Abstract
The human leucocyte antigen HLA-B27 is strongly associated with ankylosing spondylitis, a form of seronegative inflammatory arthritis. In this study aspects related to several hypothesized mechanisms of disease pathogenesis have been investigated. Blood monocyte-derived dendritic cells (DC) from a small patient cohort of 29 patients with ankylosing spondylitis and one with reactive arthritis, were compared with DC from 34 healthy control subjects, of whom four were found to be HLA-B27 positive. The ability of HLA-B27 to form heavy-chain dimers reactive with monoclonal antibody HC10 was tested, along with the induction of endoplasmic reticulum (ER) stress, assessed by splicing xbp1 mRNA and immunoblotting of Immunoglobulin Binding Protein (BiP). Additionally, the protein expression levels of the ER resident aminopeptidase gene ERAP1 in patients with ankylosing spondylitis was also determined, following its recent identification as a novel disease-associated gene. No significant difference was noted in the global levels of HC10-reactive MHC class I dimers formed in either the patient or control DC populations. Stress on the ER, as determined by xbp1 mRNA splicing, was not detected but lower levels of BiP were observed in the DC from patients. Of further potential interest, in this patient cohort the expression of ERAP1 appeared to be higher in a number of patient DC samples when compared with controls, suggesting over-expression of ERAP1 as a mechanism promoting ankylosing spondylitic pathogenesis.
Keywords: Antigen presentation, Arthritis/rheumatoidarthritis, MHC/HLA, Rheumatology/Rheumatic disease
Introduction
Ankylosing spondylitis (AS) is a form of seronegative inflammatory arthritis whose strong genetic association with the human leucocyte antigen HLA-B27 has been known for almost 40 years, with 90–95% of patients with AS expressing HLA-B27.1,2 HLA-B27 is a functional MHC class I molecule, presenting short peptides, normally about eight or nine amino acids in length, to T lymphocytes of the immune system. Initially it was thought that disease pathogenesis was driven by auto-reactive CD8+ T cells, however, in the absence of a bona fide auto-antigen and because spondyloarthritis still occurs in the absence of CD8+ T cells in disease-prone HLA-B27 transgenic rats,3 alternative features of the HLA-B27 molecule and its putative role in the aetiology of the disease have been proposed, including the formation of HLA-B27 heavy-chain dimers, and the induction of endoplasmic reticulum (ER) stress.4,5
The assembly kinetics of HLA-B27 in the ER is slower than many other MHC class I molecules, primarily as a result of the specific residues forming the B-pocket of the peptide-binding groove.6,7 This increase in the time taken for the heavy chain to fold and exit the ER appears to contribute to the accumulation of misfolded ER-resident molecules, which leads to ER stress and the initiation of the unfolded protein response (UPR).8 Associated with the initiation of the UPR there is also an up-regulation of the ER chaperone Immunoglobulin Binding Protein (BiP), which retains partially folded polypeptides in the ER until correct folding or degradation can occur.
In addition to HLA-B27 misfolding in the ER, heavy-chain dimerization both in the ER and at the cell surface have been documented.6,9,10 HLA-B27 contains a relatively rare unpaired cysteine at position 67 in the peptide-binding groove, which has been implicated in the formation of these cell surface heavy-chain dimers, generated via recycling of unstable fully folded class I heterotrimers on the cell surface of human lymphoid cell lines,10 on stimulated lymphoid cells from B27+ transgenic rats and on synovial and peripheral blood mononuclear cells of B27+ patients with AS.11–13 Additionally, we have previously shown that in both the dendritic-cell-like cell line KG1 transfected with HLA-B27 and in primary human monocyte-derived dendritic cells (DC) from HLA-B27+ individuals, dimers can be detected upon DC maturation.14
Most recently, genome-wide screens using single nucleotide polymorphisms have identified other genes with significant associations to the disease 15 including the interleukin-23 receptor, and ER aminopeptidase 1 (ERAP1/ARTS1), which trims peptides before loading onto MHC1 molecules in the ER. This study looks at MHC class I dimer formation, ER stress reporter molecules, and ERAP1 expression in a small cohort of 29 patients with AS and one patient with reactive arthritis and compares them with 34 healthy control subjects, of whom four were HLA-B27 positive.
Materials and methods
Blood samples and cell isolation
The study was ethically reviewed by the NHS National Research Ethics Service and the local medical school ethics committee. Informed written consent was obtained from donors before blood collection. For primary monocyte-derived DC, peripheral blood mononuclear cells were isolated by centrifugation over Histopaque (Sigma, Poole, UK). Monocytes were allowed to adhere to plastic dishes for 2 hr in RPMI-1640 medium plus 10% heat-inactivated fetal bovine serum and kanamycin (Gibco, Paisley, UK). Non-adherent cells were then removed and fresh medium containing 50 ng/ml interleukin 4 and 50 ng/ml granulocyte–macrophage colony-stimulating factor (R and D Systems, Abingdon, UK) were added to the culture. The DC were allowed to differentiate for 5 days before treatment with 100 ng/ml lipopolysaccharide (LPS; Sigma). Samples were harvested at 0, 24, 48 and 72 hr.
Patient and control cohorts
Twenty-nine patients with AS and one patient with reactive arthritis were recruited to the study via the rheumatology clinics of NHS Tayside. There was a 2·75 : 1 ratio of males to females within the patient group. All patients were asked to fill in Bath AS Functional Index (BASFI) questionnaires at the time of donation. BASFI scores were obtained from all but patient 30 and they varied from 0·31 to 9·34. Patient treatment regimens included non-steroidal anti-inflammatory drugs, disease-modifying anti-rheumatic drugs and anti-tumour necrosis factor-α antibody agents. A total of 95 healthy control individuals were screened for HLA-B27 by flow cytometry and confirmed using B27 gene-specific amplification (Olerup SSP B27*PCR kit, VWR). Four individuals were identified as HLA-B27 positive; these and 30 of the HLA-B27-negative healthy control subjects made up the control cohort.
Antibodies
HC10 (anti HLA-B and HLA-C) was a gift from J. Neefjes (Amsterdam, the Netherlands). Anti-ERAP1 and anti-BiP were obtained from AbCam (Cambridge, UK). Anti-actin was from Sigma. The FITC-conjugated anti-HLA-B27 was obtained from VH-Bio (Gateshead, UK). Horseradish peroxidase-coupled anti-mouse IgG was obtained from Sigma.
Flow cytometry
Cells were resuspended in PBS with 2% fetal bovine serum and 0·1% sodium azide and the cell surface was stained with FITC anti-HLA-B27. Samples were analysed on a FACScan (BD Biosciences, Oxford, UK) using CellQuest software (BD Biosciences).
Preparation of whole cell lysates and immunoblotting
Cells were harvested by centrifugation and alkylated on ice for 10 min in 50 μl PBS plus 10 mm N-ethyl maleimide (NEM). Samples were then lysed on ice in 50 μl lysis buffer (1% Nonidet P-40, 150 mm NaCl, 10 mm Tris–HCl pH 7·6, 1 mm PMSF, 10 mm NEM). Lysates were centrifuged at 20 000 g for 5 min and the supernatant was heated with an equal volume of non-reducing sample buffer at 80° for 1 min. For ERAP1 and BiP blots samples were heated in reducing sample buffer before SDS–PAGE. Samples were analysed on 8% SDS–PAGE gels, transferred to nitrocellulose (BA85, Whatman, VWR, Lutterworth, UK), blocked in 3% milk in PBS with 0·1% Tween-20 (PBST) and probed with antibodies in PBST. Detection was performed using chemiluminescence with Super Signal West Femto reagents (Perbio, Cramlington, UK) and imaging was on a Fuji LAS-3000 analyser.
RT-PCR of xbp1
The RNA was isolated from peripheral blood lymphocytes (PBL) and DC using an RNeasy Kit from Qiagen (Crawley, UK) as per the manufacturer's instructions. Then, 0·5 μg RNA was treated with DNase1 (Promega, Southampton, UK) and used as a template for the preparation of cDNA using the random priming method, followed by xbp1 PCR using the primers forward 5′-ccttgtagttgagaaccagg-3′ and reverse 5′-ggggcttggtatatatgtgg-3′. β-Actin controls were amplified using primers forward 5′-accccgtgctgctgacc-3′ and reverse 5′-aggaaggaaggctggaagagt-3′.
Results
MHC class I dimer expression on primary monocyte-derived DC
We have previously shown that MHC class I molecules can be detected in both monomeric and dimeric forms in whole cell lysates produced from monocyte-derived DC from the peripheral blood of HLA-B27-positive individuals.14 It was therefore decided to investigate whether there was any difference in dimer formation in this cell type in patients with AS compared with B27-positive and non-B27 healthy controls. Furthermore, DC from HLA-B27 transgenic rats have been shown to display defective interactions.16 In this current study, DC from patients with AS and control participants were matured using LPS and sampled at 0, 24, 48 and 72 hr after stimulation. Figure 1 shows immunoblot examples of HC10-reactive (recognizing B and C alleles) MHC class I dimers, analysed after non-reducing SDS–PAGE. Also shown is a patient sample (patient 21) analysed under both non-reducing and reducing conditions to demonstrate the loss of dimer signal after disulphide bond reduction. Although the HLA-B27 patient-derived samples displayed dimers (including a novel higher molecular species in some samples, such as patient 14), dimers were also detected in the HLA-B27-positive controls and in some of the non-HLA-B27 samples, for example controls 21 and 43. The formation of these dimer structures was further studied by immunoprecipitation of detergent-lysed DC, which isolated dimers recognized by the conformation-dependent antibody W6/32, and only low levels of HC10-reactive material (not shown). This suggests that a population of fully folded MHC class I dimers exists in these samples, which may be the cellular corollary of the dimer structures recently identified on exosome vesicles.17Figure 2 shows the relative levels of MHC class I dimers normalized to the basal level at T0 in both control and patient populations. With the exception of patients 27, 28 and 29 there is little change in the amount of dimers formed over time within the patient population. Controls 2 and 4 (HLA-B27-positive control subjects) display dimer formation over time, as do controls 21, 69 and 70. Because of the appearance of MHC class I dimer bands in both non-HLA-B27 and HLA-B27-positive control samples, these data suggest that immunoblotting with antibodies such as HC10 does not constitute a good biomarker for AS.
Figure 1.

MHC class I dimer formation in patients with ankylosing spodylitis (AS) and in control dendritic cells (DC). Representative immunoblots of HC10-reactive dimers from selected B27-positive patients with AS (patients 27, 15 and 14, top panels), B27-positive healthy controls 2 and 4 (middle panels), and non-B27-positive healthy controls 21, 40, 43 and 70 (bottom panels). Within each panel the samples represent T0, T24, T48 and T72 post-activation with LPS, from left to right. Monomeric and dimeric MHC class I species are indicated. A blot of non-reduced (NR) and reduced (R) samples of patient 21 (P21) is also included. The reduced monomer migrates more slowly because of disruption of internal structural disulphide bonds.
Figure 2.
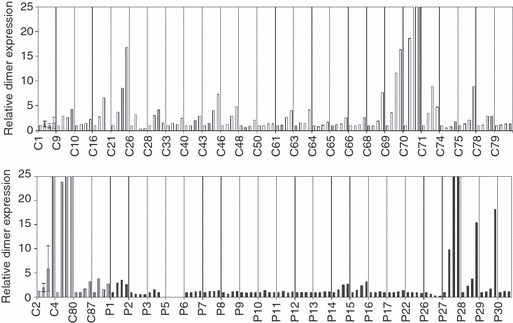
Relative increase in dimer levels with data normalized to T0. Samples were taken at T0, T24, T48 and T72 after lipopolysaccharide (LPS) activation and these are plotted for each sample. Non-B27 healthy controls (shown in white; C1, 9, 10, 16, 21, 26, 28, 33, 40, 43, 46, 48, 50, 61, 63, 64, 65, 66, 68, 70, 71, 74, 75, 78, 79); B27-positive healthy controls (shown in grey; C2, 4, 80 and 87) and patient samples (shown in black; P1, P2, P3, P5, P6, P7, P8, P9, P10, P11, P12, P13, P14, P15, P16, P17, P22, P26, P27, P28, P29, P30).
BiP levels in primary monocyte-derived DC and PBL
Studies in transgenic HLA-B27 rats have shown that HLA-B27 associates with the ER chaperone BiP,8 and is up-regulated in disease-prone HLA-B27 transgenic rats.17 We therefore investigated global expression levels of BiP by immunoblotting our patient primary monocyte-derived DC and a subset of PBL samples. Figure 3(a) shows average global BiP levels in immature (unstimulated) and mature (72-hr activation with LPS) primary monocyte-derived DC and Fig. 3(b) shows a subset of data generated from PBL. No significant difference in the average levels of BiP was observed between immature and mature (LPS-stimulated) DC samples in either the non-HLA-B27 controls or in the patient cohort. However, we noted a significant reduction in the overall BiP levels in the patient samples when compared with the non-B27 control population. No significant difference was noted in the PBL samples.
Figure 3.

Average global BiP levels in patient and control samples. (a) Data from dendritic cell (DC) cultures and (b) data from peripheral blood lymphocytes (PBL). (a) Samples marked immature DC represent samples taken at T0 whereas those marked mature DC were taken at T72 after activation with LPS; n = 24 for immature DC and n = 27 for mature DC in the non B27 control population (shown in white) and n = 24 for immature and n = 25 for mature DC in the patient population (shown in grey). P-values are calculated using Mann–Whitney U-test for both immature and mature DC. (b) Subset of the control and patient PBL samples: n = 4 for non-B27 controls and n = 5 for AS patient samples. Standard deviations are shown.
Xbp1 splicing in AS patient-derived DC and PBL
XBP1 is a constitutively expressed transcription factor that undergoes alternative mRNA splicing by phosphorylated IRE1 under conditions of ER stress, leading to the removal of a 26-bp segment and a change in reading frame. This gives rise to a potent activator of genes associated with the unfolded protein response. Spliced xbp1, has been documented to occur at enhanced levels in bone-marrow-derived macrophages in disease-prone HLA-B27 transgenic rats.18 We therefore analysed samples from our patient cohort and controls for xbp1 splicing. The cDNA was generated from AS and control samples. Induction of a strong ER stress response using the reducing agent dithiothreitol was used as a positive control (Fig. 4). Splicing of xbp1 was analysed in both PBL and a subset of DC, as spliced xbp1 has also been reported to play a role in DC maturation.19 No significant xbp1 splicing was observed in the patient or control samples, with the exception of one sample (patient 18 PBL) in whom low levels were detected.
Figure 4.

RT-PCR of xbp1mRNA splicing in patient and control peripheral blood lymphocyte (PBL) and dendritic cell (DC) samples. Under conditions of endoplasmic reticulum (ER) stress xbp1 is alternatively spliced and a second, smaller transcript is seen in addition to the unspliced form. (a) xbp1 RT-PCR of control 1 cells (PBL and DC) +/− dithiothreitol (DTT) treatment. (b) DC from patient 33. (c) Representative non-B27 and B27-positive control PBL. (d) Representative patient PBL. Patient 7 has also been included +/− DTT treatment as an additional control.
ERAP1 levels in monocyte-derived dendritic cells
ERAP1 has been implicated in the aetiology of AS through genetic association studies.15,20 Therefore we looked at the relative protein expression levels of ERAP1 in our patient and control populations by immunoblotting of DC lysates. Figure 5(a) shows ERAP1 levels in primary monocyte-derived DC activated with LPS for up to 72 hr. A number of AS patient-derived samples (notably 5, 6, 7 and 9) and the reactive arthritis sample (patient 10) appear to express higher levels of ERAP1 compared with the non-HLA-B27 controls. HLA-B27-positive control sample 87 expressed ERAP1 at levels similar to patient-derived samples 5, 6, 7, 9 and 10 but because of its size (n = 4), the B27-positive control group has not been included in the statistical analyses. Figure 5(b) shows non-B27 control and patient data ranked according to protein expression levels of ERAP1. Overall, the patient group (n = 25) has significantly higher ERAP1 expression levels than the non-B27 control group (n = 28) (P = 0·01 Mann–Whitney U-test). There was no observed correlation with either BASFI score or treatment regimen within the patient cohort.
Figure 5.

Endoplasmic reticulum aminopepidase 1 (ERAP1) protein levels in patient and control samples. (a) Both immature (T0) and mature (T72) dendritic cells (DC) were analysed for ERAP1 expression. Sample concentrations were corrected using β-actin. Immature DC are represented in white and mature DC in black. Non-B27 healthy controls (C5, 9, 10, 16, 17, 19, 20, 21, 26, 28, 33, 40, 44, 46, 48, 50, 61, 63, 64, 65, 66, 68, 69, 70, 71, 74, 75, 78, 79); B27-positive healthy controls (C2, 4, 80, 87) and patient samples (P1, 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 26, 27, 28, 29, 30). Where standard deviations are included, n > 3. (b) Mature DC samples were ranked according to ERAP1 protein expression levels; n = 28 in non-B27 control group and n = 25 in AS patient group. Means are shown as horizontal lines. P-value of 0·01 was calculated using Mann–Whitney U-test. The B27-positive control group were not included in these analyses because of the small size of the cohort.
Discussion
HLA-B27 is estimated to contribute the majority of the genetic susceptibility to AS and yet despite extensive analysis, its exact role in the aetiology of the disease still remains elusive. A number of theories have emerged relating to various aspects of the HLA-B27 molecule, from its classical role in antigen presentation to its propensity to misfold during assembly in the ER and to undergo dimerization.4
Although the formation of dimers in the ER, as a consequence of misfolding, and at the cell surface, because of unfolding, are thought to be distinct populations, their overall analysis has proven problematic, primarily because of the absence of a dimer-specific antibody. Most studies use HC10, which recognizes a wide range of HLA-B and HLA-C molecules, but which appears capable of recognizing both fully unfolded HLA-B molecules, and a population which is weakly bound to β2-microglobulin (S. Powis, unpublished observations). As such, it represents a limitation of the full characterization of HLA-B27 dimers and their role in AS. To try to understand this situation more clearly we have looked at dimer levels from whole cell lysates derived from a cohort of AS patients to determine whether this measure in itself could be useful as a biomarker for disease.
In a previous study, we have detected dimer formation in monocyte-derived DC from HLA-B27-positive individuals.14 Dimer formation occurred on maturation of the DC, after stimulation with LPS, and these dimers appeared to be transitory, indicating that they may only be visible to the immune system for specific periods of time. In contrast, in the current study, dimer appearance was more complex, and varied both quantitatively and qualitatively, with the size and resolution of the dimer band altering between individuals, a phenomenon that could reflect the specific alleles expressed and the different conformations that are generated within the oxidizing environment of the ER.21 Overall, there was no significant difference between either the increase in dimer formation over time or the basal level of dimers relative to the total amount of class I in our patient or control populations, indicating that under these experimental conditions (stimulating via Toll-like receptor 4 using LPS) this is probably not a good biomarker of disease.
The unexpected presence of HC10-reactive dimers in our non-HLA-B27 control samples led us to perform immunoprecipitation studies on lysates of several DC samples. These indicated the presence of W6/32-reactive dimers (not shown). We have previously detected a similar population of MHC class I dimers on exosomes, formed through disulphide linkage of cysteines in the cytoplasmic tail domains of many HLA-A and HLA-B molecules, and induced by a lack of a reducing environment in exosomes.17 We are currently investigating the possible source of these W6/32-reactive dimers on cells.
The induction of ER stress and subsequent up-regulation of BiP and splicing of xbp1 have been well documented in the disease-prone HLA-B27 transgenic rat model.18,22 In contrast, we found no significant up-regulation of BiP protein or xbp1 splicing in the PBL or DC of AS patient samples. In fact we detected an overall lower level of BiP protein in the patients’ samples. It is possible that the difference in our results may be related to the cells under investigation: in the rat model, bone-marrow-derived cells or spleen cells are usually used, whereas peripheral blood cells are the source cells in this human study. The PBL from patients with AS may not accurately reflect the ongoing ER stress that may be occurring elsewhere in the body or in a more specific subset of immune cells. However, the lower BiP expression may be an adaptation to stress. It was also surprising that there was no increase in xbp1 splicing in mature (LPS-stimulated) DC compared with immature DC, as was expected from the role of xbp1 in DC maturation.19 However, this too could be because of the nature of the cells under investigation, the cells in this study being heterogeneous in nature as a result of the isolation procedure. Alternatively, HLA-B27 transgenic rats express high levels of HLA-B27, therefore the lack of ER stress seen in this study may merely reflect the difficulty of detecting a stress response because of physiological levels of misfolded HLA-B27 protein.
The recently characterized disease association of ERAP1 with AS may contribute as much as 26% of attributable risk (US population). Harvey et al.20 have identified a number of non-coding non-synonymous single nucleotide polymorphisms in the upstream regulatory region of the gene in patients with AS, which could impact upon ERAP1 expression levels. It is therefore of interest that we report here a subpopulation of HLA-B27-positive individuals that express higher protein levels of ERAP1. It is known that ERAP1 knockout mice present an altered peptide repertoire to CD8+ T cells, which in turn alters the immunodominance of some viral peptides compared with wild-type mice.23,24 It is therefore possible that increased expression levels of ERAP1 could also lead to alterations in the peptide repertoire. Recently, this mechanism has been proposed as a means by which tumour cells may evade host immune surveillance with neoplastic cell lines showing highly variable levels of both ERAP1 and ERAP2, with ERAP1 correlating with MHC1 expression.25
Our patient cohort appears to split into those expressing ERAP1 protein at the same approximate level as the B27-negative control group and a subset of individuals who express ERAP1 at a higher level. Of note, however, a non-AS HLA-B27 control sample also displayed high ERAP1 protein levels. Therefore, a further study, matching ERAP1 levels to ERAP1 polymorphisms in a larger sample size would be of significant value based on our initial observations. Furthermore, the impact upon HLA-B27 in cells over-expressing ERAP1 may provide useful data on the mechanism of AS.
Acknowledgments
This work was supported by the Chief Scientist Office of the Scottish Government.
Disclosure
The authors declare no competing interests.
References
- 1.Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1:904–7. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 2.Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704–6. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- 3.Taurog JD, Dorris ML, Satumtira N, Tran TM, Sharma R, Dressel R, van den Brandt J, Reichardt HM. Spondylarthritis in HLA-B27/human beta2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis Rheum. 2009;60:1977–84. doi: 10.1002/art.24599. [DOI] [PubMed] [Google Scholar]
- 4.Colbert RA, DeLay ML, Layh-Schmitt G, Sowders DP. HLA-B27 misfolding and spondyloarthropathies. Adv Exp Med Biol. 2009;649:217–34. doi: 10.1007/978-1-4419-0298-6_16. [DOI] [PubMed] [Google Scholar]
- 5.Powis SJ, Santos SG, Antoniou AN. Biochemical features of HLA-B27 and antigen processing. Adv Exp Med Biol. 2009;649:210–6. doi: 10.1007/978-1-4419-0298-6_15. [DOI] [PubMed] [Google Scholar]
- 6.Antoniou AN, Ford S, Taurog JD, Butcher GW, Powis SJ. Formation of HLA-B27 homodimers and their relationship to assembly kinetics. J Biol Chem. 2004;279:8895–902. doi: 10.1074/jbc.M311757200. [DOI] [PubMed] [Google Scholar]
- 7.Mear JP, Schreiber KL, Munz C, Zhu X, Stevanovic S, Rammensee HG, Rowland-Jones SL, Colbert RA. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J Immunol. 1999;163:6665–70. [PubMed] [Google Scholar]
- 8.Turner MJ, Sowders DP, DeLay ML, et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol. 2005;175:2438–48. doi: 10.4049/jimmunol.175.4.2438. [DOI] [PubMed] [Google Scholar]
- 9.Allen RL, O'Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999;162:5045–8. [PubMed] [Google Scholar]
- 10.Bird LA, Peh CA, Kollnberger S, Elliott T, McMichael AJ, Bowness P. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol. 2003;33:748–59. doi: 10.1002/eji.200323678. [DOI] [PubMed] [Google Scholar]
- 11.Kollnberger S, Bird L, Sun MY, Retiere C, Braud VM, McMichael A, Bowness P. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002;46:2972–82. doi: 10.1002/art.10605. [DOI] [PubMed] [Google Scholar]
- 12.Kollnberger S, Bird LA, Roddis M, et al. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J Immunol. 2004;173:1699–710. doi: 10.4049/jimmunol.173.3.1699. [DOI] [PubMed] [Google Scholar]
- 13.Tsai WC, Chen CJ, Yen JH, Ou TT, Tsai JJ, Liu CS, Liu HW. Free HLA class I heavy chain-carrying monocytes – a potential role in the pathogenesis of spondyloarthropathies. J Rheumatol. 2002;29:966–72. [PubMed] [Google Scholar]
- 14.Santos SG, Lynch S, Campbell EC, Antoniou AN, Powis SJ. Induction of HLA-B27 heavy chain homodimer formation after activation in dendritic cells. Arthritis Res Ther. 2008;10:R100. doi: 10.1186/ar2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton PR, Clayton DG, Cardon LR, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–37. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacquard-Bouder C, Chimenti MS, Giquel B, Donnadieu E, Fert I, Schmitt A, Andre C, Breban M. Alteration of antigen-independent immunologic synapse formation between dendritic cells from HLA-B27-transgenic rats and CD4+ T cells: selective impairment of costimulatory molecule engagement by mature HLA-B27. Arthritis Rheum. 2007;56:1478–89. doi: 10.1002/art.22572. [DOI] [PubMed] [Google Scholar]
- 17.Lynch S, Santos SG, Campbell EC, Nimmo AM, Botting C, Prescott A, Antoniou AN, Powis SJ. Novel MHC class I structures on exosomes. J Immunol. 2009;183:1884–91. doi: 10.4049/jimmunol.0900798. [DOI] [PubMed] [Google Scholar]
- 18.DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–43. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 2007;204:2267–75. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey D, Pointon JJ, Evans DM, et al. Investigating the genetic association between ERAP1 and ankylosing spondylitis. Hum Mol Genet. 2009;18:4204–12. doi: 10.1093/hmg/ddp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fussell H, Nesbeth D, Lenart I, et al. Novel detection of in vivo HLA-B27 conformations correlates with ankylosing spondylitis association. Arthritis Rheum. 2008;58:3419–24. doi: 10.1002/art.23990. [DOI] [PubMed] [Google Scholar]
- 22.Turner MJ, Delay ML, Bai S, Klenk E, Colbert RA. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: implications for the pathogenesis of spondylarthritis-like disease. Arthritis Rheum. 2007;56:215–23. doi: 10.1002/art.22295. [DOI] [PubMed] [Google Scholar]
- 23.Yan J, Parekh VV, Mendez-Fernandez Y, et al. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J Exp Med. 2006;203:647–59. doi: 10.1084/jem.20052271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.York IA, Brehm MA, Zendzian S, Towne CF, Rock KL. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc Natl Acad Sci U S A. 2006;103:9202–7. doi: 10.1073/pnas.0603095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fruci D, Ferracuti S, Limongi MZ, et al. Expression of endoplasmic reticulum aminopeptidases in EBV-B cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines. J Immunol. 2006;176:4869–79. doi: 10.4049/jimmunol.176.8.4869. [DOI] [PubMed] [Google Scholar]


