and penicillin–streptomycin. The BHK-21 cultures were infected for 48 hr at 33°, sonicated and centrifuged in the cold to remove cell debris. Purified astrocytes in 75-cm2 tissue culture flasks were infected with the virus at several multiplicities of infection (MOI) in a volume of 10 ml DMEM containing 0·1% BSA at room temperature for 1 hr. After infection, cells were washed and 10 ml DMEM plus 10% FCS were added and the flasks were incubated at 37° for 2, 4, 8 or 24 hr. No changes in cell viability or in cytopathological effects were detected after infection with the virus, even at an MOI of 10. Cells used for mock infections were incubated with a virus-free BHK-21 cell lysate. For UV-light virus inactivation, viral samples were irradiated at 560 μW/cm2 for 15 min. These conditions produced inactivated stocks devoid of infectious virus, as determined by plaque assay (< 10 plaque-forming units/ml). Levels of endotoxin contamination were below 0·2 U/ml in all virus preparations.
The adenovirus Ad.βGal, belonging to serotype 5 and harbouring the LacZ gene of Escherichia coli coding for β-galactosidase under the control of the Rous sarcoma virus promoter, was used to demonstrate the virus specificity of the response studied.28 The virion particles were purified by CsCl banding, desalted in PD-10 desalting columns (Amersham Pharmacia Biotech, Uppsala, Sweden), eluted in PBS, split into aliquots and stored at −70° in PBS–10% glycerol.
cRNA target preparation, DNA hybridization and data analysis
Three replicates of SJL/J astrocyte cultures infected at an MOI of 10 were harvested 24 hr post-infection (p.i.), washed with PBS, and the total RNA was isolated by using TRIzol reagent (Gibco BRL). This was followed by further purification using the RNeasy Mini purification kit (Qiagen, Valencia, CA). Ten micrograms of RNA from each culture were converted to cDNA using the SuperScript Choice System kit (Gibco BRL). Second-strand synthesis was performed using T4 DNA polymerase, and cDNA was isolated by phenol–chloroform extraction. Isolated cDNA was transcribed using the BioArray High Yield RNA Transcript Labeling Kit (Enzo Biochem, New York, NY) with biotin-labelled UTP and CTP to produce biotin-labelled cRNA. Labelled cRNA was isolated using the RNeasy Mini kit and fragmented in 100 mm potassium acetate/30 mm magnesium acetate/40 mm Tris–acetate (pH 8·1) for 30 min at 94°. Hybridization performance was analysed using Test 2 arrays (Affymetrix, Santa Clara, CA) employing spike and housekeeping controls. Target cRNA was hybridized to the murine genome U74v2 microarray (Affymetrix) according to the manufacturer's recommendations. Briefly, 15 μg fragmented cRNA was hybridized for 16 hr at 45° with constant rotation (60 r.p.m.). The microarrays were washed and stained with streptavidin-conjugated phycoerythrin using the Affymetrix GeneChip Fluidic Station model 400. All hybridization steps were performed by Progenika Ltd. (Derio, Spain). Each gene on the U74v2 array is represented by 20 different 25-base cDNA oligonucleotides complementary to a cRNA target transcript (perfect match). As a hybridization specificity control, an oligonucleotide containing a single base substitution corresponding to each perfect-match cDNA oligonucleotide (mismatch) is represented on the array. The combination of perfect-match and mismatch cDNA oligonucleotides for each gene is termed a probe set. By using Affymetrix-defined absolute mathematical algorithms describing perfect-match and mismatch intensities, each gene was defined as absent or present. Binding intensity values were scaled to evaluate differential expression following TMEV infection. Based on Affymetrix-defined comparison mathematical algorithms, a virus-induced transcript was classified as unchanged, marginally increased, marginally decreased, increased or decreased.
Quantitative real-time PCR
Total RNA was extracted from mock-infected or TMEV-infected astrocytes using the RNeasy Mini purification kit (Qiagen). The cDNA was prepared from RNA using Moloney murine leukaemia virus reverse transcriptase (RT) from Promega Corp. (Madison, WI) and the 3′ amplimer as a template/primer. Quantitative PCR was performed using the ABI PRISM 7000 Sequence Detector System (Applied Biosystems, Weiterstadt, Germany) following the manufacturer's recommendations. Reactions were performed in 20-μl volumes, employing the SYBR® Green real-time PCR Master Mix from Applied Biosystems. All reactions were performed in quadruplicate. Standard curves were generated using serial dilutions of cDNA from astrocytes. Gene expression was normalized for β-actin expression using the standard curve method described by the manufacturer. Data were analysed using the abi prism 7000 SDS software and expressed as mean ± SEM in boxplots. Primers for mouse IL-6 were synthesized by invitrogen™ (CEDEX 95613; Cergy Pontoise, France):
forward: GAAACCGCTATGAAGTTCCTCTCTG
reverse: TGTTGGGAGTGGTATCCTCTGTGA.
ELISA determination of IL-6 and IL-18
The ELISA was performed using the Quantikine® Immunoassay kit for mouse IL-6 (cat. no. M6000B; R&D Systems, Minneapolis, MN). This has a detection limit of 1·3–1·8 pg/ml and there is no cross-reactivity with any other interleukin. The Quantikine® Immunoassay ELISA kit for mouse IL-18 (cat. no. 7625; R&D Systems) was also used. This has a sensitivity of 25·0 pg/ml. All assays were performed according to the manufacturer's recommendations.
TMEV protein purification
Virion particles of TMEV were purified by isopycnic centrifugation in a CsCl gradient.29 VP1, VP2 and VP3 virion proteins were purified by reverse-phase HPLC using an Aquapore RP-300 column (Browlee Laboratories, Santa Clara, CA).26 Elution peaks were checked for purity using SDS–PAGE.
Pro-inflammatory cytokine treatments
Cells were treated for 48 hr with 10 ng/ml of the following mouse recombinant pro-inflammatory cytokines: mouse recombinant IL-1α from R&D Systems, recombinant interferon-γ (rIFN-γ) of murine origin from Holland Biotechnology (Leiden, the Netherlands), recombinant murine tumour necrosis factor-α (TNF-α) from Innogenetics (Antwerp, Belgium), and rIL-6 from Boehringer.
IL-6 bioassay
The B9 hybridoma proliferation assay was used to detect active IL-6.30 This specific, sensitive assay can detect between 1 and 10 pg/ml IL-6. B9 cells were kindly provided by Dr L.A. Aarden (Netherlands Red Cross, Amsterdam, the Netherlands) and maintained in RPMI-1640 medium, 10% FCS, gentamicin and 5 × 10−5 m 2β-mercaptoethanol. The medium contained 50 units/ml of recombinant IL-6. To perform the assay, cells were washed three times with RPMI medium without rIL-6 and resuspended at 2 × 104 cells/ml. A total of 100 μl (2000 cells) of the cell suspension were added per well to 96-well flat-bottomed culture plates containing 100 μl culture supernatants. After 72 hr of incubation, 1 μCi [3H]thymidine (Amersham Pharmacia Biotech; Product Code TRK 758) was added per well. Cultures were harvested on glass-fibre filters 24 hr later, and radioactivity was counted in 2 ml Ecoscint A scintillation cocktail (National Diagnostics, Atlanta, GA) using a LS 6500 Beckman scintillation counter (Beckman Coulter, Fullerton, CA). A standard curve for rIL-6 was included in each assay. Experiments were performed in triplicate and repeated three times using different astrocyte culture supernatants.
Neutralization by a specific antibody
Samples (100 μl) of diluted astrocyte supernatants containing around 60 pg/ml IL-6 were incubated for 1 hr at 37° with different concentrations of either a monoclonal antibody anti-mouse IL-6 (6B4), kindly provided by Dr J. Van Snick (Ludwig Institute for Cancer Research, Brussels, Belgium), or the IgG fraction of goat anti-mouse IL-1α antiserum (Sigma Chemical Co.). Residual activity was determined in the IL-6 bioassay.
SERM inhibition of IL-6 induction
Astrocyte cultures infected at an MOI of 10 (24 hr earlier) were washed with culture medium and treated with a solution of 17β-oestradiol (10−11 m; diluted in culture medium) prepared from a 100× stock in DMSO. 17β-Oestradiol, and the SERMs tamoxifen and raloxifene were purchased from Sigma. Ospemifene was synthesized according to DeGregorio et al..31 Bazedoxifene was synthesized according to Raveendranath et al.32 All were used at a concentration of 10−9 m, and made from 10× stocks prepared in DMSO. After a further 24-hr period of incubation in the presence of the oestrogens, the supernatants were harvested, centrifuged and tested for the presence of IL-6 by ELISA. Total RNA was extracted from the same cultures using the RNeasy Mini purification kit (Qiagen) and qPCR performed as described above.
Intracerebral inoculations of virus into mice
Six-week-old SJL/J Harlan mice were anaesthetized with Fluothane® and 20 μl of a suspension of the BeAn TEMV (2 × 106 plaque-forming units) injected into the right cerebral hemisphere using a 25-μl Hamilton syringe. We used 20 μl DMEM for injection and trauma controls. Cerebrospinal fluid (CSF) was obtained on different days (n = 5 mice per point) after inoculation by puncturing the cisterna magna of anaesthetized animals with a Hamilton syringe. The extract was frozen at −80° until tested by ELISA.
Results
Microarray DNA hybridization
The Affymetrix GeneChip SE437 and SE438 microarrays revealed a total number of 533 up-regulated sequences in TMEV-infected astrocytes, with a maximum fold increase of 18·8. In addition, 1440 genes were down-regulated, with a maximal fold change of −13·3. Table 1 shows that the hybridization signal of the Affymetrix sequence 102218 was absent in sham-infected astrocytes, but present in TMEV-infected astrocytes. This sequence, located on chromosome number 5, codes for the IL-6 gene (in accordance with its UniGene databank notation). Hence, robust over-expression as the result of TMEV infection was seen, with the signal log ratio equivalent to an eightfold increase in hybridization. The sequence's Gene Ontology database cellular component notation indicated that its protein product was extracellular and involved in the inflammatory response.
Table 1.
Characteristics of the Theiler's murine encephalomyelitis virus (TMEV)-induced up-regulated sequence described as belonging to the interleukin-6 gene in the UniGene database
| Affymetrix sequence | Sham-infected astrocyte signal | TMEV-infected astrocyte signal | Signal log ratio | Fold change | Chromosome location | UniGene title |
|---|---|---|---|---|---|---|
| 102218 at | 9·9 Absent | 200·4 Present | 4·0 | 8·0 | # 5 | interleukin-6 |
The array experiments were repeated three times with different RNA preparations and the average changes in expression were recorded
Expression of IL-6 mRNA in TMEV-infected astrocytes
Owing to its accuracy and sensitivity, qPCR is the standard technique for nucleic acid quantification. This technique was used to confirm and validate the DNA chip hybridization results. The qPCR boxplots showed a significant increase in IL-6 mRNA synthesis only after infection at an MOI of 10 (P < 0·01); nothing was detected when lower MOIs were used (Fig. 1a). This increase in mRNA expression reached a maximum 2–4 hr p.i., declining by 8 hr, and falling to background levels (i.e. those corresponding to uninfected astrocytes) after 24 hr (Fig. 1b). This transient presence indicates that mRNA is labile and quickly degraded inside astrocytes.33
Figure 1.
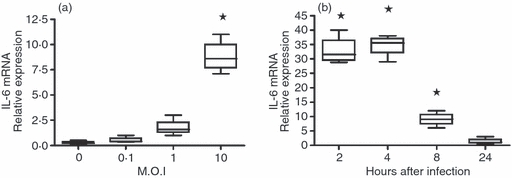
Boxplot of interleukin-6 (IL-6) mRNA synthesis after infection at different multiplicities of infection (MOI) for 8 hr (a) and at different times post-infection (p.i.) at an MOI of 10 (b), as determined by quantitative PCR. The lower boundary of the boxes represents the 25th percentile, the upper boundary the 75th, and the line inside the 50th percentile. *Significantly different compared with the mock-infected cultures (0), determined by the Student's t-test (P < 0·01).
ELISA detection of IL-6 in astrocyte supernatants
Culture supernatants of SJL/J brain astrocytes infected in vitro at different MOIs showed a statistically significant increase in IL-6 secretion, as determined using the Quantikine® (R&D Systems) mouse IL-6 ELISA kit (Fig. 2a). Maximum levels were detected at an MOI of 10. Exploring the kinetics of IL-6 production showed maximum release to the supernatant occurring at 24 hr p.i. (Fig. 2b). Supernatants of mock-infected cultures showed some spontaneous IL-6 synthesis (around 40 pg/ml). Using a specific ELISA kit, IL-18 was undetected in the supernatants of these cultures under all infection conditions (not shown).
Figure 2.
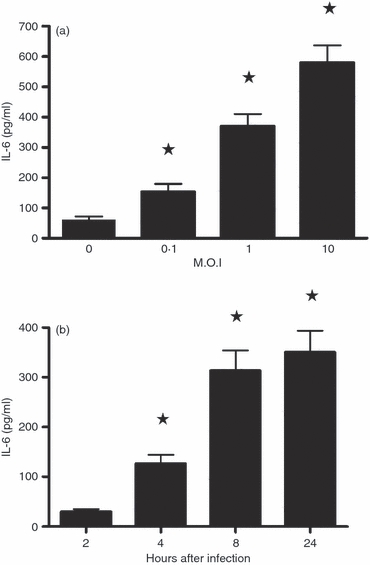
Levels of interleukin-6 (IL-6) in Theiler's murine encephalomyelitis virus (TMEV) -infected SJL/J astrocyte culture supernatants, as quantified by ELISA. (a) Centrifuged supernatants from astrocytes mock-infected (0) or infected at multiplicities of infection (MOIs) of 0·1–10 were tested at 24 hr post-infection (p.i.) (b) The kinetics of IL-6 production were measured from 2 to 24 hr p.i. at an MOI of 1. The results represent mean values ± SD of triplicate samples. * Significantly different compared with the mock-infected cultures (0), determined by the Student's t-test (P < 0·01).
IL-6 production by TMEV-infected macrophages
To compare the production of IL-6 by astrocytes with that of other cells, freshly isolated peritoneal SJL/J macrophage cultures were infected under the same conditions as for the astrocytes. Macrophages were chosen because these are the main producers of IL-634,35 and because these cells play an important role in the immune reactions that trigger TMEV-induced demyelination.36,37
Some differences between TMEV-induced cytokine synthesis in both cell types were detected. First, macrophages were more sensitive to infection: an MOI of 0·1 was almost as efficient as one of 10 at inducing IL-6. In addition, the production of and secretion into the supernatants was faster than in the macrophages, appearing as early as 2 hr p.i. and with a maximum production at 8 hr instead of 24 hr p.i. for astrocytes (Fig. 3a,b). Some spontaneous production of IL-6 was also detected in macrophages (Fig. 3, for MOI 0). Finally, maximum IL-6 secretion was around 0·7 ng/ml for astrocytes and 14 ng/ml for macrophages (notice the different scales in pg/ml and ng/ml on the y-axes of Figs 2 and 3). Therefore, the production of IL-6 by TMEV-infected macrophages was around 20 times higher than that of astrocytes. This was an expected result given the origin of the compared cell lineages. To demonstrate specific IL-6 cytokine induction in both cell lineages, the presence of IL-18 (an IFN-γ-inducing factor mainly produced by activated macrophages38,39) was sought. The empty bars in Fig. 3 show that no IL-18 production was detected, as in TMEV-infected astrocyte supernatants. Hence, a TMEV-specific over-induction of IL-6, in addition to other classical inflammatory cytokines, was seen in both cell lineages.
Figure 3.
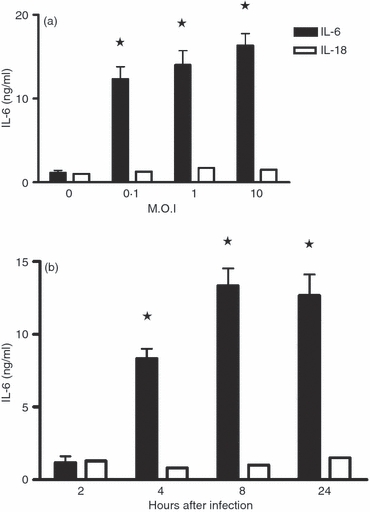
Interleukin-6 (IL-6) and IL-18 levels in Theiler's murine encephalomyelitis virus (TMEV) -infected SJL/J peritoneal macrophage culture supernatants, as quantified by ELISA. (a) Centrifuged supernatants from mock-infected macrophages (0) or infected cells at multiplicities of infection (MOI) of 0·1–10 were tested at 24 hr post-infection (p.i.). (b) The kinetics of IL-6 and Il-18 production were measured from 2 to 24 hr p.i. at an MOI of 1. The results represent mean values ± SD of triplicate samples. *Significant differences from the mock-infected controls, as determined by the Student's t-test (P < 0·01).
TMEV virion integrity and infectivity is needed for IL-6 induction
Experiments were performed to determine whether infectious TMEV or purified virion proteins alone would suffice to induce the over-expression of IL-6. When HPLC-purified TMEV capsid proteins VP-1, VP-2 and VP-3 were incubated with astrocytes in amounts corresponding to a viral inoculum of MOI 10 (a concentration of 2–4 ng/ml), no production of the cytokine over baseline was observed (Fig. 4). Further, TMEV inactivation by UV-light irradiation at 560 μW/cm2 for 15 min, produced viral particles completely devoid of infectious or IL-6-inducing properties (not shown). The specificity of viral infection was also studied, checking whether the unrelated Ad.βGal adenovirus induced the same response. Adenovirus infection (MOI 10) was unable to induce any synthesis of IL-6 (Fig. 4, Adeno). Hence, both intact and infecting TMEV virion particles are needed to induce over-expression of the IL-6 gene.
Figure 4.
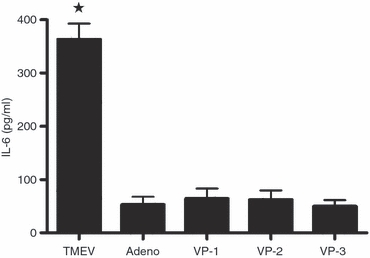
Specificity of interleukin-6 (IL-6) induction by infective, whole Theiler's murine encephalomyelitis virus (TMEV) virions. The BeAn strain purified TMEV viral particles, and VP-1, VP-2 and VP-3 native capsid proteins purified by HPLC, were added to astrocyte cultures. Astrocyte monolayers were treated with the amount of pure protein equivalent to a multiplicity of infection (MOI) of 1. The adenovirus Ad.βGal (Adeno) was used as a negative infection control. After 24 hr, the presence of IL-6 in the culture supernatants was monitored by ELISA. The results represent the mean ± SD of triplicate samples. The representative results of three experiments are shown. *Significant difference from the adenovirus-infected group (Adeno), as determined by the Student's t-test (P < 0·01).
Certain inflammatory cytokines induced IL-6 in astrocytes
Three recombinant inflammatory cytokines (IL-1α, IFN-γ and TNF-α) were tested for their possible role in the up-regulation of their family member IL-6 in SJL/J astrocytes. As shown in Fig. 5, IFN-γ (10 ng/ml) induced no up-regulation (as measured by ELISA). However, IL-1α and TNF-α (10 ng/ml) increased the release of IL-6 in the culture supernatants to 400 and 250 pg/ml above background, respectively. To determine the minimum dose required for induction, astrocyte cultures were incubated with 1, 10 and 100 ng/ml of each test cytokine for 48 hr. Release of IL-6 to supernatants was detected after stimulation with a dose of 1 ng/ml IL-1α or TNF-α, whereas IFN-γ had no effect even at the highest concentration (not shown). The ELISA used (Quantikine® mouse IL-6 assay) has no significant cross-reactivity with any of the inflammatory cytokines studied.
Figure 5.
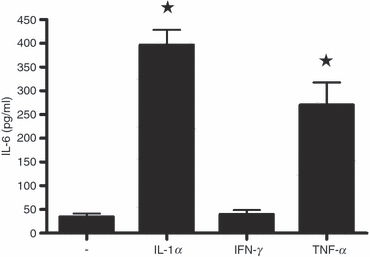
Induction of interleukin-6 (IL-6) in astrocytes by recombinant inflammatory cytokine treatment. Cultures were untreated (−) or treated with 10 ng/ml each of recombinant IL-1α, interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) for 48 hr. Supernatants were centrifuged and tested for the presence of IL-6 by ELISA. The data represent the mean ± SD of triplicate samples. *Significant differences with the untreated control group (−), as determined by the Student's t-test (P < 0·01).
Biological activity of induced IL-6
A specific and sensitive B9 hybridoma proliferation assay was used to detect IL-6 biological activity in the TMEV-infected astrocyte supernatants.30Figure 6(a) shows the biological activity of supernatant dilutions ranging from 1 : 1 to 1 : 1000. The supernatants induced clear dose-dependent proliferative activity on the IL-6-dependent B9 hybridoma. In contrast, the supernatants of cultures infected with Ad.βGal adenovirus, lacking IL-6 according to ELISA (Fig. 4), were devoid of any biological activity. To further demonstrate the specificity of this induction, the above biological activity was completely neutralized by the 6B4 anti-mouse IL-6 monoclonal antibody, but not by an anti-IL-1α antibody, used as a negative control (Fig. 6b).
Figure 6.
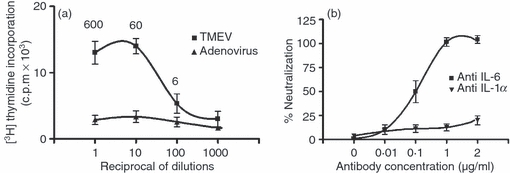
Biological activity of secreted interleukin-6 (IL-6) tested in the B-9 hybridoma assay. The mean counts/min (c.p.m) of [3H]thymidine incorporation was plotted against the dilution of the supernatants of the Theiler's murine encephalomyelitis virus (TMEV) -infected or Ad.βGal adenovirus-infected (multiplicity of infection 10, 24 hr post-infection) astrocyte cultures. The IL-6 concentrations in 600–606 pg/ml, as determined by ELISA, are also shown at each dilution point (a). (b) The neutralization of biological IL-6 activity by increasing amounts of antibody against IL-6 or IL-1α (negative control).
IL-6 in CSF of SJL/J mice after intracerebral infection
Intracerebral infection with the BeAn strain of TMEV induced transitory encephalitis in SJL/J mice followed by chronic demyelination.2 To investigate the pathological importance of these findings, the levels of IL-6 in the CSF of infected animals were measured at different times after intracerebral injection. Concentrations of IL-6 were determined by ELISA during the acute encephalitis phase (days 1–24 post-infection). A sharp CSF IL-6 peak was detected on days 2–4 (Fig. 7). On days 10–24 baseline levels were recovered. This production of IL-6 runs parallel with viral replication in the cerebral hemispheres, which peaks on day 3–4 p.i., declining by 1000-fold on days 10–11, as determined by qPCR.40
Figure 7.
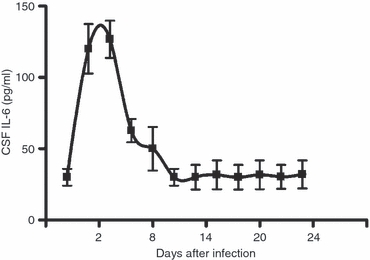
Interleukin-6 (IL-6) levels in the cerebrospinal fluid (CSF) of SJL/J mice infected intracranially with Theiler's murine encephalomyelitis virus (TMEV; 2 × 106 plaque-forming units/injection), measured from 1 to 24 days post-infection (p.i.). The data represent the mean ± SD of five individual CSF samples.
Inhibition of TMEV-induced over-expression by SERMs
Five different SERMs of proven anti-inflammatory activity were studied in this in vitro model of inflammation. Figure 8(a) shows a clear inhibitory effect of all of these SERMs on astrocyte production of IL-6 (as determined by ELISA), reducing its secretion into the culture medium by 85–95%. Quantitative PCR also showed a concomitant, highly significant reduction in TMEV-induced levels of expression of IL-6 mRNA (Fig. 8b). The SERM molecules tested almost completely abrogated TMEV-induced IL-6 over-expression, demonstrating a strong anti-inflammatory effect in this in vitro model. Both test concentrations, 10−11 m for 17β-oestradiol and 10−9 m for the others molecules, were previously determined by titration as the lowest physiological concentrations inducing strong inhibitions (not shown). The identical and total potency of all SERMs in the blockade of TMEV-induced IL-6 production was quite reproducible at the above concentrations.
Figure 8.
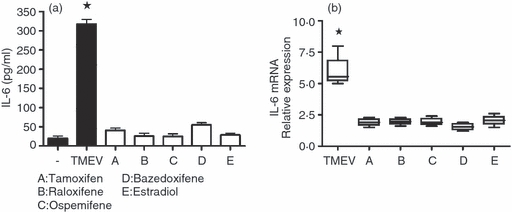
(a) Inhibition by different selective oestrogen receptor modulators (SERMs) of interleukin-6 (IL-6) secretion into the culture supernatants of astrocytes infected with Theiler's murine encephalomyelitis virus [TMEV; multiplicity of infection (MOI) of 1, 24 hr post-infection (p.i.)]. The presence of IL-6 was quantified using ELISA. Oestrogen concentrations were 10−11 m for E (oestradiol) and 10−9 m for all others. (b) Inhibition of IL-6 mRNA in TMEV-infected SJL/J astrocytes by SERMs. The relative expression of messenger RNA was determined by quantitative PCR, as explained in Fig. 1. Oestrogen concentrations were the same as in (a); the MOI was 1. The results represent mean values ± SD of quadruplicate samples. *Significantly different with respect to mock-infected cultures, as determined by the Student's t-test (P < 0·01).
Discussion
Inflammation aids in the combating of tissue injury and infection, helping to restore normal biological conditions.41 Communication between immune and resident cells at the site of inflammation is essential to the success of any inflammatory defence episode, and IL-6 is a crucial mediator of inflammatory processes.7,42 In contrast to the old idea that regarded the CNS as an immune-privileged site, it is now clear that immune cells migrate across the blood–brain barrier in pathological conditions. When foreign antigens enter the CNS, specific immune cells accumulate in the brain parenchyma. These cells, however, are also involved in chronic CNS inflammatory disorders such as multiple sclerosis.2
In the TMEV experimental model for multiple sclerosis it has been proposed that cytokines and chemokines released by astrocytes and other cells at the site of infection recruit neutrophils and virus-specific CD4+ T cells into the CNS. Unfortunately, these cells could trigger demyelination via a non-specific bystander response that induces the stripping of myelin.3 The over-production of IL-6 in infected astrocytes suggests that this cytokine is one of the candidates inducing this (although IL-6 does not recruit neutrophils43), along with CXCL1.44
In the present work, the Affymetrix U74v2 DNA microchip detected over-expression of a gene coding for IL-6 in SJL/J murine astrocytes infected with the BeAn strain of TMEV. These results were validated by qPCR, which showed a statistically significant increase in IL-6 mRNA when using IL-6-specific primers (Fig. 1). This mRNA was labile and was almost completely degraded after 4 hr. Its Gene Ontology database notation indicated that the product of the gene is transported outside the cell; IL-6 is released into the culture medium and can be detected and quantified by ELISA. Both, mRNA and ELISA analysis showed that an MOI of 10 was needed for maximum induction and that peak IL-6 mRNA production took place 4 hr p.i. Further, IL-6 released to the tissue culture supernatants for the period 8–24 hr p.i. was biologically active, fully supporting the growth of the IL-6-dependent B6 hybridoma in a proliferation assay (Fig. 6). In addition, astrocyte IL-6 production was less than that reported for macrophages, the main producers of IL-6.34,35 Infection with TMEV induced IL-6 production in macrophages with 20 times greater efficiency, but with somewhat different kinetics and infective dose requirements (Fig. 3). Induction of IL-6 appeared to be specific because no induction of IL-18 was seen in the astrocytes or macrophages. Finally, intact and infective TMEV particles were needed to induce IL-6 production; neither Ad.βGal adenovirus nor HPLC-purified capsid proteins induced any such synthesis (Fig. 4).
It has been reported that IL-6 interacts with other inflammatory mediators such as IFN-γ.43–45 The possible induction of IL-6 synthesis by this mediator and other related inflammatory cytokines, such as IL-1α and TNF-α, was therefore studied. Surprisingly, recombinant IL-1α and TNF-α were very good inducers of IL-6 in astrocyte cultures, leading to releases of 400 and 250 pg/ml to the medium, respectively. In contrast, IFN-γ had no such effect (Fig. 5). Other authors have reported similar results in rat astrocytes.46 We previously reported that the BeAn strain also induces IL-1α and TNF-α in SJL/J astrocytes,47 suggesting that interaction occurs between these and IL-6 production.
The present results confirm the over-expression of biologically active IL-6 by TMEV-infected astrocytes, which in turn could recruit immune cells to the site of infection inside the CNS. The recruited cell infiltrates, including macrophages and T cells, were found in the white matter late in the chronic infection (between 1 and 6 months, p.i.2,37,40). Therefore, the in vivo IL-6 production 2–8 days p.i. must be accomplished by a brain-resident cell population present at the moment of injection, i.e. the astrocytes.
This confirms the usefulness of this experimental model of neuroinflammation.
The use of monoclonal antibodies to neutralize the IL-6 receptor has already been proven very effective in the treatment of Crohn's disease48 and rheumatoid arthritis.49 In addition, the five SERM molecules investigated had remarkable effects on IL-6 synthesis at both the mRNA and protein levels. The similar effect of all the SERMs tested on IL-6 expression may appear surprising considering that different SERMs recruit different co-regulators to the transcriptional complex associated with the oestrogen receptors and therefore, have a dissimilar effect on oestrogen-receptor-mediated transcription. However, previous studies have shown that oestradiol and SERMs regulate the expression of pro-inflammatory molecules by a common mechanism involving the inhibition of nuclear translocation of nuclear factor-κB p65. This inhibition is mediated by oestrogen receptors but it is independent from oestrogen-receptor-mediated transcription.50,51 The SERMs would therefore appear to have an important anti-inflammatory role, although final tests in vivo would be necessary to open the possibility of using our oestrogenic substances in therapeutic roles, as suggested by other authors.19 The effect of oestradiol and SERMs may involve the oestrogen receptors expressed in naive astrocytes52 and in reactive astrocytes under different pathological conditions.53,54 Certainly, oestradiol is down-regulated in astroglial proliferation after stab wound brain lesions19,55 and in an experimental model of Parkinson's disease.56 Oestrogen-induced reduction of IL-6 production might play a neuroprotective role by neutralizing the pathological increase in IL-6 mRNA expression in wound-injured brains13 and in the spinal cords of TMEV-infected SJL/J mice.40 Finally, the SERM molecules used in the present work also significantly reduce the induction of IL-6 in astrocytes subjected to lipopolysaccharide inflammatory treatment, as recently demonstrated by our laboratory.50
Acknowledgments
This work was funded by the Ministerio de Educacion y Ciencia, Spain (grant number: BFU 2008-02950-C03-01/BFI) and the European Union (EWA Project; grant number: LSHM-CT-2005-518245). Marie Cerciat was funded by a European Erasmus Fund studentship.
Disclosures
The authors declare no conflict of interest.
References
- 1.Theiler M. Spontaneous encephalomyelitis of mice, a new virus disease. J Exp Med. 1937;65:705–19. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipton HL, Dal Canto M. Theiler's virus induced demyelination: prevention by immunosuppression. Science. 1976;192:62–4. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- 3.Clatch RJ, Lipton HL, Miller SD. Characterization of Theiler's murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986;136:920–7. [PubMed] [Google Scholar]
- 4.Roos RP, Firestone S, Wollmann R, Variakojis D, Arnason BGW. The effect of short-term and chronic immunosuppression on Theiler's virus demyelination. J Neuroimmunol. 1982;2:223–34. doi: 10.1016/0165-5728(82)90057-1. [DOI] [PubMed] [Google Scholar]
- 5.Morita E, Sundquist WI. Retrovirus budding. Annu Rev Cell Dev Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- 6.Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–94. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 7.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–8. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 8.Teranishi T, Hirano T, Arima N, Onoue K. Human helper T cell factor (s) (ThF). II. Induction of IgG production in B lymphoblastoid cell lines and identification of T cell-replacing factor- (TRF) like factor (s) J Immunol. 1982;128:1903–8. [PubMed] [Google Scholar]
- 9.Hirano T. Interleukin 6 and its receptors: ten years later. Int Rev Immunol. 1998;16:249–84. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 10.Joseph D, Grun JL, Lublin FD, Knobler RL. Interleukin-6 induction in vitro in mouse brain endothelial cells and astrocytes by exposure to mouse hepatitis virus (MHV-4, JHM) J Neuroimmunol. 1993;42:47–52. doi: 10.1016/0165-5728(93)90211-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–94. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA. 1989;86:6348–52. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu Yan H, Alcaros Banos M, Herregodts P, Hooghe R, Hooghe-Peter EL. Expression of interleukin (IL)-1β, IL-6 and their respective receptors in the normal rat brain and after injury. Eur J Immunol. 1992;22:2963–71. doi: 10.1002/eji.1830221131. [DOI] [PubMed] [Google Scholar]
- 14.Kang MH, So EY, Park H, Kim BS. Replication of Theiler's virus requires NF-κB-activation: higher viral replication and spreading in astrocytes from susceptible mice. Glia. 2008;56:942–53. doi: 10.1002/glia.20668. [DOI] [PubMed] [Google Scholar]
- 15.Van Heteren JT, Rozenberg F, Aronica E, Troost D, Lebon P, Kuijpers TW. Astrocytes produce interferon-α and CXCL10, but not IL-6 or CXCL8 in Aicardi–Goutières syndrome. Glia. 2008;56:568–78. doi: 10.1002/glia.20639. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki S, Brown SM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–15. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 18.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Kahan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2007;25:3039–46. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- 20.Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147:2263–72. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- 21.Tapia-Gonzalez S, Carrero P, Pernia O, Garcia-Segura LM, Diaz-Chaves Y. Selective oestrogen receptor (ER) modulators reduce microglia reactivity in altered peripheral inflammation: potential role of microglial ERs. J Endocrinol. 2008;198:219–30. doi: 10.1677/JOE-07-0294. [DOI] [PubMed] [Google Scholar]
- 22.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–94. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor β. Endocrinol Rev. 2005;26:456–78. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- 24.Ralston SH, Russel RG, Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990;5:983–8. doi: 10.1002/jbmr.5650050912. [DOI] [PubMed] [Google Scholar]
- 25.Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17β-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NFκB by the estrogen receptor. FEBS Lett. 1997;409:79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- 26.Rubio N, Martin-Clemente B, Lipton HL. High-neurovirulence GDVII virus induces apoptosis in murine astrocytes through tumor necrosis factor (TNF)-receptor and TNF-related apoptosis induced ligand. Virology. 2003;311:366–75. doi: 10.1016/S0042-6822(03)00157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio N, Cuesta A. Lack of cross-reaction between myelin basic proteins and putative demyelinating virus envelope proteins. Mol Immunol. 1989;26:663–8. doi: 10.1016/0161-5890(89)90049-7. [DOI] [PubMed] [Google Scholar]
- 28.Rubio N, Martin-Clemente B. Binding of adenovirus to its receptors in mouse astrocytes induces c-fos proto-oncogene and apoptosis. Virology. 2002;297:211–9. doi: 10.1006/viro.2002.1426. [DOI] [PubMed] [Google Scholar]
- 29.Lipton HL, Friedman A. Purification of Theiler's murine encephalomyelitis virus and analysis of the structural virion polypeptides: correlation of the polypeptide profile with virulence. J Virol. 1980;33:1165–72. doi: 10.1128/jvi.33.3.1165-1172.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aarden L, De Groot ER, Schaap OL, Lansdorp PM. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–6. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 31.DeGregorio M, Kangas L, Haerkoenen P, Vaeaenaenen K, Laine A, Wiebe V. Triphenylethylenes for the prevention and treatment of osteoporosis. 1996:WO9607402. [Google Scholar]
- 32.Raveendranath P, Zeldis J, Potoski JR, Ren J. Novel aryloxy-alkyl-dialkyldiamines. 1998:WO9919293. [Google Scholar]
- 33.Cisneros E, Latasa MJ, García-Flores M, Frade JM. Instability of Notch1 and Delta 1 mRNAs and reduced Notch activity in vertebrate neuroepithelial cells undergoing S-phase. Mol Cell Neurosci. 2008;37:820–31. doi: 10.1016/j.mcn.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 35.Van Snick J, Cayphas S, Vink A, Uyttenhove C, Coulie PG, Rubira MR, Simpson RJ. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci USA. 1986;83:9679–83. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jelachich ML, Bandyopadhyay P, Blum K, Lipton HL. Theiler's virus growth in murine macrophage cell lines depends on the state of differentiation. Virology. 1995;209:437–44. doi: 10.1006/viro.1995.1276. [DOI] [PubMed] [Google Scholar]
- 37.Schlitt BP, Matthew F, Jelachich ML, Lipton HL. Apoptotic cells, including macrophages, are prominent in Theiler's virus-induced inflammatory, demyelinating lesions. J Virol. 2003;77:4383–8. doi: 10.1128/JVI.77.7.4383-4388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 39.Ushio SH, Namba M, Okura T, et al. Cloning of the cDNA for human IFN-γ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–9. [PubMed] [Google Scholar]
- 40.Trottier M, Schlitt B, Kung AY, Lipton HL. Transition from acute to persistent infection requires active viral replication that drives proinflammatory cytokine expression and chronic demyelinating disease. J Virol. 2004;78:12480–8. doi: 10.1128/JVI.78.22.12480-12488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dallegri F, Ottonello L. Tissue injury and neutrophilic inflammation. Inflamm Res. 1997;46:382–91. doi: 10.1007/s000110050208. [DOI] [PubMed] [Google Scholar]
- 42.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–54. [PubMed] [Google Scholar]
- 43.McLoughlin RM, Witowski J, Robson RL, et al. Interplay between IFN-γ and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest. 2003;112:598–607. doi: 10.1172/JCI17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubio N, Sanz-Rodriguez F. Induction of CXCL1 (KC) chemokine in mouse astrocytes by infection with the murine encephalomyelitis virus of Theiler. Virology. 2007;358:98–108. doi: 10.1016/j.virol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, Nguyen VA, Gao B. Opposing roles for STAT-1 and STAT-3 in T-cell mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–13. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benveniste EN, Sparacio SM, Norris JG, Grenett HE, Fuller GM. Induction and regulation of interleukin-6 gene expression in rat astrocytes. J Neuroimmunol. 1990;30:201–12. doi: 10.1016/0165-5728(90)90104-u. [DOI] [PubMed] [Google Scholar]
- 47.Sierra A, Rubio N. Theiler's murine encephalomyelitis virus induces tumor necrosis factor-α in murine astrocyte cell cultures. Immunology. 1993;78:399–404. [PMC free article] [PubMed] [Google Scholar]
- 48.Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989–96. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Choy EHS, Isenberg DA, Garrood T, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002;46:3143–50. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 50.Cerciat M, Unkila M, Garcia-Segura LM, Arévalo M-A. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-γ-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia. 2009;58:93–102. doi: 10.1002/glia.20904. [DOI] [PubMed] [Google Scholar]
- 51.Ghisletti S, Meda C, Maggi A, Vegeto E. 17β-estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Mol Cell Biol. 2005;25:2957–68. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Ovejero D, Azcoitia I, DonCarlos LL, Melcangi RC, Garcia-Segura LM. Glia–neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Rev. 2005;48:273–86. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi N, Tonchev AB, Koike K, Muramaki K, Yamada K, Yamashima T, Inoue M. Expression of estrogen receptor-β in the postischemic monkey hippocampus. Neurosci Lett. 2004;369:9–13. doi: 10.1016/j.neulet.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 54.Sakuma S, Tokuhara D, Hattori H, Matsuoka O, Yamano T. Expression of estrogen receptor α and β in reactive astrocytes at the male hippocampus after status epilepticus. Neuropathology. 2008;29:55–62. doi: 10.1111/j.1440-1789.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Estrada J, Luquin S, Fernandez AM, Garcia-Segura LM. Dehydroepiandrosterone, pregnenolone and sex steroids down-regulate reactive astroglia in male rat brain after a penetrating brain injury. Int J Dev Neurosci. 1999;17:145–51. doi: 10.1016/s0736-5748(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 56.Tripanichkul W, Sripanichkulchai K, Finkelstein DI. Estrogen down-regulates glial activation in male mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication. Brain Res. 2006;1084:28–37. doi: 10.1016/j.brainres.2006.02.029. [DOI] [PubMed] [Google Scholar]


