Abstract
Background and aims
Coeliac disease (CD) is triggered by an abnormal reaction to gluten. Peptides resulting from partially digested gluten of wheat, barley or rye cause inflammation of the small intestinal mucosa. Previous contradictory studies suggest that oats may trigger the abnormal immunological response in patients with CD. Monoclonal antibodies (moAbs) against the main immunotoxic 33-mer peptide (A1 and G12) react strongly against wheat, barley and rye but have less reactivity against oats. The stated aim of this study is to test whether this observed reactivity could be related to the potential toxicity of oats for patients with CD.
Methods
In the present study, different oat varieties, controlled for their purity and by their distinct protein pattern, were used to examine differences in moAb G12 recognition by ELISA and western blot. Immunogenicity of oat varieties was determined by 33-mer concentration, T cell proliferation and interferon γ production.
Results
Three groups of oat cultivars reacting differently against moAb G12 could be distinguished: a group with considerable affinity, a group showing slight reactivity and a third with no detectable reactivity. The immunogenicity of the three types of oats as well as that of a positive and negative control was determined with isolated peripheral blood mononuclear T cells from patients with CD by measurement of cell proliferation and interferon γ release. A direct correlation of the reactivity with G12 and the immunogenicity of the different prolamins was observed.
Conclusions
The results showed that the reactivity of the moAb G12 is proportional to the potential immunotoxicity of the cereal cultivar. These differences may explain the different clinical responses observed in patients suffering from CD and open up a means to identify immunologically safe oat cultivars, which could be used to enrich a gluten-free diet.
Keywords: Coeliac disease, oats, moAb, T cells
Significance of this study.
What is already known about this subject?
The safety of oats in a gluten-free diet (GFD) has been a topic of debate for several years. At the nutritional level, oats are an important source of proteins, fat, vitamins, minerals, and fibres, and could therefore be beneficial for people with coeliac disease (CD). In addition, the palatability of oats and their wide availability could also contribute to the acceptability of a diet without wheat, barley and rye.
The difference in type of oats used, the purity and study design have not allowed a clear answer as to whether oats are safe or not for all patients with CD. Current beliefs are that pure oats are safe for most people with CD, and contamination with other cereal sources is the main problem facing people with CD.
What are the new findings?
In this study we show, using a new antibody raised against the toxic fragment, that oat immunogenicity for patients with CD varies according to the cultivars. We could distinguish three groups of oat cultivars reacting differently against the antibody: a group with considerable affinity, a group showing slight reactivity and another with no detectable reactivity.
The potentially immunotoxicity of the three types of oats was determined by T cell proliferation and interferon γ release. We have proved that the reactivity of this antibody with cereal storage proteins of different varieties of oats was correlated with the immunotoxicity of the dietary grains.
This gives a rational explanation for why only some oats trigger an immunological response.
Significance of this study.
How might it impact on clinical practice in the foreseeable future?
The incorporation of some varieties of oats in food products not only may improve the nutritional quality but may provide a treatment for various illnesses and would be welcomed by patients with CD. Our work may explain the apparently contradictory previous studies relating to the safety of oats for patients with CD. Our study provides new insights about the oat dilemma in CD and suggests practical methods to select tolerable varieties of oats for patients with CD.
Our results suggested that the specific antibody is a reliable tool for detecting varieties of oats potentially safe for patients with CD, permitting the enrichment of a GFD. This work should also be taken into consideration in food safety regulations, in particular labelling of gluten-free products that may contain oats. The design of clinical research about oat effect on patients with CD should consider this study to remark the importance of the oat source.
Introduction
Coeliac disease (CD) is defined as an alteration of the mucosa of the proximal small intestine, associated with a permanent intolerance to gluten. Currently, the only effective treatment is a strict gluten-free diet (GFD).1 The main immunogenic peptides of gluten belong to a family of closely related proline- and glutamine-rich proteins called prolamins. CD is triggered by peptides resulting from the fragmentation of prolamins, which are not digested by human proteases.
The identification of a 17 amino acid peptide corresponding to the partially deamidated peptide of A-gliadin amino acids 57–73 and of the 33-mer peptide of α-gliadin residues 57–89 contributed to the demonstration that the highly antigenic gluten epitopes are located in the proline-rich regions of gliadin.2–5 The 33-mer immunogenic peptide in other prolamins such as hordein and secalin has been demonstrated.6 This oligopeptide is usually the most bioactive gluten peptide recognised by gluten-specific T cell lines from HLA-DQ2+ CD donors; however, it is not the only gluten peptide sequence which is harmful for patients with CD and, furthermore, patients with CD may react against glutenins found in these cereals.4 Attempts to demonstrate 33-mer peptides in oats have not been successful yet, although some patients with CD have HLA-DQ2-reactive mucosal T cells that recognise peptides in oats which may cause mucosal inflammation.7
The presence of oats in a GFD is still a subject of debate. Oats differ from other cereals in their prolamin content. The percentage of proline and glutamine (amino acids abundant in toxic regions) in avenin is lower than in other toxic cereals. Some clinical researchers state that patients with CD tolerate oats without signs of intestinal inflammation.8–10 Several countries permit the use of oats in ‘gluten-free’ products—for example, Gluten Free Oats. According to the Codex Standard for food for special dietary use for persons intolerant to gluten, CODEX STAN118-1979 (revised 2008, http://www.codexalimentarius.net/web/more_info.jsp?id_sta=291), oats can be tolerated by most but not all people who are intolerant to gluten. Therefore, whether or not oats that are not contaminated with wheat, rye or barley in foods covered by this standard can be allowed may be determined at the national level. Moreover, according to the Commission Regulation (EC) No 41/2009 (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:016:0003:0005:EN:PDF) concerning the composition and labelling of foodstuffs suitable for people intolerant to gluten, a major concern is the contamination of oats with wheat, rye or barley that can occur during grain harvesting, transport, storage and processing. Therefore, the risk of gluten contamination in products containing oats should be taken into consideration with regrad to labelling of those products. Some cross-reactivity with gliadin-specific antibody has been attributed to wheat contamination in oat-based food.10
In contrast, other studies confirmed the toxicity of oats in certain types of patients with CD. Arentz-Hansen et al7 described the intestinal deterioration suffered by some patients with CD following the consumption of oats while on a GFD. Avenin can trigger an immunological response in these patients similar to the response produced by the gluten of wheat, rye or barley. The monitoring of 19 adult patients with CD who consumed 50 g/day of oats over 12 weeks showed that one of the subjects was sensitive to oats.11 Therefore, it is critical to clarify either qualitatively or quantitatively the potential immunotoxicity of oats to patients with CD.7 10
In a previous work, we obtained two monoclonal antibodies (moAbs) G12 and A1 against the 33-mer.12 Our results suggested that the reactivity of these moAbs was correlated with the potential immunotoxicity of the dietary grains from which the proteins were extracted.6 13 These antibodies are able to recognise with great sensitivity peptides (besides the 33-mer peptide) immunotoxic for patients with CD. The sensitivity and epitope preferences of these antibodies were found to be useful for detecting gluten-relevant peptides to infer the potencial toxicity of food for patients with CD.
The moAb G12 showed cross-reactivity that served to detect a certain amount of oat avenins, although with lower sensitivity than hordein, gliadin and secalin.12 The oat controversy appeared to be a unique case to explore that issue, because cases of intermediated immunotoxicity could be expected and correlation of potential toxicity and analytical signals could be assessed.
With this background, the aim of the present study was to determine whether the most sensitive antibody for 33-mer, G12, could be used to identify varieties of oats that are potentially toxic for patients with CD by establishing a possible correlation between the reactivity of the different varieties and the capacity to activate coeliac T cells from patients with CD.12
We have shown that the reactivity of moAb G12 with cereal storage proteins of different varieties of oats was correlated with the immunotoxicity of the dietary grains from which the proteins were extracted. We showed that the intensity of the signal obtained with this antibody was proportional to the responses in terms of cell proliferation and interferon γ (IFN-γ) release. Our results suggested that the specific moAb G12 is a reliable tool for detecting oat varieties potentially safe for patients with CD, permitting the enrichment of a GFD.
Materials and methods
Plant materials
Oats (Avena sativa L.) from different cultivars, accessions OM719, OE717, OL715, OA729, OH727, OC723, OF720, OR721 and OP722, were obtained from Spanish and Australian commercial sources. Rice (J. Sendra variety, Spain) and wheat (Triticum durum, Don Pedro variety, Spain) were used as a negative and positive control, respectively.
Preparation of prolamin solutions
Oat and rice flours were prepared by grinding the kernels of the oat varieties and rice, and extracted as described in Cornell et al.14 Gliadin (Sigma, St Louis, Missouri, USA) was prepared in 60% (v/v) ethanol.
33-mer peptide and antigliadin 33-mer moAb
The 33-mer peptide, the G12 moAb and its derived horseradish peroxidase (HRP)-conjugated moAb (G12–HRP) were supplied by Biomedal (Sevilla, Spain).
DNA extractions and PCR amplification
Extraction of DNA was performed using a modified cetyltrimethylammonium bromide (CTAB) method. DNA concentrations were determined by UV absorption. The purity of the DNA solution was assessed by the 260/280 nm absorption ratio.
Oligonucleotides used in this work were provided by Biomedal and are listed in Supplementary table S1. PCR (Biotools B&M Labs, Madrid, Spain) was performed following the manufacturer's protocol.
Competitive ELISA
We used R5 ELISA according to the supplier's instructions RIDASCREEN kit (Gliadin competitive R-Biopharm AG, Darmstadt, Germany). MoAb G12 competitive ELISA was carried out according to Morón et al.12
Protein analysis by sodium dodecyl sulfate–polyacylamide gel electrophoresis (SDS–PAGE) and western blotting
SDS–PAGE and immunoblotting were performed under standard conditions. SDS–PAGE was prepared with 12.5% acrylamide. Proteins in the gel were stained with silver staining or transferred to a polyvinylidene fluoride (PVDF) membrane. The PVDF membranes were incubated with G12. After washing, antimouse immunoglobulin G (IgG) phosphatase antibody (Sigma) was added.
Matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS) analysis
The MALDI-TOF MS analysis was performed as described by Hernando et al.15 Identification of avenin by MALDI-TOF MS showed characteristic protonated mass patterns.
Peptic–trypsic–chymotrypsin digestion
The alcohol-soluble protein fraction was extracted from whole flour and subjected to peptic, trypsic and chymotrypsin sequential digestion according to Ehren et al.13
Transglutaminase deamidation
The avenin peptides of the different oat varieties as well as the gliadin and oryzein control were treated with guinea pig liver tissue transglutaminase (tTG) (Sigma) in the presence of 2 mM CaCl2, at 37°C for 4 h.16
Histological and serological analysis of subjects
Ten patients with biopsy-proven active CD were included in this study and five healthy patients were considered as the control group. The diagnosis of CD in the patients was determined by serological screening tests accompanied by biopsy of the small intestine according to the criteria of Marsh17 and confirmation of a clinical response to gluten elimination from the diet. Subjects were prospectively screened for CD using antiendomysial antibodies, antigliadin antibodies, tTG antibodies and CD-specific HLA (human leucocyte antigen) typing (table 1). Venous blood was taken at the time of index biopsy. The healthy control patients were serologically negative for antiendomysial, antigliadin and tTG antibodies. The study was approved by the ethic committee of the ‘Virgen de las Nieves’ Hospital, Granada (Spain), and informed consent was obtained.
Table 1.
Clinical data of patients with coeliac disease
| Patient | Sex | Age (years) | Atrophy grade (Marsh criteria) | AAEM | AATG | HLA-DQB1 | HLA-DRB1 |
| Coeliac 1 | Male | 6 | III b | + | 252 | 0201–0202 | 3–7 |
| Coeliac 2 | Female | 3 | III a | + | 20 | 0201–0202 | 3–7 |
| Coeliac 3 | Male | 13 | III b | – | 2 | 0202–0301 | 7–11 |
| Coeliac 4 | Female | 5 | III b | + | 102 | 0201–0604 | 3–11 |
| Coeliac 5 | Female | 9 | III b | + | 139 | 0201–0202 | 3–7 |
| Coeliac 6 | Female | 2 | III b | + | 15 | 0302–0301 | 4–4 |
| Coeliac 7 | Female | 4 | III b | + | 28 | 0201–0501 | 1–3 |
| Coeliac 8 | Female | 7 | II | + | 111 | 0201–0202 | 3–7 |
| Coeliac 9 | Male | 10 | III b | + | 165 | 0202–0301 | 7–11 |
| Coeliac 10 | Male | 5 | III b | + | 118 | 0201–0202 | 3–7 |
AAEM, antiendomysial antibody; AATG, antitransglutaminase antibody, expressed as U/ml; HLA, human leucocyte antigen.
Peripheral blood mononuclear cells (PBMCs) and cell cultures
PBMCs from patients with active CD on a gluten-containing diet were isolated from 6 ml of heparinised blood by Histopaque gradient centrifugation and cultured at a density of 1×106 cells/ml in RPMI-1640 culture medium. After 48 h, PBMCs were incubated with avenin, gliadin and oryzein peptides (50 μg/ml).
Cell proliferation analysis
T cell proliferation was determined after 48 h of incubation using the ELISA 5-bromo-2-deoxyuridine cell proliferation test (Millipore Chemicon, Temecula, California, USA). The stimulation index (SI) value was calculated by dividing the mean absorbance/10 at 450 nm after stimulation by the mean absorbance of T cells exposed to the culture medium alone (negative control) and divided by 10. The proliferation of PBMCs exposed to the different fragments of avenins was expressed as the mean fluorescent intensity 48 h after exposure.
IFN-γ production
Supernatants from PBMC culture were collected after 48 h and stored at −80°C for IFN-γ determination using a commercial ELISA kit in accordance with the manufacturer's instructions (Thermo Scientific, Madrid, Spain). Standards were run on each plate. The sensitivity of the assay was <2 pg/ml.
Statistical analysis of T cell and IFN-γ assays
Each experiment was carried out in duplicate on separate days. Data are expressed as mean±SD. All statistical analysis was performed with the STATGRAPHICS program. When the interaction was significant, the differences between groups were examined by one-factor analysis of variance (ANOVA). A Bonferroni-corrected Student t test was used to compare the individual means. A statistical probability of p<0.05 was considered significant.
Results
Determination of the purity of oat samples
In the present study, the purity of the oat samples was carefully controlled, first by a visual examination to isolate individual grains and avoid the presence of grains from other cereals, and secondly by PCR18 for the detection of different cereal DNAs (wheat, rye and barley) in oat samples.
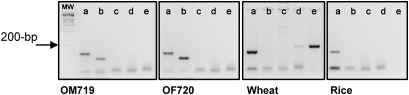
To detect cereal contamination (wheat, rye and barley) in the different oat varieties, specific target sequences encoding fragments of gliadin, secalin, hordein and avenin were chosen for amplification. The amplicon length of the different cereals varied from 104 bp for oats, to 164 bp for barley and 181 bp for wheat and rye. In these experiments, negative results were obtained with the PCR specific for wheat, rye and barley in oat samples; however, positive results were obtained when the oat- and 18S-specific primers were used (figure 1). Wheat amplification showed positive results with the PCR specific for 18S and gliadin, and rye DNA was present in lower amounts in this sample. As shown in figure 1, the analysis of the DNA amplification products by agarose gel electrophoresis confirmed that none of the oat samples was contaminated with wheat, barley, rye or mixtures of these cereals.
Figure 1.
Evaluation of the presence of amplifiable DNA in oat varieties, wheat and rice by PCR. An ethidium bromide-stained agarose gel with oat, barley, rye and wheat PCR amplification products of two representative oat samples and other cereals (wheat and rice). MW, DNA molecular weight marker. Positive controls: DNA of these cereals amplified with 18S primers. The corresponding primers used were: (a) 18S; (b) ω-avenin; (c) ω-hordein; (d) ω-secalin; and (e) ω-gliadin. A representative example from the two biological replicates performed is shown.
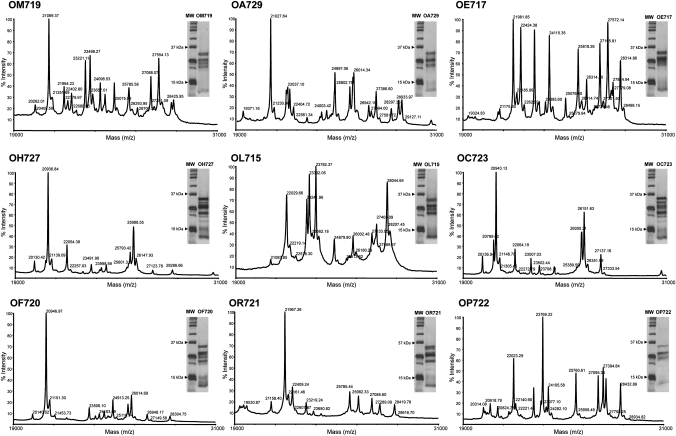
Analysis of avenin variability of the oat cultivars by MALDI-TOF MS and SDS–PAGE
We used MALDI-TOF MS to analyse the prolamin fractions in oats, and to find ‘molecular signatures’ to identify and characterise the different oat varieties. The spectra of the avenin fractions of the different oat varieties obtained by MALDI-TOF MS and the electrophoretic distribution of the prolamins by SDS–PAGE revealed distinct patterns that could allow their further identification (figure 2). In both MALDI-TOF MS and SDS–PAGE, the distribution of molecular weights ranged between 19 and 31 kDa, in agreement with previous work describing avenins.18 19 The number and relative intensity of the peaks obtained by MALDI-TOF MS in the nine oat cultivars were very variable, indicating that oats differ in their avenin complement. Variability in protein length and distribution was also evident by SDS–PAGE. These results indicate the presence of different prolamin subunits in the oat varieties, which differ in both their amino acid composition and length.
Figure 2.
Comparison of avenin fractions extracted from the oat varieties studied. Avenin spectra were determined by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of the nine oat varieties. Analysis of proteins extracted from the oat varieties by sodium dodecyl sulfate–polyacrylamide gel electrophoreis (SDS–PAGE). MW, protein molecular weight marker.
Relative affinity of the moAb G12 for different varieties of oats
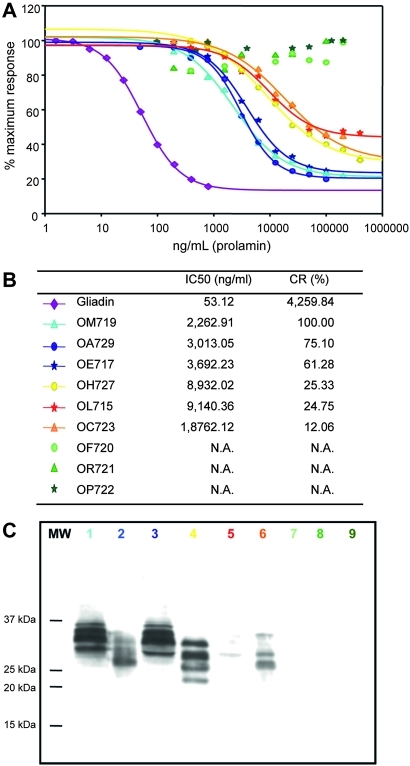
To determine whether the moAb G12 had distinct reactivity to the different oat varieties, the affinity of the moAb G12 was determined by competitive ELISA. The oat varieties tested showed different affinities for the moAb G12 (figure 3A). Therefore, three groups of oat varieties could be clearly distinguished depending on their recognition by the moAb G12: a group with high affinity towards the antibody (OM719, OA729 and OE717), a group with intermediate recognition (OH727, OL715 and OC723) and another group comprising oats that were not recognised by moAb G12 (OF720, OR721 and OP722). The alternative anti-33mer moAb A1 also provided equivalent results (data not shown).
Figure 3.
Relative affinity of the monoclonal antibody (moAb) G12 for different oat varieties and gliadin. (A) Competitive ELISA using the anti-33-mer horseradish peroxidase-conjugated G12 to determine the relative affinity of this antibody for the different varieties of oat. Three assays were performed, with three replicates of each. (B) IC50 and CR of the different oat varieties. N.A., not applicable. (C) Western blot analysis of toxic fractions from different oat prolamins. Membranes were stained with the moAb G12. The colour code for labelling the varieties is the same as that used in A and B. MW, protein molecular weight marker.
In order to quantify the affinity of the oat varieties for the moAb G12, the IC50 and the CR were determined for each variety (figure 3B). The IC50 is defined as the concentration that produces a reduction of 50% in the peak signal in the ELISA. The CR was determined as (IC50 of the oat variety that presents the greatest affinity for the antibody/IC50 of each variety assayed)×100. Varieties OE717 and OA729 showed a CR of ∼60% and 75%, respectively, with respect to the most sensitive variety (OM719). Varieties OH727, OL715 and OC723, with a CR of 25, 24 and 12%, respectively, were recognised by the moAb G12, but with a lower sensitivity. The avenins of OF720, OR721 and OP722 were not recognised by the moAb G12, as was the case for the negative control (rice).
In order to confirm the ELISA results with another immunological technique and to identify the protein pattern with cross-reactivity to the anti-33-mer antibody, immunoblotting electrophoresis analyses were performed. The results (figure 3C) showed that the moAb G12 had affinity for the varieties OM719, OA729, OE717, OH727, OL715 and OC723. However, the antibody did not react with the varieties OF720, OP722 and OR721. The variability in reactivity demonstrated by western blot thus correlated with the previously presented ELISA results. These results suggested the presence of different prolamin subunits in the oat varieties, differing in both their amino acid composition and length.
Another antibody specific for the sequence QQPFP (R5) was used to analyse the different oat varieties. The results obtained with R5 demonstrate that this antibody, like G12, was able to detect the same six oat varieties as the anti-33-mer antibodies. In the varieties OM719, OA729, OE717 and OH727 the values for the peptide QQPFP ranged between 25 180 and 97 860 μg of peptide per gram of cereal, which according to the instructions of the supplier (Gliadin competitive R-Biopharm AG, Darmstadt, Germany) would correspond to values >100 ppm of gliadin. The varieties OL715 and OC723 were also recognised by the R5 antibody, although with lower sensitivity than the aforementioned varieties. In contrast, the varieties OF720, OR721 and OP722 presented levels of gliadin below 5 ppm. Therefore, the results obtained with R5 were similar to those reported in this work with the anti-33-mer antibodies. In order to avoid false positives, the purity of the samples was monitored molecularly. Earlier works stated that the reactivity of R5 against certain foods prepared with oats is due to cross-contamination with wheat, barley or rye, rather than a direct recognition of epitopes of this antibody in the oat prolamins.18 However, the results obtained in this work clearly demonstrated that R5 was able to recognise, although with differing affinity, different oat varieties of verified purity.
Determining the concentration of immunoreactive peptides in oats
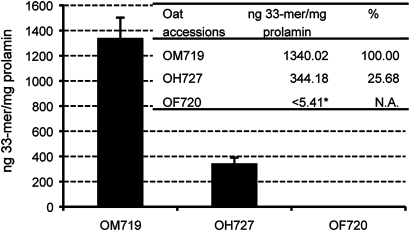
To evaluate the relative amount of immunotoxic epitopes present in the prolamins of different oat varieties, one variety was chosen from each of the three groups previously identified for their affinity towards the moAb G12. Thus, OM719 represented the group with greatest affinity towards the moAb G12, OH727 those of intermediate reactivity and OF720 those not recognised by this antibody. The presence of immunoreactive peptides was determined by the moAb G12 competitive ELISA, using the 33-mer peptide as the standard curve. The presence of 33-mer and close analogues in the most reactive oat variety, OM719, was of the order of 1340 ng/mg of avenin (figure 4). In OH727, the levels of 33-mer were some fourfold lower than in OM719. However, in the case of OF720, the concentration of 33-mer was reduced >1300-fold with respect to OM719, reaching levels undetectable by this method. This result was consistent with earlier results obtained using IC50 and CR, and by western blot. At the same time, these results indicated the enormous difference between some varieties regarding the presence of sequences that are immunoreactive for patients with CD.
Figure 4.
Detection of the concentration of the 33-mer peptide in different oat varieties. The concentration of 33-mer was determined by competitive ELISA using the monoclonal antibody (moAb) G12 conjugated to horseradish peroxidase. Different independent dilutions were tested for each oat variety, each with three repetitions. The percentage of 33-mer of the variety assayed with respect to that of the most reactive variety (OM719). *The concentration of the 33-mer was lower than the limit of quantification of the competitive ELISA for the detection of 33-mer (5.4 ng/ml). N.A., not applicable.
Correlation between moAb G12 reactivity and immunogenicity of different oat varieties
To determine whether the variations in the reactivity of the anti-33-mer G12 in the different oat varieties were correlated with the greater or lesser immunogenicity of the cereal, we directly challenged the cereal extracts with relevant cells obtained from patients with CD. The clinical and immunological characteristics of patients with CD are presented in table 1.
Immunogenicity was determined by T cell proliferation and IFN-γ production. Three cultivars of oats were selected; one variety was chosen from each previously identified group (OM719, OH727 and OF720). We tested whether there was a correlation between their potential immunotoxicity for patients with CD and their reactivity with the moAb G12.
The avenin of the oat varieties, gliadin and oryzein were subjected to peptic, trypsic and chymotrypsin sequential digestion and treated with tTG.
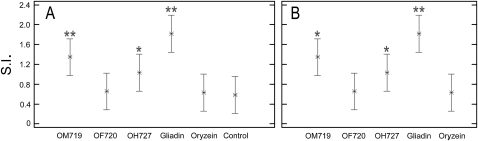
Cell proliferation and IFN-γ release in the culture medium were measured as indexes of lymphocyte activation. We found a significant increase in the T cell proliferation in cultures incubated with OM719 and gliadin (SI=1.3±0.7 and 1.8±0.9, respectively), and also with OH727 (SI=1.02±0.5). These results clearly showed that gliadin and OM719 displayed the highest activity, being the most potentially immunogenic (figure 5). The incubation with OF720 increased cell proliferation (SI=0.65±0.4) similar to incubation with oryzein (SI=0.62±0.3). We included the values presented in healthy patients (control) as reference values to compare the effect of peptides in patients with CD under the same conditions of cell culture.
Figure 5.
Proliferative responses of T cells to deamidated peptides of prolamin from three different oat varieties. Peripheral blood mononuclear cells were stimulated by tissue transglutaminase-treated prolamin digest for 48 h. Gliadin and oryzein were used as the positive and negative control, respectively. The experiments were performed in duplicate and the mean stimulation index (SI) ±SD is shown. The SI values of T cells exposed to prolamin digests were statistically significant with respect to (A) the control (healthy patients) and (B) oryzein. *p<0.05; **p<0.005.
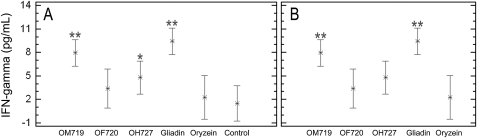
Release of IFN-γ in the culture medium after the exposure of coeliac peripheral T lymphocytes to deamidated avenin peptides was assessed (figure 6). According to this assay, gliadin and prolamins from OM719 were very immunogenic, with the highest values of IFN-γ release (9.4±0.76 and 7.9±0.57 pg/ml, respectively), whereas the exposure to OH727 induced a lower mean value of IFN-γ (4.8±0.95 pg/ml). Finally, OF720 and oryzein were the least immunogenic (3.4±1.09 and 2.3±0.89 pg/ml, respectively).
Figure 6.
Interferon γ (IFN-γ) production by T cells with prolamin digests from three different oat varieties. T lymphocytes were stimulated with digested prolamins after treatment with tissue transglutaminase. IFN-γ production was evaluated by ELISA after 48 h of incubation. The results are shown as the means of duplicate wells and expressed as pg/ml. Gliadin and oryzein were used as the positive and negative control, respectively. Significant with respect to (A) healthy controls and (B) oryzein. *p<0.05; **p<0.005.
Discussion
In this work, we have shown that there is a wide range of reactivity of different oat cultivars to the anti-33-mer G12.12 Furthermore, the reactivity of isolated coeliac T cells to three different oat varieties ranging from none to maximal moAb G12 recognition showed a direct correlation of the T cell reactivity and moAb G12 reactivity. Traditionally, treatment with a GFD has excluded wheat, barley, rye and also oats.20 However, there is still some debate about the safety of oats.10 The safety of oats in individuals with CD has been widely investigated.10 Several in vivo and in vitro studies indicated that the majority of patients with CD could tolerate moderate amounts of pure oats.8 10 21 In contrast, other authors showed clear evidence suggesting that avenins have the ability to induce the activation of mucosal T cells, causing gut inflammation and villous atrophy.7 11 22 23 These results suggested that intolerance to oats exists in some patients with CD.7
Comparison of the different studies are complicated by the different study designs, the different conditions used in the testing, the number of subjects included in each study and the reporting of the purity control of the oat material used in the clinical trials. Another relevant factor in different designs is the absence of information on the oat variety used. Silano et al24 investigated the immunogenic effect of avenins from four oat cultivars using peripheral lymphocytes from patients with CD. The results obtained in this work show that there are in fact differences in immunogenicity in the oat varieties studied. In contrast to those authors, we found that some varieties were not reactive against the T cells of patients with CD.
In our work, first we have examined whether the low but significant reactivity of the moAb G12 to oats was due to any potential cross-contamination, as suggested elsewhere, by PCR analysis, and secondly, we have studied the importance of the cultivar in moAb G12 recognition.18 For this study, the purity of the oat samples was carefully controlled and shown to be free from contamination by other cereals that was reported as an intermittent problem that could potentially skew trial results.
Three oats groups could be distinguished based on their recognition by moAb G12: a group with considerable affinity towards the antibody, a group with moderate–low recognition and another group that was not recognised at all by the antibody. We confirmed these results by MALDI-TOF, SDS–PAGE and western blot by showing that the number, relative intensity of the peaks and protein profile obtained for the nine oat varieties differ from one another.
A direct correlation of the reactivity with moAb G12 and the immunogenicity of the different prolamins was observed by in vitro studies. In fact, prolamin peptides from OM719, and to a lesser degree OH727, could be potentially immunotoxic, whereas those from OF720 proved to be non-active in triggering both T cell proliferation and IFN-γ release. These results could also explain previous contradictory results described elsewhere relating to the potential oat toxicity for patients with CD. Future experiments based on the method described by Anderson et al2 and Tye-Din et al4 will be carried out. The next logical set of experiments would involve an acute 3 day oat challenge of patients on a GFD, with blood sampling at 6 days.
It is worth mentioning that gliadin was at least 40- to 400-fold more reactive to moAb G12 than any of the reactive tested avenins. Catassi et al25 demonstrated that the ingestion of contaminated gluten should be kept lower than 50 mg/day in the treatment of CD. Based on the reactivity of the G12 antibody against the different oat varieties and gliadin and on the results published by Catassi et al,25 the tolerance to the most toxic oats might be in the range of 2–20 g/day. Based on previous literature it appears that the amount of pure oats considered within the range of safe limits is a minimum of 20 g/day.10 Therefore, the limits for potential immunotoxicity inferred from moAb G12 oat reactivity were of the same order of magnitude as clinical assays with no consideration of the intrinsic potential of the oat cultivar. Furthermore, the oat varieties identified as non-immunogenic in T cell assays and by moAb G12 could be proposed for their use as well-tolerated cereals recommended for a GFD.
The interest in oats for human consumption has increased in recent years because of the widely recognised nutritional and health benefits of this cereal.10 The incorporation of some oat varieties in food products may not only improve the nutritional quality but could act also a treatment for various illnesses.
By dissecting the reactivity of moAb G12 to different oat cultivars and its correlation in biological assays with T cells, we have shown that there is a wide range of variation of potential immunotoxicity of oat cultivars. We also demonstrated that there is no strict correlation between total gluten amount and potential immunotoxicity due to the fact that some 33-mer analogue epitopes may be much less immunogenic and require higher doses to provoke an equivalent toxic effect. Our observations suggest that moAb G12-based immunotechniques appear to be a pragmatic method to evaluate potential immunotoxicity. Our approach offers a new tool to conduct proper clinical trials in order to confirm the in vitro results.
Acknowledgments
The authors are grateful to Dr Amado Salvador Peña and Sandra van Zeeland for their helpful discussions and critical reading of the manuscript. We thank Catherine N. Torgler for comments on the manuscript. We are grateful to Dr Marco Silano for his expert advice and to Manuel Márquez for his help in the design of the figures. We also thank Dr Manuel Gómez for his advice regarding the plant materials.
Footnotes
Funding: This work was supported by Asociación de Celíacos de Madrid (to SC) by grants PET2008_0055 from VI Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (Ministerio de Ciencia e Innovación), IAB (Instituto Andaluz de Biotecnología) (to SC) and by AGR2009-4966M (Proyecto de Excelencia, Junta de Andalucía) (to TM). Biomedal thanks Corporación Tecnológica de Andalucía and Agencia IDEA for co-founding this study (to CA).
Competing interests: None.
Patient consent: Obtained.
Ethics approval: This study was conducted with the approval of the Hospital Virgen de las Nieves, Granada, Spain.
Contributors: Conceived and designed the experiments: CI, TM, CA, SC. Performed the experiments: CI, RA, LL, LAM, BF, LP. Analysed the data: CI, LL, TM, CA, SC. Contributed reagents/materials/analysis tools: CH, LAM, BF, CA, CS. Wrote the paper: CI, LL, BF, TM, CA, SC.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bethune MT, Khosla C. Parallels between pathogens and gluten peptides in celiac sprue. PLoS Pathogens 2008;4:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RP, Degano P, Godkin AJ, et al. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med 2000;6:337–42 [DOI] [PubMed] [Google Scholar]
- 3.Shan L, Molberg Ø, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science 2002;297:2275–9 [DOI] [PubMed] [Google Scholar]
- 4.Tye-Din JA, Stewart JA, Dromey JA, et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med 2010;2:41ra51. [DOI] [PubMed] [Google Scholar]
- 5.Shan L, Qiao SW, Arentz-Hansen H, et al. Identification and analysis of multivalent proteolytically resistant peptides from gluten: implications for celiac sprue. J Proteome Res 2005;4:1732–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morón B, Bethune M, Comino I, et al. Toward the assessment of food toxicity for celiac patients: characterization of monoclonal antibodies to a main immunogenic gluten peptide. PloS One 2008;3:e2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arentz-Hansen H, Fleckenstein B, Molberg Ø, et al. The molecular basis for oat intolerance in patients with celiac disease. PLoS Medic 2004;1:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janatuinen EK, Pikkarainen PH, Kemppainen TA, et al. A comparison of diets with and without oats in adults with celiac disease. N Engl J Med 1995;333:1033–7 [DOI] [PubMed] [Google Scholar]
- 9.Thompson T. Oats and the gluten-free diet. J Am Diet Assoc 2003;103:376–9 [DOI] [PubMed] [Google Scholar]
- 10.Pulido O, Gillespie Z, Zarkadas M, et al. Introduction of oats in the diet of individuals with celiac disease: a systematic review. Adv Food Nutr Res 2009;57:235–85 [DOI] [PubMed] [Google Scholar]
- 11.Lundin KE, Nilsen EM, Scott HG, et al. Oats induced villous atrophy in coeliac disease. Gut 2003;52:1649–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morón B, Cebolla A, Manyani H, et al. Sensitive detection of cereal fractions that are toxic to celiac disease patients by using monoclonal antibodies to a main immunogenic wheat peptide. Am J Clin Nutr 2008;87:405–14 [DOI] [PubMed] [Google Scholar]
- 13.Ehren J, Morón B, Martin E, et al. A food-grade enzyme preparation with modest gluten detoxification properties. PLoS One 2009;4:e6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornell HJ, McLachlan A, Cullis PG. Extraction of cereal prolamins and their toxicity in celiac disease. J Biochem Mol Biol Biophys 2002;6:151–8 [DOI] [PubMed] [Google Scholar]
- 15.Hernando A, Valdés I, Méndez E. New strategy for the determination of gliadins in maize- or rice-based foods by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: fractionation of gliadins from maize or rice prolamins by acidic treatment. J Mass Spectrom 2003;38:862–71 [DOI] [PubMed] [Google Scholar]
- 16.Vincentini O, Borrelli O, Silano M, et al. T-Cell response to different cultivars of farro wheat, triticum turgidum ssp. Dicoccum, in celiac disease patients. Clin Nutr 2009;28:272–7 [DOI] [PubMed] [Google Scholar]
- 17.Marsh MN, Crowe PT. Morphology of the mucosal lesion in gluten sensitivity. Baillieres Clin Gastroenterol 1995;9:273–93 [DOI] [PubMed] [Google Scholar]
- 18.Hernando A, Mujico JR, Mena MC, et al. Measurement of wheat gluten and barley hordeins in contaminated oats from Europe, the United States and Canada by Sandwich R5 ELISA. Eur J Gastroenterol Hepatol 2008;20:545–54 [DOI] [PubMed] [Google Scholar]
- 19.Chesnut RS, Shotwell MA, Boyer SK, et al. Analysis of avenin proteins and the expression of their mRNAs in developing oat seeds. The Plant Cell 1989;1:913–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shewry PR, Tatham AS, Kasarda DD. Cereal proteins and coeliac disease. In: Marsh M, ed. Coeliac Disease Oxford: Blackwell Scientific Publications, 1992:305–48 [Google Scholar]
- 21.Srinivasan U, Jones E, Carolan J, et al. Immunohistochemical analysis of coeliac mucosa following ingestion of oats. Clin Exp Immunol 2006;144:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vader LW, Stepniak DT, Bunnik E, et al. Characterization of cereal toxicity for celiac disease patients based on protein homology in grains. Gastroenterol 2003;125:1105–13 [DOI] [PubMed] [Google Scholar]
- 23.Peräaho M, Kaukinen K, Mustalahti K, et al. Effect of an oats-containing gluten-free diet on symptoms and quality of life in coeliac disease. A randomized study. Scand J Gastroenterol 2004;39:27–31 [DOI] [PubMed] [Google Scholar]
- 24.Silano M, Di Benedetto R, Maialetti F, et al. Avenins from different cultivars of oats elicit response by coeliac peripheral lymphocytes. Scand J Gastroenterol 2007;42:1302–5 [DOI] [PubMed] [Google Scholar]
- 25.Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 2007;85:160–6 [DOI] [PubMed] [Google Scholar]