Abstract
Mouse models that perturb homocysteine metabolism, including genetic mouse models that result in deficiencies of methylenetetrahydrofolate reductase, methionine synthase, methionine synthase reductase, and cystathionine β-synthase, and a pharmaceutically induced mouse model with a transient deficiency in betaine-homocysteine methyl transferase, have now been characterized and can be compared. Although each of these enzyme deficiencies is associated with moderate to severe hyperhomocyst(e)inemia, the broader metabolic profiles are profoundly different. In particular the various models differ in the degree to which tissue ratios of S-adenosylmethionine to S-adenosylhomocysteine are lowered in the face of elevated plasma homocyst(e)ine, and in the distribution of the tissue folate pools. These different metabolic profiles illustrate the potential complexities of hyperhomocyst(e)inemia in humans, and suggest that comparison of the disease phenotypes of the various mouse models may be extremely useful in dissecting the underlying risk factors associated with human hyperhomocyst(e)inemia.
INTRODUCTION
Hyperhomocyst(e)inemia has been correlated with increased risk for cardiovascular disease [(5), and references cited therein], development of Alzheimer’s disease (6, 36, 56), neural tube defects (39, 51, 59, 60), and other adverse pregnancy outcomes including congenital cardiac defects (26, 51, 57, 69), preeclampsia (11, 44–46), and orofacial clefts (70). It is now well established that administration of folic acid results in significant lowering of plasma total homocyst(e)ine (2, 47, 64, 67), but the results of several large folate therapy clinical trials to reduce the progression of cardiovascular disease have been disappointing (1, 12, 31, 34, 43, 64), while showing promise in others (48, 53, 58). These findings argue against the hypothesis that homocysteine itself is the causative agent of disease, per se. To address this dilemma, it has been proposed that hyperhomocyst(e)inemia may merely be a marker for these disease processes, unrelated to the etiology of disease. However, we also need to consider an alternate hypothesis, namely that hyperhomocyst(e)inemia can result from a number of different genetic and physiological conditions, e.g. dietary, disease-related, or clinically induced, and that the associated pathologies will be directly linked to the particular conditions giving rise to the elevation in plasma total homocyst(e)ine in the first place.
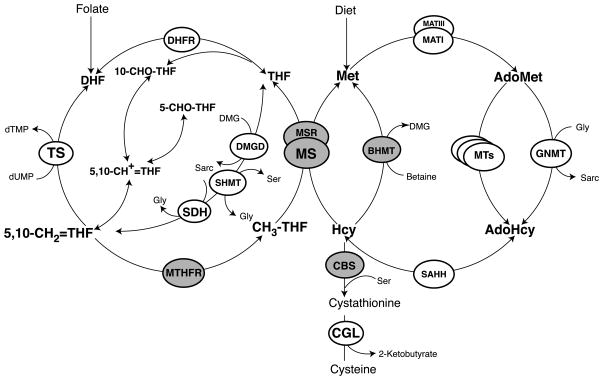
In considering this alternate hypothesis, the various transgenic mouse models for hyperhomocyst(e)inemia are particularly useful, because the detailed metabolite measurements that are possible in the mouse serve to illustrate the very different profiles of hyperhomocyst(e)inemia that are possible in mammals, and may suggest possible approaches to treating human conditions. Each of these mouse genes has a known orthologous human counterpart believed to play an identical role. Interestingly, each of these genes specifies an enzyme that utilizes a vitamin-derived cofactor and/or substrate/product, and methionine itself is an essential amino acid, so that diet becomes an important factor in the development and progression of disease. There are now four transgenic mouse models involving disruption of genes specifying enzymes directly involved in homocysteine metabolism: methylenetetrahydrofolate reductase (4), cystathionine β-synthase (68), methionine synthase (61), and methionine synthase reductase (13). A final mouse model created a transient deficiency in betaine-homocysteine methyltransferase induced by the inhibitor S-(δ-carboxybutyl)-DL-homocysteine (7). Figure 1 shows the reactions catalyzed by each of these enzymes and places them in perspective in mammalian one carbon metabolism.
Figure 1.
Overview of mammalian one carbon metabolism. Four genetic mouse models involving disruptions of enzymes in this pathway have been highlighted in grey as has betaine-homocysteine methyltransferase, where transient enzyme deficiencies have been induced by treatment of mice with an inhibitor. Abbreviations: BHMT, betaine-homocysteine methyltransferase; CBS, cystathionine β-synthase; CGL, cystathionine γ-lyase; DHFR, dihydrofolate reductase; DMGD, dimethylglycine dehydrogenase; GNMT, glycine N-methyltransferase; MAT, methionine adenosyltransferase; MS, cobalamin-dependent methionine synthase; MSR, methionine synthase reductase; MTHFR, methylenetetrahydrofolate reductase; MTs, AdoMet-dependent methyltransferases; SAHH, S-adenosylhomocysteine hydrolase; SDH, sarcosine dehydrogenase; SHMT, serine hydroxylmethyltransferase; TS, thymidylate synthase.
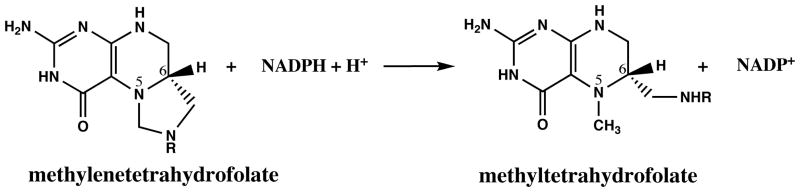
Methylenetetrahydrofolate reductase catalyzes the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, and the mammalian enzyme uses electrons derived from NADPH for this reduction (Figure 2). The electrons flow from NADPH to the flavin adenine dinucleotide cofactor of the enzyme, and thence from the reduced flavin to methylenetetrahydrofolate. This reaction, which is irreversible in vivo (20), commits tetrahydrofolate-bound one carbon units to use in the remethylation of homocysteine to form methionine and removes them from the pool of one carbon units that are used for de novo purine and thymidylate biosynthesis. The enzyme is allosterically regulated by the ratio of S-adenosylmethionine (AdoMet) to S-adenosylhomocysteine (AdoHcy) in the cell (25, 30); AdoMet inhibits the enzyme, while AdoHcy competes with AdoMet but does not affect enzyme activity.
Figure 2.
Reaction catalyzed by methylenetetrahydrofolate reductase.
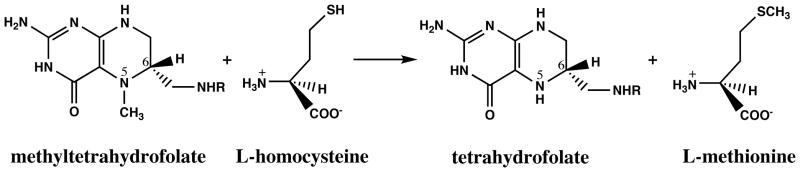
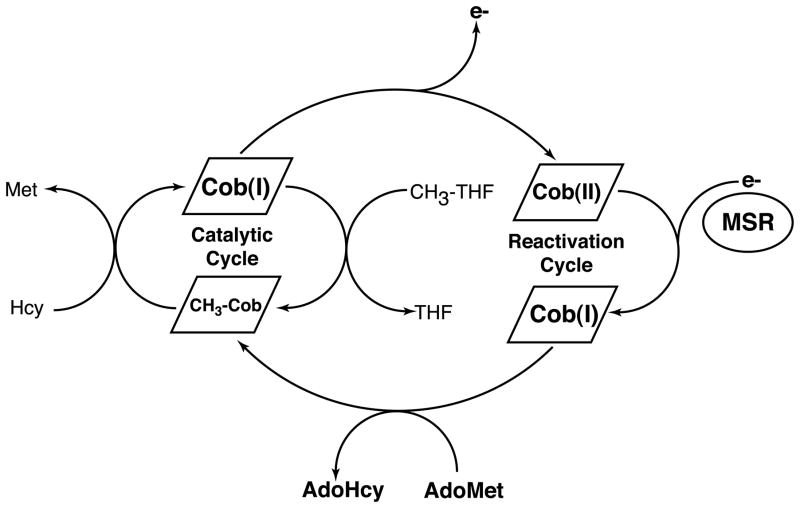
Cobalamin-dependent methionine synthase catalyzes the next step in the regeneration of the methyl group of methionine (Figure 3). In the course of this overall reaction, the methyl group of methyltetrahydrofolate is transferred to the cobalamin cofactor to form methylcobalamin and tetrahydrofolate and then from methylcobalamin to homocysteine to form methionine and to regenerate the cobalamin cofactor in the highly reduced cob(I)alamin form (Figure 4). The cob(I)alamin form of the enzyme is susceptible to oxidation, and about once in every thousand turnovers, forms an inactive cob(II)alamin species. Return of this inactive species to the catalytic cycle requires reduction of cob(II)alamin to cob(I)alamin and remethylation. In mammals, the electron needed for reduction is supplied by methionine synthase reductase, a dual flavoprotein with one flavin adenine dinucleotide and one flavin mononucleotide cofactor (32, 41). The flavin adenine dinucleotide cofactor of methionine synthase reductase accepts electrons from NADPH. The methyl group for reactivation of methionine synthase is supplied by AdoMet, rather than by methyltetrahydrofolate. AdoMet binds to the C-terminal activation domain of methionine synthase. Either dietary deficiency in cobalamin or impaired methionine synthase reductase activity may lead to reduced methionine synthase activity.
Figure 3.
Reaction catalyzed by methionine synthase.
Figure 4.
Reactions catalyzed by mammalian methionine synthase. During catalysis, the cobalamin cofactor of methionine synthase cycles between the methylcobalamin and cob(I)alamin forms, as the cofactor is alternately methylated by methyltetrahydrofolate (CH3-THF) and demethylated by homocysteine to form methionine. Cob(I)alamin is occasionally oxidized to form an inactive cob(II)alamin species; return of this species to the catalytic cycle requires a reductive remethylation of the cofactor in which an electron is provided by the auxiliary protein methionine synthase reductase (MSR) and a methyl group is provided by AdoMet, which is bound to the reactivation module of methionine synthase itself.
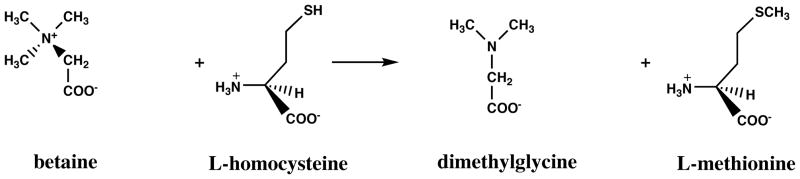
Methionine synthase and methionine synthase reductase are ubiquitously expressed, and in many cells they catalyze the only pathway for the regeneration of the methyl group of methionine. However, in some tissues an alternative pathway for remethylation of methionine is catalyzed by betaine-homocysteine methyltransferase (Figure 5) (37). This enzyme uses betaine, a derivative formed from the metabolism of choline and also present in the diet, as the methyl donor to homocysteine, and does not require methyltetrahydrofolate. Its distribution is limited; the enzyme is abundant in human liver and kidney, but absent in heart and brain.
Figure 5.
Reaction catalyzed by betaine-homocysteine methyltransferase.
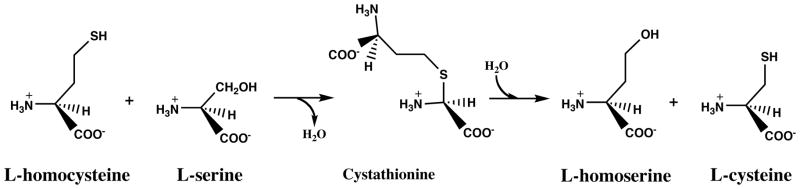
Homocysteine is generated as a result of biological methylation reactions that use AdoMet as a methyl donor (Figure 1). When excess dietary methionine is present, homocysteine can be converted to cystathionine by cystathionine β-synthase (Figure 6); cystathionine is then broken down to form cysteine by cystathionine γ-lyase. Humans with cystathionine β-synthase deficiency develop severe hyperhomocyst(e)inemia and homocystinuria, and their intracellular [AdoMet]:[AdoHcy] ratios are dramatically lowered due to the accumulation of AdoHcy in their tissues.
Figure 6.
Reactions catalyzed by cystathionine β-synthase and cystathionine γ-lyase.
In this review, we will compare the metabolic and physiological profiles associated with hyperhomocyst(e)inemia in each of the five mouse models, pointing out where further experiments might be informative, and we will discuss their relevance to human disease. We will begin with a brief discussion of the published literature on each of these five mouse models:
Cystathionine β-synthase
Homozygous cystathionine β-synthase deficiency in humans leads to extreme hyperhomocysteinemia and elevated plasma methionine. Homozygotes also develop skeletal abnormalities, dislocation of the ocular lens, and thromboembolic disease (40). Heterozygotes typically have metabolite levels that overlap with those of controls and are generally assymptomatic.
The first transgenic mouse model of hyperhomocyst(e)inemia to be developed was a targeted disruption of the Cbs gene specifying cystathionine β-synthase (68). The disruption led to a complete absence of cystathionine β-synthase activity in Cbs(−/−) mice. Nonetheless, matings of Cbs(+/−) pairs resulted in the Mendelian 1:2:1 distribution of wild-type:heterozygous:homozygous genotypes; thus deficiencies of cystathionineβ-synthase are not associated with embryonic lethality. However, Cbs(−/−) animals exhibited ~80% mortality during the third and fourth weeks postpartum. Prior to two weeks of age, when viability becomes an issue, Cbs(−/−) animals showed decreased glutathione concentrations in liver and brain tissues. Cysteine concentrations were also low in liver and brain tissues, and in kidneys of males (66). While surviving homozygous males are able to reproduce, female homozygotes are infertile (68).
Due to the high mortality in Cbs(−/−) mice, most studies have been conducted with heterozygotes. In Cbs(+/−) mice derived from matings of heterozygotes, plasma total homocyst(e)ine levels were found to be elevated 2.5-fold over Cbs(+/+) littermates at 7 weeks, and 1.5-fold at 15 weeks in mice fed a standard lab chow. At 15 weeks, plasma methionine levels were elevated 1.3-fold in Cbs(+/−) versus wild-type mice, while total plasma folate levels were unaffected. Levels of AdoHcy were elevated approximately 1.3-fold in the liver of Cbs(+/−), while the AdoMet levels were the same. Similar results were observed in Cbs(+/−) brain, although the decrease in the [AdoMet]:[AdoHcy] ratio was somewhat more pronounced (9).
Many physiological studies have been conducted on cystathionine β-synthase heterozygotes fed high methionine-low folate or methyl-deficient diets, but for the purposes of comparison with other mouse models of hyperhomocyst(e)inemia this review will focus on those studies performed with mice fed a standard lab chow. No differences in acetylcholine-dependent relaxation of the aorta were seen on comparison of Cbs heterozygotes with wild-type litter mates fed standard lab chow at either 7 or 15 weeks, despite differences in plasma total homocyst(e)ine (9). After 15 weeks, thrombomodulin coagulation activity was about 20% lower in Cbs(+/−) mice than in wild-type mice. The authors noted that endothelial dysfunction was associated not only with elevation in plasma total homocyst(e)ine but also with elevated AdoHcy in tissues. Endothelial dysfunction was exacerbated by a low folate-high methionine diet; endothelial cells lack cystathionine β-synthase and betaine-homocysteine methyltransferase and therefore must rely on the folate-dependent enzyme methionine synthase for homocysteine remethylation (23). In another study, DNA hypomethylation was not seen in the liver or kidney of heterozygotes on a standard lab chow, and in these tissues, AdoHcy was only slightly elevated (3).
Methylenetetrahydrofolate reductase
The most common genetic cause of mild human hyperhomocyst(e)inemia is the 677C>T mutation in the MTHFR gene specifying methylenetetrahydrofolate reductase (24). Patients with severe methylenetetrahydrofolate reductase deficiency exhibit homocystinuria and hyperhomocyst(e)inemia, but to a much lesser degree than patients with complete cystathionine β-synthase deficiency. Plasma methionine levels are uniformly low. A decreased proportion of their intracellular folate is present as methyltetrahydrofolate (19). The clinical severity associated with severe deficiency is variable, and may be manifested as developmental delay, and motor and gait abnormalities. They do not exhibit megaloblastic anemia. (50).
A transgenic mouse model of hyperhomocyst(e)inemia induced by methylenetetrahydrofolate reductase disruption was developed in Rima Rozen’s laboratory (4). Her mouse strain incorporated a targeted disruption of the Mthfr gene. When heterozygous Mthfr-deleted mice are mated, the offspring show the Mendelian distribution at 6 days, indicating that neither the heterozygous nor the homozygous genotype results in increased embryonic lethality (4). However, there is significant postnatal mortality of homozygotes, with 83% dying at an average age of 9 days post partum (54).
Mthfr homozygous null mice completely lacked methylenetetrahydrofolate reductase activity in all tissues, while in Mthfr heterozygotes, the measured activity was 60–70% of that found in wild-type littermates. Body weights of the surviving homozygotes were about 80% that of wild-type littermates at two weeks, but at later times, the surviving homozygotes showed nearly normal body weight. Plasma total homocyst(e)ine concentrations in Mthfr(−/−) mice were ~ten times those of wild-type littermates, while Mthfr heterozygotes had ~twice wild-type levels. The livers of surviving three week old Mthfr(−/−) mice showed severe fatty infiltration of the liver with microvesicular lipid droplets (steatosis), while liver pathology was absent from Mthfr(+/−) littermates.
Plasma total homocyst(e)ine, in μmol/L, was 3.2 in Mthfr(+/+), 5.3 in Mthfr(+/−), and 32 in Mthfr(−/−) mice at 5 weeks of age (4). AdoMet levels were lower in all tissues examined in Mthfr(−/−) mice than in their wild-type littermates, and AdoHcy levels were significantly elevated, resulting in [AdoMet]:[AdoHcy] ratios that were sharply decreased (4). In agreement with this finding, DNA hypomethylation was observed in all tissues analyzed (4).
Methylenetetrahydrofolate reductase converts methylenetetrahydrofolate to methyltetrahydrofolate, the only ubiquitous one-carbon donor for the remethylation of homocysteine to form methionine in mammals (Figure 5). In homozygotes, the lack of methylenetetrahydrofolate reductase activity results in severe depletion of methyltetrahydrofolate pools, especially in liver (4). The residual methyltetrahydrofolate is presumably derived from the diet.
Mthfr heterozygotes develop normally and appear outwardly healthy. In contrast, Mthfr homozygous mutants exhibit developmental delays, and their body weights are significantly lower than those of their wild-type littermates throughout the first five weeks post partum. Many of the homozygous Mthfr-null mice that will later die prematurely show protrusive eyes, facial abnormalities, and shorter tails. Some show a “kinked” tail, a feature also seen in the curly tail (ct) mouse, a model for neural tube defects. In contrast to female Cbs-null mice, the majority of surviving Mthfr homozygotes could reproduce. Homozygous Mthfr-deleted mice also exhibit lipid accumulation in the aorta, although the lesions are not typical of the advanced atherosclerotic lesions seen in, for example, the apoE knockout mouse.
Betaine-homocysteine methyltransferase provides an alternate route for remethylation of homocysteine, independent of methyltetrahydrofolate. The enzyme is located primarily in liver and kidney. Betaine supplementation of the dams during gestation dramatically decreases neonatal mortality of Mthfr nullizygotes, from 83% to 26% (54). Offspring of betaine-supplemented dams also show decreased levels of homocysteine in both liver and brain, increased methionine, and more normal [AdoMet]:[AdoHcy] ratios. Cerebellar abnormalities are also ameliorated by betaine supplementation, although the cerebellum was still smaller than in wild-type littermates and its architecture showed remaining abnormalities. These observations suggest that much of the pathology associated with Mthfr deficiency is the result of defective remethylation. Withdrawal of betaine supplementation after weaning had no effect on the survival of the nullizygotes, suggesting that the most critical need for remethylation may be in the first few weeks after birth.
Methionine synthase
Severe deficiency of methionine synthase (MTR, the cblE genetic complementation group) is very rare in humans. Patients typically present in the first few months of life with vomiting, poor feeding, and lethargy. They show severe neurologic dysfunction, megaloblastic anemia and homocystinuria and/or homocyst(e)inemia, sometimes accompanied by hypomethioninemia (15).
A targeted deletion of the methionine synthase gene in mice results in embryonic lethality in Mtr-deleted homozygotes (61). The authors suggested that embryos implant normally, as judged by the appearance of homozygous embryos undergoing resorption at embryonic day 8.5 (E8.5). Supplementation of the dams with folic acid, folinic acid, and/or methionine and choline failed to rescue the phenotype. The authors suggest that the lethality is the result of methyl trapping in the embryo, i.e. the accumulation of methyltetrahydrofolate at the expense of methylenetetrahydrofolate and 10-formyltetrahydrofolate in the absence of a functional methionine synthase. Because embryonic death occurs prior to the development of the placenta, they hypothesize that maternal to fetal transfer of folinic acid is insufficient to meet the needs of the developing embryo. The authors also demonstrated that methionine synthase is probably expressed in wild-type embryos by day E9.5, as judged by detection of methionine synthase mRNA.
The phenotype of Mtr(+/−) mice is relatively mild (10, 61). Mtr heterozygotes have about 60% of the methionine synthase activity measured in wild-type littermates. Plasma total homocyst(e)ine is mildly elevated, and plasma methionine concentrations are normal (10) or slightly elevated (61). Liver total folates are the same in wild-type and heterozygous animals fed a control diet, liver AdoHcy levels are decreased about 10% in the heterozygotes compared to the wild-type animals, and AdoMet levels are increased about 1.3-fold. In brain, AdoMet levels are the same in both genotypes within experimental error, while AdoHcy levels are slightly lower in the Mtr(+/−) mice. While the authors did not emphasize these somewhat surprising metabolite levels, which did not reach statistical significance, we will see that similar results are seen in a mouse with a partial deficiency of methionine synthase reductase (see below).
In mice fed a standard lab chow, Mtr heterozygotes did not show any difference in relaxation of aortic rings in response to acetylcholine or nitroprusside, but higher levels of superoxide were detected in sections of heterozygotic cerebral arterioles using the oxidative fluorescent dye dihydroethidium (10). The evidence for oxidative stress in mice that have little or no elevation in plasma total homocyst(e)ine is somewhat surprising, and the authors hypothesize that methyl trapping and the resultant redistribution of folate species might be related to the observed oxidative stress in cerebral vessels. However, this study did not directly examine folate distributions.
Methionine synthase reductase
Humans with severe methionine synthase reductase deficiency (MTRR, the cblG genetic complementation group) show symptoms that are very similar to those with severe methionine synthase deficiency (15).
Preliminary studies of a mouse model with a targeted disruption of the Mtrr gene specifying methionine synthase reductase indicated that a complete lack of methionine synthase reductase also results in embryonic lethality (unpublished data of Roy Gravel, cited in (13)). A mouse model in which the Mtrr gene was interrupted by a gene-trap insertion has been constructed (MtrrGt(pGT1Lxf)XG334Byg; hereafter abbreviated MtrrGt) (13). Intercross mating of Mtrr(+/Gt) mice resulted in the Mendelian distribution of offspring and tissue-specific skipping of the gene trap’s cryptic exon was shown, leading to the presence of some wild-type Mtrr mRNA in Mtrr(Gt/Gt) mice. Levels of wild-type mRNA in homozygotes ranged from 37% in brain tissue to 0.8% in heart tissue, and specific activities of methionine synthase reductase were significantly lower in Mtrr(Gt/Gt) than in wild-type animals.
Mtrr(+/Gt) mice developed normally and showed only slightly elevated plasma total homocyst(e)ine and mildly decreased plasma methionine concentrations (13). Mtrr(Gt/Gt) males gained weight more slowly than their wild-type littermates, while female homozygotes gained weight normally. Mtrr(Gt/Gt) animals also showed a four-fold elevation in plasma total homocyst(e)ine and a 35% decrease in plasma methionine. These values indicate that the Mtrr(Gt/Gt) mice are more severely affected than the Mtr(+/−) mice. Despite elevated plasma total homocyst(e)ine and decreased plasma methionine, Mtrr(Gt/Gt) mice show normal to elevated intracellular AdoMet and reduced to normal intracellular AdoHcy, except in heart tissue. The [AdoMet]:[AdoHcy] ratio measured in the livers of wild-type Mtrr mice was 0.9 while that in the Mtrr(Gt/Gt) mice was 2.4. Both of these measurements were made in mice that had been fasted overnight to eliminate variability related to dietary intake of methionine.
Although total folate pool sizes in the liver, kidney and heart of Mtrr(Gt/Gt) animals were the same as in their wild-type littermates, there was a trend towards increases in the contribution of methyltetrahydrofolate to the pool. While not statistically significant, these observations suggest that mild methyl trapping may be occurring in these animals (13).
The gene-trap insertion containing a β-galactosidase-neomycin phosphotransferase gene fusion permits staining of Mtrr(+/Gt) embryos for β-galactosidase activity driven by the Mtrr promoter. At E9.5, a stage of development at which the neural tube is closing, the reporter is ubiquitously expressed, with particularly high levels in neural tissues, including the optic eminence, forebrain, midbrain, the 2nd and 4th rhombomeres of the hindbrain and the neural tube (32).
Betaine-homocysteine methyltransferase
We are not aware of any published studies on humans with a betaine-homocysteine methyltransferase deficiency.
A transgenic mouse model for betaine-homocysteine methyl transferase (BHMT) has not yet been constructed. However, transient BHMT deficiency can be induced in wild-type mice by injection of the BHMT inhibitor S-(δ–carboxybutyl)-DL-homocysteine (7). These injections lead to 87% lower BHMT activity and a 2.7-fold increase in plasma total homocyst(e)ine. After repeated injections, the liver [AdoMet]:[AdoHcy] ratio was reduced.
DISCUSSION
Table 1 provides a comparison of the metabolite levels associated with the different mouse models for hyperhomocyst(e)inemia. It is important to bear in mind that the absolute values may not be strictly comparable, both because different methods and standards were used in different studies, and because of differences in the protocols, e.g. in some studies animals were fasted overnight prior to sample collection, while in others they were not. Despite these caveats, comparisons of the wild-type mouse values with those of the heterozygous and homozygous mutant mice (or treated mice in the case of betaine-homocysteine methyltransferase) are informative. In the section that follows, we will attempt to relate the very different metabolite profiles of these mouse models with some of the phenotypes associated with hyperhomocyst(e)inemia in mice and humans.
Table 1.
Metabolite concentrations in plasma and liver tissue of mouse models for hyperhomocyst(e)inemia
| Mouse modela | Reference | Hcy (μM) | Met (μM) | AdoMet (nmol/g liver) | AdoHcy (nmol/g liver) | [AdoMet]: [AdoHcy] | 5-MethylTHF (% liver folates) |
|---|---|---|---|---|---|---|---|
| genotype/treatment | |||||||
| Mtrr | (13) | ||||||
| +/+ | 4.6±0.8 | 59.4±7.4 | 45.9±4.5 | 60.6±8.3 | 0.9±0.2 | 27±4 | |
| +/Gt | 5.5±1.8 | 48.9±10.5 | ND | ND | ND | ND | |
| Gt/Gt | 18.4±5.5 | 40.4±6.6 | 64.1±6.4 | 30.7±4.7 | 2.9±0.6 | 34±10 | |
| Mtr | (61) (9) |
||||||
| +/+ | 4.1 | 78 | 33.1±4.7 | 22.4±2.2 | 1.5±0.2 | ND | |
| +/− | 6.1 | 102 | 42.1±4.4 | 20.2±2.2 | 2.3±0.3 | ND | |
| −/− | NA | NA | NA | NA | NA | NA | |
| Mthfr | (4) | ||||||
| +/+ | 3.3±1.0 | ND | 27.7±0.3 | 13.8±0.3 | 2.0 | 23±12 | |
| +/− | 5.3±0.8 | ND | 36.3±0.7 | 36.3±0.5 | 1.0 | 13±6 | |
| −/− | 32.3±5.5 | ND | 19.0±0.7 | 55.4±0.9 | 0.3 | 0.9±1.3 | |
| Cbs | (68) (9) |
||||||
| +/+ | 6.1±0.8 | 22.8±1.6 | 42.9±8.8 | 27.4±2.9 | 1.7±0.2 | ND | |
| +/− | 13.5±3.2 | 30.6±2.5 | 41.9±3.1 | 45.7±5.1 | 1.0±0.1 | ND | |
| −/− | 203.6±65.3 | ND | ND | ND | ND | ND | |
| BHMT | (7) | ||||||
| −CBHcy | 2.3±0.2 | ND | 16.4±2.0 | 15.1±1.5 | 1.1 | ND | |
| +CBHcy | 18.5±1.0 | ND | 8.1±0.9 | 19.4±1.1 | 0.4 | ND |
Embryonic lethality
Of the five mouse models for hyperhomocyst(e)inemia discussed here, only two, those due to deficiencies in methionine synthase or methionine synthase reductase, cause embryonic lethality. In such cases, the metabolism of the dam is unable to sustain the viability of the embryo, suggesting that these enzymes are required for embryonic survival. In the case of methionine synthase deficiency, lethality occurs before day E8.5, indicating that the embryo dies before the placental circulation becomes fully functional starting at day E9.5 (61). The unique feature of both the methionine synthase and the methionine synthase reductase mouse models is that the associated enzyme deficiency is thought to lead to methyl trapping, or the accumulation of intracellular methyltetrahydrofolate at the expense of 10-formyltetrahydrofolate and methylenetetrahydrofolate. Mild methyl trapping is likely in the gene-trap model for methionine synthase reductase deficiency (13). It is well established that deficiencies in formyltetrahydrofolate and methylenetetrahydrofolate are associated with the apoptosis of rapidly growing cells in the adult (29) and it is plausible that they are also associated with the embryonic lethality, although this remains to be established rigorously. During the early stages of development, the growing embryo must rely primarily on folate stores deposited in the egg by the mother, and subsequently on exchange of nutrients with the mother through the yolk sac. It is plausible that yolk sac-mediated exchange becomes more and more limiting as the embryo grows in size, reaching a critical juncture just prior to the initiation of placental exchange, at a time when the neural tube is closing. The ability of the embryo to rebalance folate stores in response to metabolic demands may be essential for viability during this critical period. Embryos lacking methionine synthase or methionine synthase reductase may be particularly sensitive to folate pool imbalances.
Other mouse models for hyperhomocyst(e)inemia due to perturbations of one-carbon metabolism are associated with a deficiency rather than an excess of intracellular methyltetrahydrofolate (e.g. Mthfr deficiency) or are likely to have no effect on folate pool distribution (Cbs or Bhmt). Thus hyperhomocyst(e)inemia in and of itself is not likely to be responsible for the embryonic lethality observed in mice with severe deficiencies in methionine synthase activity.
Embryonic lethality is also observed in mice with defective folate transport. Deficiencies in the folate binding protein 1 (Folbp1) (42) or the reduced-folate carrier (Rfc) (35) lead to embryonic death by day E10. Supplementation of the Folbp1 dams with folinic acid reverses this phenotype but leads to a high incidence of neural tube defects in Folbp1(−/−) embryos (63), while supplementation of the Rfc dams with high levels of folic acid partially prevents the embryonic lethality associated with Rfc deficiency (73). The embryonic lethality due to Rfc deficiency has been shown to be associated with impaired hematopoiesis (73).
It is of considerable interest that impaired folate transport and deficiencies of methionine synthase or methionine synthase reductase may be associated with embryonic lethality, while deficiencies of methylenetetrahydrofolate reductase are not. While it might be thought that folate deficiency would affect all components of the folate pool equally, studies on the in vitro catalytic properties of the enzymes that compete for methylenetetrahydrofolate have suggested that methylenetetrahydrofolate reductase has a much lower Michaelis constant (Km) for this substrate than thymidylate synthase or methylenetetrahydrofolate dehydrogenase (21), which would be expected to result in methyl trapping. Thus embryos with folate deficiency and those with reduced methionine synthase activity may share a propensity for apoptosis due to insufficient supplies of 10-formyltetrahydrofolate and methylenetetrahydrofolate.
Postnatal mortality
In Mthfr-deficient mice and those lacking cystathionine β-synthase, homozygous mutants are born at the normal frequency from heterozygote matings, indicating that the dam can compensate for the absence of the enzyme in the fetus. However, in both cases, postnatal mortality is severe over the first few weeks. Homozygous mutants in both these models exhibit severe hyperhomocyst(e)inemia and methylation defects stemming from the lowered [AdoMet]:[AdoHcy] ratios in their tissues. The greatly reduced postnatal mortality of homozygous Mthfr-deficient pups seen when the dams are supplemented with betaine until weaning is a strong indication that the mortality is directly related to the inability to recycle homocysteine to methionine. Betaine supplementation does not appear to have been attempted to reduce the postnatal mortality in the cystathionine β-synthase-deficient mouse, but supplementation of heterozygotes with betaine produced marked decreases in plasma total homocyst(e)ine (55).
Deficient methylation
DNA hypomethylation has been demonstrated in methylenetetrahydrofolate reductase-deficient and cystathionine β-synthase-deficient mice with decreased intracellular [AdoMet]:[AdoHcy] ratios. It might have been anticipated that methylation defects would also be observed in those mouse models that have impaired methionine synthase activity, caused either by deficiencies in methionine synthase or methionine synthase reductase, or dietary deficiency of cobalamin, the cofactor for methionine synthase, or possibly riboflavin, the precursor for the flavin mononucleotide and flavin adenine dinucleotide cofactors of methionine synthase reductase. To our surprise, we found no evidence of decreased [AdoMet]:[AdoHcy] ratios in the liver, brain or kidney of the methionine synthase reductase-deficient mouse, despite elevated plasma total homocyst(e)ine and lowered plasma methionine. While the [AdoMet]:[AdoHcy] ratio was decreased about 3-fold in heart, in the liver the ratio of [AdoMet]:[AdoHcy] was actually increased 3-fold in the Mtrr(Gt/Gt) animals as compared to their wild-type littermates (32). This unexpected finding suggests that compensatory mechanisms to spare AdoMet exist, especially in liver.
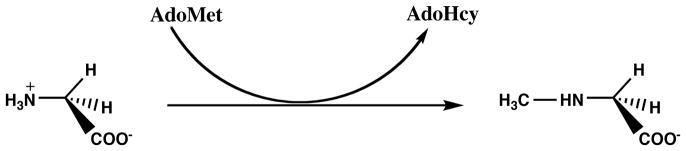
Mammalian liver contains a large amount of glycine N-methyltransferase activity, which normally serves to convert excess AdoMet to AdoHcy by methylation of glycine to produce sarcosine (Figure 7). Tissues like liver that contain high levels of glycine N-methyltransferase typically exhibit lower [AdoMet]:[AdoHcy] ratios than do tissues like heart that lack this enzyme (27, 72). In a wild-type mouse after overnight fasting, the [AdoMet]:[AdoHcy] ratio was 0.9 in liver and 47 in heart (13). Glycine N-methyltransferase activity is inhibited by methyltetrahydrofolate and activated by AdoMet (71). Thus, in an Mthfr-deficient mouse, with extremely low levels of methyltetrahydrofolate, glycine N-methyltransferase should be maximally active in the liver, while in a methionine synthase reductase-deficient mouse or a methionine synthase-deficient mouse, glycine N-methyltransferase would be expected to be substantially inhibited in liver, resulting in a sparing of the [AdoMet]:[AdoHcy] ratio, and perhaps, in the case of methyl trapping, even leading to an increase in the [AdoMet]:[AdoHcy] ratio.
Figure 7.
Reaction catalyzed by glycine N-methyltransferase.
Methylation defects would be expected in all tissues of methylenetetrahydrofolate reductase-deficient and cystathionine β-synthase-deficient mice, but only in tissues lacking glycine N-methyltransferase in the methionine synthase-deficient and methionine synthase reductase-deficient mouse. We would expect cobalamin-deficiency to induce similar effects in rodents, although to our knowledge, the results of such studies have not yet been published.
Endothelial dysfunction and cardiovascular disease
Endothelial dysfunction has been proposed to represent a condition predisposing those affected to the development of cardiovascular disease and is often used as a surrogate for more expensive long-term studies on the incidence of cardiovascular disease in mouse models. The cerebral microcirculation appears to be particularly susceptible to endothelial dysfunction during hyperhomocyst(e)inemia (10). Among the techniques used to demonstrate endothelial dysfunction are measurements of relaxation of aortic rings or cerebral arterioles in response to administration of acetylcholine or nitroprusside (10), detection of superoxide in sections of vessels using a fluorescent dye (10), and measurements of thromobomodulin activity (9). Evidence for endothelial dysfunction in the cerebral arterioles has been demonstrated in Cbs(+/−) mice with mild cystathionine β-synthase deficiency (9, 33), in heterozygotic Mthfr mice (65), and in heterozygotic Mtr mice (10). The degree of dysfunction correlated with the level of plasma total homocyst(e)ine, regardless of the combination of genetic and dietary factors used to induce elevated homocyst(e)ine (14). Thus, endothelial dysfunction may be a consequence of the elevation in plasma total homocyst(e)ine itself, rather than resulting from indirect sequellae of the condition giving rise to hyperhomocyst(e)inemia. We look forward to long-term studies on the incidence of cardiovascular disease in various mouse models to establish that endothelial dysfunction is in fact a good surrogate.
Neural tube defects
Failure of the embryonic neural tube to close results in neural tube defects, including spina bifida and anencephalus. In humans, about 70% of these devastating malformations can be prevented by periconceptual folate supplementation of the mother (38). However, plasma folate and red cell folate levels in pregnant mothers who later have an infant with a neural tube defect, although lower than those in controls, are in the normal range for the majority of affected mothers (8). Homocyst(e)ine levels in early pregnancy are significantly higher in affected mothers than in controls suggesting impairment of cystathionine synthesis or homocysteine remethylation [(28) and references cited therein].
Recent studies have identified maternal autoantibodies to the folate receptor in the placenta in a significant number of women with a pregnancy complicated by a neural tube defect (52). There is a high incidence of neural tube defects in murine Folbp1(−/−) embryos with defective folate transport, even when the diet of the dam is supplemented with folinic acid (63). Folbp1-nullizygous mice show an approximately four-fold increase in plasma total homocyst(e)ine levels as compared to their wild-type littermates (42). In liver and brain tissues of Folbp(+/−) mice, the [AdoMet]:[AdoHcy] ratio is maintained, with a trend towards an increase (16). These data reinforce the striking finding that functional folate deficiency actually leads to sparing of AdoMet in at least some tissues, even under conditions of hyperhomocyst(e)inemia. These studies certainly implicate folate deficiency as a potential causative agent for neural tube defects in mice. They are also very important in that they reveal that severe folate depletion leads to embryonic death, while the milder depletion induced when the dam is supplemented with folinic acid leads to embryonic survival and the development of neural tube defects.
However, we still do not know which folate-dependent pathways are required for normal neural tube closure. Is the requirement for remethylation of homocysteine, or for one-carbon units for the de novo biosynthesis of purines and pyrimidines, or both or neither? Do the folate pool imbalances associated with some causes of hyperhomocyst(e)inemia exacerbate mild folate deficiency in pregnant women? Studies with the splotch (Pax3) mouse, with a high incidence of neural tube defects, used deoxythymidine suppression tests to demonstrate a metabolic deficiency in the supply of folate for thymidylate biosynthesis (18). However, it is not clear whether this mutant mouse is a good surrogate for the etiology of spontaneous neural tube defects in wild-type animals.
None of the models of disrupted one carbon metabolism that cause hyperhomocyst(e)inemia in mouse models has thus far been associated with a significant increase in the incidence of neural tube defects. That said, spontaneous neural tube defects in the mouse, as in humans, are rarely encountered. The incidence in wild-type mice is estimated to be 0.3–4.2 per 1000 pups (22, 62). Furthermore, severe dietary folate deficiency in mice results in failure to implant, or in embryonic resorption, rather than in viable embryos with neural tube defects (22, 62), similar to the phenotype seen when folate transport is defective due to the absence of Folbp1. Even assuming that an enzyme deficiency were associated with a 10-fold increase in the incidence of neural tube defects, few studies of sufficient statistical power have been conducted to allow detection of such an increase.
An initial approach to such studies would be to determine which enzymes involved in homocysteine and folate metabolism are expressed in mouse embryos during the period of neural tube closure. Cystathionine β-synthase is initially expressed only in liver, skeletal, cardiac and nervous systems (49), but the studies were performed at E12.5, after neural tube closure is complete. Reverse transcriptase-polymerase chain reaction studies indicate that betaine-homocysteine methyltransferase is not expressed in either yolk sac or embryonic tissue until E10, when neurulation is nearly complete, so the activity of this enzyme is unlikely to be required for neural tube closure (17). Methionine synthase is expressed in Mtr(+/−) embryos at E9.5 (61), as is methionine synthase reductase (13). The latter enzyme was shown to be highly expressed in neural tube as well as in the second and fourth rhombomeres, where migrating neural crest cells are located at this stage of development. Studies do not appear to have been performed on expression of methylenetetrahydrofolate reductase in the embryo, but Mthfr(−/−) embryos survive until the postnatal period. Since the dam should be able to provide methyltetrahydrofolate to the developing embryo, methylenetetrahydrofolate reductase may not be required until after birth.
The initial studies on folate deficiency in wild-type mice examined only 300 embryos, and thus lacked the statistical power to detect a modest increase in the incidence of neural tube defects. It would be extremely interesting to compare the incidence of neural tube defects in Mthfr-deficient and Mtrr-deficient mice of the same strain background, since the former have extremely low levels of methyltetrahydrofolate and reduced cellular [AdoMet]:[AdoHcy] ratios while the latter have elevated or normal levels of methyltetrahydrofolate and normal to high levels of [AdoMet]:[AdoHcy] in several tissues, including brain. It would also be valuable to examine the effect of supplementation of the dam with various forms of folic acid on the incidence of neural tube defects, e.g. with folinic acid or methyltetrahydrofolate.
The complexity of the etiology of hyperhomocyst(e)inemia suggests that folic acid supplementation alone is unlikely to be a “magic bullet” for all associated pathology in humans. The folate and methionine biochemical pathways are intertwined (Figure 1) such that defects in one pathway disturb homeostasis of the other pathway. In designing future studies, the underlying causes of hyperhomocyst(e)inemia should be considered as potential confounders. This may prove critical for the development of successful treatments, where a one-size-fits-all approach will likely fail. Although folic acid supplementation might lead to an across the board decrease in the levels of homocyst(e)ine in the blood, it is conceivable that imbalances elsewhere in the folate and methionine pathways may yet persist that gave rise to the increase in plasma total homocyst(e)ine in the first place.
Acknowledgments
Work performed in this laboratory has been supported in part by National Institutes of Health Grants GM24908 and HL58955
ABBREVIATIONS
- AdoHcy
S-adenosylhomocysteine
- AdoMet
S-adenosylmethionine
- Hcy
homocysteine
- Met
methionine
- 5-methylTHF
5-methyltetrahydrofolate
- MtrrGt
the MtrrGt(pGT1Lxf)XG334Byg allele of the Mtrr gene specifying methionine synthase reductase
References
- 1.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 2.Brouwer IA, van Dusseldorp M, Thomas CM, Duran M, Hautvast JG, Eskes TK, Steegers-Theunissen RP. Low-dose folic acid supplementation decreases plasma homocysteine concentrations: a randomized trial. Am J Clin Nutr. 1999;69:99–104. doi: 10.1093/ajcn/69.1.99. [DOI] [PubMed] [Google Scholar]
- 3.Caudill MA, Wang JC, Meinyk S, Pogribny IP, Jernigan S, Collins MD, Santos-Guzman J, Swendseid ME, Cogger EA, James SJ. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine-β-synthase heterozygous mice. J Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Meinyk S, Lussier-Cacan S, Chen MF, Pai A, John SWM, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 5.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 6.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 7.Collinsova M, Strakova J, Jiracek J, Garrow TA. Inhibition of betaine-homocysteine S-methyltransferase causes hyperhomocysteinemia in mice. J Nutr. 2006;136:1493–1497. doi: 10.1093/jn/136.6.1493. [DOI] [PubMed] [Google Scholar]
- 8.Daly LE, Kirke PN, Molloy AM, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. J Am Med Assoc. 1995;274:1698–1702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- 9.Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, Heistad DD, Faraci FM, Lentz SR. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res. 2001;88:1203–1209. doi: 10.1161/hh1101.092180. [DOI] [PubMed] [Google Scholar]
- 10.Dayal S, Devlin AM, McCaw RB, Liu ML, Arning E, Bottiglieri T, Shane B, Faraci FM, Lentz SR. Cerebral vascular dysfunction in methionine synthase-deficient mice. Circulation. 2005;112:737–744. doi: 10.1161/CIRCULATIONAHA.104.529248. [DOI] [PubMed] [Google Scholar]
- 11.Dekker GA, de Vries JI, Doelitzsch PM, Huijgens PC, von Blomberg BM, Jakobs C, van Geijn HP. Underlying disorders associated with severe early-onset preeclampsia. Am J Obstet Gynecol. 1995;173:1042–1048. doi: 10.1016/0002-9378(95)91324-6. [DOI] [PubMed] [Google Scholar]
- 12.Doshi SN, McDowell IF, Moat SJ, Payne N, Durrant HJ, Lewis MJ, Goodfellow J. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002;105:22–26. doi: 10.1161/hc0102.101388. [DOI] [PubMed] [Google Scholar]
- 13.Elmore CL, Wu X, Leclerc D, Watson ED, Bottiglieri T, Krupenko NI, Krupenko SA, Cross JC, Rozen R, Gravel RA, Matthews RG. Metabolic derangement of methionine and folate metabolism in mice deficient in methionine synthase reductase. Mol Genet Metab. 2007;91:85–97. doi: 10.1016/j.ymgme.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke. 2004;35:345–347. doi: 10.1161/01.STR.0000115161.10646.67. [DOI] [PubMed] [Google Scholar]
- 15.Fenton WA, Rosenberg LE. Inherited disorders of cobalamin transport and metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw Hill; 1995. pp. 3129–3150. [Google Scholar]
- 16.Finnell RH, Spiegelstein O, Wlodarczyk B, Triplett A, Pogribny IP, Melnyk S, James JS. DNA methylation in Folbp1 knockout mice supplemented with folic acid during gestation. J Nutr. 2002;132:2457S–2461S. doi: 10.1093/jn/132.8.2457S. [DOI] [PubMed] [Google Scholar]
- 17.Fisher MC, Zeisel SH, Mar M-H, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J. 2002;16:619–621. doi: 10.1096/fj.01-0564fje. [DOI] [PubMed] [Google Scholar]
- 18.Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280:2107–2109. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- 19.Ghandour H, Chen Z, Selhub J, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit tissue-specific distribution of folates. J Nutr. 2004;134:2975–2978. doi: 10.1093/jn/134.11.2975. [DOI] [PubMed] [Google Scholar]
- 20.Green JM, Ballou DP, Matthews RG. Examination of the role of methylenetetrahydrofolate reductase in incorporation of methyltetrahydrofolate into cellular metabolism. FASEB J. 1988;2:42–47. doi: 10.1096/fasebj.2.1.3335280. [DOI] [PubMed] [Google Scholar]
- 21.Green JM, MacKenzie RE, Matthews RG. Substrate flux through methylenetetrahydrofolate dehydrogenase: predicted effects of the concentration of methylenetetrahydrofolate on its partitioning into pathways leading to nucleotide biosynthesis or methionine regeneration. Biochemistry. 1988;27:8014–8022. doi: 10.1021/bi00421a007. [DOI] [PubMed] [Google Scholar]
- 22.Heid MK, Bills ND, Hinrichs SH, Clifford AJ. Folate deficiency alone does not produce neural tube defects in mice. J Nutr. 1992;122:888–894. doi: 10.1093/jn/122.4.888. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen DW. Hyperhomocysteinemia and oxidative stress: time for a reality check? Arterioscler Thromb Vasc Biol. 2000;20:1182–1184. doi: 10.1161/01.atv.20.5.1182. [DOI] [PubMed] [Google Scholar]
- 24.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R. Relationship between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- 25.Jencks DA, Matthews RG. Allosteric inhibition of methylenetetrahydrofolate reductase by adenosylmethionine: Effects of adenosylmethionine and NADPH on the equilibrium between active and inactive forms of the enzyme and on the kinetics of approach to equilibrium. J Biol Chem. 1987;262:2485–2493. [PubMed] [Google Scholar]
- 26.Kapusta L, Haagmans ML, Steegers EA, Cuypers MH, Blom HJ, Eskes TK. Congenital heart defects and maternal derangement of homocysteine metabolism. J Pediatr. 1999;135:773–774. doi: 10.1016/s0022-3476(99)70102-2. [DOI] [PubMed] [Google Scholar]
- 27.Kerr SJ. Competing methyltransferase systems. J Biol Chem. 1972;247:4248–4252. [PubMed] [Google Scholar]
- 28.Kirke PN, Mills JL, Scott JM. Homocysteine metabolism in pregnancies complicated by neural tube defects. Nutrition. 1997;13:994–995. doi: 10.1016/s0899-9007(97)82079-5. [DOI] [PubMed] [Google Scholar]
- 29.Koury MJ, Horne DW, Brown ZA, Pietenpol JA, Blount BC, Ames BN, Hard R, Koury ST. Apoptosis of late-stage erythroblasts in megaloblastic anemia: association with DNA damage and macrocyte production. Blood. 1997;89:4617–4623. [PubMed] [Google Scholar]
- 30.Kutzbach C, Stokstad ELR. Mammalian methylenetetrahydrofolate reductase: partial purification, properties, and inhibition by S-adenosylmethionine. Biochim Biophys Acta. 1971;250:459–477. doi: 10.1016/0005-2744(71)90247-6. [DOI] [PubMed] [Google Scholar]
- 31.Lange H, Suryapranata H, De Luca G, Borner C, Dille J, Kallmayer K, Pasalary MN, Scherer E, Dambrink JH. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350:2673–2681. doi: 10.1056/NEJMoa032845. [DOI] [PubMed] [Google Scholar]
- 32.LeClerc D, Wilson A, Dumas R, Gafuik C, Song D, Watkins D, Heng HHQ, Rommens JM, Scherer SW, Rosenblatt DS, Gravel RA. Cloning and mapping of a cDNA for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc Natl Acad Sci USA. 1998;95:3059–3064. doi: 10.1073/pnas.95.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lentz SR, Erger RA, Dayal S, Maeda N, Malinow MR, Heistad DD, Faraci FM. Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine beta-synthase-deficient mice. Am J Physiol Heart Circ Physiol. 2000;279:H970–975. doi: 10.1152/ajpheart.2000.279.3.H970. [DOI] [PubMed] [Google Scholar]
- 34.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J., Jr Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 35.Maddox DM, Manlapat A, Roon P, Prasad P, Ganapathy V, Smith SB. Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, craniofacial region, eye, limb buds and heart. BMC Devel Biol. 2003;3:6. doi: 10.1186/1471-213X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13:235–239. doi: 10.1002/(sici)1099-1166(199804)13:4<235::aid-gps761>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 37.McKeever MP, Weir DG, Molloy A, Scott JM. Betaine-homocysteine methyltransferase: organ distribution in man, pig and rat and subcellular distribution in the rat. Clinical Science. 1991;81:551–556. doi: 10.1042/cs0810551. [DOI] [PubMed] [Google Scholar]
- 38.Medical Research Council Vitamin Study Research Group. Prevention of neural tube defects by periconceptual vitamin supplementation. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 39.Mills JL, McPartlin JM, Kirke PN, Lee YJ, Conley MR, Weir DG, Scott JM. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet. 1995;345:149–151. doi: 10.1016/s0140-6736(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 40.Mudd SH, Levy HL, Skovby F. Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw-Hill; 1995. pp. 1279–1327. [Google Scholar]
- 41.Olteanu H, Banerjee R. Human methionine synthase reductase, a soluble P-450 reductase-like dual flavoprotein, is sufficient for NADPH-dependent methionine synthase activation. J Biol Chem. 2001;276:35558–35563. doi: 10.1074/jbc.M103707200. [DOI] [PubMed] [Google Scholar]
- 42.Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, Lacey SW, Anderson RGW, Finnell RH. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999;23:228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- 43.Potena L, Grigioni F, Magnani G, Ortolani P, Coccolo F, Sassi S, Koessels K, Marrozzini C, Marzocchi A, Carigi S, Musuraca AC, Russo A, Magelli C, Branzi A. Homocysteine-lowering therapy and early progression of transplant vasculopathy: a prospective, randomized, IVUS-based study. Am J Transplant. 2005;5:2258–2264. doi: 10.1111/j.1600-6143.2005.01015.x. [DOI] [PubMed] [Google Scholar]
- 44.Powers RW, Evans RW, Majors AK, Ojimba JI, Ness RB, Crombleholme WR, Roberts JM. Plasma homocysteine concentration is increased in preeclampsia and is associated with evidence of endothelial activation. Am J Obstet Gynecol. 1998;179:1605–1611. doi: 10.1016/s0002-9378(98)70033-x. [DOI] [PubMed] [Google Scholar]
- 45.Raijmakers MT, Roes EM, Zusterzeel PL, Steegers EA, Peters WH. Thiol status and antioxidant capacity in women with a history of severe pre-eclampsia. BJOG. 2004;111:207–212. doi: 10.1111/j.1471-0528.2004.00051.x. [DOI] [PubMed] [Google Scholar]
- 46.Rajkovic A, Catalano PM, Malinow MR. Elevated homocyst(e)ine levels with preeclampsia. Obstet Gynecol. 1997;90:168–171. doi: 10.1016/S0029-7844(97)00223-8. [DOI] [PubMed] [Google Scholar]
- 47.Riddell LJ, Chisholm A, Williams S, Mann JI. Dietary strategies for lowering homocysteine concentrations. Am J Clin Nutr. 2000;71:1448–1454. doi: 10.1093/ajcn/71.6.1448. [DOI] [PubMed] [Google Scholar]
- 48.Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24:379–386. doi: 10.1159/000093680. [DOI] [PubMed] [Google Scholar]
- 49.Robert K, Vialard F, Thiery E, Toyama K, Sinet P-M, Janel N, London J. Expression of the cystathionine-β-synthase (CBS) gene during mouse development and immunolocalization in adult brain. J Histochem Cytochem. 2003;51:363–371. doi: 10.1177/002215540305100311. [DOI] [PubMed] [Google Scholar]
- 50.Rosenblatt DS. Inherited disorders of folate transport and metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw Hill; 1995. pp. 3111–3128. [Google Scholar]
- 51.Rosenquist TH, Ratashak SA, Selhub J. Homocysteine induces congenital defects of the heart and neural tube: effect of folic acid. Proc Natl Acad Sci USA. 1996;93:15227–15232. doi: 10.1073/pnas.93.26.15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothenberg SP, da Costa MP, Sequeira JM, Cracco J, Roberts JL, Weedon J, Quadros EV. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. N Engl J Med. 2004;350:134–142. doi: 10.1056/NEJMoa031145. [DOI] [PubMed] [Google Scholar]
- 53.Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA. 2002;288:973–979. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 54.Schwahn BC, Laryea MD, Chen Z, Melnyk S, Pogribny I, Garrow T, James SJ, Rozen R. Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency. Biochem J. 2004;382:831–840. doi: 10.1042/BJ20030822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwahn BC, Wendel U, Lussier-Cacan S, Mar MH, Zeisel SH, Leclerc D, Castro C, Garrow TA, Rozen R. Effects of betaine in a murine model of mild cystathionine-beta-synthase deficiency. Metabolism. 2004;53:594–599. doi: 10.1016/j.metabol.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 56.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 57.Shaw GM, O’Malley CD, Wasserman CR, Tolarova MM, Lammer EJ. Maternal periconceptional use of multivitamins and reduced risk for conotruncal heart defects and limb deficiencies among offspring. Am J Med Genet. 1995;59:536–545. doi: 10.1002/ajmg.1320590428. [DOI] [PubMed] [Google Scholar]
- 58.Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin Intervention For Stroke Prevention trial: an efficacy analysis. Stroke. 2005;36:2404–2409. doi: 10.1161/01.STR.0000185929.38534.f3. [DOI] [PubMed] [Google Scholar]
- 59.Steegers-Theunissen RP, Boers GH, Blom HJ, Nijhuis JG, Thomas CM, Borm GF, Eskes TK. Neural tube defects and elevated homocysteine levels in amniotic fluid. Am J Obstet Gynecol. 1995;172:1436–1441. doi: 10.1016/0002-9378(95)90474-3. [DOI] [PubMed] [Google Scholar]
- 60.Steegers-Theunissen RP, Boers GH, Trijbels FJ, Eskes TK. Neural-tube defects and derangement of homocysteine metabolism. N Engl J Med. 1991;324:199–200. doi: 10.1056/NEJM199101173240315. [DOI] [PubMed] [Google Scholar]
- 61.Swanson DA, Liu M-L, Baker PJ, Garrett L, Stitzel M, Wu J, Harris M, Banerjee R, Shane B, Brody LC. Targeted disruption of the methionine synthase gene in mice. Mol Cell Biol. 2001;21:1058–1065. doi: 10.1128/MCB.21.4.1058-1065.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szabo KT. Congenital Malformations in Laboratory and Farm Animal. New York, NY: Academic Press; 1989. pp. 95–143. [Google Scholar]
- 63.Tang LS, Finnell RH. Neural and orofacial defects in Folbp1 knockout mice. Birth Defects Res (Part A) 2003;67:209–218. doi: 10.1002/bdra.10045. [DOI] [PubMed] [Google Scholar]
- 64.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 65.Virdis A, Iglarz M, Neves MF, Amiri F, Touyz RM, Rozen R, Schiffrin EL. Effect of hyperhomocystinemia and hypertension on endothelial function in methylenetetrahydrofolate reductase-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1352–1357. doi: 10.1161/01.ATV.0000083297.47245.DA. [DOI] [PubMed] [Google Scholar]
- 66.Vitvitsky V, Dayal S, Stabler S, Zhou Y, Wang H, Lentz SR, Banerjee R. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am J Physiol Regul Integr Comp Physiol. 2004;287:R39–R46. doi: 10.1152/ajpregu.00036.2004. [DOI] [PubMed] [Google Scholar]
- 67.Ward M, McNulty H, McPartlin J, Strain JJ, Weir DG, Scott JM. Plasma homocysteine, a risk factor for cardiovascular disease, is lowered by physiological doses of folic acid. QJM. 1997;90:519–524. doi: 10.1093/qjmed/90.8.519. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine-beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wenstrom KD, Johanning GL, Johnston KE, DuBard M. Association of the C677T methylenetetrahydrofolate reductase mutation and elevated homocysteine levels with congenital cardiac malformations. Am J Obstet Gynecol. 2001;184:806–812. doi: 10.1067/mob.2001.113845. [DOI] [PubMed] [Google Scholar]
- 70.Wong WY, Eskes TK, Kuijpers-Jagtman AM, Spauwen PH, Steegers EA, Thomas CM, Hamel BC, Blom HJ, Steegers-Theunissen RP. Nonsyndromic orofacial clefts: association with maternal hyperhomocysteinemia. Teratology. 1999;60:253–257. doi: 10.1002/(SICI)1096-9926(199911)60:5<253::AID-TERA4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 71.Yeo E-J, Briggs WT, Wagner C. Inhibition of glycine-N-methyltransferase by 5-methyltetrahydrofolate pentaglutamate. J Biol Chem. 1999;274:37559–37564. doi: 10.1074/jbc.274.53.37559. [DOI] [PubMed] [Google Scholar]
- 72.Yeo EJ, Wagner C. Tissue distribution of glycine N-methyltransferase, a major folate- binding protein of rat liver. Proc Natl Acad Sci USA. 1994;91:210–214. doi: 10.1073/pnas.91.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao R, Russell RG, Wang Y, Liu L, Gao F, Kneitz B, Edelmann W, Goldman ID. Rescue of embryonic lethality in reduced folate carrier-deficient mice by maternal folic acid supplementation reveals early neonatal failure of hematopoietic organs. J Biol Chem. 2001;276:10224–10228. doi: 10.1074/jbc.c000905200. [DOI] [PubMed] [Google Scholar]