Abstract
The sensation of knee instability (shifting, buckling. and giving way) is common in people with medial knee osteoarthritis (OA). Its influence on knee stabilization strategies is unknown. This study investigated the influence of knee instability on muscle activation during walking when knee stability was challenged. Twenty people with medial knee OA participated and were grouped as OA Stable (OAS) (n = 10) and OA Unstable (OAU) (n = 10) based on self-reported knee instability during daily activities. Quadriceps strength, passive knee laxity, and varus alignment were assessed and related to knee instability and muscle cocontraction during walking when the support surface translated laterally. Few differences in knee joint kinematics between the groups were seen; however, there were pronounced differences in muscle activation. The OAU group used greater medial muscle cocontraction before, during, and following the lateral translation. Self-reported knee instability predicted medial muscle cocontraction, but medial laxity and limb alignment did not. The higher muscle cocontraction used by the OAU subjects appears to be an ineffective strategy to stabilize the knee. Instability and high cocontraction can be detrimental to joint integrity, and should be the focus of future research.
Keywords: knee, osteoarthritis, instability, muscle activity, walking
Osteoarthritis (OA) is a world-wide problem1,2 that commonly develops in the knee,3 most often in the medial compartment.4 Studies of knee OA often focus on biomechanical factors associated with the development and progression of OA,5–7 while others focus on knee function. Strong quadriceps can help maintain or improve function in people with knee OA;8–10 however, recent work suggests that strong quadriceps muscles increase the likelihood of OA progression in people with malalignment or excessive frontal plane laxity,11 which are common in this population.12,13 Thus, progressive OA may be related not only to local factors (e.g., ligament laxity, malalignment) and quadricep strength, but also to the manner in which muscles are activated around the knee. Studies have shown14–16 that people with knee OA use higher magnitudes of knee muscle cocontraction during walking. High cocontraction can increase joint compression,17 which could have important influences on function and joint integrity.
Recently, investigators have identified that self-reported knee instability (knee instability), defined as the sensation of shifting buckling or giving way in the knee, is a common complaint of people with knee OA.18,19 We have demonstrated that knee instability is a sensation that is not related to frontal plane knee laxity or quadricep strength, and further, knee instability affects daily function over and above laxity and quadricep weakness.19 The sensation of knee instability is likely associated with abnormal arthrokinematics that subject articular surfaces to shear forces that are particularly detrimental to articular cartilage.20,21 Because knee instability is not solely related to weakness or laxity, it is plausible that knee instability is related to neuromuscular activation patterns in individuals with knee OA. A better understanding of neuromuscular activation patterns associated with knee instability will help to elucidate factors that could increase patient function but may also influence OA progression.
The purpose of this study was to investigate the influence of knee instability on muscle activation strategies during a disturbed walking paradigm designed to challenge knee stability. We hypothesized that individuals with OA and knee instability would use higher muscle cocontraction that was influenced by quadricep strength but not medial knee laxity or varus alignment.
MATERIALS AND METHODS
Subjects
Persons with isolated medial knee OA [Kellgren-Lawrence (K-L) grades II–IV] were recruited from local physicians and from the community (see Table 1). Relevant exclusion criteria included OA in the lateral tibiofemoral or patellofemoral compartments greater than K-L grade I or a history of knee ligament injury. Subjects with a history of atraumatic meniscal injury or meniscectomy were included. All subjects signed an informed consent approved by the Institutional Review Board.
Table 1.
Group Characteristics, Strength, and Radiographic Data [mean and SD]
| OA Stable (OAS) n = 10 | OA Unstable (OAU) n = 10 | p-value | |
|---|---|---|---|
| Age (years) | 64.5 (range 44–78) | 64.7 (range 49–77) | 0.967 |
| Gender | ♀ n = 5 | ♀ n = 4 | |
| ♂ n = 5 | ♂ n = 6 | ||
| Height (m) | 1.71 (0.127) | 1.69 (0.105) | 0.835 |
| Body mass (kg) | 93.45 (17.47) | 87.98 (16.83) | 0.485 |
| Quadriceps MVIC (N) | 757 (231) | 651 (208) | 0.309 |
| Lateral Laxity (mm) | 3.08 (1.42) | 2.55 (1.28) | 0.406 |
| Medial Laxity (mm) | 4.31 (1.16) | 4.54 (2.28) | 0.790 |
| Alignment * (degrees) | 176.8 (1.69) | 173.2 (3.39) | 0.008a |
| KL Grade | |||
| II | n = 7 | n = 4 | |
| III | n = 2 | n = 4 | 0.403 |
| IV | n = 1 | n = 2 |
Significant at p ≤ 0.05.
Participants were classified based on knee instability that was assessed from the Knee Outcome Survey—Activities of Daily Living Scale instability question (instability score), which is scored on a six-point scale (5 = instability does not impact daily function, 0 = instability prevents daily function).18,22 Ten subjects reported having no instability (OA Stable (OAS), instability score = 5) and 10 subjects were classified as having instability that affects function (OA Unstable (OAU). The OAU group included seven subjects with instability score = 3 (instability affects daily function slightly) and three subjects with instability score = 2 (instability affects daily function moderately).
Radiograph Assessment
Tibiofemoral Joint Alignment
Alignment was assessed using a standing, anterior–posterior radiograph in which the hip, knee, and ankle joints were visible. Alignment was determined by the angle (varus <180°, valgus >180°) of the mechanical axes of the femur and tibia.23
Frontal Plane Laxity
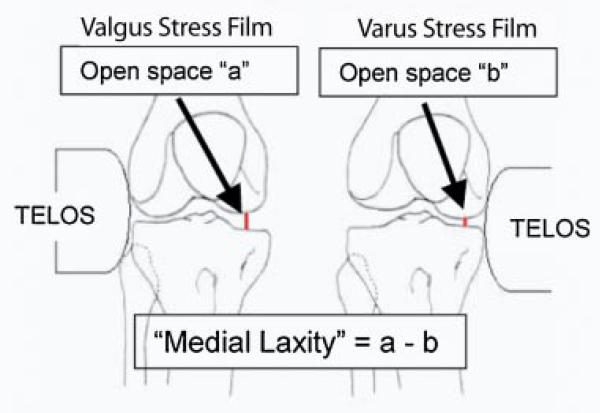
Medial and lateral joint laxities were measured using the “open space” technique during varus and valgus stress radiographs24 (Fig. 1). Subjects were positioned in a Telos Stress device (Austin Assoc., Fallston, MD) with the knee flexed 20° and the patella facing anteriorly. Varus or valgus stress was created by 150 N of force applied by the Telos device at the joint line. Radiographs were adjusted for magnification. Joint spaces were measured and laxity was calculated as shown in Figure 1.
Figure 1.
Calculation of medial laxity. A similar calculation was used for the lateral laxity. Repeat testing using these techniques revealed high test–retest reliability (ICC, 0.96 for medial laxity and ICC, 0.97 for lateral laxity).
Volitional Quadricep Strength Measurement
Quadricep femoris force output (N) was determined during a maximal voluntary isometric contraction (MVIC) with electrical burst superimposition to ensure maximum effort.25 Subjects were seated and stabilized in an isokinetic dynamometer (KinCom Isokinetic International, Harrison, TN) with the knee flexed to 90°. A supramaximal burst of electrical stimulation (100 pulses/s, 600-μs pulse duration, 10 pulse tetanic train, 130 volts) was delivered (Grass S48 stimulator, Astro-Med Inc., West Warwick, RI) during the MVIC. If the subject's volitional force was <0.95 of the force during the electrical burst, the test was repeated up to three times and the highest volitional force was used for analysis.
Motion Analysis
The position of reflective markers on the pelvis and legs was tracked by a passive, three-dimensional motion analysis system (Vicon 512, Vicon Peak, Oxford, UK) at 120 Hz. The cameras tracked retroreflective (15.5 mm) markers from both legs during walking. Marker data were filtered at 6 Hz with a fourth order, phase-corrected Butterworth filter.
Electromyography (EMG)
EMG data were sampled at 1080 Hz and collected simultaneously with preamplified surface electrodes (MA-16, Motion Lab Systems, Baton Rouge, LA) with an 18-mm interelectrode distance. EMG signals were recorded from the medial and lateral quadriceps, medial and lateral hamstrings, and medial and lateral gastrocnemius muscles.26
Testing Procedures—Disturbed Walking Paradigm
Subjects walked at a self-selected speed along a 13-m walkway across a custom-built, moveable platform (NSK Ltd, Tokyo, Japan). When unlocked, the platform translated laterally (5.8 cm at 40 cm/s) at initial contact (lateral trials), and was triggered by a switch mat on the surface of the platform. For safety, subjects were allowed to perform several practice trials. A total of 20 trials (10 lateral trials and 10 locked trials, presented in a random order) were collected. Subjects were unaware of which condition they were going to experience in each trial. Only data from the lateral trials are included in this analysis. Subjects rated how unstable their knee felt during the lateral trials using a 0–10 visual analog scale (0 = extreme instability, 10 = no instability).
Data Management
Sagittal and frontal plane knee joint angles were calculated (Visual3D, C-Motion, Rockville, MD) across the stance phase (initial contact to toe off). Sagittal and frontal plane knee motion excursions were calculated over two intervals: weight acceptance (angular motion from initial contact through peak knee flexion) and midstance (angular motion from peak knee flexion angle through peak knee extension). Data were time normalized and averaged across the 10 lateral trials, and compared between groups.
EMG data were processed using custom-written software (Labview, National Instruments, Austin, TX). A linear envelope was created with full wave rectification and filtering with a 10 Hz low pass, phase-corrected, eighth-order Butterworth filter. The linear envelope was normalized by peak muscle activity for each muscle during undisturbed walking. Data from the undisturbed walking trials were collected prior to the randomized trials but within the same testing session and have been reported elsewhere.16 Cocontraction was calculated between four opposing muscle groups: lateral quadriceps–lateral hamstrings (LQH), lateral quadriceps–lateral gastrocnemius (LQG), medial quad-riceps–medial hamstrings (MQH), and medial quadriceps– medial gastrocnemius (MQG) using Equation 1 developed in our laboratory.27
| (1) |
in which i is the sample number and n is the number of data samples in the interval. This method accounts for both timing and magnitude of muscle activity at each time point in the interval. The resulting cocontraction curve was then averaged across three intervals: preparation (over the 100 ms prior to initial contact), weight acceptance, and midstance.
Statistical Analysis
Group means and standard deviations were compared between the groups with independent samples t-tests for all measures except those related to knee motion. Knee flexion and knee adduction excursions during weight acceptance, and knee extension excursion during midstance were analyzed with analysis of covariance (ANCOVA) using walking speed as a covariate (SPSS 13.0, SPSS Inc., Chicago, IL). Significance was established when p ≤ 0.05. We evaluated the relationships between disease severity and group assignment by determining the frequency of people with KL Grade II, III, or IV in each group using a chi-square test of independence.
Influences on Knee Motion
Knee stabilization strategies used by the groups were evaluated using separate stepwise linear regressions to evaluate the relationships between group assignment (OAS vs. OAU), muscle cocontraction, and sagittal plane knee motion excursion. Separate regression models were evaluated for weight acceptance and midstance intervals. First, the correlations between muscle cocontraction values and knee motion excursion during each interval were determined with Pearson's correlation coefficients (Table 2). Muscle cocontraction variables that correlated with knee excursions over weight acceptance and midstance were used in the regression models. The models used cocontraction variables and group assignment to predict knee excursion over each of the two intervals of the stance phase.
Table 2.
Correlations between Knee Motion and Muscle Cocontraction Variables
| Cocontraction Variables | r | p-value |
|---|---|---|
| Cocontraction and knee flexion excursion during preparatory phase | ||
| MQH (P) | –0.620 | 0.006 |
| LQH (P) | –0.554 | 0.014 |
| Cocontraction and knee extension excursion during Midstance | ||
| MQH (MS) | –0.559 | 0.016 |
| MQG (MS) | –0.784 | <0.001 |
| LQH (MS) | –0.687 | 0.001 |
| LQG (MS) | –0.811 | <0.001 |
Influence of Instability on Muscle Cocontraction
To evaluate the unique contribution of knee instability on muscle cocontraction we first determined which cocontraction variables were different between the groups. Those cocontraction variables were then used in separate hierarchical regressions were used to determine the contribution of quad-riceps MVIC, instability score, varus alignment, and knee laxity (independent variables) in predicting muscle cocontraction (dependent variables). Hierarchical regression is an incremental approach to multiple regression analysis that allows investigators to enter variables into the model in a specific order based on a priori hypotheses to assess the unique contribution of each independent variable in predicting the dependent variable. The order in which variables were entered into the regression model (MVIC, instability score, alignment, laxity) was based on our previous work demonstrating that quadriceps MVIC and instability score predicted the knee motion in people with medial knee OA.16
Spearman's Rho was used to investigate the relationships between instability reported during the lateral trials and muscle cocontraction variables that were found to be different between the groups.
RESULTS
Between the groups, there were no differences in medial laxity (p = 0.790), lateral laxity (p = 0.406), or quadricep strength (p = 0.309) (Table 1). The OAU group was in greater varus alignment (p = 0.008). There were no differences between groups in terms of severity of medial knee OA (chi-square = 1.818, p = 0.403). After experiencing the lateral trials, the OAU group also reported greater knee instability on visual analog scale than those who did not experience instability in their daily lives (OAU = 7.8±2.68, OAS = 9.8±0.42, p = 0.031).
Differences in Knee Motion
The OAU group walked more slowly than the OAS group (OAU = 1.34 m/s±0.17, OAS = 1.54±0.14, p = 0.009). In the sagittal plane, after adjusting for walking speed, no difference was observed in the knee flexion excursion during weight acceptance (OAU = 9.8°±4.2, OAS = 12.5°±5.4, p = 0.220) or knee extension excursion during midstance (OAU = 12.5°±5.8, OAS = 16.6°±8.0, p = 0.207). In the frontal plane, knee adduction excursion during weight acceptance was no different between the groups (p > 0.10).
Differences in Muscle Activation
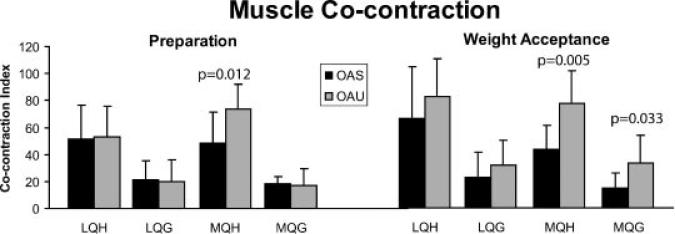
Despite relatively few differences in joint kinematics the perturbation elicited differences in muscle activation strategies. In preparation for the potential perturbation, the OAU group used higher MQH cocontraction compared to the OAS group (p = 0.012) (Fig. 2). The OAU group used higher medial muscle cocontraction (MQH and MQG) as the limb accepted weight (p = 0.005 and p = 0.033, respectively) (Fig. 2). During single limb support (midstance), it appeared that the OAU group continued to use higher MQH cocontraction, although this did not reach statistical significance (p = 0.086).
Figure 2.
Average muscle cocontraction during preparation (left) weight acceptance (right) (+standard deviation).
Influences on Knee Motion
Across all subjects higher muscle cocontraction correlated with less knee motion (Table 2). Regression analysis showed that medial muscle cocontraction (MQH) during preparation significantly predicted knee flexion excursion during weight acceptance (R2 = 0.389, p = 0.007) and an even stronger relationship was observed in midstance during which lateral (LQG) cocontraction predicted a significant portion of the variance in knee extension excursion (R2 = 0.671, p < 0.001). Group assignment did not significantly influence knee motion excursion in either regression.
Influence of Instability on Muscle Cocontraction
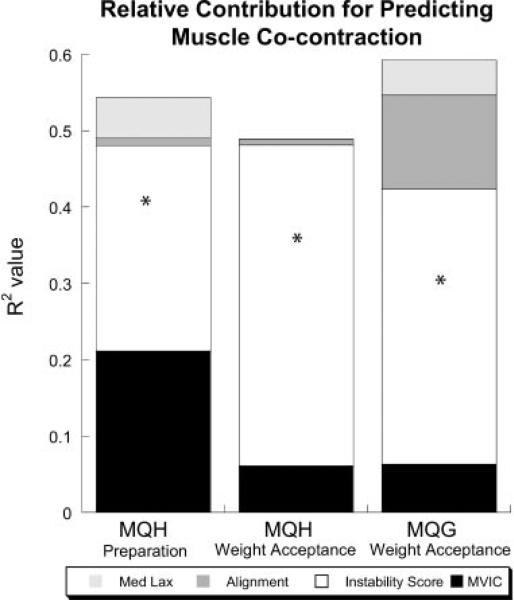
Prior to initial contact during the preparation phase, hierarchical regression showed that instability score (change in R2 = 0.269, p = 0.022) and quadriceps MVIC (change in R2 = 0.211, p = 0.074) contributed to MQH cocontraction (Fig. 3). During weight acceptance, instability score was the only variable with a unique contribution to medial muscle cocontraction (MQH: change in R2 = 0.420, p = 0.006, MQG: change in R2 = 0.360, p = 0.018) (Fig. 3).
Figure 3.
Results of hierarchial regression to predict cocontraction. Relative contribution (R2 value) of quadricepts MVIC, instability score, alignment, and medial laxity in predicting medial cocontraction during preparation and weight acceptance. The value of the segments of each bar represents the change in R2 value for each of the four independent variables. *Indicates that the addition of that independent variable to the model significantly increased the R2 value at p ≤ 0.05.
Instability experienced during the testing session did not correlate with MQH cocontraction in the preparation (r = –0.292, p = 0.256) or weight acceptance (r = –0.202, p = 0.436) phases, but did correlate with MQG co-contraction during weight acceptance (r = –0.500, p = 0.048).
DISCUSSION
The purpose of this investigation was to evaluate the influence of knee instability on movement and muscle activation patterns during a disturbed walking paradigm. Our previous work has shown that knee insta bility, a sensation that is common in people with medial knee OA, is not related to frontal plane joint laxity or limb alignment, and has an unfavorable affect on daily function.18 The current study is the first to find, as we hypothesized, that people with OA whose knees feel unstable activate their muscles differently than those whose knees feel stable. Despite relatively few differences in kinematic patterns, the difference in muscle activation underscores the importance of looking beyond joint kinematic and kinetics to investigate the mechanisms underlying the influence of knee instability on movement patterns in people with knee OA.
We used the instability score from the Knee Outcome Survey to classify subjects because it is a simple and reliable clinical measure of knee instability,22 and it represents a sensation that is experienced by patients during their everyday lives. The visual analog scale was used to record the experience of the subjects during the disturbed walking paradigm. The finding that the OAU subjects reported lower scores on the visual analog scale (indicating worse instability during the lateral trials) indicates that the lateral translation was effective in challenging knee control and was likely to elicit differences in movement and muscle activation patterns between the groups.
Indeed, the OAU group used higher muscle cocontraction in preparation for, during, and after the lateral translation despite no differences in knee motion excursions. Before and during weight acceptance, cocontraction between the quadriceps and hamstrings muscles is expected, as these muscles work together to control hip and knee motion.28 Thus, it was expected that cocontraction between the quadriceps and hamstrings muscles would significantly correlate with knee flexion motion during weight acceptance. However, it was somewhat surprising that subjects with instability used higher muscle cocontraction prior to contacting the ground yet they achieved knee flexion excursions comparable to OAS subjects. It is plausible that higher cocontraction used by the OAU subjects was used to control joint motions associated with knee instability that a motion capture system that relies on skin mounted markers cannot detect. Instability score was the only variable in the hierarchical regression that contributed significantly to predicting muscle cocontraction during preparation and weight acceptance. If a strategy of higher muscle cocontraction was successful in stabilizing the knee, then we would expect a significant relationship between cocontraction and instability that was experienced during the testing session that was not observed for MQH. The significant relationship between instability experienced during the testing session and MQG cocontraction during weight acceptance suggests that the cocontraction is an attempt to stabilize the knee. However, despite the higher levels of medial cocontraction, the OAU group continued to experience knee instability indicating that the strategy of higher MQG cocontraction is unsuccessful in achieving a sense of stability. This type of stabilization strategy may have important consequences on long-term joint integrity as cocontraction of antagonist muscles can increase joint compression.17
High muscle cocontraction, particularly on the medial side of the joint, coupled with abnormal arthrokinematics presumably associated with unstable knees can be particularly detrimental to articular cartilage due to the confluence of high compressive forces (from increased cocontraction) and excessive shear forces (due to repeated shifting or buckling in the knee).21,29 Cocontraction of strong quadriceps may lead to higher joint compression, which may explain why strong quadriceps have been associated with higher likelihood of OA progression.11
To the best of our knowledge, this study is the first to investigate how knee instability contributes to the altered muscle activation strategies observed in persons with knee OA; however, there are limitations to this study. We acknowledge the potential limitations of the small sample used in this investigation. The addition of more subjects would have improved power of the statistical analyses and may have identified more differences between the stable and unstable subjects. The addition of more subjects may also have enabled us to investigate instability on a continuum from mild to severe instability rather than the dichotomous grouping of stable and unstable. We also cannot make any inferences about how muscle cocontraction might influence joint contact forces, because EMG and force do not correlate during nonisometric contractions. Another limitation of this study is the effect of anxiety and/or fatigue experienced by our subjects. We provided practice trials and rest breaks for the subjects, and no subject reported fear or fatigue during the testing; however, both could have occurred, and may have influenced the results.
The findings from this preliminary investigation show the important influence of knee instability on muscle activation patterns around the knee and these findings warrant further investigation. We chose to investigate the influence of common factors that are typically altered in persons with medial knee OA; however, we acknowledge that there are other factors that may also contribute to muscle activation patterns. Nonetheless, our results suggest that people with knee OA and instability use higher magnitudes of medial muscle cocontraction, which appears to be an ineffective stabilization strategy. Verification of these results with larger sample sizes as well as longitudinal investigations of the impact of instability and high muscle cocontraction on joint function and OA progression should be the focus of future research.
ACKNOWLEDGMENTS
Funding was provided by the Foundation for Physical Therapy through the Promotion of Doctoral Studies Program (PODS II) and by NIH T32-HR7490 and NIH P20 RR16458.
REFERENCES
- 1.Carmona L, Ballina J, Gabriel R, et al. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis. 2001;60:1040–1045. doi: 10.1136/ard.60.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannus P, Jarvinen M, Kontiala H, et al. Occurrence of symptomatic knee osteoarthrosis in rural Finland: a prospective follow up study. Ann Rheum Dis. 1987;46:804–808. doi: 10.1136/ard.46.11.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 4.Thomas RH, Resnick D, Alazraki NP, et al. Compartmental evaluation of osteoarthritis of the knee. A comparative study of available diagnostic modalities. Radiology. 1975;116:585–594. doi: 10.1148/116.3.585. [DOI] [PubMed] [Google Scholar]
- 5.Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am. 1994;25:395–403. [PubMed] [Google Scholar]
- 6.Baliunas AJ, Hurwitz DE, Ryals AB, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz DE, Ryals AB, Case JP, et al. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20:101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 8.Hurley MV, Scott DL. Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. Br J Rheumatol. 1998;37:1181–1187. doi: 10.1093/rheumatology/37.11.1181. [DOI] [PubMed] [Google Scholar]
- 9.Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359–3370. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- 10.Miller ME, Rejeski WJ, Messier SP, et al. Modifiers of change in physical functioning in older adults with knee pain: the Observational Arthritis Study in Seniors (OASIS). Arthritis Rheum. 2001;45:331–339. doi: 10.1002/1529-0131(200108)45:4<331::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Sharma L, Dunlop DD, Cahue S, et al. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138:613–619. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Sharma L, Lou C, Felson DT, et al. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42:861–870. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Wada M, Imura S, Baba H, et al. Knee laxity in patients with osteoarthritis and rheumatoid arthritis. Br J Rheumatol. 1996;35:560–563. doi: 10.1093/rheumatology/35.6.560. [DOI] [PubMed] [Google Scholar]
- 14.Childs JD, Sparto PJ, Fitzgerald GK, et al. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech (Bristol, Avon) 2004;19:44–49. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:745–751. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt LC, Rudolph KS. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arthritis Rheum. 2007;57:1018–1026. doi: 10.1002/art.22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodge WA, Fijan RS, Carlson KL, et al. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci USA. 1986;83:2879–2883. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51:941–946. doi: 10.1002/art.20825. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt L. Biomechanics and movement science program. University of Delaware; Newark, DE: 2006. Knee stabilization and medial knee osteoarthritis. [Google Scholar]
- 20.Herzog W, Adams M, Matyas J, et al. A preliminary study of hindlimb loading, morphology, and biochemistry of articular cartilage in the acl-deficient cat knee. Osteoarthritis Cartilage. 1993;1:243–251. doi: 10.1016/s1063-4584(05)80330-9. [DOI] [PubMed] [Google Scholar]
- 21.Setton LA, Mow VC, Howell DS. Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament. J Orthop Res. 1995;13:473–482. doi: 10.1002/jor.1100130402. [DOI] [PubMed] [Google Scholar]
- 22.Irrgang JJ, Snyder-Mackler L, Wainner RS, et al. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80:1132–1145. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Hsu RW, Himeno S, Coventry MB, et al. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop. 1990;255:215–227. [PubMed] [Google Scholar]
- 24.Moore TM, Meyers MH, Harvey JP., Jr Collateral ligament laxity of the knee. Long-term comparison between plateau fractures and normal. J Bone Joint Surg Am. 1976;58:594–598. [PubMed] [Google Scholar]
- 25.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19:861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Delagi E, Iazzetti J, Perotto A, et al. Anatomic guide for the electromyographer, the limbs. Charles C. Thomas; Springfield, IL: 1981. [Google Scholar]
- 27.Rudolph KS, Axe MJ, Buchanan TS, et al. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 2001;9:62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 28.Perry J. Normal and pathological function. McGraw-Hill; New York: 1992. Gait analysis. [Google Scholar]
- 29.Wu JZ, Herzog W, Epstein M. Joint contact mechanics in the early stages of osteoarthritis. Med Eng Phys. 2000;22:1–12. doi: 10.1016/s1350-4533(00)00012-6. [DOI] [PubMed] [Google Scholar]