Abstract
A mushroom body extrinsic neuron, the Pe1 neuron, connects the peduncle of the mushroom body (MB) with two areas of the protocerebrum in the honeybee brain, the lateral protocerebral lobe (LPL) and the ring neuropil around the α-lobe. Each side of the bee brain contains only one Pe1 neuron. Using a combination of intracellular recording and neuroanatomical techniques we analyzed its properties of integrative processing of the different sensory modalities. The Pe1 neuron responds to visual, mechanosensory, and olfactory stimuli. The responses are broadly tuned, consisting of a sustained increase of spike frequency to the onset and offset of light flashes, to horizontal and vertical movements of extended objects, to mechanical stimuli applied to the antennae or mouth parts, and to all olfactory stimuli tested (29 chemicals). These multisensory properties are reflected in its dendritic organization. Serial reconstructions of intracellularly stained Pe1 neurons using confocal microscopy reveal that the Pe1 neuron arborizes throughout all layers of MB peduncle with finger-like, vertically oriented dendrites. The peduncle of the MB is formed by the axons of Kenyon cells, whose dendritic inputs are organized in modality-specific subcompartments of the calyx region. The peduncular arborization indicates that the Pe1 neuron receives input from Kenyon cells of all calycal subcompartments. Because the Pe1 neuron changes its odor responses transiently as a consequence of olfactory learning, we hypothesize that the multimodal response properties might have a role in memory consolidation and help to establish contextual references in the long-term trace.
The mushroom bodies (MBs) are prominent brain structures in the central brain of insects. MBs are regarded as high order integrative centers of the insect brain. Their principal organization was described at the turn of the century (Kenyon 1896). Since then, there has been speculation about their functional role (Vowles 1955; Howse 1974, for review, see Erber et al. 1987; Menzel et al. 1994). Molecular analysis, electrophysiological recordings, elimination of MBs during ontogenetic development, and reversible interference of function with cooling probes showed that these properties have an important role in olfactory learning and memory processes, both in Drosophila and the honeybee (Heisenberg et al. 1985; Menzel 1990; Davis 1993; de Belle and Heisenberg 1994). The MBs in the honeybee are highly differentiated. They are located along the midline in the central brain, and each MB is formed by ∼170,000 intrinsic elements, the Kenyon cells (Witthöft 1967). Their dendritic arborizations give rise to concentrically arranged neuropils that form the median and lateral calyces. Each calyx is subdivided into lip, collar, dorsobasal, and basal ring subcompartments. Axonal projections of the Kenyon cells originating in the calycal zones project in bundles into the midbrain, thus forming the peduncle and the α- and β-lobes of the MB (Kenyon 1896; Howse 1974; Mobbs 1982; Rybak 1994). Because of the highly ordered, parallel arrangement of the Kenyon cell axons, the MB in each brain hemisphere is partitioned into two subsystems: the median and lateral subcompartment. The calyces of the MB are modality-specific input stations. The lip and collar neuropils are innervated exclusively by antennal and visual tracts, respectively (Mobbs 1982; Menzel et al. 1994). The basal ring receives mixed input from various brain regions (Mobbs 1982; R. Abel, unpubl.). This compartmentalized organization of Kenyon cells is maintained within the peduncle and maps onto specific regions of the α- and β-lobes. Proto- and deutocerebral interneurons connect the axonal system of the MB at different levels along the peduncle and the lobes, with distinct areas in the protocerebral lobe and the antennal lobe (Schürmann 1974; Mobbs 1982; Rybak and Menzel 1993).
In the honeybee brain, interneurons associated with the MBs have been identified (Rybak and Menzel 1993). The pedunculus extrinsic Pe1 neuron changes its response properties to olfactory stimuli specifically when the animal is sensitized or conditioned to an odor (Mauelshagen 1993). The single Pe1 neuron in each MB represents a pair of large, identified neurons in the bee brain that form a major output pathway from the peduncle of the MBs (Mauelshagen 1993; Rybak 1994). The Pe1 neuron is positioned at a strategic site in the bee brain. It receives input from within the MB neuropil and transmits it to the protocerebrum, the lateral protocerebral lobe (LPL), and the ring neuropil around the α-lobe. It also sends a few branches to the ventral α-lobe, where it forms output synapses (Rybak and Mauelshagen 1994). The Pe1 neuron arborizes widely in the peduncle at a level where α- and β-lobes branch off. Because of the dendritic arbors span the whole cross section area of the peduncle we asked whether the Pe1 neuron might receive input from Kenyon cells of all calycal subcompartments, leading to multisensory convergence of Kenyon cells on a single, unique extrinsic neuron.
The question arises as to whether and how a multisensory integrating neuron like Pe1 might both serve the function of reading out specific (e.g., olfactory) memory and at the same time respond to a large variety of stimuli. We conceptualize that any form of associative learning needs to be related to other stimuli, thus setting the learning of a cue stimulus to the context in which it occurs. To approach this question, we concentrate in an initial step on the study of the response properties of the Pe1 neuron and its dendritic arborization with the aim of setting the background for an analysis of the learning-induced changes in its multisensory properties. Convergence of multisensory input on an adaptive neuron may represent a neural strategy of encoding context-dependent plasticities. Before we can address this question, we need a better understanding of the structural and functional aspects of the multisensory properties of the Pe1 neuron.
Materials and Methods
ANIMALS
Honeybees (Apis mellifera carnica) were collected from the hive of the laboratory the day before they were used for electrophysiological recordings. Bees were fixed to a metal tube, and the heads were glued to a support spanning the neck region. Bees were kept overnight in a cool, dark, and moist compartment after having been fed with 2 m sucrose solution to satiation.
ELECTROPHYSIOLOGY
Conventional intracellular recording techniques were applied to sample membrane voltage fluctuations (synaptic potentials and action potentials) from Pe1 neurons in either isolated heads as performed by Mauelshagen (1993), or in whole animal preparations. The electrophysiological setup was the same as described by Mauelshagen (1993). Most results were gained by whole animal recordings, which in our experience allow more frequent data collection. For this, bees were fixed in a tube, as has been described previously for proboscis extension conditioning (Menzel et al. 1974; Menzel 1990). The head of the bee was glued to the tube by a drop of a wax–collophonium mixture, allowing the proboscis and antennae to move unhindered. A window was cut in the cuticle of the head capsule between the bases of the antennae, the median ocellus, and the inner borders of the compound eyes. The esophagus was pulled out through a second incision ventrally to the bases of the antennae by inserting a very fine forceps into the esophageal hole. A thin layer of vaseline was used to prevent dehydration of the brain. Animals in which the hemolymph level fell too low were discarded. Also, animals that showed strong brain movements, caused by hemolymph pumping or muscle contractions, were not used. Recording electrodes (outer diam., 1 mm) were pulled by a Flaming/Brown microelectrode puller (P87, Sutter Instruments). The resistance in the tissue ranged between 60 and 100 mΩ for electrodes filled with 2.5 m potassium acetate and 140–180 mΩ for electrodes filled with 4% lucifer yellow in 0.1 m LiCl. The Pe1 neuron was impaled at its neurite near the transition between peduncle and α- and β-lobe (ventrally to the β-exit point at a depth of 140–180 μm; Mobbs 1982). The Pe1 neuron could be identified reliably with high probability during the process of recording because of its spike form and response properties (see Results). However, in about half of the recordings evaluated here (5 of a total of 11 recordings lasting longer than 15 min), the recording site was confirmed by intracellular staining and subsequent morphological analysis. Neurons were iontophoretically filled with lucifer yellow by hyperpolarizing DC currents (2–4 nA) for several minutes. The brains were dissected under saline solution after 20–30 min diffusion time, thus allowing the dye to stain all parts of the neuron. The specimens were fixed in 4% paraformaldehyde in Millonig buffer (pH 7.3), dehydrated in an ascending ethanol series, and cleared in methylsalicylate. The whole-mount preparations were viewed under epifluorescence and analyzed with the confocal microscope (Leica). Reconstructions were made from a series of optical sections at the confocal microscope.
STIMULATION AND DATA EVALUATION
Twenty-two different odor compounds were tested in all preparations (see Fig. 3, below); 7 additional stimuli consisting of mixtures of citral, 2-hexanol, benzaldehyde in different combinations were used in only three preparations. Olfactory stimuli were provided either by directing the tip of a 20-ml plastic syringe containing a piece of filter paper soaked with the undiluted odorant toward the antennae and blowing its content over the head or by moving a glass vessel containing the undiluted compound (or mixture) into the pathway of a continuos slow airstream. In the first case, a sharp onset of the odor stimulus and a lasting stimulation with a rather constant odor stream was acquired, but the odor stimulation was also combined with a slight mechanical stimulation (Fig. 2a,b, below). In the latter case the odorant was added to a constant airstream produced by an exhaustion piper (diam., 10 cm) that was mounted behind the preparation. This procedure avoided the additional mechanical stimulus, but it did not allow the precise determination of the onset, duration, and concentration of the compounds, because the odor plumes were carried across the antennae not in a constant stream but in eddies. However, this stimulation technique allowed the testing of a large number of chemicals in quick succession (one stimulus of 5–7 sec duration every 10 sec) during the recording of the Pe1 neuron. The time point when an odor eddy reached the antennae was indicated by a clearly detectable phasic ON excitation of the Pe1 neuron (Fig. 2c,b, below). Mechanical stimuli were applied to the antennae, mouth parts, and the dorsal region of the head between the compound eyes using a tiny brush with only a few hairs attached.
Figure 3.
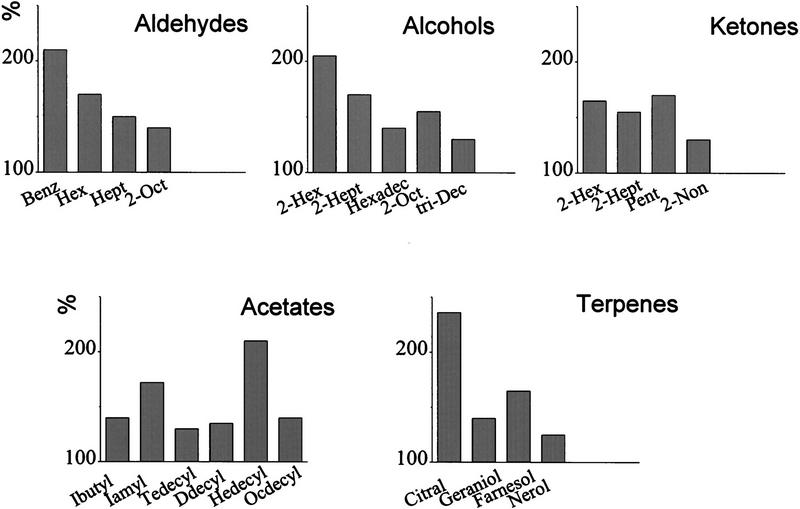
Pe1 responses to 23 different pure compounds of five groups of chemicals. The animal was stimulated with a glass vessel introduced into an airstream carrying odor plumes across the antennae. This stimulation leads to responses as shown in Fig. 2, c–e. Spike frequency was evaluated for the strongest response component during a 5-sec-long stimulation indicative for the arrival of an eddy of highest odor concentration. The ordinate gives the change of spike frequency relative to spontaneous frequency (100%). Aldehydes: (Benz) benzaldehyde; (Hex) hexanal; (Hept) heptanal; (2-Oct) 2-octanal. Alcohols: (2-Hex) 2-hexanol; (2-Hept) 2-heptanol; (Hexadec) hexadecanol; (2-Oct) 2-octanol; (tri-Dec) tri-decanol. Ketones: (2-Hex) 2-hexanon;(2-Hept) 2-heptanon; (Pent) pentanon; (2-Non) 2-nonanon. Acetates: (Ibutyl) isobutylacetate; (Iamyl) isoamylacetate; (Tedecyl) tetradecylacetate; (Ddecyl) dodecylacetate; (Hedecyl) hexadecylacetate; (Ocdecyl) octadecylacetate. Terpenes: As indicated.
Figure 2.
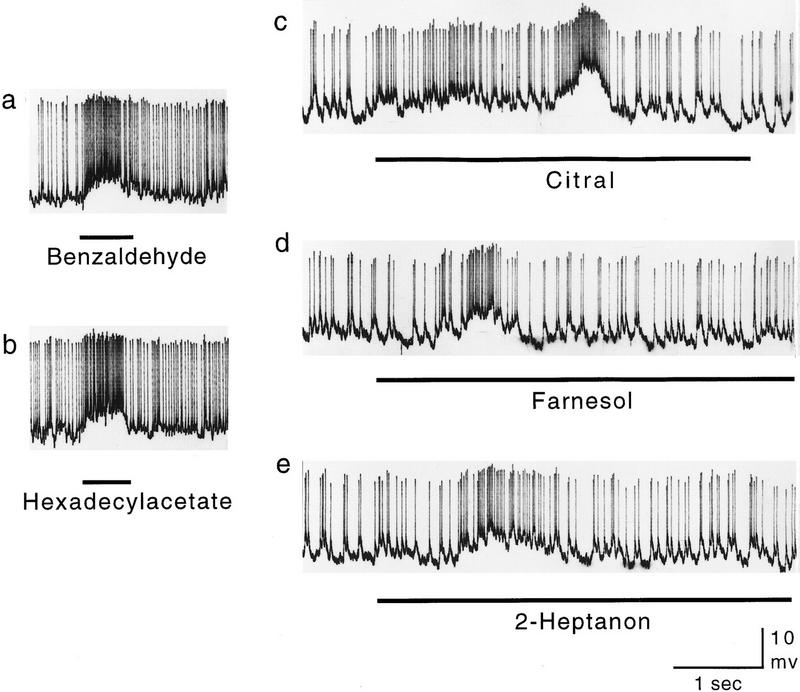
Examples of Pe1 responses to different chemical compounds (a) benzaldehyde; (b) hexadecylacetate; (c) citral; (d) farnesol; (e) 2-heptanone. Stimulation in a and b was 1 sec using a syringe for fast stimulus onset; in c–e, a glass vessel containing the undiluted odorant was brought into a constant airstream (see Materials and Methods). Because of the latter stimulation procedure, stimulus onset is rather gradual, and both stimulus strength and duration may vary. Fluctuations in spike activity as seen in c–e may thus indicate eddies of odorants.
Visual light on–off stimuli were produced by switching the illumination of the dissection microscope on and off, which focused the light via two light guides onto the dorsofrontal parts of both compound eyes. Moving stripes were presented to the left compound eyes using a handheld 10 × 10-cm-wide screen illuminated from behind. In front of the screen, a grating of black-and-white bars moved from left to right or in the opposite direction. The screen appeared at a visual angle of 90°, and the spatial wavelength of the grating was 9°. Depending on the orientation, the screen was moved front to back, back to front, or up- and downward. Sucrose stimuli were applied to the antennae (ipsi- or contralateral to the recording site) by slightly touching the flagellum with a wooden toothpick soaked in 2 m sucrose solution. To stimulate the glossa of the proboscis selectively, only the middle part of the toothpick was soaked. By gently moving the dry tip of the toothpick under the proboscis and advancing sideways slightly, the glossa was maneuvered to the sucrose part of the toothpick. Combined stimuli were tested for odor–mechanical antennal stimulation. The antennae were touched gently with a piece of filter paper from a used odor syringe.
All intracellular recordings were stored by an FM tape recorder and later recorded on a Brush pen writer. Frequencies of action potentials (APs) were evaluated by counting the number of APs in 0.5-sec intervals. The analysis indicated that the responses to odorants were rather variable. Because of the stimulation procedure it was not possible to separate clearly stimulus variability from response variability. Because the Pe1 neuron did not give any indication of odor-specific responses and because the aim of this study was to test as many different stimuli as possible, we also categorized the responses of the Pe1 neuron with respect to the AP frequency using an audio monitor and distinguished three categories—no response, weak response (∼20%–50% increase of spontaneous activity), and strong responses (approximately >50% increase of spontaneous activity). These categories were used only to detect any strong deviations from the general broad response profile to a large number of odorants and to decide during the experiment whether additional stimulations were needed to clarify the response profile.
HISTOLOGY
Bodian staining was used according to the protocol from Gregory‘s modified Bodian protargol technique (Clark 1973). Briefly, brains were fixed for 3–4 hr in AAF, a mixture of acetic acid, ethanol, and formalaldehyde (Blest 1961), dehydrated in an ascending ethanol series, cleared in xylene, embedded in paraffin, and sectioned at 12 μm thickness. Sectioned material was deparaffinized in xylene and hydrated in a descending ethanol series. Staining was performed with Protargol S (Laboratoire Roques, France). A mixture of hydroquinone and sodium sulfite was used for reduction of silver. In the final steps, the sections were toned with gold chloride, developed in oxalic acid, and fixed with sodium thiosulfate. After dehydrating, the sections were mounted in Entellan (Merck, Germany). The staining cycle was repeated to enhance staining. For Golgi preparations the brains were incubated using a modified Golgi–Collonier technique (Strausfeld 1980). They were immersed in a Karnowsky fixative containing 2% paraformaldehyde/2% glutaraldehyde in sodium cacodylate buffer (pH 7.4) followed by a wash in the same buffer. The osmolarity of the fixative was adjusted by adding sucrose. The chromation was done in a mixture of one part 2% glutaraldehyde and four parts 4% potassium dichromate for 4–5 days in vials placed in the dark at room temperature. Following several washes in Holmes Borax–Borat buffer (pH 7.4) (Nässel and Seyan, cited in Strausfeld 1980) the specimens were washed several times in 0.1% aqueous silver nitrate solution until no more precipitates were formed. The final silver impregnation was done in 0.75% silver nitrate. Preparations were viewed as whole mounts under the microscope.
IMAGING
Images were obtained with a confocal microscope (Leica, Germany) using 16× and 40× primary magnification. Whole-mount preparations of the Pe1 neuron, intracellularly filled with lucifer yellow, were scanned in a frontal view. Reconstructions were made from printouts of 0.5-μm thick optical sections.
Results
PHYSIOLOGY
GENERAL PHYSIOLOGICAL CHARACTERISTICS
As reported by Mauelshagen (1993), the Pe1 neuron (Fig. 1) can be recognized reliably by its physiological features, which are clearly distinguishable from neurons running in the anterodorsal protocerebral commissure [a.d.p.c. neurons (Mobbs 1982; Homberg 1984)] or A7 neurons (Rybak and Menzel 1993) whose neurites also leave the MB close to the Pe1 neuron at the transition of the peduncle to the lobes, the so called β-exit (Mobbs 1982). Summating excitatory postsynaptic potentials (EPSPs) are recorded during spontaneous activity and occur more frequently during stimulation (Fig. 2). APs (20–40 mV) ride on top of the EPSPs, but the variable temporal relationship between EPSPs and AP occurrence indicates that APs are conducted passively over some distance from the spike-initiating zone. Spontaneous AP activity with frequencies of 5–30 Hz occurs rather irregularly; however, in some cases, bursts of two or three spikes with regular interburst intervals occur. The intervals between the short bursts range between 150 and 250 msec, and AP frequency during the burst is ∼60–200 Hz. The change from an irregular to a bursting pattern is abrupt and occurs unpredictably from time to time, confirming observations by Mauelshagen (1993); this change was not found to be correlated with any obvious external stimuli (e.g., low level of stimulation by chemical, mechanosensory, or optical stimuli) or behavioral parameters (antennal position or movement, movement of the mouth parts). When the spontaneous AP activity was high (>20 Hz), no grouping of APs in bursts was recorded. Slight hyperpolarization of the membrane potential (−3 to −7 mV below resting potential of −35 to −45 mV) reduced the AP frequency, with spikes occurring typically in bursts of two or three spikes (in 3 of the 11 preparations). These characteristic patterns allow the identification of the Pe1 neuron based on physiological parameters, because no other neuron recorded and stained in the β-exit region showed similar properties.
Figure 1.
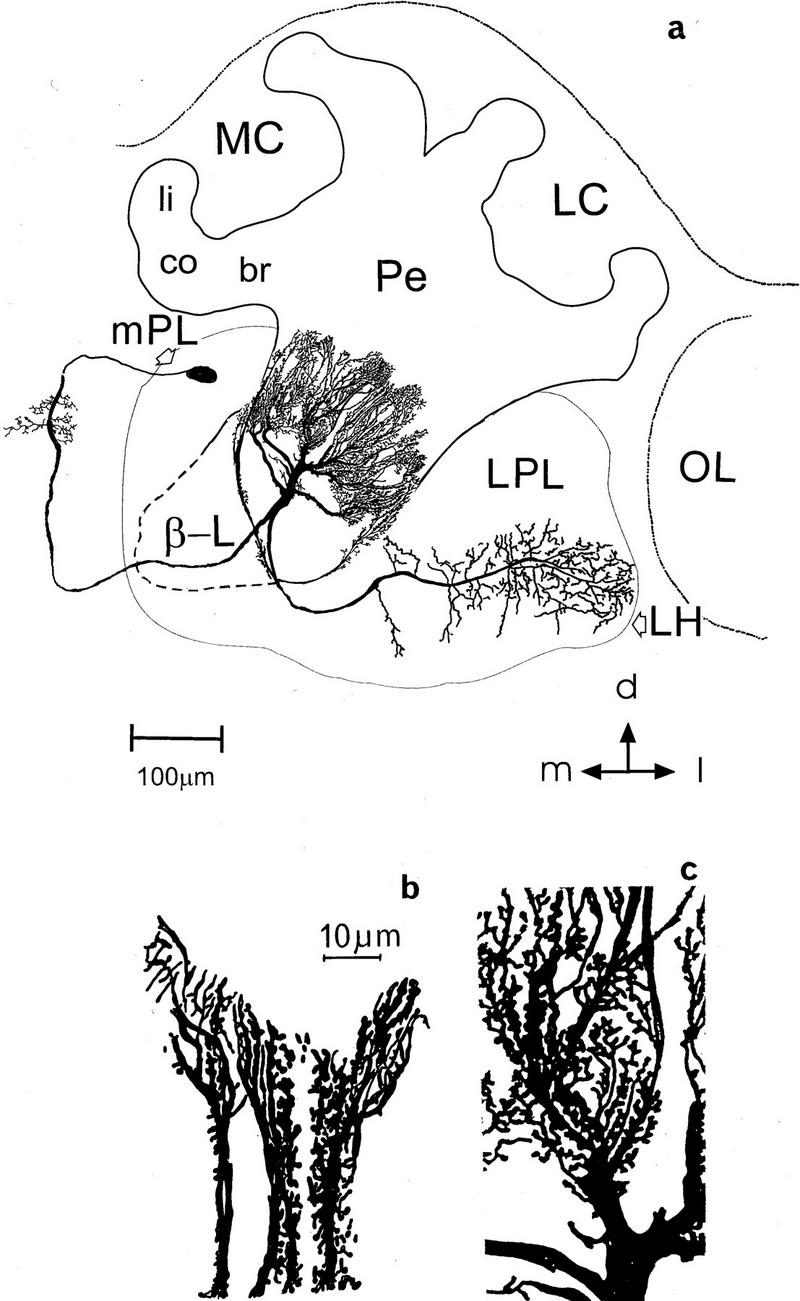
(a) Reconstruction of the Pe1 neuron within the protocerebrum of the honeybee from a cell filled intracellularly with lucifer yellow. The Pe1 neuron enters the MB at the border from the β-lobe (β-L) to the peduncle (Pe) and arborizes extensively in the peduncle. The soma is located below the anterior brain surface and gives rise to a neurite that arborizes in the median contralateral protocerebral lobe (mPL). In the protocerebral lobe one branch courses lateral, gives rise to arborizations in the lateral protocerebral lobe (LPL), and terminates in the lateral horn (LH). (MC) Median calyx; (LC) lateral calyx; (li) lip; (co) collar; (br) basal ring; (OL) optic lobe. Scale, 100 μm. (d) Dorsal; (l) lateral; (m) median. (b,c) Magnified parts of the Pe1 neuron in the posterior (b) and more anterior peduncle (c). Note the spiny processes along the course of the dendrites. Scale in b and c, 10 μm.
OLFACTORY RESPONSES
Responses to lasting odor stimuli (>2 sec) are typically phasic-tonic with onset frequencies in the range of 100–300 Hz and sustained responses in the range of 50–100 Hz. The responses to odor pulses of 1 sec give only the phasic component (Fig. 2a,b), and responses to the stimulation procedure applied most frequently in our study (presentation of a glass vessel containing the undiluted odorant into the pathway of a continuous airstream) indicate the arrival of odor jetties at irregular intervals, differing in concentration and duration. Such a stimulation allows testing of a large number of compounds in quick succession, and in addition, it resembles more closely the natural form of odor stimulation. In the natural environment, odor eddies reach the antennae in packages of varying frequencies. The frequency of odor packages might be indicative of the distance to the odor source, as air turbulence separates the packages with increasing distance. It was, therefore, interesting to see whether the Pe1 neuron can monitor such temporal structures. Figure 2, c–e, shows that the responses to such stimuli are highly structured, indicating a sensitivity to the sequential arrival of odor packages and a capacity to code the sequential arrival of odor eddies at a time scale well below 1 Hz. Different forms of complex spiking patterns were elicited by step-like odor stimulation (see Fig. 4D in Mauelshagen 1993) indicating that long-lasting and constant odor stimulation induces additional intrinsic response dynamics. It has yet to be analyzed how these different forms of temporal dynamics interact, for example, whether the Pe1 neuron is tuned to particular stimulus frequencies.
Twenty-nine different odorants were tested. Figure 3 shows a representative example for a recording, where 22 odorants were applied. As in all other cases, this Pe1 neuron responds with excitation to all odors. Strong responses are found for the aldehydes benzaldehyde and hexanal, the alcohol 2-hexanol, the ketone pentanon, the acetate hexadecylacetate, and the terpen citral. In 11 Pe1 recordings we did not find an odorant that was not responded to with at least a weak increase in AP frequency. Other odor stimuli included mixtures of citral, 2-hexanol, and benzaldehyde. No differences in the AP patterns to the tested compounds were found. This applies also to the mixtures tested. The mixing of two components leads sometimes to an increase of the response, but in most cases no difference was seen when compared to the response to the most effective single component. It is therefore likely that the concentration dependence, which we did not test specifically, may have reached response saturation of the Pe1 neuron or at any stage upstream of Pe1 (see also below). Repetitions of stimulation indicated that the differences between strong and weak responses were odor specific, but the variability between different neurons and from stimulation to stimulation of the same neuron was rather large. That the variability of responses is at least partially due to the stimulation procedure cannot therefore be excluded.
RESPONSES TO OTHER SENSORY MODALITIES
Contact chemoreceptive, mechanosensory, and visual stimuli evoke excitatory responses in the Pe1 neuron as well (Fig. 4). Mechanical stimulation at one or both antennae and proboscis including the glossa (but not at other locations on the head) induce phasic ON responses (Fig. 4c). The same is true for sucrose stimulation of the antennae or glossa. Both stationary visual stimuli (ON, OFF) and moving stripes lead to phasic excitatory responses. The response to light OFF is stronger than that to light ON (Fig. 4a). In both test situations adaptation time was about equal (several minutes light or dark). Motion stimuli are effective but are not discriminated: All four directions tested (front to back, back to front, up-, and downward) elicit similar response patterns (Fig. 4b). We tested also stimulus combinations, for example, an odor (citral or 2-hexanol) combined with mechanical stimulation to the antennae. The responses were always stronger than to the mechanical stimulus alone. However, the variability in the response did not allow us to determine whether the stronger response was just the linear addition of the individual responses or more. At least this result indicates that the failure to detect an additive effect in odor mixtures does not result from a saturated response of the Pe1 but might indicate response saturation in the olfactory pathway upstream of Pe1.
Figure 4.
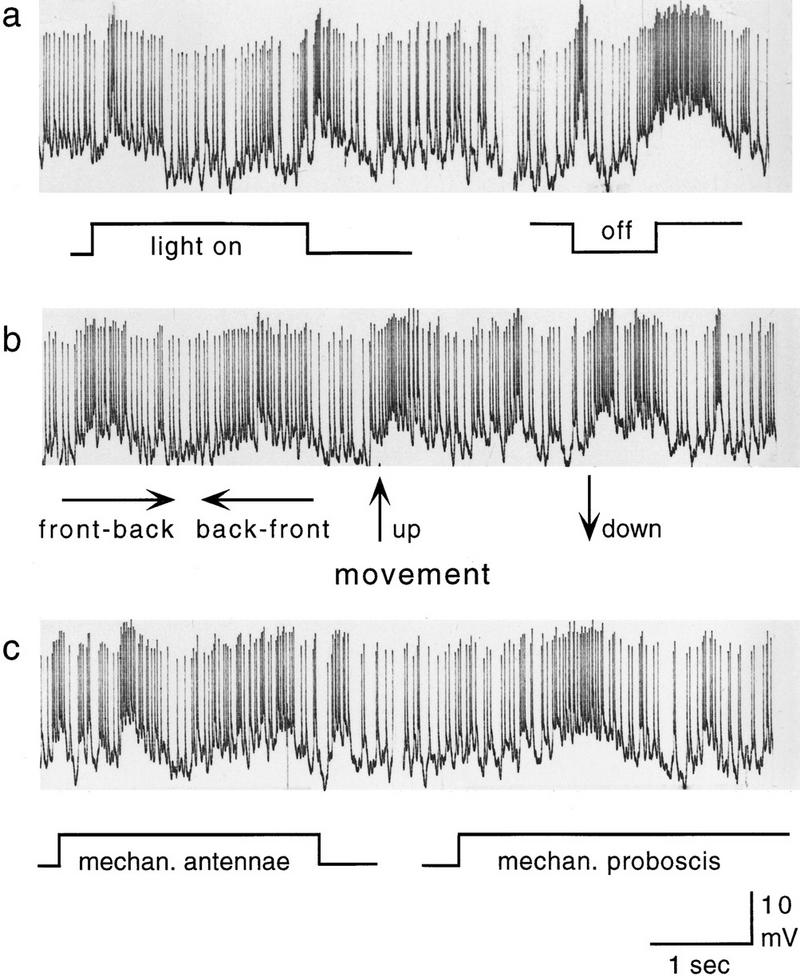
Pe1 responses to different modalities: (a) Light ON, light OFF; (b) moving stripes; (c) mechanical stimuli to antennae and proboscis.
It thus appears that the Pe1 neuron integrates not only olfactory stimuli but also contact chemosensory, mechanosensory, and visual stimuli. We may therefore predict that in principle, the Pe1 neuron is synaptically connected with Kenyon cell subpopulations that derive from all modality-specific calycal subcompartments.
MORPHOLOGY
If one of the basic characteristics of the Pe1 neuron would be to integrate information from different sensory modalities, this should be implemented in its morphological organization within the Kenyon cell bundles arranged in parallel in the peduncle. To test this hypothesis we studied the arborization pattern of the Pe1 neuron within the peduncle [for an account of Pe1 synaptic connectivity on the electron microscopy (EM) level, see Rybak and Mauelshagen 1994]. To allocate the projections of the Pe1 neuron in the peduncle to calycal subcompartments and reveal its structure at the light microscopy level, we performed a confocal analysis of lucifer yellow-filled cells. Additionally, we compared the results of Bodian- and Golgi-stained preparations (Fig. 5a,b).
Figure 5.
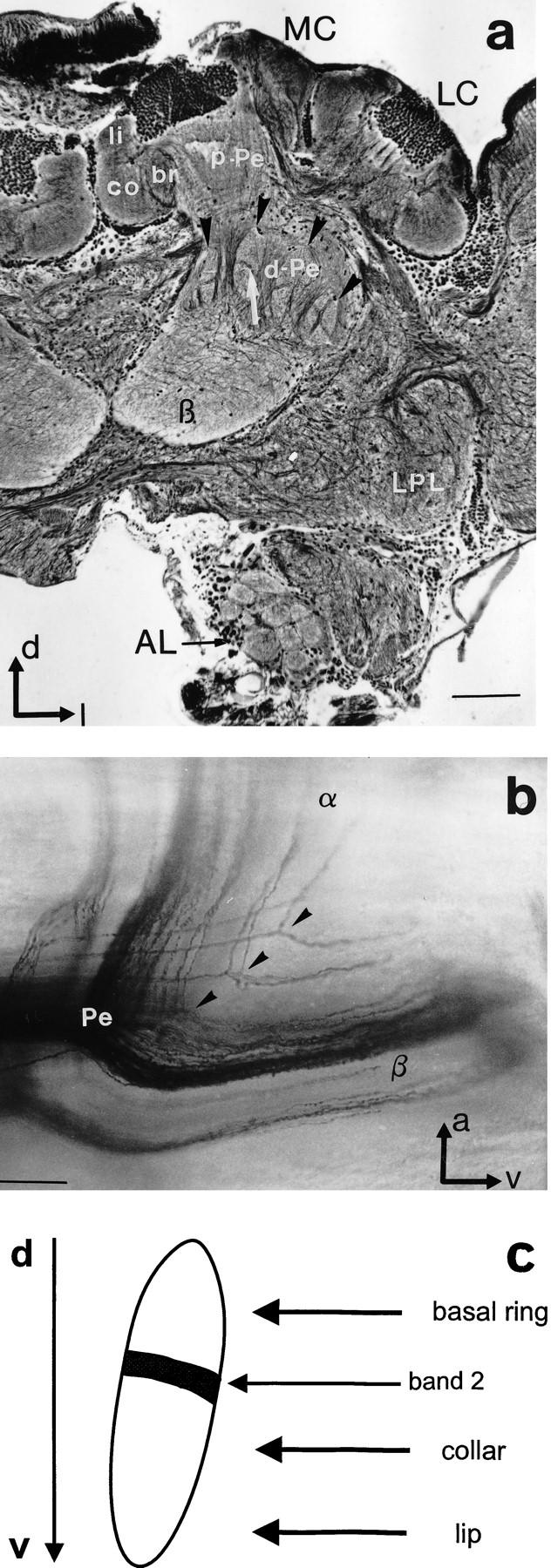
Structural organization of the MBs and Pe1 innervation. (a) Bodian staining reveals subcompartments of MBs at a depth of ∼250 μm (frontal view). The calyces (MC, LC) are composed of ring neuropils, lip, collar, and basal ring (li, co, br). Axonal projections of the Kenyon cells form the layered proximal peduncle (p-Pe). Axons in the distal peduncle (d-Pe) split into collaterals more distal to the calyces. The finger-like structures (black arrowheads) are collaterals that form more anteriorly the α-lobe, which extends into more frontal sections. Longitudinal areas extend into the β-lobe (β). Note the banding pattern in the vertical structures (arrow). (AL) Antennal lobe; (LPL) lateral protocerebral lobe. Scale, 100 μm. (d) Dorsal; (l) lateral. (b) Sagittal view of a Golgi–Collonier staining of Kenyon cell axon bundles at the splitting point [transition from peduncle (P) to α- and β-lobe, α and β]. Scale, 50 μm. (a) Anterior; (v) ventral. (c) Allocation of calycal subcompartments (basal ring, collar, and lip) to zones in the peduncle. The black bar in the finger-like structure represents the thin band shown in a (arrow).
INTRINSIC PATTERN OF THE MBs
The complex pattern of Kenyon cell axonal projection in the MB stems from the fact that bundles of axons deriving from different calycal zones split off at different depths throughout the peduncle (see below) and form bands in the α- and β-lobes. This structural organization is described as the transformation of circular coordinates (dendritic arborizations of Kenyon cells in the calyces) into the Cartesian coordinate of the peduncle and lobes (Howse 1974; Mobbs 1982). The analysis of the matching of intrinsic and extrinsic neuronal elements is facilitated by the fact that Kenyon cell axon bundles are organized in a highly ordered fashion without the crossing of fibers. Also, because each MB consists of two parts that are separated anatomically at the level of the calyx (median and lateral calyx; Fig. 1), but otherwise equally built (Rybak 1994), the same topographical principle applies to axonal projections originating in the median and lateral calyx. Thus, if the Pe1 neuron arborizes across the peduncle it should sample a dual set of rather similar information coming from the two calyces.
Proximal to the calyx, axon bundles of the Kenyon cells form a three-layered proximal peduncle (p-Pe in the median calyx in Fig. 5a). More ventrally, where the most distal dendrites of the Pe1 neuron are found, the pedunculi of both calyces fuse and Kenyon cell axons begin to split. In this part of the peduncle, which we name distal peduncle (d-Pe in Fig. 5a), groups of axon bundles with different directions can be distinguished. Longitudinal strands project medioventrally to form the β-lobe; axons grouped in vertically oriented, finger-like zones run anteriorly to build up the α-lobe (arrows in Fig. 5a). This organization is the consequence of the splitting of axons into two collaterals projecting into the α-lobe and β-lobe, respectively (Fig. 5b). The α- and β-lobes are defined here as neuropils, in which all of the axon collaterals that are separated in the distal peduncle are fused to form the relative homogenous texture of the lobes. For the α-lobe, this is a cylinder that extends from the anterior brain surface to a depth of ∼200 μm.
ZONE ALLOCATION IN THE PEDUNCLE AND LOBES TO CALYX SUBCOMPARTMENTS
The ordered arrangement of parallel-running axons allows the allocation of zones in the peduncle and lobes to calycal zones. The distal peduncle (d-Pe in Fig. 5a) extends from the fusion of both pedunculi at a depth of 400 μm and ends with the base of the α-lobe ∼200 μm depth. The specific texture caused by the intermingling and dividing axons is clearly distinguishable from the layered organization in the proximal peduncle and the lobes as seen in Bodian preparations (Fig. 5a), as well as in preparations with intracellularly labeled cells (Fig. 6). Throughout the peduncle Kenyon cell axons from all calycal subcompartments are split in a sequential order; most posteriorly Kenyon cell axons from the basal ring subcompartment divide and send collaterals to the dorsal α-lobe and posterior β-lobe. Kenyon cells from the lip subcompartment split more anteriorly (close to the α-lobe) and send fibers into the ventral α-lobe and anterior β-lobe (see arrows in Fig. 5b). Collaterals that project toward the α-lobe are visible as finger-like structures in the distal peduncle (Fig. 5a). These vertically oriented zones begin to form posteriorly and become gradually larger until all axons are split. The pattern that finally forms the α-lobe is seen most clearly at a depth of ∼200 μm, at the tripartition of lobes and distal peduncle. Thus, the stratifications at this level can be allocated to the calycal zones (Fig. 5c) and can be interpreted as a preformation of the α-lobe.
Figure 6.
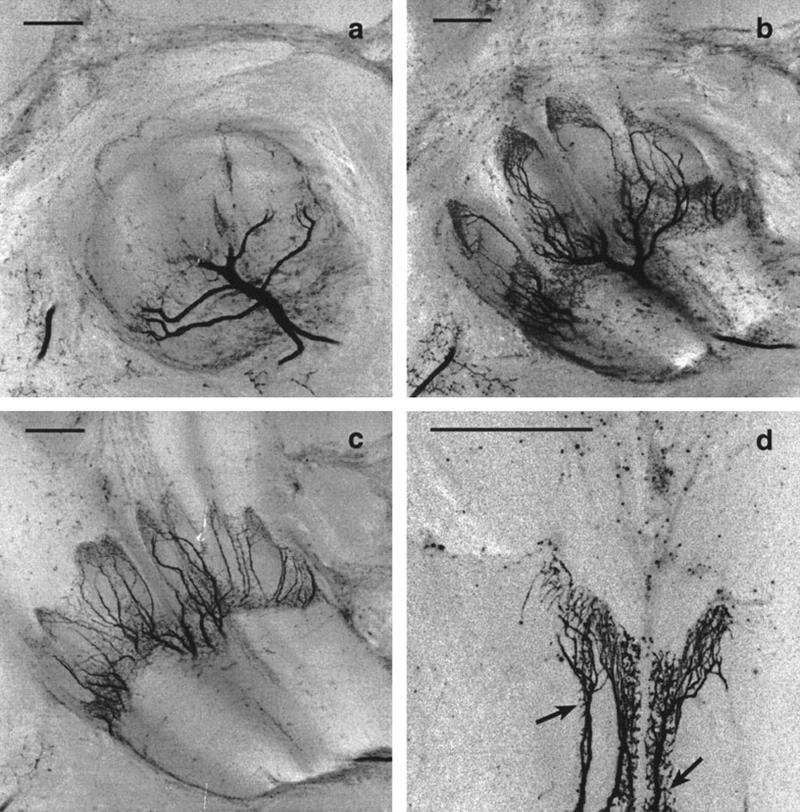
(a–c) Confocal images at different depth of the peduncle. The main dendrite enters the MB at the transition of the peduncle to α- and β-lobe, and its fine arborizations are restricted to vertically oriented zones. Compare with Fig. 5a. Scale, 50 μm. (d) Fine structure of the Pe1 neuron in the ventral peduncle at a higher resolution. Note the spiny endings (arrows). Scale, 50 μm.
ARBORIZATION OF THE PE1 NEURON WITHIN THE PEDUNCLE
How do the arborizations of the Pe1 neuron fit into the scheme described above? The Pe1 neuron enters the MB neuropil with a large diameter neurite (12–15 μm) at the tripartition of peduncle and lobes (Figs. 1a and 6a). Its entrance point lies at a depth of 180 μm. The neurite splits off and gives rise to finger-like ramifications within the peduncle (Figs. 1a and 6). The fine arborizations bear spine-like processes (Figs. 1, b and c, and 6d), indicating postsynaptic structures. Pe1 arborizations in the peduncle cover a large area ranging anteriorly from the base of the α-lobe (∼200 μm depth) to the beginning of the proximal peduncle (∼400 μm depth). Comparison of the regions innervated by the Pe1 neuron as revealed with Bodian stain (Fig. 5a) indicates that Pe1 arborizations cover the whole distal peduncle, projecting more anteriorly within the part of the peduncle that comprises axons of Kenyon cells originating in the lateral calyx. This corresponds to the fact that in the lateral calyx axons begin to split off more posteriorly. The Pe1 dendrites do not enter the proximal peduncle or the lobes (but see below for fine axon projections of the Pe-1 to the α-lobe). Because the Pe1 neuron arborizes exclusively in vertical areas of the “splitting zone” of the distal peduncle, it contacts primarily Kenyon cell collaterals that form the α-lobe. The collaterals of the Pe1 neuron form narrow bands in different depths of the peduncle and show spiny processes along the course of thicker axons. Thus, the Pe1 neuron is connected with Kenyon cell projections stemming from all calycal subcompartments and is therefore anatomically well suited for integrating sensory information present in the MB system.
PE1 ARBORIZATIONS IN THE PROTOCEREBRAL LOBE
Outside the MB, the Pe1 neuron has extensive arborizations in the protocerebral lobe (Figs. 1 and 6). It sends branches in the ring neuropil around the α-lobe and the LPL, where they arborize extensively in the lateral horn (LPL and LH in Fig.1a). The soma (diam., 30 μm) gives rise to the primary neurite that courses contralaterally into the medial protocerebral lobe (mPL) and posterior. The neurite describes a posteriorly directed loop through the mPL, where it sends off small branches (Fig. 1a). In a depth of ∼210 μm from the anterior brain surface the primary neurite crosses back ipsilaterally to the MB. A major branch enters the MB at the β-exit as described above (Fig. 1a).
Ventrally to the β-exit in the protocerebral lobe, the main neurite gives rise to a second large process that follows the ventrolateral margin of the α-lobe. Here lies the origin of several branches that invade the ring neuropil around the α-lobe. Originating from processes in the ring neuropil, one median and one lateral branch innervate in a depth of 100–120 μm the ventral α-lobe with fine and also bleb-like varicosities (not shown in Fig. 1a). This region was described as the A4 region and is assumed to carry preferentially olfactory information (Rybak and Menzel 1993; Rybak 1994, see also Fig. 7). The β-lobe is not innervated by Pe1 profiles.
Figure 7.
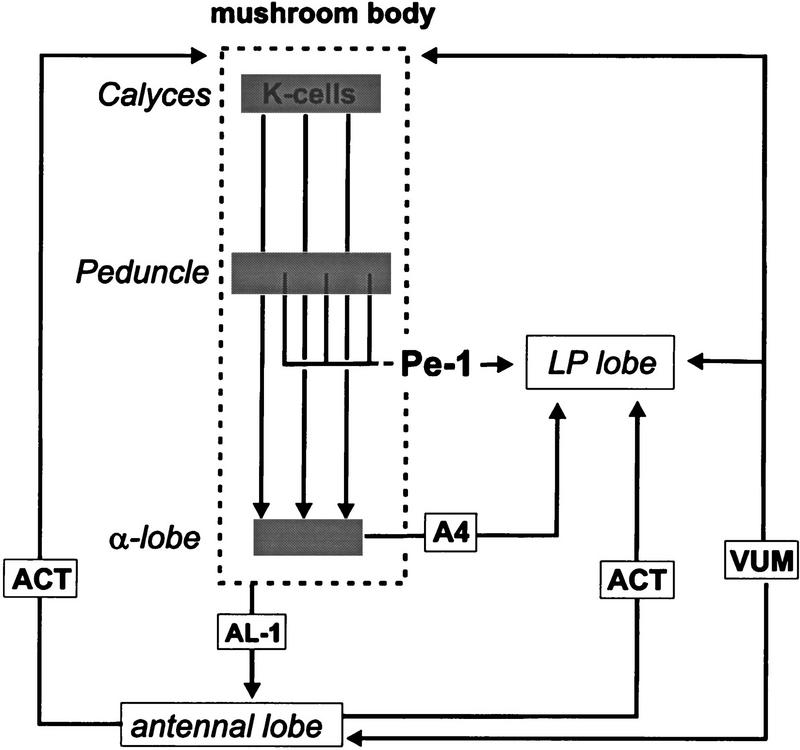
Summary of Pe1 neuron projections and other neuronal pathways involved in olfactory processing and learning in the honeybee brain. The Kenyon cells of the MB project onto the extrinsic Pe1 neuron in the peduncle. Outside the MB the Pe1 neuron projects to premotor centers of the protocerebrum (LP lobe), which includes the LH. In the LP lobe the Pe1 neuron converges with the VUMmx1 neuron (VUM), which also projects to the antennal lobe and the calyces. Furthermore, Pe1 overlaps with the A4 cells (output neurons of the α-lobe), and the AL-1, a feedback neuron from the MB to the antennal lobe. (K-cells) Kenyon cells; (ACT) antennocalycal tract. (Adapted from Rybak and Menzel 1993; Rybak 1994; Hammer and Menzel 1995).
At the lateral margin of the α-lobe one large axon turns lateroventrally and posteriorly into the LPL to terminate with bleb-like endings in two large projection areas at a depth ranging between 30 and 300 μm (Fig. 1a). One of them is located more anteriorly, and the other directed posteriorly, thus spanning the whole area of the so-called LH. This area is known as a projection area of antennocerebralis tracts (ACTs) originating in the antennal lobes (e.g., Mobbs 1982). Terminals of the Pe1 in the protocerebral lobe and A4 band of the MB are swollen bleb-like varicosities.
Discussion
The Pe1 neuron is a unique neuron in the central brain of the honeybee. Its impressive size, its particular shape, and its location inspired physiological (Mauelshagen 1993) and anatomical (Rybak 1994) studies. The Pe1 neuron responds to odor stimuli and changes its response properties as a consequence of sensitizing and conditioning trials (Mauelshagen 1993). Sensitization leads to an increase of subsequent odor responses. A single conditioning trial results in a reduction of the sustained response to the conditioned odor; multiple differential conditioning trials enhance responses to the CS+ (odor stimulus paired with sucrose) but leave response to the CS− (specifically unpaired odor stimulus) unchanged. These associative changes of responsiveness appear not to last long (∼10 min). Thus the Pe1 neuron implements learned olfactory information transiently.
The extended dendritic arbors of the PE1 neuron within the MB indicate that it might be sensitive to multiple sensory input. We find that all odors tested lead to excitatory responses without any indication of odor-specific response patterns. The recordings were also carefully inspected for any rhythmicity of action potentials. We found that only spontaneous activity may show rhythmic spiking with bursts of two to three APs at 80–200 Hz separated by intervals in the range of 150–250 msec. Odor stimuli did not induce any rhythmic spiking. Simultaneous recording of field potentials are necessary to test whether there is any temporal component of the odor-induced response as was found for projection neurons in the antennal lobe of the locust (Laurent and Davidowitz 1994; Wehr and Laurent 1996) and the honeybee (Stopfer et al. 1997). No motor patterns of the antennae or the proboscis could be related to the occurrence of rhythmic spiking during spontaneous activity. In addition to its broad sensitivity to odorants, the Pe1 neuron is also sensitive to other sensory modalities. Visual sensitivity appears to be rather unspecific with respect to the stimuli tested (light ON and OFF, moving black-and-white stripes). However, it may well be that certain complex features could lead to much stronger responses. Although we have tried to search for particular stimulus combinations using rather simple forms of stimuli, no particularly effective combinations were found. A combination of olfactory and mechanical stimulation induced stronger responses than the single stimuli, but the variability of the responses did not allow evaluation of whether the responses to the combined stimuli exceeded a simple summation of excitation induced by the single stimuli.
How is the multisensory property of the Pe1 neuron reflected in its anatomy? The Pe1 neuron is a MB extrinsic neuron. According to Mobbs (1982) MB extrinsic neurons can be distinguished into broad- and narrow-field neurons. The Pe1 neuron belongs to a group of broad-field MB extrinsic cells that project extensively within the MB neuropil. Other examples of this type of neurons are the A6 neurons that arborize throughout the whole anterior α-lobe (Rybak and Menzel 1993). In contrast, α-lobe extrinsic neurons like the A4 cells, occupy relatively small bands in the ventral α-lobe (Rybak and Menzel, 1993).
Arborizations in the protocerebral lobe bear mostly swollen varicosities, indicating their presynaptic nature. This is supported by preliminary results on the EM level (Rybak and Mauelshagen 1994). Therefore, the existing terminal fields of the Pe1 in the ring neuropil around the α-lobe and in the LPL, including the LH, indicate that the Pe1 neuron receives most or all input from within the Kenyon cells of the MB. The Pe1 neuron does not resemble the type of complex MB extrinsic neurons with dendritic and terminal fields outside the MB, which supply afferent information to the lobes as described in Periplaneta (Li and Strausfeld 1997). However, the Pe1 forms collaterals that project back into the A4 band of the α-lobe and form presumably output synapses here (Rybak and Mauelshagen 1994). The importance of the A4 zone for processing olfactory information is substantiated by the fact that it receives information via the Kenyon cell axons that originate primarily from the lip subcompartment of the calyces (Rybak 1994). Optical recordings using a Ca2+-sensitive dye indicated that odor stimulation elicits activity in the calyces and the A4 zone of the α-lobe (J. Joerges, G. Galizia, and R. Menzel, unpubl.). A4 neurons project to the LPL, where they overlap partially with the Pe1 neuron projections. It thus appears that the multisensory responses of Pe1 are forwarded onto neurons apparently selectively devoted to olfactory coding both in MBs and LPLs.
The analysis of the dendritic Pe1 neuron pattern within the MB reveals further important properties of this neuron. The comparison with the internal structure of Kenyon cell projections substantiates the multimodal sensitivity of the Pe1 neuron (see Fig. 5). The main branch of the Pe1 neuron enters at the tripartition of the peduncle and both lobes, and gives rise to extensive arborizations throughout the vertically oriented zones of the peduncle (Figs. 5 and 6). Thus, the dendritic arbors of the Pe1 neuron cover areas in the peduncle that consist of Kenyon cell axons from all calycal subcompartments, as the vertical stripes as a whole represent all calycal subcompartments. Ultrastructural studies confirm that the Pe1 neuron receives synaptic input from Kenyon cells (Rybak and Mauelshagen 1994). Therefore, the anatomy indicates that the Pe1 neuron would be ideally suited to integrate information across the sensory modalities reaching the MB. The ordered input into the MB calyx from olfactory, visual, and mechanosensory systems is maintained throughout the peduncle and the lobes by the parallel Kenyon cell bundles with only a few exceptions to this rule (Rybak 1994). For example, Kenyon cells were found that arborize in an unusual manner in both collar and lip subcompartments. Feedback neurons of the protocerebro calycal tract [p.c.t. (Bicker et al. 1985; Gronenberg 1987)], which connect output regions of the MB with the calycal input sites, mix information processed in different compartments of the MB and may also be responsible for the multimodal properties of a variety of extrinsic neurons (for review, see Erber et al. 1987). However, p.c.t. neurons do not innervate the ventral α-lobe (to which the A4 zone belongs; B. Grünewald, unpubl.). Multisensory integration might therefore mainly be performed by neurons like the Pe1 neuron or A6 neurons.
The presumable output areas of the Pe1 neuron are in the LPL, mainly in the LH and in the ring neuropil around the α-lobe (Fig. 7). In the LPL, a presumable premotor area (e.g., Li and Strausfeld 1997), it overlaps with neurons of the ACT, the VUMmx1 neuron (Hammer 1993), and a recurrent neuron from the MB to the antennal lobes, the AL-1 (Rybak and Menzel 1993). The convergence with the modulatory VUM neurons may indicate that the Pe1 neuron is under the control of presynaptic modulatory action. The convergence with the AL-1 neuron feeding back to the antennal lobe may indicate that output from the MB is provided to the antennal lobe, possibly with the contribution of the Pe1 neuron, and thus in a relationship to Pe1’s specific properties. Therefore, we forward the hypothesis that such a contribution may be viewed in the context of memory consolidation.
According to its response properties and its connectivity, the Pe1 neuron can be incorporated in a hypothetical model of the neural substrate of learning and memory formation in the honeybee brain. Multisensory convergence at the Pe1 neuron may be a potential neural substrate for contextual coding of olfactory stimuli and may be related to synaptic processes that underlie context-dependent learning of olfactory stimuli. Contextual learning is a well-documented property of honeybee learning under natural conditions (Menzel 1990; Menzel et al. 1996).
The information conveyed by the Pe1 neuron is multisensory and MB processed. In the short-term range following learning, the Pe1 neuron reports about a learned odor possibly in relationship to contextual stimuli. A comparison with the information transmitted directly from the antennal lobe to the LPL via olfactory projection neurons (ACTs in Fig. 7) may lead to a signal that indicates the difference between the information coming from the antennal lobe and the MB. This signal may indicate the amount of learned information as processed in the MB, thus not only with respect to the olfactory stimulus but also the context in which the odor occurred. Furthermore, such a signal may be evaluated in reference to the information provided by the VUM neurons, because VUM neurons project also to the LPL. The hypothetical signal may be transmitted not only to premotor neurons but via neurons like the AL-1 also back to the antennal lobe. It is conceivable that this signal serves the function of memory consolidation at the level of the antennal lobe and instructs the antennal lobe about the transient learning processes occurring in the Pe1. A consequence of this model is that important aspects of olfactory memory including context dependencies may influence the process of memory formation in the antennal lobe under the control of the MB. Such a hypothesis can readily be tested in the bee (see also Hammer and Menzel 1998), because the specific components of memory formation in the antennal lobe and MB are experimentally accessible.
Acknowledgments
We are most grateful to Dr. B. Grünewald for carefully commenting on earlier versions of the manuscript, Astrid Klawitter for help with the Bodian procedure, and Sybille Schaare and Dr. Robert Brandt for their most valuable help with the figures. The reconstruction of Pe1 presented in Figure 1 is based on intracellular marking performed by Janna Klein, whose contribution we gratefully acknowledge. The work presented here was supported by the Sonderforschungsbereich 515.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Bicker G, Schäfer S, Kingan TG. Mushroom body feedback interneurons in the honeybee show GABA-like immunoreactivity. Brain Res. 1985;360:394–397. doi: 10.1016/0006-8993(85)91262-4. [DOI] [PubMed] [Google Scholar]
- Blest AD. Some modifications of Holme’s silver method for insect nervous system. Q J Microsc Sci. 1961;102:413–417. [Google Scholar]
- Clark G. Neurological staining methods: Bodian’s protargol method. In: Clatk G, editor. Staining procedures used by the biological stain commission. Baltimore, MD: Williams & Wilkins; 1973. pp. 98–100. [Google Scholar]
- Davis RL. Mushroom bodies and Drosophila learning. Neuron. 1993;11:1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- Erber J, Homberg U, Gronenberg W. Functional roles of the mushroom bodies in insects. In: Gupta AP, editor. Arthropod brain: Its evolution, development, structure, and functions. New York, NY: John Wiley and Sons; 1987. pp. 485–511. [Google Scholar]
- Gronenberg W. Anatomical and physiological properties of feedback neurons of the mushroom bodies in the bee brain. Exp Biol. 1987;46:115–125. [PubMed] [Google Scholar]
- Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Learning and memory in the honeybee. J Neurosci. 1995;15:1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. Multiple sites of associative odor learning as revealed by local injections of octopamine in honeybees. Learn. & Mem. (this issue). [PMC free article] [PubMed]
- Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- Homberg U. Processing of antennal information in extrinsic mushroom body neurons of the bee brain. J Comp Physiol A. 1984;154:825–836. [Google Scholar]
- Howse PE. Design and function in the insect brain. In: Barton-Browne L, editor. Experimental analysis of insect behaviour. Berlin, Germany: Springer; 1974. pp. 180–194. [Google Scholar]
- Kenyon FC. The brain of the bee—A preliminary contribution to the morphology of the nervous system of the Arthropoda. J Comp Neurol. 1896;6:134–210. [Google Scholar]
- Laurent G, Davidowitz H. Encoding of olfactory information with oscillating neural assemblies. Science. 1994;265:1872–1875. doi: 10.1126/science.265.5180.1872. [DOI] [PubMed] [Google Scholar]
- Li Y, Strausfeld NJ. Morphology and sensory modality of mushroom body extrinsic neurons in the brain of the cockroach, Periplaneta americana. J Comp Neurol. 1997;387:631–650. [PubMed] [Google Scholar]
- Mauelshagen J. Neural correlates of olfactory learning in an identified neuron in the honey bee brain. J Neurophysiol. 1993;69:609–625. doi: 10.1152/jn.1993.69.2.609. [DOI] [PubMed] [Google Scholar]
- Menzel R. Learning, memory, and “cognition” in honey bees. In: Kesner RP, Olten DS, editors. Neurobiology of comparative cognition. Hillsdale, NJ: Erlbaum Inc.; 1990. pp. 237–292. [Google Scholar]
- Menzel R, Erber J, Masuhr T. Learning and memory in the honeybee. In: Barton-Browne L, editor. Experimental analysis of insect behaviour. Berlin, Germany: Springer; 1974. pp. 195–217. [Google Scholar]
- Menzel R, Durst C, Erber J, Eichmüller S, Hammer M, Hildebrandt H, Mauelshagen J, Müller U, Rosenboom H, Rybak J, Schäfer S, Scheidler A. The mushroom bodies in the honeybee: From molecules to behavior. Neural basis of behavioral adaptations. Fortschr Zool. 1994;39:81–102. [Google Scholar]
- Menzel R, Geiger K, Chittka L, Joerges J, Kunze J, Müller U. The knowledge base of bee navigation. J Exp Biol. 1996;199:141–146. doi: 10.1242/jeb.199.1.141. [DOI] [PubMed] [Google Scholar]
- Mobbs PG. The brain of the honeybee Apis mellifera I. The connections and spatial organization of the mushroom bodies. Philos Trans R Soc Lond B Biol Sci. 1982;298:309–354. [Google Scholar]
- Rybak J. Germany: Freie Universität Berlin; 1994. [Google Scholar]
- Rybak J, Menzel R. Anatomy of the mushroom bodies in the honey bee brain: The neuronal connections of the alpha-lobe. J Comp Neurol. 1993;334:444–465. doi: 10.1002/cne.903340309. [DOI] [PubMed] [Google Scholar]
- Rybak J, Mauelshagen J. Proceeding of the Twenty-second Göttingen Neurobiology Conference. II. Stuttgart, Germany: Thieme Verlag; 1994. The Pe1-neuron of the honeybee—An afferent pathway from the mushroom bodies to the protocerebral lobe; p. 830. [Google Scholar]
- Schürmann FW. Bemerkungen zur Funktion der Corpora pedunculata im Gehirn der Insekten aus morphologischer Sicht. Exp Brain Res. 1974;19:406–432. doi: 10.1007/BF00234464. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. The Golgi method: Its application to the insect nervous system and the phenomen of stochastic impregnation. In: Strausfeld NJ, Miller TA, editors. Neuroanatomical techniques. Insect nervous system. Berlin, Germany: Springer Verlag; 1980. pp. 131–203. [Google Scholar]
- Vowles DM. The structure and connections of the corpora pendunculata in bees and ants. Q J Microsc Sci. 1955;96:239–255. [Google Scholar]
- Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384:162–166. doi: 10.1038/384162a0. [DOI] [PubMed] [Google Scholar]
- Witthöft W. Absolute Anzahl und Verteilung der Zellen im Hirn der Honigbiene. Z Morph Tiere. 1967;61:160–184. [Google Scholar]