Abstract
Learning to contend with threats in the environment is essential to survival, but dysregulation of memories for traumatic events can lead to disabling psychopathology. Recent years have witnessed an impressive growth in our understanding of the neural systems and synaptic mechanisms underlying emotional memory formation. As a consequence, interest has emerged in developing strategies for suppressing, if not eliminating, fear memories. Here I review recent work employing sophisticated behavioral, pharmacological, and molecular tools to target fear memories, placing these memories firmly behind the crosshairs of neurobiologically informed interventions.
Learning to fear threats in the environment is highly adaptive; it allows animals, whether rats or humans, to anticipate harm and organize appropriate defensive behaviors in response to threat (Bolles, 1970; Fanselow and Lester, 1988; Ohman and Mineka, 2001). However, this form of learning can also lead to pathological fear memories that fuel disorders of fear and anxiety, such as panic disorder and post-traumatic stress disorder (PTSD) in humans (Bouton et al., 2001; Rasmusson and Charney, 1997; Wolpe and Rowan, 1988). What determines an individual’s vulnerability to developing pathological fear after a traumatic experience is not clear; simply experiencing trauma does not appear to be sufficient (Jovanovic and Ressler, 2010; Yehuda and Ledoux, 2007). Less than 10% of individuals that experience a traumatic event, such as a natural disaster, will develop symptoms of PTSD. Nonetheless, in those individuals that develop PTSD, an important clinical concern is how to limit pathological fear once it has been established (Powers et al., 2010; Rothbaum and Davis, 2003).
Yet limiting pathological fear is a considerable challenge insofar as fear memories are evolutionarily programmed to be rapidly acquired, temporally enduring, and broadly generalized across both familiar and novel contexts. Moreover, behavioral methods to reduce fear and anxiety, such as exposure therapy, tend to produce fear suppression that is often slow to develop, short-lived, and context-dependent (Bouton, 1988; Craske et al., 2008; Hermans et al., 2006). Apparently, the extinction learning that occurs when overt cues or mental images associated with trauma are presented alone in a safe and therapeutic setting only temporarily suppresses the dominant memory that trauma-related stimuli are fearful. This stands to reason when one considers that an incorrect attribution of safety to a dangerous place, object, or animal may result in death or injury, whereas an incorrect attribution of danger to an otherwise safe stimulus protects one from harm.
Given the resilience of fear memory, there has been considerable interest in understanding the neurobiological mechanisms that mediate the long-term storage and retrieval of fear memories, as well as the mechanisms underlying the safety memories acquired during extinction. Fortunately, there are several rich experimental animal models that have been developed to study emotional learning and memory, and these have yielded considerable new information concerning the neurobiology of fear conditioning and extinction. The purpose of this review is to consider how these models have contributed to recent advances in understanding the molecular and cellular mechanisms and neural circuits in the brain involved in learning new fears and inhibiting, even erasing, old ones.
Neural Substrates of Fear Conditioning
The quintessential model for the neuroscientific study of aversive learning and memory is Pavlovian fear conditioning. In this form of learning, an innocuous stimulus (conditioned stimulus or CS), such as a tone or light, is paired with a noxious stimulus (unconditioned stimulus or US), such as an electric footshock. After one or more such trials, animals rapidly learn that the CS predicts the aversive US and consequently produce a learned fear response (conditioned response or CR) to the CS. This form of learning is ubiquitous in the animal kingdom, and is now routinely used in mice, rats, cats, rabbits, primates, and humans to probe the neural systems and cellular mechanisms underlying emotional learning and memory. Importantly, nearly a century of fundamental work by experimental psychologists on the behavioral processes involved in associative learning have established conditioning methods as sophisticated tools for disentangling the brain mechanisms of sensation, memory, and action (Fanselow and Poulos, 2005).
Decades of research into the neural substrates of Pavlovian fear conditioning has revealed the essential neural circuit required for the acquisition and expression of fear memory (Figure 1) (Davis, 2006; LeDoux, 2000; Maren, 2001; Pape and Pare, 2010). The core of this fear circuit is centered on the amygdala, which is a heterogeneous collection of nuclei buried deep within the temporal lobe. Two regions within the amygdala are particularly important for fear conditioning: the basolateral complex (BLA, consisting of lateral, basolateral, and basomedial nuclei) and the central nuclear group [CEA, consisting of the medial (CEm) and lateral (CEl) nuclei]. Anatomically, the BLA represents a nuclear extension of the temporal neocortex and the CEA represents a ventrocaudal extension of the striatum (Pitkanen et al., 1997; Swanson and Petrovich, 1998). The flow of information between the BLA and CEA is largely unidirectional with LA neurons projecting to CEl directly, and indirectly to CEm via the basolateral nucleus (BL) and through a network of inhibitory interneurons in the intercalated cell masses (ITC) (Krettek and Price, 1978; Pare and Smith, 1993, 1998; Pare et al., 1995). CEm projects to several brain regions that mediate fear responses, such as freezing, tachycardia, and stress hormone release, and axonal projections from BLA to CE are critical for the expression of these responses after fear conditioning (Ciocchi et al., 2010; Haubensak et al., 2010; Jimenez and Maren, 2009; Paré et al., 2004). That is, after fear conditioning, it has recently been shown that aversive CSs come to suppress the inhibitory influence of CEl on CEm and drive the expression of conditional fear responses (Ciocchi et al., 2010; Haubensak et al., 2010). This reveals that CEl normally inhibits CEm and the regulation of this inhibition appears to be essential for the expression of fear and anxiety (Tye et al., 2011).
Figure 1.
Neuronatomy of conditioned fear. A simplified neuroanatomical schematic outlining some of the major brain regions and their anatomical connections invoved in Pavlovian fear conditioning. Shading indicates major brain regions (brown, midbrain; yellow, thalamus; orange, basal forebrain; blue, neocortex; red, amygdala; violet, hippocampus). Sensory information asends to the amygdala through the midbrain and thalamus; auditory information also reaches the amygdala via the cortex. Antomical convergence and association of of the conditioned stimulus (CS) and unconditioned stimulus (US) occurs in the amygdala; contextual information processed by the hippocampus can also enter into association with the US in the amygdala. Conditioned and unconditioned fear responses (CRs and URs) are mediated by projections from the amygdala to an array of brain areas involved in autonomic and somatic defensive responses. Abbreviations: AC, auditory cortex; BA, basal nuclei of the amygdala; BNST, bed nuclei of the stria terminalis; CEl, lateral division of central nucleus of the amygdala; CEm, medial divsion of the central nucleus of the amygdala; HIP, hippocampus; IC, inferior colliculus; IL, infralimbic division of the medial prefrontal cortex; ITC, intercalated cellsof the amygdala; LA, lateral nucleus of the amygdala; LH, lateral hypothalamus; MGdv, dorsal and ventral divisions of the thalamic medial geniculate nucleus; MGm, medial division of the thalamic medial geniculate nucleus; NAcc, nucleus accumbens, dlPAG, dorsolateral division of the periaqueductal gray; vPAG, ventral division of the periaquedcutal gray; PIN, posterior intralaminar nucleus of the thalamus; PL, prelimbic division of the medial prefrontal cortex; PRh, perirhinal cortex; PVN, paraventricular nucleus of the hypothalamus.
Multimodal sensory information reaches both regions of the amygdala, and this affords an opportunity for the convergence of CS and US information within these areas. Indeed, substantial data indicate that the lateral nucleus (LA) is a critical sensory interface of the amygdala that mediates CS-US association formation during fear conditioning (Blair et al., 2001; Maren, 1999). For example, auditory and somatic stimuli excite LA neurons at short latencies (Johansen et al., 2010; Romanski et al., 1993), and fear conditioning greatly augments responses of LA neurons to auditory CSs (Goosens et al., 2003; Goosens and Maren, 2004; Herry et al., 2008b; Hobin et al., 2003; Johansen et al., 2010; Maren, 2000; Quirk et al., 1997; Repa et al., 2001). Bernstein and colleagues have also recently shown that individual LA neurons exhibit increases in the expression of the immediate early gene Arc that reflects CS-US convergence in these cells (Barot et al., 2009). The convergence of CS and US information in the LA engenders associative plasticity that increases the efficacy of CS inputs onto LA neurons (Blair et al., 2001; Maren, 1999). For example, fear conditioning increases CS-evoked extracellular field potentials in the LA in vivo (Rogan and LeDoux, 1995; Tang et al., 2001), and LA neurons exhibit conditioning-related changes in synaptic transmission measured ex vivo (McKernan and Shinnick-Gallagher, 1997; Rumpel et al., 2005; Tsvetkov et al., 2002).
In addition to these electrophysiological correlates of conditioning, LA neurons exhibit changes in gene expression and protein phosphorylation after fear conditioning (Lamprecht et al., 2009; Ploski et al., 2010; Ploski et al., 2008; Shumyatsky et al., 2005). Indeed, fear conditioning regulates several proteins importantly involved in the induction and maintenance of synaptic plasticity, including AMPA receptors, PI-3 kinase, and A-kinase anchoring proteins, nexin protease, and mitogen-activated protein kinase among others (Apergis-Schoute et al., 2005; Lamprecht et al., 2002; Lin et al., 2001; Meins et al., 2010; Migues et al., 2010; Moita et al., 2002; Schafe et al., 2000). Accordingly, inhibiting synaptic plasticity in the LA with a variety of manipulations including NMDA receptor antagonists (Fendt, 2001; Goosens and Maren, 2003; Lee and Kim, 1998; Maren et al., 1996b; Miserendino et al., 1990), protein synthesis inhibitors (Maren et al., 2003; Nader et al., 2000; Schafe and LeDoux, 2000), protein kinase inhibitors (Apergis-Schoute et al., 2005; Goosens et al., 2000; Lamprecht et al., 2002; Lin et al., 2001; Merino and Maren, 2006; Migues et al., 2010), or antisense oligonucleotides for plasticity-related genes (Malkani et al., 2004; Ploski et al., 2008) impairs the acquisition of fear memory. Similarly, inactivating LA neurons prevents fear expression (Helmstetter and Bellgowan, 1994; Maren et al., 2001; Muller et al., 1997). Genetically modified mice that lack proteins essential for amygdala synaptic plasticity exhibit deficits in fear conditioning (Brambilla et al., 1997; Shumyatsky et al., 2002).
Yet despite the focus on synaptic plasticity in the LA as a mechanism for fear conditioning, emerging evidence suggests that fear conditioning is likely mediated by distributed synaptic plasticity within the amygdala (Wilensky et al., 2006; Zimmerman et al., 2007). For instance, central nucleus neurons exhibit conditioning-related plasticity in spike firing and changes in gene expression after conditioning (Ciocchi et al., 2010; Pascoe and Kapp, 1985). Moreover, NMDA receptor antagonism in the CEA prevents the acquisition of conditioned fear (Goosens and Maren, 2003) and blocks synaptic plasticity in CEA neurons (Lopez de Armentia and Sah, 2007; Samson and Paré, 2005). Inhibition of protein synthesis in the CEA prevents the consolidation of fear memory (Wilensky et al., 2006). Within the amygdala, the CEA receives its primary excitatory input from the basolateral nucleus (BL), which in turn receives projections from LA. Hence, a cascade of NMDA receptor-dependent plasticity among these glutamatergic synapses may be essential for fear conditioning (Maren, 2008). Plasticity among inhibitory neurons in the intercalated cell masses that are interposed between the LA and CEA may also functionally disinhibit LA neurons to promote fear expression (Amano et al., 2010; Royer and Pare, 2003).
It is apparent that a considerable amount of work implicates the amygdala in fear memory. Yet which population of neurons in the amygdala is essential for fear conditioning and where are they located? This question is beginning to be addressed by powerful new molecular genetic methodologies that allow visualization of neurons involved in encoding and retrieving fear memory. For example, Mayford and colleagues (2007) have used a transgenic mouse (TetTag) expressing a doxycycline-insensitive tetracycline-transactivator (tTA*) coupled to a tauLacZ reporter to visualize neurons activated by a fear conditioning experience (Reijmers et al., 2007). In this mouse, activation of the tTA* requires a standard doxycycline-sensitive tTA, which is under the control of the immediate early gene (IEG) Fos promoter to index neuronal activity. Hence, once activated (off doxycycline), the tTA* transgene drives tauLacZ reporter expression even in the presence of doxycycline. This allows neurons that were active in a particular time window (when animals are off doxycycline) to be persistently tagged. Combining this method with immunohistochemistry for Zif (an IEG protein that also indexes activity), the authors were able to determine whether neurons active at the time of conditioning (tauLacZ -positive) were also active at the time of memory retrieval (Zif-positive) three days after conditioning. Indeed, the authors found that a significant subset (roughly 12%) of neurons in the BLA (CEA was not reported) co-expressed tauLacZ and Zif, and that the number of co-labeled neurons correlated with the expression of fear. Similarly, Josselyn and colleagues (2007) found that CREB-overexpressing neurons in LA were preferentially incorporated into the fear memory network insofar as those neurons were more likely to co-express Arc (an IEG protein that also indexes activity) upon memory retrieval (Han et al., 2007). Hence, these approaches have the potential to define specific neuronal networks involved in encoding and retrieving fear memories.
Once acquired, the amygdala has a long-term role in maintaining fear memory. Unlike hippocampal-dependent memories that undergo systems consolidation in neocortex (Bontempi et al., 1999; Frankland and Bontempi, 2005; Frankland et al., 2004; Squire and Alvarez, 1995), CS-US associations encoded in the amygdala appear to reside there permanently. Postconditioning lesions of the BLA yield equivalent impairments in conditional fear independent of when they are made after training (Cousens and Otto, 1998; Lee et al., 1996; Maren et al., 1996a). In fact, BLA lesions impair fear memory when made up to one year after conditioning (Gale et al., 2004), suggesting that the amygdala maintains fear memory for the life of the animal. Although we do not yet understand the nature of the permanent changes in brain circuitry that maintain fear memory for the life of the organism, we now have an anatomical locus to target interventions to either suppress or reverse fear memories.
Extinction and the Contextual Regulation of Fear
During Pavlovian conditioning, animals learn that a CS predicts the occurrence of a US. This predictive association fosters adaptive, anticipatory learned responses when the CS occurs. Given that conditioning depends on the CS-US contingency, it follows that breaking the contingency might reduce conditional responding. To break the CS-US contingency, Pavlov developed an experimental procedure in which the CS was presented alone (without the US) for several trials after the completion of conditioning (Pavlov, 1927). Not surprisingly, the earliest CS-alone trials produced a robust CR, but the CR gradually faded with subsequent CS presentations. Pavlov termed this phenomenon “extinction”, and it is now apparent that this form of learning is an important component of behavioral interventions for patients with pathological fear memories. For example, exposure therapy involves the use of mental imagery and exposure to trauma-relevant cues in a safe environment to suppress the fear associated with the memory of the traumatic event (Craske et al., 2008; Powers et al., 2010; Rothbaum and Davis, 2003).
Given the importance of extinction learning as a mechanism for suppressing fear memory, there has been an explosion of work into the neural mechanisms of extinction (Bouton et al., 2006a; Herry et al., 2010; Myers and Davis, 2002; Pape and Pare, 2010; Quirk and Mueller, 2008). Not surprisingly, much of this work has focused on the contribution of the amygdala to fear extinction and several reports indicate that the BLA is critical for the acquisition of extinction memories. For example, infusing NMDA receptor antagonists into the BLA disrupts the acquisition of extinction (Falls et al., 1992; Laurent et al., 2008; Zimmerman and Maren, 2010), whereas blockade of NMDA receptors in the CEA does not affect extinction learning (Zimmerman and Maren, 2010). Intracellular signaling pathways downstream of BLA NMDA receptors are also critical for extinction learning (Herry et al., 2006; Lin et al., 2003a; Lin et al., 2003c; Lu et al., 2001; Yang and Lu, 2005). In addition to the glutamatergic system, recent work indicates that other neurotransmitter systems contribute to extinction learning. For example, mice lacking endocannabinoid receptors (CB1 receptors, specifically) exhibit impairments in extinction learning and systemic administration of a CB1 antagonist (SR141716, rimonabant) inhibits extinction learning (Chhatwal et al., 2009; Marsicano et al., 2002). Endocannabinoids modulate inhibitory GABAergic synaptic transmission in the amygdala, which is also essential for extinction learning (Chhatwal et al., 2005b; Harris and Westbrook, 1998; Laurent et al., 2008; Laurent and Westbrook, 2008; Makkar et al., 2010). Collectively, these data suggest that changes in the synaptic transmission within the BLA contribute to the suppression of conditional fear after extinction training. Indeed, depotentiation of amygdaloid synaptic transmission has been reported to occur after extinction training (Kim et al., 2007).
Curiously, however, extinction produces a relatively transient suppression of fear; conditioned fear responses return under a variety of conditions including after the mere passage of time (i.e., spontaneous recovery) or if the extinguished CS is presented outside the extinction context (e.g., renewal) (Bouton, 1993). This suggests that memories of both fear conditioning and extinction are encoded in the amygdala, and contextual retrieval cues determine which memory is expressed in behavior. The medial prefrontal cortex and hippocampus have rich connections with the amygdala and are involved in processing contextual information. Not surprisingly, considerable work now implicates these brain areas in the regulation of fear expression after extinction (Maren and Quirk, 2004; Quirk et al., 2006; Quirk and Mueller, 2008; Quirk et al., 2000; Sotres-Bayon et al., 2006; Sotres-Bayon and Quirk, 2010).
Anatomically, the infralimbic (IL) division of the vmPFC projects to a network of inhibitory interneurons in the amygdala; these neurons are located in the intercalated cell masses (ITC) interposed between the BLA and CEA (Figure 1). ITC neurons send massive inhibitory projections to CEA, and are therefore well positioned to limit excitatory input from the BLA and reduce CEA-mediated fear responses (Berretta et al., 2005; Paré and Smith, 1993). Paré and colleagues recently demonstrated the important role for ITC neurons in the expression of extinction using selective lesions of ITC neurons (Likhtik et al., 2008). In this study, rats received intra-amygdala infusions of a selective immunotoxin against ITC neurons after extinction training; ITC lesions produced a significant loss of extinction (i.e., the expression of freezing was increased by the lesion). Other work has shown that pharmacological manipulation of the vmPFC influences the consolidation of extinction memory (Hugues et al., 2006; Laurent and Westbrook, 2008, 2009; Mueller et al., 2010; Sierra-Mercado et al., 2011), suggesting that the vmPFC may also play a role in establishing extinction memories (as opposed to merely regulating extinction recall). Extinction learning and recall induces Fos in vmPFC neurons (Hefner et al., 2008; Herry and Mons, 2004; Knapska and Maren, 2009) and electrical stimulation of the vmPFC facilitates extinction (Milad et al., 2004; Vidal-Gonzalez et al., 2006). Prefrontal cortical neurons also exhibit physiological changes, including increased bursting, during extinction learning (Burgos-Robles et al., 2007; Chang et al., 2010; Milad and Quirk, 2002). Interestingly, several studies report intact extinction after vmPFC lesions (Farinelli et al., 2006; Garcia et al., 2006; Gewirtz et al., 1997); some of these disparities may arise from strain differences in the effects of PFC lesions on extinction (Chang and Maren, 2010).
In addition to the mPFC, the hippocampus has been implicated in both the acquisition and expression of fear extinction (Bouton et al., 2006b). A number of groups have shown that disrupting hippocampal function prior to extinction training impairs the acquisition of extinction (Corcoran et al., 2005; Fischer et al., 2007; Fischer et al., 2004; Sananbenesi et al., 2007; Szapiro et al., 2003; Tronson et al., 2009; Vianna et al., 2001). Moreover, the hippocampus is involved in the context-dependence of extinction, particularly for regulating the renewal of fear to an extinguished CS outside of the extinction context (Maren and Holt, 2000). Lesions or reversible inactivation of the hippocampus prevents the renewal of fear to an extinguished CS outside of the extinction context (Corcoran et al., 2005; Corcoran and Maren, 2001, 2004; Hobin et al., 2006; Ji and Maren, 2008a; Ji and Maren, 2005), and a recent report implicates electrical synapses in the hippocampus in this function (Bissiere et al., 2011). Both cortical and subcortical projections of the hippocampus are important for renewal insofar as fornix or entorhinal cortical lesions reproduce the effects of hippocampal lesions (Ji and Maren, 2008b). Inactivation of the hippocampus also eliminates “neuronal renewal” of CS-evoked neuronal activity that is observed in the LA when an extinguished CS is presented outside the extinction context (Hobin et al., 2003; Maren and Hobin, 2007). Collectively, these data reveal that extinction yields a new inhibitory memory that competes with the fear memory for expression in behavior. Disruption of the hippocampal system prevents the return of fear normally observed when an extinguished CS is presented outside of the extinction context. Animals without a functional hippocampus are unable to contextualize their fear and extinction memories and therefore respond according to the net experience with the CS. Hence, disrupting hippocampal function actually promotes the generalization of extinction memories to many contexts.
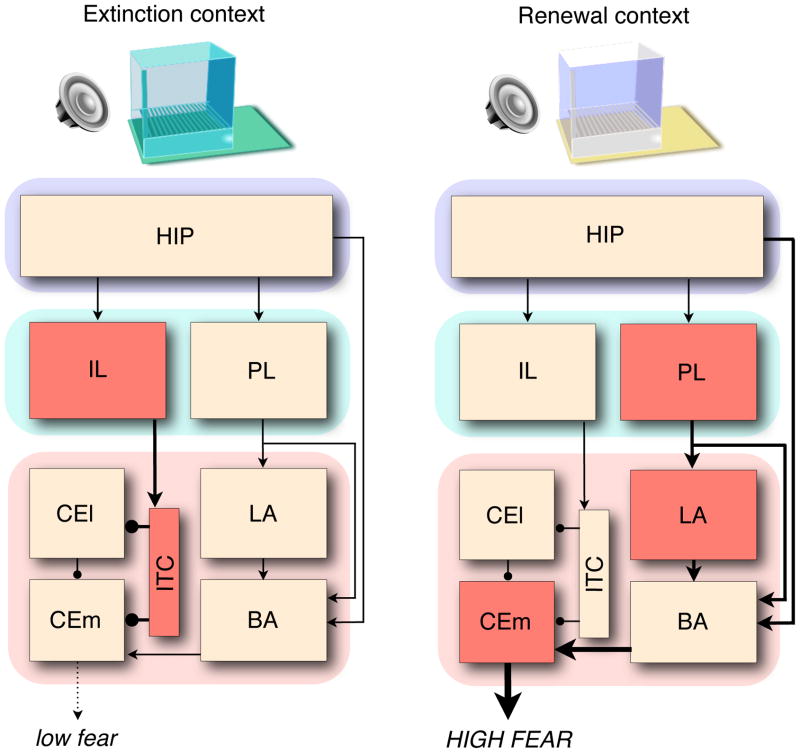
The hippocampus projects to both the BLA and the vmPFC and is therefore well positioned to gate the expression of fear and extinction memories. Indeed, a recent study in our laboratory showed that different prefrontal-amygdala circuits were engaged during the retrieval of fear and extinction memories (Knapska and Maren, 2009). Figure 2 highlights the brain regions (in red) that exhibited differential c-fos expression during retention tests in which an extinguished CS was presented either in the extinction context or in another context. Interestingly, neurons in the IL and ITC were active during the retrieval test in the extinction context, when conditioned freezing was suppressed, but not outside the extinction context when conditioned fear was high. Conversely, neurons in the PL, LA, and CEm were active outside the extinction context (when fear was high), but not in the extinction context (when fear was low). The hippocampus was engaged under both conditions, suggesting that it may uniquely process where and when a CS is experienced, independent of the valence of that memory.
Figure 2.
Contextual control of extinction. A simplfied neuroantaomical schematic illustrating differential c-fos expression in brain structures involved in the expression of fear after extinction. Suppression of fear to a CS in the extinction context (left) is associated with activity in the infralimbic division of the medial prefrontal cortex (IL) and inhibitory intercalated neurons (ITC) in the amygdala. Inhibition of the medial division of the central nucleus (CEm) by the ITC limits the expression of conditioned fear. The return of fear to a CS presented outside of the extinction context (Renewal context, right) is associated with activity in the prelimbic division of the medial prefrontal cortex (PL), the lateral nucleus of the amygdala (LA), and CEm. The hippocampus (HIP) basal nuclei of the amygdala (BA) are engaged in both situations, and may therefore gate the expression of conditioned fear through their connections with the the unique prefrontal-amygdala networks associated with extinction and renewal.
It is possible that contextual processing by the hippocampus gates fear through direct projections to the amygdala, or through indirect projections through vmPFC. To this point, recent work by Luthi and colleagues has shown that there are anatomically distinct populations of neurons in the basolateral amygdaloid nucleus that respond when animals express either express or suppress conditional fear (Herry et al., 2008a). “Fear” neurons responded to non-extinguished CSs or extinguished CSs presented outside the extinction context, whereas extinction neurons only responded to extinguished CSs presented in their extinction context. Interestingly, the majority of fear neurons were orthodromically activated by electrical stimulation of the ventral hippocampus, whereas extinction neurons received their afferent input from the vmPFC. Hence, the contextual retrieval of fear memory might involve a hippocampo-prefrontal cortical network that regulates the balance of excitation and inhibition in the amygdala to foster or suppress, respectively, fear to an extinguished CS (Maren, 2005). It is also conceivable that the balance of activity among inhibitory CEl neurons that are either excited (“CS on” neurons) or inhibited (“CS off” neurons) by a CS (e.g., Ciocchi et al., 2010) regulates the suppression or renewal, respectively, of fear after extinction; this possibility has not yet been explored.
Preventing the Return of Fear
Reducing the expression of fear memory with extinction procedures, such as exposure therapy, is fundamental to therapeutic interventions for fear and anxiety disorders in humans. Unfortunately, the suppression of conditional responding that follows extinction is transient (Bouton, 1993; Bouton and Bolles, 1979a; Rescorla, 2004). In his early work, Pavlov noted that an extinguished CR would return if the animal was presented with a novel stimulus, a phenomenon termed “disinhibition” (Pavlov, 1927). He also showed that extinguished CRs would spontaneously return with the mere passage of time, a phenomenon termed “spontaneous recovery”. As previously described, extinguished CRs are also highly specific to the experimental context in which they are acquired. In other words, an extinguished CR exhibits “renewal” when the CS is presented outside the extinction context. Similarly, unsignaled USs can restore extinguished responding when the CS is presented in the context in which the US was delivered. This phenomenon is termed “reinstatement” (Bouton and Bolles, 1979b; Rescorla and Heth, 1975). These phenomena indicate that extinction does not erase the conditioning memory, rather it causes new learning about the CS. Indeed, it appears that extinction training yields a new “safety” memory that inhibits retrieval of the fear memory. Unlike fear memory, the expression of this safety memory is limited by context and time (Bouton, 1993). “Context” is defined broadly to include the experimental environment and interoceptive state of the animal, as well as the actual (time of day) and relative time (how long ago) the events were learned. A challenge then for clinicians is to develop therapeutic interventions that yield fear suppression not only in the treatment context, but also in every context in which a fear stimulus is encountered. A challenge for neuroscientists is to understand not only how safety memories are encoded alongside fear memories in the brain, but also how these different memories come to be regulated by time and context.
Given that considerable progress has been made in elucidating the neural substrates of extinction, several investigators have examined whether extinction learning can be facilitated. Some of the early work in this arena focused on the FDA-approved drug, D-cycloserine (DCS), which is an allosteric modulator of the NMDA receptor that facilitates agonist binding therefore increasing NMDA receptor function. It has been reported that either systemic or intra-amygdaloid administration of DCS facilitates extinction learning (Ledgerwood et al., 2003, 2005; Walker et al., 2002). Interestingly, there have been reports that the extinction memory acquired under DCS is less likely to exhibit recovery (e.g., reinstatement) (Ledgerwood et al., 2004), although this outcome has not been universally reported (Bouton et al., 2008; Woods and Bouton, 2006). Moreover, administering DCS prior to extinction in rats appears to promote the reversal of some of the synaptic changes in the lateral amygdala that accompany fear conditioning (Mao et al., 2008). Preliminary work using DCS as a pharmacological adjunct to exposure therapy has shown some promise (Davis et al., 2006). For example, administration of DCS along with controlled exposure therapy improved outcomes for patients with fear of heights (Ressler et al., 2004) and social anxiety disorder (Guastella et al., 2008), although it did not improve therapeutic outcomes for spider phobics (Guastella et al., 2007a) or effect the extinction of fear conditioning (Guastella et al., 2007b).
Another compound that has been reported to enhance extinction learning is yohimbine, an alpha2-adrenergic agonist that has been used in humans to treat erectile dysfunction. Cain and colleagues reported that systemic yohimbine administration prior to extinction training in mice increased the long-term retention of extinction the following day (Cain et al., 2004). However, the effects of yohimbine on extinction are quite variable, in some cases even impairing extinction learning (Holmes and Quirk, 2010). Nonetheless, a recent report in humans suggests that yohimbine administration enhances the efficacy of exposure therapy in claustrophobic patients (Powers et al., 2009). The role for the adrenergic system in extinction learning is likely to be quite complex, however, insofar as prazosin, an alpha1-adrenoceptor antagonist, has recently been reported to impair fear extinction in mice after systemic (Bernardi and Lattal, 2010; Do-Monte et al., 2010) and intra-vmPFC administration (Do-Monte et al., 2010). Prazosin is currently used in the treatment of nightmares and sleep disorders in patients with PTSD (Raskind et al., 2007; Van Liempt et al., 2006), but its effect on extinction learning suggests that it may have limited efficacy as an adjunct to exposure therapy.
Endocannabinoids provide another potential route for enhancing extinction (Lutz, 2007). CB1 receptors are localized on inhibitory interneurons in the amygdala (Azad et al., 2004), and may regulate the activity of these neurons during extinction learning (Chhatwal et al., 2005a; Chhatwal et al., 2009). Systemic administration of drugs that enhance cannabinoid signaling, such as the reuptake inhibitor AM404 and the CB1 receptor agonist WIN55212-2, have been reported to facilitate extinction learning under some conditions (Marsicano et al., 2002), although chronic administration of WIN55212-2 has recently been reported to impair extinction learning (Lin et al., 2008). Moreover, there is recent data suggesting that CB1 receptors may not have a specific role in long-term fear extinction, but may be more generally involved in behavioral habituation (Plendl and Wotjak, 2010). These drugs have not been approved for use in humans, however, so it is not known whether increasing activity at endocannabinoid receptors would facilitate exposure therapy, for example.
Recently, Quirk and colleagues have reported that they can produce a pharmacologically-induced extinction without any behavioral training (Peters et al., 2010). They infused brain-derived neurotrophic factor (BDNF) into the infralimbic cortex twenty-four hours after fear conditioning and found that the expression of fear to the auditory CS was greatly diminished the following day. A series of control experiments ruled out the possibility that the infusion disrupted performance or the fear memory itself; notably, the fear memory was readily reinstated by additional unsignaled footshock. Analysis of BDNF levels in brain revealed animals that successfully extinguished fear showed elevated levels of BDNF in the hippocampus. Hippocampal infusions of BDNF were found to reproduce the effects of IL BDNF infusions, and infusing a BDNF-sequestering antibody into the IL disrupted this effect. These results extend other studies that have implicated BDNF in extinction learning (Chhatwal et al., 2006) and may explain why genetic variation in the gene encoding BDNF correlates with extinction in humans (Soliman et al., 2010). Indeed, they reveal a novel pharmacological target for either enhancing fear extinction during exposure therapy or even inducing fear extinction without formal exposure therapy. Ultimately, combining behavioral strategies to optimize extinction learning (Craske et al., 2008) with pharmacological adjuncts such as BDNF or DCS may yield even greater fear suppression in patients with anxiety disorders than has been achieved with traditional therapeutic interventions.
Erasing the Trace
Despite the promise of enhancing extinction with behavioral and pharmacological approaches, the lability of extinction memory suggests that, at best, it can only temporarily quell traumatic fear. A more effective way to eliminate unwanted fears would be to erase the fear memory itself. It has long been appreciated that new memories undergo a period of consolidation in which they are labile and sensitive to disruption (McGaugh, 2000). Long-term synaptic plasticity in the brain requires de novo protein synthesis (Deadwyler et al., 1987; Krug et al., 1984; Stanton and Sarvey, 1984), and administration of protein synthesis inhibitors soon after learning produces memory impairments (Agranoff et al., 1965; Agranoff and Klinger, 1964; Davis and Squire, 1984). Therefore, one strategy for reducing pathological fear would be to prevent the consolidation of long-term fear memories soon after a traumatic experience. Consistent with this aim, several investigators have now shown that fear memory is inhibited by systemic post-training protein synthesis inhibition (Bourtchouladze, 1998; Lattal and Abel, 2001). Moreover, infusion of the protein synthesis inhibitor anisomycin into the BLA within hours of fear conditioning disrupts the consolidation of long-term fear memories and reduces conditional fear responses (Maren et al., 2003; Parsons et al., 2006; Schafe and LeDoux, 2000; Schafe et al., 1999).
Immediate extinction
In addition to protein synthesis inhibitors, administering behavioral interventions soon after fear conditioning might also disrupt long-term fear memory by interfering with consolidation processes. For example, it has been reported that administering low-frequency stimulation soon after fear conditioning eliminates conditioning-related changes in MAPK phosphorylation in the BLA, a biochemical correlate of long-term synaptic plasticity and fear memory, as well as fear memory (Lin et al., 2003b). Based on this evidence, Davis and colleagues explored whether administering extinction trials soon after fear conditioning would yield a permanent loss of fear, rather than the temporary inhibition of fear typically observed with delayed extinction training (Myers et al., 2006). To test this, they administered extinction trials shortly (i.e., 10 min) after fear-potentiated startle conditioning in rats and examined whether fear suppression was more durable than that produced by extinction 24 hours after conditioning. They found that this immediate extinction procedure resulted in a loss of fear that was quite durable and exhibited little spontaneous recovery, reinstatement, or renewal. The implication of these findings is that early extinction training resulted in a permanent fear loss, which is not typical when extinction training is conducted one day after conditioning. The lack of fear recovery in this report suggested that immediate extinction might disrupt the consolidation of fear memory, yielding a relatively permanent loss of fear.
Although promising, several laboratories have now found that immediate extinction does not always reduce the recovery of fear (Archbold et al., 2010; Huff et al., 2009), and in fact may not suppress fear at all under some conditions (Chang and Maren, 2009; Maren and Chang, 2006; Woods and Bouton, 2008). For example, we have found that extinction training soon after fear conditioning produces short-term suppression of conditional freezing during the extinction session, but this suppression is not long lasting and fully recovers the following day (Maren and Chang, 2006). In fact, recently acquired fear memories appear to be particularly resistant to extinction insofar as we failed to obtain long-term fear loss even after over 200 extinction trials. In our hands, this “immediate extinction deficit” was obtained up to 6 hours after fear conditioning (Chang and Maren, 2009), suggesting that there is a substantial time window after fear conditioning in which fear memory is resistant to extinction. Interestingly, two recent papers suggest that immediate extinction does not engage medial prefrontal cortical circuits involved in extinction learning (Chang et al., 2010; Kim et al., 2010b). Interestingly, either electrical (Kim et al., 2010b) or pharmacological (Chang and Maren, in press) activation of the prefrontal cortex were shown to alleviate the immediate extinction deficit. Although recent fear is resistant to extinction, interventions targeting enhancement of medial prefrontal cortical activity may facilitate extinction, particularly under conditions in which it normally fails (Thompson et al., 2010). In sum, although post-conditioning protein synthesis inhibition effectively impairs the consolidation of fear memory, immediate extinction does not.
Post-retrieval deconsolidation
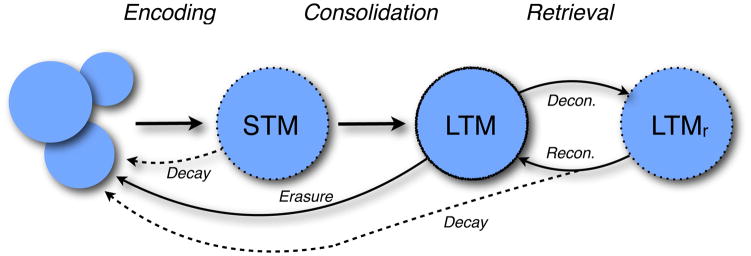
The brief time window after acquisition that memory is susceptible to disruption produces logistical challenges for intervention. However, another temporal window in which fear memory is sensitive to disruption is shortly after retrieval (Misanin et al., 1968; Nader and Hardt, 2009a; Sara, 2000). Like new memories, older memories appear to become labile yet again once they are retrieved and reactivated (Figure 3). This suggests that consolidated fear memories might be vulnerable to disruption soon after they have been retrieved. Consistent with this possibility, Nader and colleagues have shown in an influential series of experiments that manipulations that interfere with the consolidation of fear memory also disrupt fear memory when administered shortly after retrieval of that memory (Nader et al., 2000). In these experiments, rats underwent standard auditory fear conditioning in which a tone CS is paired with a footshock US. The next day a single CS was presented to retrieve the fear memory, and this was followed immediately by an infusion of the protein synthesis inhibitor anisomycin into the BLA. Although anisomycin spared short-term retention of the fear memory, it severely impaired the long-term retention of that memory. Although debate continues about the nature of this deficit (Lattal and Abel, 2004; Miller and Matzel, 2000; Rudy et al., 2006), there is considerable evidence that it results from a failure to maintain the fear memory in a consolidated state.
Figure 3.
Stabilization and destabilization of memory. Memory formation is associated with initial encoding to establish a short-term memory (STM) followed by a time-dependent consolidation phase to establish a stable long-term memory (LTM). Retrieval of LTM destabilizes or deconsolidates (decon.) the memory (LTMr) rendering it labile once more; the persistence of LTM after retrieval requires reconsolidation (recon.). Failure to reconsolidate the memory trace results in decay, much as STM decays in the absence of consolidation. In the absence of retrieval, LTM may be actively erased by a variety of manipulations by interfering with the molecular mechanisms involved in memory maintenance.
This work suggests that memory consolidation is a dynamic process that is not unique to the encoding of new memory. In fact, memory retrieval appears to “deconsolidate” established memory traces returning them to a labile and destabilized state that requires protein synthesis-dependent reconsolidation for long-term retention. The mechanisms of deconsolidation are not known, and it is unclear whether memory reactivation actually reverses the outcome of consolidation or renders the consolidated trace labile in some other way. In either case, interfering with reconsolidation after retrieval leads to memory loss: the deconsolidated memory fails to stabilize and decays much as short-term memory decays in the absence of consolidation to long-term memory (Figure 3). Although reconsolidation has been described in many memory systems, it is bounded (Nader, 2006). For example, the sensitivity of reactivated memories to protein synthesis inhibitors is related to many factors including the age and strength of the memory (Milekic and Alberini, 2002; Wang et al., 2009). In addition, not all forms of memory appear to undergo protein synthesis-dependent reconsolidation (Nader and Hardt, 2009b). Nonetheless, the sensitivity of long-term fear memories to retrieval-based manipulations provides a much more tractable time window for therapeutic intervention insofar patients with anxiety disorders often seek treatment long after trauma.
As a consequence, several groups have attempted to disrupt consolidated fear memories by interfering with reconsolidation processes after reactivation. Because there is strong interest in developing effective interventions for patients with anxiety disorders, the focus has been on developing interventions that can be safely administered to humans. For example, in rats systemic administration of the beta-adrenergic receptor antagonist, propranolol, disrupts the reconsolidation of fear memories under some conditions (Debiec and LeDoux, 2004; Muravieva and Alberini, 2010). A pair of studies in humans similarly suggests that propranolol administration can influence the reconsolidation of fear memory. In one report (Kindt et al., 2009), healthy subjects underwent a fear-potentiated startle conditioning procedure followed by oral propranolol administration and memory reactivation the day after conditioning. Interestingly, propranolol disrupted the retention of one index of fear memory (i.e., the conditioned acoustic startle response), but spared declarative memory of the CS-US relationship (i.e., shock expectancy). This effect was not due to propranolol administration alone, insofar as administering propranolol without reactivating the memory did not dampen startle. In other words, reactivating the fear memory after propranolol administration appeared to remove the emotional tone of the aversive experience while sparing the memories of the stimulus contingencies and the conditioning experience itself. In another study, patients with PTSD were given oral propranolol after recalling events related to their trauma (Brunet et al., 2008; Pitman et al., 2006). One week later physiological responses to those trauma-relevant memories were assessed. Relative to placebo controls, patients administered propranolol exhibited lower heart rate and skin conductances when recalling trauma-related memories. It is not clear in this case, however, whether propranolol administration alone would produce a similar outcome (i.e., a non-reactivated propranolol group was not run). Nonetheless, these results suggest that pharmacological disruption of fear memory reconsolidation may be an effective intervention for reducing some indices of fear and anxiety.
In addition to pharmacological approaches to reducing fear memory, it has recently been argued that delivering extinction trials shortly after reactivation of fear memory might erase those memories. In these experiments, extinction trials were delivered from 10 minutes to an hour after reactivation of a fear memory conditioned twenty-four hours earlier (Monfils et al., 2009; Schiller et al., 2010). Under these conditions, the extinction of fear in the reactivated subjects did not exhibit renewal (Monfils et al., 2009), reinstatement (Monfils et al., 2009; Schiller et al., 2010), or spontaneous recovery (Monfils et al., 2009; Schiller et al., 2010); extinction in non-reactivated subjects exhibited recovery. Only one of the studies examined the duration of the effect, and in that case it was reported to last at least one year (Schiller et al., 2010). Hence, the failure of fear to recover under these conditions suggests that administering extinction trials during the reconsolidation window leads to a permanent disruption of the fear memory. This suggests that extinction can disrupt the reconsolidation of fear under some circumstances (e.g., soon after retrieval), and lead to loss of the fear memory itself. It should be noted, however, that the generality of this effect is not yet clear. McNally and colleagues recently examined post-reactivation extinction using procedures nearly identical to those used in the previous experiments (Chan et al., 2010). Unlike the previous reports, McNally and colleagues failed to observe impaired renewal and reinstatement in rats receiving extinction trials shortly after reactivation of the fear memory. In fact, there was a trend for more robust renewal when extinction was conducted after reactivation, suggesting that extinction after memory retrieval does not impair fear memories as previously proposed. Clearly, further work is necessary to understand the conditions under which extinction training yields impairments in long-term fear memory.
However, given the considerable therapeutic potential of eliminating fear memories, there is obvious interest in defining the molecular processes governing reconsolidation (Tronson and Taylor, 2007). In fact, recent work indicates that metabotropic glutamate receptors (mGluR1) receptors may confer susceptibility of fear memories to disruption by extinction (Clem and Huganir, 2010). In these studies, mice that underwent fear conditioning were found to exhibit significant increases in AMPA-receptor mediated synaptic transmission in the lateral amygdala in vitro. A portion of this increased AMPA-receptor mediated current was maintained by AMPA receptors lacking GluA2 receptors, which are also calcium-permeable (CP-AMPARs). Interestingly, low-frequency electrical stimulation of LA synapses induced mGluR1-dependent long-term depression (LTD) that was mediated by a reduction in CP-AMPA receptor-mediated current. Similarly, delivering extinction trials 30 minutes after reactivation of the fear memory also led to a decrease in CP-AMPA receptor-mediated current in the LA in vitro and reduced the recovery of fear that normally occurs after extinction. The behavioral effect of post-retrieval extinction was impaired after systemic administration of an mGluR1 antagonist, suggesting that mGluR1-mediated synaptic depression mediates fear memory erasure. NMDA receptors also play a role in inducing synaptic depression, and previous work indicates that NMDA antagonists also prevent fear memories from becoming labile after a reminder (Ben Mamou et al., 2006).
A critical remaining question, however, is why a CS reminder was required to deconsolidate LA synapses to render them susceptible to mGluR1-mediated removal of CP-AMPA receptors in the first place. Others have reported that extinction without memory reactivation also yields mGluR1- and NMDA-dependent depotentiation of lateral amygdala synaptic transmission and a reduction in the surface expression of GluA1- and GluA2-containing AMPA receptors (Kim et al., 2007). In fact, consolidated, rather than labile, memories appear to be more sensitive to GluR1-mediated depotentiation (Kim et al., 2010a). Clearly, the regulation of AMPA receptor expression in the lateral amygdala is involved in both the acquisition and extinction of fear, but the behavioral modulation of AMPA receptor endocytosis after fear conditioning is poorly understood. Nader and colleagues have shown that NMDA receptors are required for a CS reminder to render fear memory sensitive to subsequent protein synthesis inhibition (Ben Mamou et al., 2006), so it is conceivable that an NMDA-receptor dependent process induces susceptibility to mGluR1-mediated LTD involved in reversing conditioning-related changes in the LA. Yet how particular behavioral experiences confer susceptibility of synapses to AMPA receptor endocytosis is unknown.
It is noteworthy that the sensitivity of fear memories to “reconsolidation update” (i.e., extinction after reactivation) appears to be time-limited. For example, Clem and Huganir (2010) found that fear memory erasure and CP-AMPA receptor removal was maximal 24 hours after conditioning, reduced 48 hours after conditioning, and not possible one week after fear conditioning. It has also been suggested that the passage of time limits the sensitivity of fear memories to protein synthesis inhibition after reactivation (Anokhin et al., 2002; Milekic and Alberini, 2002). Collectively, these results reveal that memories at the earliest stages of consolidation are the most sensitive to disruption, whether by post-conditioning or post-retrieval protein synthesis inhibitors or post-retrieval extinction manipulations. A continued challenge is how to lengthen the window of susceptibility such that even the most enduring fear memories can be eliminated.
Erasure
Preventing the reconsolidation of fear memory leads to a reduction in fear behavior, but there is some debate about the nature of this impairment. On the one hand, many authors have found that post-retrieval manipulations yield a non-recoverable loss of performance, suggesting that destabilized memory traces vanish if they are not reconsolidated. On the other hand, others have found that performance impairments after these manipulations are transient, suggesting that temporary retrieval failures, rather than disruption of the memory trace per se, underlie the effects of post-retrieval manipulations of memory (Lattal and Abel, 2004; Power et al., 2006). Indeed, it is perhaps not surprising that reactivation approaches would spare at least some aspects of the original memory insofar as the typical reactivation procedure may not retrieve the entire memory (Debiec et al., 2006; Doyère et al., 2007). Failing to reactivate the entire associative network of a memory might protect that memory from the influence of post-retrieval manipulations. In essence, complete erasure of a memory would require that the entire associative network containing that memory be eliminated.
To this end, Josselyn and colleagues have made use of an innovative molecular genetic approach to recruit and then disable a network of neurons in the amygdala mediating conditioned fear (Han et al., 2009). To recruit a network of amygdala neurons during fear conditioning, they used a viral vector to overexpress CREB, a transcription factor previously shown to bias amygdala neurons for inclusion in the neural network underlying fear memory (Han et al., 2007). To selectively target these neurons, they used transgenic mice (iDTR) that express the simian diphtheria toxin receptor under the control of Cre-recombinase (cre). In these mice, infusion of a replication-deficient herpes simplex virus expressing CREB-cre into the lateral amygdala renders neurons overexpressing CREB sensitive to apoptosis by systemic injection of diphtheria toxin. In an elegant series of experiments, Josselyn and colleagues found that ablating CREB-cre neurons recruited during fear conditioning severely and selectively impaired the expression of fear memory. The deficits in fear memory were long lasting, and several control experiments ruled out the possibility that non-specific damage to the amygdala contributed to the memory impairments. In fact, the most parsimonious interpretation of these results is that the investigators selectively erased the neuronal network in the amygdala harboring the memory trace.
Another approach to erasing memory targets the molecules within neurons that maintain long-term memories. Although there are several candidate molecules involved in memory maintenance (Kandel, 2009; Martin et al., 2000), one molecule in particular has received considerable attention as a substrate for long-term memory (Sacktor, 2011). Protein kinase M zeta (PKMzeta), which is a constitutively active isoform of protein kinase C, is involved in both the maintenance of synaptic long-term potentiation (Ling et al., 2002; Osten et al., 1996) as well as several forms of learning and memory (Pastalkova et al., 2006; Sacktor, 2011; Serrano et al., 2008). Within the amygdala, for example, it has been shown that inhibition of PKMzeta with a pseudosubstrate of the kinase (zeta inhibitory peptide or ZIP) impairs the expression of consolidated fear memories (Kwapis et al., 2009; Migues et al., 2010; Serrano et al., 2008). Recent data suggest that ZIP impairs memory by interacting with GluA2-containing AMPA receptors in the amygdala. Like CP-AMPA receptors (that lack GluA2), GluA1/2 receptors appear to be driven into LA synapses after fear conditioning (Kim et al., 2007; Mao et al., 2006; Rumpel et al., 2005) and PKMzeta appears to have a role in maintaining the surface expression of these receptors after learning (Migues et al., 2010). The precise regulation of GluA2-lacking and GluA-2 containing AMPA receptors is likely to be quite complex. Nonetheless, it appears that both types of glutamate receptors are upregulated at amygdala synapses after fear conditioning and pulling down either class of receptor after learning influences the retention of fear memories.
Turning back the clock
Clearly, the stability of fear memory represents presents a major challenge to manipulations designed to eliminate fear memories. But are fear memories necessarily resistant to erasure? Recent studies on the ontogeny of fear extinction have provided some interesting insight into the stability of fear memory across the lifespan. Recent studies by Richardson and colleagues have examined whether age influences the properties of extinction in rats (Kim and Richardson, 2007, 2008, 2010). Like adults, recently weaned 23-day old exhibit both contextual and auditory fear conditioning and extinction of that fear exhibits renewal, reinstatement, and spontaneous recovery. Surprisingly, however, 17-day old preweanling rats exhibited an unusual form of extinction that does not exhibit any of the hallmark recovery phenomena (e.g., renewal, reinstatement, and spontaneous recovery) that are associated with extinction in older rats. In other words, extinction may erase conditioned fear in preweanling rats. Interestingly, preweanling rats do not exhibit contextual fear conditioning (Rudy and Morledge, 1994), suggesting that they may also have global deficits in contextual processing functions required for renewal, spontaneous recovery, and reinstatement. Indeed, the nature of the renewal deficits in young rats is similar to that in adult rats with hippocampal lesions (Corcoran et al., 2005; Corcoran and Maren, 2001, 2004; Hobin et al., 2006; Ji and Maren, 2008a; Ji and Maren, 2005): both young rats and adult rats with hippocampal lesions fail to renew fear to an extinguished CS outside of the extinction context. Hence, the development of the hippocampus may afford a flexible memory system that allows a CS to mean different things in different contexts.
This explanation of how extinction comes to be resistant to erasure emphasizes the development of neural systems that allow the flexible representation of information. Another possibility is that the synaptic network that encodes fear memory is fundamentally different in young animals. In fact, Gogolla and colleagues (2010) have recently observed in mice that the development of the amygdala extracellular matrix, in particular perineuronal nets composed of chondroitin sulfated proteoglycans (CSPGs), parallels the development of extinction learning (Gogolla et al., 2009). Interestingly, infusion of a CSPG-degrading enzyme (chondroitinase ABC or chABC) into the amygdala of an adult mouse digested perineuronal nets and produced a fear extinction phenotype like that of a young mouse. That is, chABC-treated mice exhibited normal conditioning and consolidation of fear conditioning, and also showed normal decreases in conditioned fear after extinction training. Remarkably, however, chABC-treated rats did not show spontaneous recovery or renewal of fear. This suggests that perineuronal nets, while not necessary for the acquisition of fear memory, may prevent those memories for destabilizing after extinction training. The mechanism by which degradation of perineuronal nets alters the stability of fear memory is not known, although chABC impairs several forms of synaptic plasticity in the amygdala and hippocampus. Independent of the precise mechanism, however, these data suggest that molecular factors at the synapse are not only involved in the long-term maintenance of memory, but in protecting those memories from the destabilizing influences of other behavioral experiences. Unfortunately, though, removing perineuronal nets in the amygdala only promotes a non-recoverable extinction of fear when chABC is applied before, but not after fear conditioning. This obviously limits the therapeutic potential of compounds targeting perineuronal nets insofar as they would have to be administered before a traumatic experience, and would presumably be ineffective at promoting the suppression of old fears.
Conclusions
After decades of research aimed at how fears are learned, the last five years have witnessed an explosion of work on the neural mechanisms underlying how fear memories are suppressed and, ultimately, erased. Although fear memories are typically long-lived, there is now considerable evidence that they can be erased under some conditions. For instance, pharmacological disruption of molecules critical for memory reconsolidation and memory maintenance produce enduring fear loss. Moreover, behavioral manipulations, such as extinction, appear to yield fear erasure under some circumstances. These phenomena provide insight not only into the mechanisms underlying the encoding and regulation of fear and extinction memories, but also illuminate novel clinical interventions in patients with pathological fear memories.
Acknowledgments
Supported by a grant from the National Institutes of Health (R01MH065961).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agranoff BW, Davis RE, Brink JJ. Memory Fixation in Goldfish. P Natl Acad Sci USA. 1965;54:788. doi: 10.1073/pnas.54.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agranoff BW, Klinger PD. Puromycin Effect on Memory Fixation in Goldfish. Science. 1964;146:952. doi: 10.1126/science.146.3646.952. [DOI] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–U112. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin KV, Tiunova AA, Rose SPR. Reminder effects - reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. Eur J Neurosci. 2002;15:1759–1765. doi: 10.1046/j.1460-9568.2002.02023.x. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Debiec J, Doyere V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: A role for presynaptic plasticity in the fear system. J Neurosci. 2005;25:5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archbold GEB, Bouton ME, Nader K. Evidence for the persistence of contextual fear memories following immediate extinction. Eur J Neurosci. 2010;31:1303–1311. doi: 10.1111/j.1460-9568.2010.07161.x. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barot SK, Chung A, Kim JJ, Bernstein IL. Functional imaging of stimulus convergence in amygdalar neurons during Pavlovian fear conditioning. PLoS ONE. 2009;4:e6156. doi: 10.1371/journal.pone.0006156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM. A Role for alpha(1)-Adrenergic Receptors in Extinction of Conditioned Fear and Cocaine Conditioned Place Preference. Behav Neurosci. 2010;124:204–210. doi: 10.1037/a0018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Paré D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S, Zelikowsky M, Ponnusamy R, Jacobs NS, Blair HT, Fanselow MS. Electrical Synapses Control Hippocampal Contributions to Fear Learning and Memory. Science. 2011;331 doi: 10.1126/science.1193785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: A cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Bolles R. Species-specific defense reactions and avoidance learning. Psychol Rev. 1970;77:32–48. [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and ambiguity in the extinction of emotional learning: implications for exposure therapy. Behaviour Research & Therapy. 1988;26:137–149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979a;10:445–466. [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol: Anim Behav Process. 1979b;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry. 2006a;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006b;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, Ramsey M, Wolfer DP, Cestari V, Rossi-Arnaud C, et al. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WYM, Leung HT, Westbrook RF, Mcnally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem. 2010;17:512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Maren S. Early extinction after fear conditioning yields a context-independent and short-term suppression of conditional freezing in rats. Learn Mem. 2009;16:62–68. doi: 10.1101/lm.1085009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Berke JD, Maren S. Single-Unit Activity in the Medial Prefrontal Cortex during Immediate and Delayed Extinction of Fear in Rats. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Strain Difference in the Effect of Infra limbic Cortex Lesions on Fear Extinction in Rats. Behav Neurosci. 2010;124:391–397. doi: 10.1037/a0019479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacol. 2005a;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, Ressler KJ. Functional Interactions between Endocannabinoid and CCK Neurotransmitter Systems May Be Critical for Extinction Learning. Neuropsychopharmacol. 2009;34:509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005b;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–U239. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav Neurosci. 1998;112:1092–1103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Amer Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2006;3:82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Dunwiddie T, Lynch G. A critical level of protein synthesis is required for long-term potentiation. Synapse. 1987;1:90–95. doi: 10.1002/syn.890010112. [DOI] [PubMed] [Google Scholar]
- Debiec J, Doyere V, Nader K, LeDoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. P Natl Acad Sci USA. 2006;103:3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Do-Monte FHM, Allensworth M, Carobrez AP. Impairment of contextual conditioned fear extinction after microinjection of alpha-1-adrenergic blocker prazosin into the medial prefrontal cortex. Behav Brain Res. 2010;211:89–95. doi: 10.1016/j.bbr.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Doyère V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and Learning. Hillsdale, N. J: Erlbaum; 1988. pp. 185–211. [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psych. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M. Injections of the NMDA receptor antagonist aminophosphonopentanoic acid into the lateral nucleus of the amygdala block the expression of fear-potentiated startle and freezing. J Neurosci. 2001;21:4111–4115. doi: 10.1523/JNEUROSCI.21-11-04111.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol Learn Mem. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci. 2004;24:1962–1966. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Chang CH, Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem. 2006;13:14–17. doi: 10.1101/lm.60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Falls WA, Davis M. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medical prefrontal cortex in rats. Behav Neurosci. 1997;111:712–726. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal Nets Protect Fear Memories from Erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Hobin JA, Maren S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: mnemonic code or fear bias? Neuron. 2003;40:1013–1022. doi: 10.1016/s0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Holt W, Maren S. A role for amygdaloid PKA and PKC in the acquisition of long-term conditional fear memories in rats. Behav Brain Res. 2000;114:145–152. doi: 10.1016/s0166-4328(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci. 2003;117:738–750. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. NMDA receptors are essential for the acquisition, but not expression, of conditional fear and associative spike firing in the lateral amygdala. Eur J Neurosci. 2004;20:537–548. doi: 10.1111/j.1460-9568.2004.03513.x. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. Journal of Psychiatric Research. 2007a;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007b;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HLL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology (Berl) 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai HJ, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–U230. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biol Psychiatry. 2006;60:361–368. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008a;454:600–U628. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008b;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Holmes A, Quirk GJ. Pharmacological facilitation of fear extinction and the search for adjunct treatments for anxiety disorders - the case of yohimbine. Trends Pharmacol Sci. 2010;31:2–7. doi: 10.1016/j.tips.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Hernandez JA, Blanding NQ, Labar KS. Delayed extinction attenuates conditioned fear renewal and spontaneous recovery in humans. Behav Neurosci. 2009;123:834–843. doi: 10.1037/a0016511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Chessel A, Lena I, Marsault R, Garcia R. Prefrontal infusion of PD098059 immediately after fear extinction training blocks extinction-associated prefrontal synaptic plasticity and decreases prefrontal ERK2 phosphorylation. Synapse. 2006;60:280–287. doi: 10.1002/syn.20291. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn Mem. 2008a;15:244–251. doi: 10.1101/lm.794808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Lesions of the entorhinal cortex or fornix disrupt the context-dependence of fear extinction in rats. Behav Brain Res. 2008b;194:201–206. doi: 10.1016/j.bbr.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JZ, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez SA, Maren S. Nuclear disconnection within the amygdala reveals a direct pathway to fear. Learn Mem. 2009;16:766–768. doi: 10.1101/lm.1607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, Ledoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci. 2010:1–10. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the Neurocircuitry and Genetics of Fear Inhibition May Inform Our Understanding of PTSD. Amer J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The Biology of Memory: A Forty-Year Perspective. J Neurosci. 2009;29:12748–12756. doi: 10.1523/JNEUROSCI.3958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, et al. Amygdala depotentiation and fear extinction. Proceedings of the National Academy of Sciences. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Song B, Hong I, Kim J, Lee J, Park S, Eom JY, Lee CJ, Lee S, Choi S. Reactivation of fear memory renders consolidated amygdala synapses labile. J Neurosci. 2010a;30:9631–9640. doi: 10.1523/JNEUROSCI.0940-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007;121:131–139. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. J Neurosci. 2008;28:1282–1290. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kim SC, Jo YS, Kim IH, Kim H, Choi JS. Lack of medial prefrontal cortex activation underlies the immediate extinction deficit. J Neurosci. 2010b;30:832–837. doi: 10.1523/JNEUROSCI.4145-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with obervations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–279. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Krug M, Lossner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Protein Kinase Mzeta Maintains Fear Memory in the Amygdala but Not in the Hippocampus. Behav Neurosci. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Dracheva S, Assoun S, LeDoux JE. Fear conditioning induces distinct patterns of gene expression in lateral amygdala. Genes Brain and Behavior. 2009;8:735–743. doi: 10.1111/j.1601-183X.2009.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Farb CR, LeDoux JE. Fear memory formation involves p190 RhoGAP and ROCK proteins through a GRB2-mediated complex. Neuron. 2002;36:727–738. doi: 10.1016/s0896-6273(02)01047-4. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Abel T. Different requirements for protein synthesis in acquisition and extinction of spatial preferences and context-evoked fear. J Neurosci. 2001;21:5773–5780. doi: 10.1523/JNEUROSCI.21-15-05773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. P Natl Acad Sci USA. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 2008;15:304–314. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: Consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-Cycloserine facilitates extinction of learned fear: Effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Walker D, Davis M. Lack of a temporal gradient of retrograde amnesia following NMDA-induced lesions of the basolateral amygdala assessed with the fear-potentiated startle paradigm. Behav Neurosci. 1996;110:836–839. doi: 10.1037//0735-7044.110.4.836. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol. 2003a;63:44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol. 2003b;63:44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003c;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Chen PS, Gean PW. Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learn Mem. 2008;15:876–884. doi: 10.1101/lm.1081908. [DOI] [PubMed] [Google Scholar]
- Ling DSF, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Bidirectional synaptic plasticity at nociceptive afferents in the rat central amygdala. J Physiol-London. 2007;581:961–970. doi: 10.1113/jphysiol.2006.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:art. no.-RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. The endocannabinoid system and extinction learning. Mol Neurobiol. 2007;36:92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacol. 2010;35:1625–1652. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning. Learn Mem. 2004;11:617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao SC, Hsiao YH, Gean PW. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. J Neurosci. 2006;26:8892–8899. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao SC, Lin HC, Gean PW. Augmentation of Fear Extinction by D-Cycloserine is Blocked by Proteasome Inhibitors. Neuropsychopharmacol. 2008;33:3085–3095. doi: 10.1038/npp.2008.30. [DOI] [PubMed] [Google Scholar]
- Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22:561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur J Neurosci. 2000;12:4047–4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996a;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996b;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Maren S, Chang C-h. Recent fear is resistant to extinction. Proc Natl Acad Sci USA. 2006;103:18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21:RC135. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang JR, Hofmann C, Zieglgansberger W, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature (Lond) 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Meins M, Herry C, Muller C, Ciocchi S, Moreno E, Luthi A, Monard D. Impaired fear extinction in mice lacking protease nexin-1. Eur J Neurosci. 2010;31:2033–2042. doi: 10.1111/j.1460-9568.2010.07221.x. [DOI] [PubMed] [Google Scholar]
- Merino SM, Maren S. Hitting Ras where it counts: Ras antagonism in the basolateral amygdala inhibits long-term fear memory. Eur J Neurosci. 2006;23:196–204. doi: 10.1111/j.1460-9568.2005.04546.x. [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. Memory involves far more than ‘consolidation’. Nat Rev Neurosci. 2000;1:214–216. doi: 10.1038/35044578. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde Amnesia Produced by Electroconvulsie Shock after Reactivation of a Consolidated Memory Trace. Science. 1968;160:554. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature (Lond) 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Moita MAP, Lamprecht R, Nader K, LeDoux JE. A-kinase anchoring proteins in amygdala are involved in auditory fear memory. Nat Neurosci. 2002;5:837–838. doi: 10.1038/nn901. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Bravo-Rivera C, Quirk GJ. Infralimbic D2 Receptors Are Necessary for Fear Extinction and Extinction-Related Tone Responses. Biol Psychiatry. 2010;68:1055–1060. doi: 10.1016/j.biopsych.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem. 2010;17:306–313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K. Identifying the neural mechanisms by which boundary conditions inhibit reconsolidation from occurring. Molecular Pain. 2006;2 [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009a;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009b;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Osten P, Valsamis L, Harris A, Sacktor TC. Protein synthesis-dependent formation of protein kinase Mzeta in long- term potentiation. J Neurosci. 1996;16:2444–2451. doi: 10.1523/JNEUROSCI.16-08-02444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic Synaptic Networks of the Amygdala for the Acquisition, Expression, and Extinction of Conditioned Fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. Intrinsic circuitry of the amygdaloid complex: common principles of organization in rats and cats. Trends Neurosci. 1998;21:240–241. doi: 10.1016/s0166-2236(98)01240-5. [DOI] [PubMed] [Google Scholar]
- Paré D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y, Pare JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience. 1995;69:567–583. doi: 10.1016/0306-4522(95)00272-k. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci. 2006;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res. 1985;16:117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of Fear Extinction with Hippocampal-Infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Brunet A, Orr SP, Tremblay J, Nader K. A novel treatment for post-traumatic stress disorder by reconsolidation blockade with propranolol. Neuropsychopharmacol. 2006;31:S8–S9. [Google Scholar]
- Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J Neurosci. 2010;30:4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploski JE, Park KW, Ping JL, Monsey MS, Schafe GE. Identification of plasticity-associated genes regulated by Pavlovian fear conditioning in the lateral amygdala. J Neurochem. 2010;112:636–650. doi: 10.1111/j.1471-4159.2009.06491.x. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The Activity-Regulated Cytoskeletal-Associated Protein (Arc/Arg3.1) Is Required for Memory Consolidation of Pavlovian Fear Conditioning in the Lateral Amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: The role of re-exposure duration. Learn Mem. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010;30:635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JAJ, Otto MW, Sanders C, Emmelkamp PMG. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: A randomized placebo controlled trial of yohimbine augmentation. Journal of Anxiety Disorders. 2009;23:350–356. doi: 10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacol. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O’Connell J, Taylor F, Gross C, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Charney DS. Animal models of relevance to PTSD. Ann N Y Acad Sci. 1997;821:332–351. doi: 10.1111/j.1749-6632.1997.tb48290.x. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learn Mem. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol: Anim Behav Process. 1975;1:88–96. [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy - Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiat. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Royer S, Pare D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature. 2003;422:518–522. doi: 10.1038/nature01530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, Moineau J, Bolding K. Anisomycin and the reconsolidation hypothesis. Learn Mem. 2006;13:1–3. doi: 10.1101/lm.157806. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. How does PKM zeta maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Samson RD, Paré D. Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J Neurosci. 2005;25:1847–1855. doi: 10.1523/JNEUROSCI.3713-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Wang XY, Schrick C, Neve R, Radulovic J, Tsai LH. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10:1012–1019. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, et al. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. Plos Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumyatsky GP, Malleret G, Shin RM, Takizawa S, Tully K, Tsvetkov E, Zakharenko SS, Joseph J, Vronskaya S, Yin DQ, et al. stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacol. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing DQ, Tottenham N, Amso D, Somerville LH, et al. A Genetic Variant BDNF Polymorphism Alters Extinction Learning in Both Mouse and Human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk G. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde-Amnesia and Memory Consolidation - a Neurobiological Perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Szapiro G, Vianna MRM, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Tang JR, Wotjak CT, Wagner S, Williams G, Schachner M, Dityatev A. Potentiated amygdaloid auditory-evoked potentials and freezing behavior after fear conditioning in mice. Brain Res. 2001;919:232–241. doi: 10.1016/s0006-8993(01)03020-7. [DOI] [PubMed] [Google Scholar]
- Thompson BM, Baratta MV, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. Activation of the infralimbic cortex in a fear context enhances extinction learning. Learn Mem. 2010;17:591–599. doi: 10.1101/lm.1920810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Guedea AL, Gao C, Radulovic J. Segregated Populations of Hippocampal Principal CA1 Neurons Mediating Conditioning and Extinction of Contextual Fear. J Neurosci. 2009;29:3387–3394. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Liempt S, Vermetten E, Geuze E, Westenberg H. Pharmacotherapeutic treatment of nightmares and insomnia in posttraumatic stress disorder - An overview of the literature. Psychobiology of Posttraumatic Stress Disorder: A Decade of Progress. 2006;1071:502–507. doi: 10.1196/annals.1364.053. [DOI] [PubMed] [Google Scholar]
- Vianna MRM, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. P Natl Acad Sci USA. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Alvares LD, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009;12:905–U116. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe J, Rowan VC. Panic disorder: a product of classical conditioning. Behav Res Ther. 1988;26:441–450. doi: 10.1016/0005-7967(88)90138-6. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-Cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. Immediate extinction causes a less durable loss of performance than delayed extinction following either fear or appetitive conditioning. Learn Mem. 2008;15:909–920. doi: 10.1101/lm.1078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by D-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Ledoux J. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci. 2010;31:1664–1670. doi: 10.1111/j.1460-9568.2010.07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]