Abstract
Purpose
The magnitude of intestinal adaptation is considered to correlate with the extent of small bowel resection (SBR). However, this association has never been tested in mice. We sought to test the hypothesis that a greater SBR will induce a greater adaptation response.
Methods
C57/B6 mice underwent 50% SBR, 75% SBR, or sham operation and were sacrificed on postoperative day 7. The magnitude of adaptation was compared between 50% SBR and 75% SBR as changes in villus height, crypt depth, as well as rates of apoptosis and proliferation.
Results
75% SBR led to decreased survival and increased weight loss compared to 50% SBR. The remnant ileum of both 50% SBR and 75% SBR displayed similar crypt expansion, enhanced villi, and increased apoptotic indices. Proliferation rates increased after 50% and 75% SBR equally.
Conclusion
Models of resection greater than 50% in mice result in greater morbidity and mortality and do not magnify the adaptation response to massive SBR. The use of more extreme resection models does not appear to provide added benefit for investigating mechanisms of intestinal adaptation.
Keywords: Small Bowel Resection, Adaptation, Extent of Resection, Proliferation, Apoptosis
Short Bowel Syndrome (SBS) is a condition in which there is insufficient nutrient absorptive capacity to sustain life, resulting in malnutrition, dehydration and need for parenteral nutrition. This condition frequently ensues following massive small bowel resection (SBR), which is required to treat many clinical conditions, including necrotizing enterocolitis, midgut volvulus, inflammatory bowel disease, mesenteric vascular disease, trauma, and cancer. Intestinal adaptation is a process whereby the remaining bowel undergoes compensatory changes resulting in increased absorptive surface area (1). Adaptation is characterized by augmented morphologic parameters including longer villi and deeper crypts, which serve to increase intestinal mucosal surface area for nutrient absorption and digestion (2, 3). In addition, it is believed to be driven by altered cellular kinetics, such as increased rates of enterocyte proliferation and apoptosis (2-4).
Several animal models exist to study intestinal adaptation after massive intestinal resection, including mice (5), rats (6), pigs (7), dogs (8), and, even, pythons (9). Of these, the mouse and rat models are most commonly used due to their lower cost and relative ease of genetic manipulation. Our lab has primarily utilized the mouse model (for details, see 5) in order to study the mechanisms that underlie adaptation after a 50% proximal SBR on a cellular and molecular level.
It is generally accepted that more extensive small bowel resections lead to increased adaptive responses in the remnant bowel (2, 10-12), despite the fact that there is very little primary data to support this claim (12-14). In hopes of being able to generate both a more consistent and profound adaptation response after massive intestinal resection, we sought to test the hypothesis that a 75% SBR would generate augmented adaptive changes compared with a traditional 50% SBR model. Furthermore, we hoped that an increased adaptive response after 75% SBR would correlate with amplified cellular and, perhaps, more obvious genetic changes that could lead to new discoveries about the mechanisms responsible for intestinal adaptation.
1. Methods
1.1. Animals
The protocol for this study was approved by the Washington University Institutional Animal Care and Use Committee (Protocol 20070145; Washington University School of Medicine, St Louis, MO). C57/B6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Male mice aged 8 to 10 weeks were used within a weight range of 20 to 26 g. Mice were housed in a standard facility with a 12-hour day-night cycle. Mice were allowed to acclimate for at least 7 days before experimentation.
1.2. Experimental design
Mice were randomly assigned to either 75% proximal small bowel resection (75% SBR, n =10) or sham operation (75% sham, n = 10). These mice were compared to another group of mice that had been randomly assigned to either 50% proximal SBR (50% SBR, n = 24) or sham operation (50% sham, n = 13). All mice were harvested on postoperative day 7. Parameters of adaptation (villus height, crypt depth, proliferation, and apoptosis) were compared between intraoperative (IO) bowel (removed at the time of intestinal resection) and postoperative (PO) tissue (removed 7 days following SBR) as well as between the two SBR groups to determine if the extent of bowel resection resulted in different adaptation responses.
1.3. Operative procedure
Specific details of the protocol for 50% SBR and sham procedures have been described previously (5). The 75% SBR was performed by transecting the small intestine 6 cm proximal to the cecum. The intervening proximal small bowel (about 18 cm) was removed and a piece of the distal-most resected bowel was fixed in formalin for histologic analysis. The remaining small bowel was anastomosed using 10-0 nylon suture. The 75% sham operation was performed by transecting the small bowel 6 cm proximal to the cecum and immediately creating an anastomosis.
One day before surgery, the diet was changed from solid chow (Lab diet) to liquid rodent diet (Micro-Stabilized Rodent Liquid Diet LD101; Purina Mills, St. Louis, MO). Following operation, mice were allowed only water ad libitum for the first 24 hours. On postoperative day one they were fed liquid rodent diet ad libitum until sacrifice. Mice that died, appeared ill, or had evidence of intestinal obstruction at the time of sacrifice were excluded from further analyses.
1.4. Small bowel harvest
All mice were sacrificed on the seventh postoperative day. Mice were anesthetized with a subcutaneous injection of ketamine, xylazine, and acepromazine (4:1:1 proportion). The abdominal cavity was opened and the remaining small bowel was excised. The intestine was immediately flushed with and placed in ice-cold phosphate-buffered saline (PBS). The first centimeter of bowel distal to the anastomosis was discarded, while the next 2 cm were fixed in 10% formalin for histologic analyses. Mice were sacrificed by cervical dislocation.
1.5 Histology
Formalin fixed specimens of intestine were embedded in paraffin and five-micrometer-thick longitudinal sections were mounted on glass slides and used for morphologic analyses. Hematoxylin- and eosin–stained sections were used to measure villus height and crypt depth with a video-assisted computer program (Metamorph, UIC, Downingtown, PA). Crypts were considered well oriented and counted only if the crypt-villus junctions on both sides of the crypt were intact and Paneth cells were present at the base of the crypt. Villi were considered well oriented and counted only if the central lymphatic channel extended from the villus base to the tip and if their mucosal surface was in continuity with an intact crypt. At least twenty crypts and villi were counted per slide by investigators who were blinded to the experimental group.
1.6 Crypt cell proliferation
At 90 minutes before sacrifice, mice received a subcutaneous injection of 5-bromodeoxyuridine (BrdU; 0.1 ml/10 gm body weight; Zymed Laboratories Inc., San Francisco CA). Incorporation of BrdU into proliferating crypt cells was detected in paraffin-embedded tissue sections by immunohistochemistry using a biotinylated monoclonal antibody system with streptavidin-peroxidase as a signal generator. The staining reagents and methods were provided in kit form (Zymed Laboratories Inc., San Francisco, CA). The number of cells staining positive (incorporating BrdU) per crypt were counted along with the total number of cells per crypt. A proliferative rate was calculated from the ratio of these measurements. Twenty well-oriented crypts were counted for each mouse by blinded scoring.
1.7. Crypt cell apoptosis
Apoptotic indices were calculated from H&E stained slides. Apoptotic bodies were determined by the presence of pyknotic nuclei, condensed chromatin, and nuclear fragmentation. An apoptotic index was defined as the number of apoptotic bodies per 100 crypts. One hundred well-oriented crypts were counted for each mouse by blinded scoring.
1.8 Statistics
The magnitude of adaptation was compared between the 50% SBR and 75% SBR groups. For morphologic parameters (villus length and crypt depth) and apoptotic indices the absolute change between postoperative (PO) and intraoperative (bowel removed at time of resection; IO) values was calculated. Absolute Change = PO value - IO value. The percent change was calculated by dividing the absolute change by the IO value. Percent Change = (PO value – IO value) / IO value. In this way, the morphologic adaptive change for 50% SBR was compared to 75% SBR. All results are presented as a mean ± standard error of measure. Student t-tests were performed to determine statistical difference using SigmaStat software (SPSS, Chicago, IL). P < 0.05 was considered significant.
2. Results
The overall survival for mice that underwent 75% SBR was 73% (16 of 23 mice). A group of 10 mice was chosen for inclusion in subsequent analyses. In this group, 10 of 10 mice survived to postoperative day 7. All 10 mice that underwent the 75% sham operation survived to sacrifice. The historical survival for a 50% SBR in our 15 year experience is roughly 90%.
2.1 Animal Weights
Mice that underwent 75% SBR lost an average of 4.05 +/− 0.38 grams from operation to sacrifice (Fig. 1) while the weight loss was less in the 50% SBR group (1.55 +/− 0.30g). The difference in weight loss between the two SBR groups was highly significant (p < 0.0001; Fig. 1).
Figure 1.
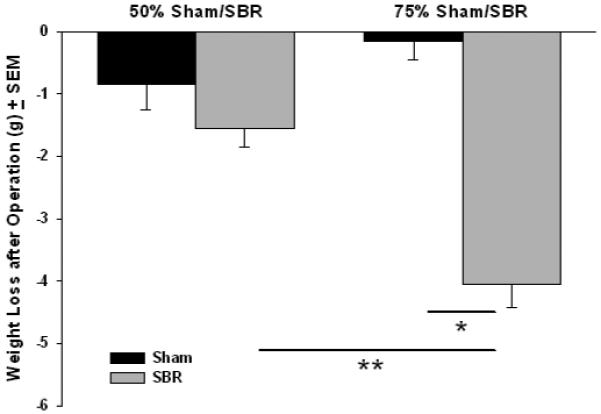
Mean weight loss between operation and sacrifice in mice undergoing either 50% or 75% small bowel resection (SBR) or transection of the bowel with reanastomosis alone (sham). Mice in the 75% SBR group lost significantly more weight than shams and 50% SBR’s. N=10 for all groups * = p<.000001, ** = p<.0001.
2.2 Ileal Morphology: IO vs PO
There is a natural decline in villus height and crypt depth along the proximal-to-distal axis of the small bowel (1). Because the tissue harvested postoperatively after 50% SBR and 75% SBR necessarily were from different sites along the proximal-to-distal axis of the small intestine, we could not compare raw values for various morphologic parameters between these groups. To account for site-related variation in adaptive parameters, PO crypt depth and villus length were normalized to their IO values as described in section 1.8. There was no statistical difference in either the absolute or percentage increase in morphologic parameters between 50% SBR and 75% SBR groups: % increase in crypt depth (+17.3% +/− 5.7% vs +27.3% +/− 5.3%; p = NS); % increase in villus length (+23.8% +/− 4.5% vs +25.7% +/− 5.3%; p = NS; Fig. 2).
Figure 2.
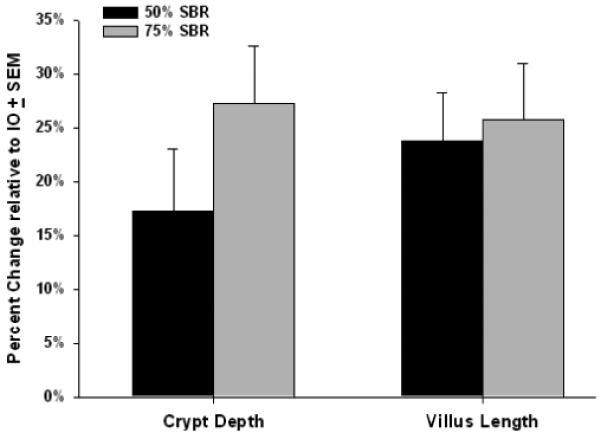
Percent change in crypt depth and villus length for 50% (n=24) and 75% small bowel resection (SBR; n=10) groups. PO – postoperative measurements in the remnant bowel at 7 days following SBR; IO – intraoperative bowel removed at the time of intestinal resection. Percent Change = (PO-IO) / IO. No significant differences in magnitude of morphometric parameters of was observed between 50% SBR and 75% SBR groups (p=NS).
2.3 Proliferation
Mice that underwent 75% SBR (n = 9) had greater proliferative rates than shams (n = 10) as measured by the percentage of crypt cells staining positively for BrdU. Compared to shams, mice that had 50% SBR also had increased rates of proliferation (Fig. 3A). With regard to the effect of the extent of resection on proliferation, we normalized the SBR groups to the mean proliferative rate of their respective shams (see section 1.8 for details). There was no difference between the rate of crypt proliferation relative to sham values between the 50% (n = 13) and 75% SBR groups (n = 9) (4.35% +/− 0.86% vs 6.91% +/− 1.37%; p = NS; Fig. 3B).
Figure 3.
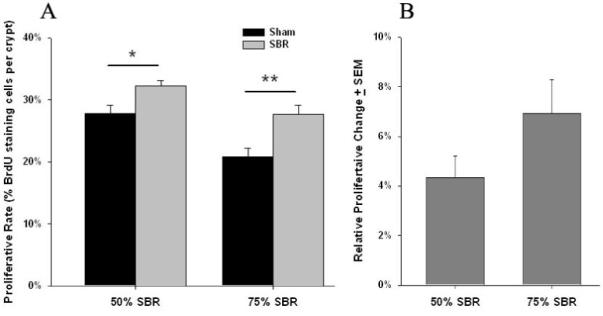
A Proliferative rates measured by the percentage of BrdU staining cells per crypt in mice undergoing either 50% or 75% small bowel resection (SBR) or transection of the bowel with reanastomosis alone (sham). Both SBR groups showed increased proliferative rates compared to shams. * = p<.01, ** = p<.005.
B Percent change in rates of enterocyte proliferation after small bowel resection (SBR). No significant difference was found for the relative proliferative change between 50% SBR and 75% SBR groups after normalizing for sham values (p=NS).
2.4 Apoptosis
Mice in the 75% SBR group had greater PO rates of apoptosis in crypts than IO. Similarly, mice that had 50% SBR had enhanced apoptotic rates after SBR (Fig. 4A). Comparing 50% SBR to 75% SBR, no difference was observed in IO or PO apoptotic rates. Similarly, there was no difference in the change in apoptotic rates from IO to PO for 50% SBR vs 75% SBR (+4.79 +/− 0.86 vs +4.84 +/− 0.75 apoptotic bodies per 100 crypts; p = NS; Fig. 4B).
Figure 4.
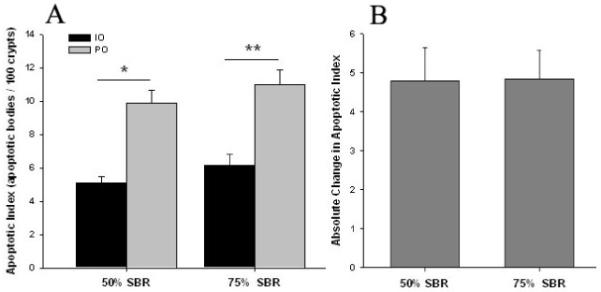
A Apoptotic indices measured by the number of apoptotic bodies per 100 crypts in mice undergoing either 50% or 75% small bowel resection (SBR) or transection of the bowel with reanastomosis alone (sham). Both SBR groups showed increased apoptotic rates PO compared to IO values. * = p<.000001, ** = p<.001.
B No statistical difference was found for the absolute change in apoptotic indices (PO-IO) between 50% SBR and 75% SBR groups (p=NS).
3. Discussion
In the current study, we have extended our model of SBS in the mouse to a greater magnitude of resection with the intent of demonstrating greater adaptive changes. If this were the case, we would anticipate that genetic alterations responsible for adaptation would be magnified, thereby leading to easier detection of target genes responsible for this important response. Unfortunately, this was not the case as changes in both mucosal morphology and kinetics of enterocyte turnover were similar despite the greater amount of bowel resected.
As expected, we noted an increased mortality associated with increased resection. Others have found 75% SBR in the mouse to be even more lethal. For example, our group has previously published a 16% survival in mice 3 weeks after 75% SBR, with most of the mice dying within the first three days after operation (5). Other researchers in our lab have recorded a 55% survival 7 days after 75% SBR (unpublished data). The 75% SBR model is likely more morbid due to the more advanced degree of SBS and dehydration that ensues. Indeed, we have verified that the animals have noticeably worse diarrhea and lose more weight following 75% SBR than after 50% SBR.
As expected, the remnant bowel adapted after mice underwent 75% SBR relative to intraoperative levels. The findings of augmented crypt depth, villus length, proliferative rate, and apoptotic indices all parallel previously reported measures of adaptation following 50% SBR in mice (4, 5). These data represent the first study regarding the extent of small intestinal resections and the magnitude of adaptation in the mouse. Prior studies had been done in rats (12-14). Perhaps the best evidence that greater bowel resections induce increasingly more robust adaptation responses comes from Hanson’s work published in 1977 (13). They performed resections ranging from 10%-80% in rats (n=5 in each group). Similar to our findings, they reported that greater resections led to increased weight loss after 30 days.
On the other hand, this group found that greater resections resulted in enhanced intestinal adaptation as gauged by increased remnant bowel wet weights and proliferative rates in crypts. Hanson et al sampled tissue 3cm distal to the anastomosis, a comparable location to our PO tissue harvest site in mice. However, they performed a mid-SBR while we have performed a proximal-SBR. Although seemingly unlikely, this difference may account for the different findings between groups. They also sampled tissue from another, fixed point – 3 cm proximal to the cecum. Interestingly, in the distal ileum they found a plateau-effect seen for proliferative rates between 10-70% SBR’s; only 80% SBR led to greater proliferative rates. Perhaps our findings were not too dissimilar from Hanson’s, as our tissue from 75% SBR mice was harvested in the distal ileum, as well.
We identified two other studies that directly tested the impact different resection lengths had on adaptation (12, 14). Our lab has published on 25%, 50%, and 75% SBR’s in rats (12). We showed that increasingly greater resections lead to increased ileal wet weights, but no change in proliferative rates in the remnant bowel. Intriguingly, serum taken from these rats was capable of stimulating enterocyte proliferation in vitro, but there was no effect of the extent of resection on the magnitude of proliferation. Lanzoni et al tested 60%, 70%, 80%, and 90% SBR’s in rats (14). They found that the 80% and 90% SBR’s caused more weight loss than the lesser resections. Although they claim that adaptive parameters increased with greater resections, no statistical analyses were performed to support this claim. In addition, villus growth calculated from IO to PO groups appears similar across groups.
Our inability to demonstrate enhanced adaptation after SBR could be due to the increased technical limitations of performing the intestinal anastomosis during the 75% SBR. Due to the reduced size of the distal ileum, these mice are more likely to develop bowel obstruction postoperatively. Although no animals exhibited overt evidence of obstruction at harvest, it is possible that mice after 75% SBR have subclinical partial obstruction that does not manifest, but, nevertheless, impairs the distal bowel’s adaptive response. We observed a more profound state of SBS in mice that underwent 75% SBR. Perhaps, these mice lack the nutritional reserve to mount a more robust adaptation response than the 50% SBR mice. It must be considered that adaptation responses may be species-specific. As such, the findings of the present study may not be directly transferable to the clinical setting. Indeed, little is understood regarding adaptation in humans. Most knowledge has been derived from various animal studies with different species, sites, and extents of intestinal resection.
The present study does have limitations. First of all, the 50% SBR and 50% sham operations were preformed by a different surgeon (SWL) than the 75% SBR and 75% sham procedures (DW). On the other hand, the magnitude of adaptive change in the 50% group of the present study is no different than multiple other prior studies done in our laboratory. The ability to perform greater lengths of intestinal resection in the present report undoubtedly reflects the large experience gained in our laboratory over the past several years. Despite improved operative techniques and survival with this model, the extent of adaptation remains fixed. Another factor to consider is the timing for adaptation to occur. While we have previously demonstrated that adaptation reaches a significant peak after seven days following a 50% SBR (5), we have not performed the same time course study following a 75% SBR. It is possible that adaptation plateaus after 50% SBR before it does after 75% SBR and, therefore, seven days may not be long enough to observe different adaptation responses between the groups.
Since our murine model affords the opportunity to directly test the significance of various genes in the genesis of adaptation, we sought to characterize the influence of resection specifically in this model. Regardless of its limitations, this study does indicate that SBR greater than 50% in mice is complicated by increased morbidity and mortality. This is accompanied by seemingly little evidence of enhanced parameters of adaptation. We therefore conclude that performing resections greater than 50% in mice to study the mechanisms underlying adaptation is not useful. Although we have yet to test if any of the signaling cascades associated with adaptation are altered by greater resections, it seems doubtful given the similar morphometric scale of adaptation.
Acknowledgements
The authors wish to acknowledge the work of Hongbo Liu and Susan Shi for their excellent technical support.
This work was supported by National Institutes of Health Grant R01 DK 059288 (Dr Warner), T32 CA009621 (Drs Wakeman and Longshore), and P30DK52574 - Morphology and Murine Models Cores of the Digestive Diseases Research Core Center of the Washington University School of Medicine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longshore SW, Wakeman D, et al. Bowel resection induced intestinal adaptation: progress from bench to bedside. Minerva Pediatr. 2009 Jun;61(3):239–251. [PubMed] [Google Scholar]
- 2.Williamson RC. Intestinal adaptation. Structural, functional and cytokinetic changes. N Engl J Med. 1978;298:1393–1402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]
- 3.Williamson RC. Intestinal adaptation. Mechanisms of control. N Engl J Med. 1978;98:1444–1450. doi: 10.1056/NEJM197806292982604. [DOI] [PubMed] [Google Scholar]
- 4.Helmrath MA, Erwin CR, et al. Enterocyte apoptosis is increased following small bowel resection. J Gastrointest Surg. 1998;2:44–49. doi: 10.1016/s1091-255x(98)80102-9. [DOI] [PubMed] [Google Scholar]
- 5.Helmrath MA, VanderKolk WE, et al. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996 Nov;183(5):441–449. [PubMed] [Google Scholar]
- 6.Chaet MS, Arya G, et al. Epidermal growth factor enhances intestinal adaptation after massive small bowel resection. J Pediatr Surg. 1994 Aug;29(8):1035–1039. doi: 10.1016/0022-3468(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 7.Sigalet DL, Lees GM, et al. The physiology of adaptation to small bowel resection in the pig: an integrated study of morphological and functional changes. J Pediatr Surg. 1990 Jun;25(6):650–657. doi: 10.1016/0022-3468(90)90356-e. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JS, Quigley EM, Adrian TE. Effect of intestinal tapering and lengthening on intestinal structure and function. Am J Surg. 1995 Jan;169(1):111–119. doi: 10.1016/s0002-9610(99)80118-4. [DOI] [PubMed] [Google Scholar]
- 9.Secor SM, Lane JS, et al. Luminal nutrient signals for intestinal adaptation in pythons. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1298–G1309. doi: 10.1152/ajpgi.00194.2002. [DOI] [PubMed] [Google Scholar]
- 10.Drozdowski L, Thomson AB. Intestinal mucosal adaptation. World J Gastroenterol. 2006 Aug 7;12(29):4614–4627. doi: 10.3748/wjg.v12.i29.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiesen A, Drozdowski L, et al. Adaptation following intestinal resection: mechanisms and signals. Best Pract Res Clin Gastroenterol. 2003 Dec;17(6):981–995. doi: 10.1016/s1521-6918(03)00097-0. [DOI] [PubMed] [Google Scholar]
- 12.Juno RJ, Knott AW, et al. A serum factor(s) after small bowel resection induces intestinal epithelial cell proliferation: effects of timing, site, and extent of resection. J Pediatr Surg. 2003 Jun;38(6):868–874. doi: 10.1016/s0022-3468(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 13.Hanson WR, Osborne JW, Sharp JG. Compensation by the residual intestine after intestinal resection in the rat. I. Influence of amount of tissue removed. Gastroenterology. 1977 Apr;72(4 Pt 1):692–700. [PubMed] [Google Scholar]
- 14.Lanzoni N, Martins JL, et al. Repercussions of extensive small bowel resections in growing rats. Transplant Proc. 2004 May;36(4):1009–1011. doi: 10.1016/j.transproceed.2004.03.048. [DOI] [PubMed] [Google Scholar]


