Abstract
In ancient times, physicians had a limited number of therapies to provide pain relief. Not surprisingly, plant extracts applied topically often served as the primary analgesic plan. With the discovery of the capsaicin receptor (TRPV1), the search for ‘new’ analgesics has returned to compounds used by physicians thousands of years ago. One such compound, capsaicin, couples the paradoxical action of nociceptor activation (burning pain) with subsequent analgesia following repeat or high-dose application. Investigating this ‘paradoxical’ action of capsaicin has revealed several overlapping and complementary mechanisms to achieve analgesia including receptor desensitization, nociceptor dysfunction, neuropeptide depletion and nerve terminal destruction. Moreover, the realization that TRPV1 is both sensitized and activated by endogenous products of inflammation including bradykinin, H+, ATP, fatty acid derivatives, NGF and trypsins, has renewed interest in TRPV1 as an important site of analgesia. Building on this foundation, a new series of preclinical and clinical studies targeting TRPV1 have been reported. These include trials using brief exposure to high-dose topical capsaicin in conjunction with prior application of a local anesthetic. Clinical use of resiniferatoxin (RTX), another ancient but potent TRPV1 agonist, is also being explored as a therapy for refractory pain. The development of orally-administered high affinity TRPV1 antagonists hold promise for pioneering a new generation of analgesics capable of blocking painful sensations at the site of inflammation and tissue injury. With the isolation of other members of the TRP channel family such as TRPA1, additional opportunities are emerging in the development of safe and effective analgesics.
Keywords: Analgesics, Non-Narcotic, Pain, Sensory Receptors, transient receptor potential cation channel, TRP channels, TRPV1, capsaicin receptor
Introduction
Since ancient times, physicians have faced a common dilemma: How does one effectively relieve a patient’s pain? Long before the advent of randomized double-blinded clinical trials, early physicians successfully used native plant derivatives to provide pain relief. Although their preparations may have been crude by today’s standards, they set in motion a path of discovery that has resulted in the current revolution in novel analgesic development. Now there is evidence that compounds used by ancient physicians to treat painful conditions from arthritis to toothaches contain unique chemicals that can block a peripheral sensory neuron’s ability to detect painful stimuli. Moreover, the molecular identity of these compounds is helping to reveal how noxious thermal, mechanical and chemical stimuli are detected and signal persistent states of tissue injury and inflammation. As discussed later, the capsaicin receptor, also known as TRPV1, is an archetype for a broader family of ion channels that likely transduce virtually all modalities of painful stimuli. As such, progress has been made both at the bench and bedside to develop therapeutics that selectively target the TRP-family of pain-transducing channels providing an exquisite alternative to opioid-based analgesics.
What are the features of an ideal analgesic? A clinician’s viewpoint likely includes: acts selectively on the “pain-sensing” nerves, does not depress the central nervous system or respiration, maintains an analgesic effect over time, is easy to administer, is not addictive and is inexpensive. Will such a compound ever be found or synthesized? Perhaps, the future of the ‘ideal’ analgesic has been with us all along.
Ancient analgesics
Some of the earliest written accounts in Western civilization describing analgesic compounds, especially topical agents, date back to Roman times more than 2000 years ago (50 BC – 23 AD). Although popular legend contends that the Roman physician, Euphorbius, first used the resin from Euphorbia resinifera to treat the arthritic pain suffered by Emperor Augustus, it was actually King Juba II of Mauretania, that scholars believe is responsible for its discovery 1. E. resinifera is a cactus like plant indigenous to the Anti-Atlas Mountains of North Africa (Morocco). This spurge contains a toxic latex that when dried, resolves into a potent resin capable of inducing topical analgesia and reducing the pain of a toothache. Surprisingly, its medicinal use continued for hundreds of years before largely vanishing in the 1700’s. Predating the accounts of E. resinifera during Roman times was the medicinal use of hot chilies in South America dating as far back as 4000 BC. However, much of our modern accounts and written records of chili’s irritant properties and medicinal use are derived from Aztec culture beginning in the 12th century. Aztec’s were a highly disciplined culture where a child’s inappropriate behavior could result in being held over a pile of burning chili peppers! Fortunately, Aztec physicians of that time also realized chili’s usefulness to treat painful maladies. Nevertheless, it was not until Columbus returned to Europe with chili ‘peppers’ in the late 1400s that its culinary and medicinal attributes began to spread throughout the modern world.
Where does pain start?
In the peripheral nervous system, somatosensory detection of tissue damaging stimuli occurs at the peripheral terminals of primary afferent neurons whose cell bodies reside in the trigeminal and dorsal root ganglia. These specialized nociceptive neurons innervate essentially all tissues within the body with the exception of the brain parenchyma. Those neurons that respond to tissue damage (intense mechanical, thermal or noxious chemical stimuli) express specialized proteins in their nerve terminals capable of pain transduction and have been termed primary afferent nociceptors or ‘nociceptors’. Nociceptors have many distinguishing features when compared with other peripheral neurons. Importantly, they are relatively small in size, and are either unmyelinated (C-type) or thinly myelinated (A delta-type) explaining their relatively slow conduction velocities. Under conditions of noxious stimuli, the nociceptor terminals detect impending or actual tissue injury and elicit a complex barrage of electrochemical activity which subsequently signals second order neurons in lamina I, II and V of the dorsal horn of the spinal cord. Ultimately, nociceptive signaling is transmitted to higher centers of the central nervous where it is perceived as a harmful or unpleasant experience 2.
Within the trigeminal (V) and dorsal root ganglia (DRG) reside the cell bodies for the majority of nociceptive fibers that populate cranial nerves V (innervation of the majority of the face, conjunctiva, mouth and dura mater) as well as cranial nerves VII, IX and X (innervation of the skin of the external ear, and mucous membranes of the larynx and pharynx) 3. Likewise, nociceptor terminals derived from the spinal dorsal root ganglia (cervical, thoracic, and lumbar) innervate the somatotopic dermatomes of the skin and underlying tissue and visceral organs. Vagal afferents provide a second source of visceral innervation from cell bodies located within the nodose (inferior vagal) ganglion. Despite dual sensory innervation of the majority of internal organs, afferents involved with the transduction of painful visceral stimuli (ischemia, stretch, distension) are primarily derived from the dorsal root ganglia 4. Nevertheless, vagal afferents still play a role in pain transduction even though their function may be more ‘global’ in nature, providing feedback loops to the brain and neuroendocrine systems resulting in systemic pain modulation and associated perceptions of nausea, malaise or impending doom 5.
Nociceptors
Nociceptors are also characterized based on their threshold for evoking a sensation of pain including noxious chemical, thermal (temperatures ≥ 43–45 °C) or mechanical stimuli. Recent work has focused on further subdividing nociceptive neurons based on their adult expression of associate neuropeptides and receptor proteins. Specific antibody staining is now available for the detection of subtypes of epidermal nociceptive fibers in human volunteers. In general, this has revealed at least two additional subcategories of small-diameter nociceptive neurons: Peptidergic, containing substance P and CGRP, with co-expression of the nerve growth factor (NGF) receptors TrkA and p75, or Non-peptidergic sensory neurons that lack neuropeptides and TrkA receptors but express the antigen Isolectin - B4 6–8. Although both subtypes initially required NGF during development, their adult phenotypes differ based on the down regulation and loss of TrkA receptors on the nonpetidergic nociceptors. Despite this elegant classification of nociceptor subtypes, discharge patterns of polymodal nociceptors do not correlate with stimulus-induced pain sensation 7. Therefore, central processing of nociceptor impulses must be required for the discrimination of painful sensations.
As neuroscientists investigated the characteristics of nociceptor physiology in cultured sensory neurons, inward current responses to noxious heat (I heat) 9–12, mechanical stimuli (I mech) 13 and chemical stimuli were observed in small-diameter sensory neurons 14. In particular, a subset of sensory neurons were activated by capsaicin, the principle pungent component in hot chili peppers. Structurally, capsaicin contains a homovanillic acid group that is important for its pungent activity. Therefore capsaicin and its related derivatives are usually referred to as ‘vanilloid compounds’. In mammals, exposure to capsaicin produces excitation of nociceptors with secondary release of inflammatory and vasoactive peptides 15. In humans, intradermal injection of capsaicin produces immediate burning pain, similar to that reported with noxious thermal stimuli or in certain painful neuropathies 2.
The Capsaicin Receptor (TRPV1)
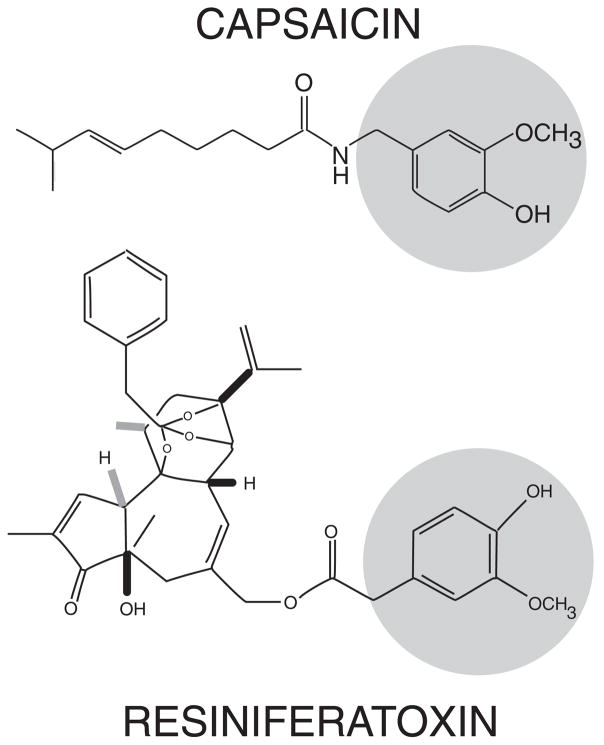
Prior to the isolation of a cDNA encoding a functional capsaicin (vanilloid) receptor, evidence that the effects of capsaicin may be mediated by a receptor began with measuring the dose-dependent effects of capsaicin and its analogues on protective eye wiping behavior in the rat. Subsequently, dose response experiments measuring calcium influx and current responses in cultured sensory neurons were completed 16. In addition, resiniferatoxin (RTX), a diterpine derived from the latex of the plant Euphorbia resinifera used by the ancient physicians of Roman times, was found to share structural similarity to capsaicin by containing a common vanilloid moiety essential for activity (Figure 1). Both capsaicin and RTX induce a dose-dependent influx of calcium in cultured sensory neurons. RTX has an apparent nanomolar binding affinity in DRG membranes and was originally utilized as a high affinity radioligand for the characterization of purported vanilloid binding sites 17. Several comprehensive reviews have been published encapsulating early work on vanilloid receptor biology 17, 18.
Figure 1.
Chemical structure of capsaicin (top) and resiniferatoxin (RTX) (bottom) illustrating common active moieties including –methoxy and hydroxyl groups (circled). Although both function as agonists at the TRPV1 receptor, RTX has higher potency and a characteristically slow but persistent activation.
TRPV1 is an ion channel that integrates multiple noxious stimuli in nociceptors
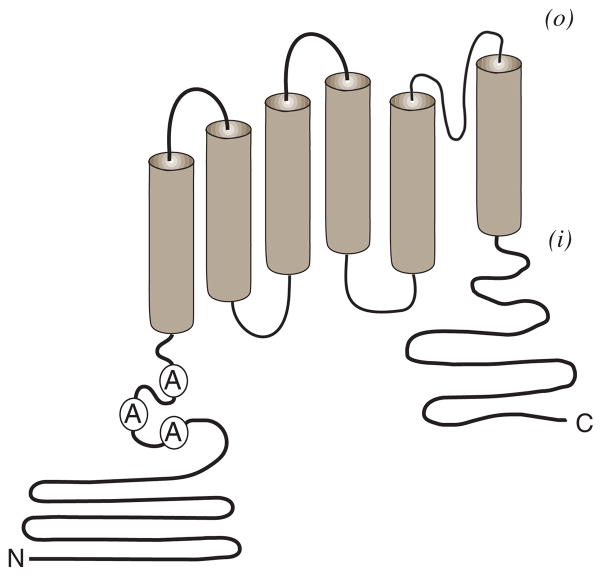
With the isolation of a cDNA clone encoding a capsaicin-activated ion channel in 1997, the molecular basis of the vanilloid receptor -VR1 (TRPV1) was finally realized 19. Now termed TRPV1 (transient receptor potential cation channel, subfamily V, member 1), it encodes a nonselective cation channel subunit of approximately 95kDa that is highly expressed in the small-diameter sensory neurons of dorsal root, trigeminal and vagal ganglion. Its structure (Figure 2) most resembles that of members of the Kv 1.2 and store operated channel family 19. The TRPV1 subunit spans the plasma membrane six times containing large N- and C-terminal intracellular regions and is proposed to form tetrameric and/or heteromeric channel complexes 20–22. It is activated by capsaicin and RTX on the intracellular surface in a dose-dependent manner. Once activated, TRPV1 is not selective for monovalent cations; rather it preferentially conducts calcium through its channel pore resulting in an increase in intracellular calcium and cellular depolarization.
Figure 2. TRPV1 protein topology.
TRPV1 is distinguished by six transmembrane spanning regions flanked by two intracellular domains (N) amino-terminal and (C) carboxyl-terminal. The N-terminal domain includes three ankyrin (A) repeat domains that may function in receptor modulation. A pore loop domain is predicted between the fifth and sixth transmembrane spanning region. It is proposed that at least four such subunits assemble to form a functional channel complex. Formation of hetermomeric channel complexes incorporating TRPV1 plus other TRPV1 splice variant and/or TRP – channel subunits have been proposed.
The role of TRPV1 on Inflammatory Pain and Hyperalgesia
The pain and disability from chronic inflammatory conditions remains widespread and difficult to manage despite a variety of available pharmacologic therapies. With the realization that TRPV1 could be activated by thermal stimuli, it was initially considered to serve a restricted role for nociceptive transduction - the acute detection of heat in the noxious range. Importantly, TRPV1 was not found to be simply a thermal detector but also a critical part of a system designed to signal potential and/or ongoing pathophysiological conditions that if left uncorrected, could lead to irreversible cellular injury.
Nociceptors have the ability to adjust their sensitivity following repetitive noxious stimuli or tissue injury. Sensitization encompasses an increase in spontaneous nociceptor activity, a lowered threshold for activation, and an increase in action potential firing after suprathreshold stimuli 2. Under these circumstances, prolonged nociceptor activation may be warranted to ensure protective behavioral responses. Together with plasticity changes in the dorsal horn of the spinal cord, local nociceptor sensitization contributes an essential role in the initiation and maintenance of hyperalgesia. A turning point in the realization that TRPV1 was critical to the signaling of inflammatory pain and hyperalgesia was the finding that TRPV1 −/− null mice failed to develop thermal hyperalgesia following exposure to peripheral inflammation 23, 24. Therefore, TRPV1 serves as a critical molecular site of nociceptor sensitization where the action of both an inflammatory mediator and a noxious stimulus (heat) are required for nociceptor activation.
Endogenous Activation and Sensitization of TRPV1
Nociceptive ion channels and, by definition, the nociceptors expressing them, may serve more to signal ongoing tissue injury and inflammation rather than acute noxious stimuli. This suggests a sensory system that can distinguish between an acute noxious stimulus and a chronic painful condition such as inflammation. It also suggests that compounds capable of selectively blocking the activation of this class of receptor/channels could theoretically spare normal sensations of touch or extremes of heat. Although the identity of endogenous agents capable of activating TRPV1 continues to emerge, a number of these have been shown to either act directly or sensitize TRPV1 through secondary messengers and/or protein modification. As summarized (Table 1), multiple agents and pathways are acting in concert to modulate TRPV1 under conditions of tissue injury/inflammation and nerve injury.
Table 1.
| Stimulus/Agent | Mechanism | Response | Ref |
|---|---|---|---|
| Capsaicin | Intracellular N & C terminal | Activation, Increased Ca++ I Desensitization Cell death | 19, 25,26 |
| Resiniferatoxin (RTX) | Transmembrane domain | Slow irreversible activation, Increased Ca++I Depolarizing block Cell death | 27–31 |
| Heat | Multiple sites – C-terminal; PIP2 | Activation at Temp >43 °C Increased Ca++i | 19, 32–34 |
| Acid/Base | Extracellular (H+) Intracellular (−OH) |
Activation & sensitization | 19,32, 35,36 |
| Anandamide | Direct intracellular binding | Vasodilation, Activation, Sensitization | 37–40 |
| Bradykinin (BK) | 12-HPETE Leukotriene B4; PKC | Activation & Sensitization | 41–43 |
| ATP | P2Y2/PKC | Sensitization & Activation | 44–46 |
| Nerve Growth Factor (NGF) | TrkA/PI3K | Activation & Sensitization Increased expression | 47, 48 |
| Trypsins | PAR2/PKC | Sensitization | 49, 50 |
Protons
Protons (H+) as found in excess under acidic conditions (low pH) have been shown to potentiate both the vanilloid and noxious thermal response of TRPV1 through direct activation of TRPV1 in vitro 19, 32. Moreover, an extracellular site essential for proton-induced activation of TRPV1 has been identified and apparently differs from the sites that mediate capsaicin and heat activation 35, 51. Although the detection of ‘mild’ acidic conditions, pH 7.0–7.4, may be mediated by a family of acid-sensing sodium channels 52, the ability of hydrogen ions to potentiate or directly activate TRPV1 suggests a nociceptive role under pathophysiologic conditions of ischemia or infection. Additional behavioral studies should help determine the degree to which TRPV1 participates in vivo to proton-mediated nociception 23, 24.
Bradykinin
(BK) a naturally occuring inflammatory nonapeptide, has been shown to directly activate nociceptors as well as to produce nociceptor sensitization through several mechanisms 53. The effects of bradykinin are mediated through two receptor subtypes: B1 and B2. The B2 receptor is widely expressed being responsible for the majority of BK-induced effects including those in nociceptors. In contrast, the B1 subtype is expressed in lower abundance and has a higher affinity for the BK metabolite, Des [Arg9] bradykinin. B1 receptors are upregulated under conditions of injury/inflammation and have been shown to contribute to inflammatory hyperalgesia 54. Activation of PKC produces C-fiber type nociceptor depolarization and activated forms of PKC are associated with the phosphorylation of selective domains of receptors and ion channels 55. A PKC isozyme (epsilon), PKCe, has been implicated in nociceptor function as it may mediate a component of NGF-mediated hyperalgesia 56. Furthermore, PKCe mutant mice have reduced mechanical and thermal hyperalgesia but have normal baseline thresholds for noxious stimuli 56. Nevertheless, BK activation of nociceptors was eliminated in B2 deficient mice, reaffirming the role of the B2 receptor as the predominant target for BK action on nociceptors 57. Moreover, at least two potential pathways have been described that could link BK to TRPV1 activation in nociceptors: 1) BK activation of phospolipase A2 with subsequent metabolism of arachidonic acid into products of the lipoxygenase pathway 41, and 2) BK-mediated production of diacylglycerol and inositol 1,4,5 trisphosphate with subsequent activation of PKC 58.
ATP
The hypothesis that adenosine triphosphate (ATP) plays an important role in the synaptic transmission of sensory neurons began with the early observations of Holton and Holton 59. Cellular activation in response to ATP revealed that one or more ‘fast’ ATP gated channels may exist in various tissues, including nociceptors. Furthermore, it is plausible that ATP released from injured cells functions as a signal of tissue injury. Of importance is the ATP gated channel subtype P2X3, which is predominantly expressed in small-diameter sensory neurons and is proposed to be one mechanism that mediates ATP-induced activation of nociceptors 60. P2X3 may play a role in enhancing thermal and/ormechanical transduction under inflammatory or pathophysiologic conditions 61. More recently, the metabotropic G-protein coupled receptor, P2Y2, has been shown to have a direct link to TRPV1 activation and sensitization 44–46.
Lipids: Fatty acid metabolites
Nociceptors are sensitized by a wide range of inflammatory products of arachidonic acid (AA) metabolism. These include certain products of the cyclooxygenase pathway (PGE2, PGI2) that are known to exert their biological action through G-protein coupled receptors and more recently isoprostanes, compounds that are formed by nonenzymatic peroxidation of AA such as 8-iso PGE2 and 8-iso PGF2a. Alternately, AA is metabolized via the lipoxygenase pathway, producing a multitude of products including LTB4, and 15-S-di HETE that have been shown to sensitize nociceptors. Although lipoxygenase products have a wide range of biological activities and their receptor targets were previously minimally characterized, 12-S-HPETE and LTB4 have been recently shown to directly activate TRPV1 41. More recently, two derivatives of dopamine (N-arachidonoyl-dopamine (NADA) and N-oleoyl-dopamine) have also been found to activate TRPV1 and are associated with experimental hyperalgesia 62, 63.
Nerve Growth Factor (NGF)
Since its identification by Levi-Montalcini and Calissano, NGF has been distinguished from other neurotrophin family members (BDNF, NT-3 and NT-4/5) as being essential for normal nociceptor development and function 64. NGF is synthesized and secreted by a wide variety of tissues including Schwann cells located within sensory ganglion and importantly in the end-target tissues of nociceptive terminals - epidermal fibrobroblasts and keratinocytes. NGF is intimately involved in maintaining and modifying the phenotype of the nociceptor population. Adult sensory neurons lose their dependency on NGF for survival but retain expression of its high affinity receptor-TrkA, primarily on the small-diameter primary afferent nociceptors (C and A-delta) 65. Conditions of inflammation that are characterized by inflammatory cell migration, cytokine release, edema, erythema, pain and hyperalgesia, can be experimentally modeled by injection of Complete Freund’s Adjuvant (CFA) into the hind paw of the rat. Following CFA injection, one finds increased NGF production and content at the site of injury serving as the driving signal for the associated pain and hyperalgesia 66–68.
Use of IgG-Trk fusion protein and anti-NGF antibodies have been shown to block inflammatory models of pain and thermal hyperalgesia despite continued evidence of erythema and edema 65. Human studies also corroborate a role for NGF in peripheral pain transduction. Intradermal injection of NGF in human volunteers induces thermal hyperalgesia and mechanical allodynia at the site of injection, beginning as early as three hours and lasting up to 21 days (66). NGF is also detected in the synovial fluid of patients with rheumatic disease or other types of chronic arthritis 69. Patients with congenital insensitivity to pain with anhydrosis (CIPA) have an absence of reaction to noxious stimuli and have been shown to contain mutations within the gene that encodes the NGF – TrkA receptor 70.
A major consequence of NGF production in peripheral inflammation is TRPV1 -mediated pain and thermal hyperalgesia. It is now emerging that NGF has at least three principle actions on TRPV1 in nociceptors: 1) Modification of the TRPV1 channel structure changing its sensitivity towards activation - lowering its threshold of thermal activation 47, 2) Increasing the transport of the TRPV1 channel protein to the plasma membrane, thereby making more TRPV1 receptor immediately available for a greater cellular response under activating conditions 48, 3) Increasing both TRPV1 translation (protein) 71 and transcription (RNA) 22, 72, 73 to sustain over-expression of TRPV1 in C-type nociceptors and to facilitate de novo expression of TRPV1 in A-delta-type nociceptors 74, 75.
Therefore long-term exposure of nociceptive terminals to inflammatory mediators such as NGF can result in long-term phenotypic changes in the repertoire of nociceptive transducing elements 76. Extending these observations to TRPV1, experiments from several laboratories have found that NGF directs both early and long-term increases in capsaicin-mediated responses 47, 64, 77, 78. Since it has been established that TRPV1 −/− null mice fail to develop thermal hyperalgesia following exposure to peripheral inflammation 23, 24, the relative level of expressed TRPV1 at the site of inflammation (and probably at the spinal cord) will have a profound impact on the magnitude of inflammatory-induced pain and hyperalgesia.
Vanilloid – based therapies for the treatment of pain
Although the use of vanilloid – like creams and salves have their therapeutic origins to treat painful conditions thousands of years ago 1, their scientific and clinical uses in Western societies has only emerged since the 1800’s with the isolation of the principle agent, capsaicin, from hot chili peppers. Building on the realization that the experience of pain is based on nerves (nociceptors) that respond to specific noxious stimuli that can cause tissue damage (Sherington 1906) 79, Hungarian investigators in the 1940’s observed that capsaicin can both activate and inactivate sensory nerves. Following the confirmation of the ‘nociceptor’ hypothesis by Bessou and Perl in 1969, the existence of a ‘capsaicin receptor’ expressed on C-polymodal nociceptors was hypothesized. The phenomenon of nociceptor ‘desensitization’ due to repetitive exposure to capsaicin was finally investigated in the 1970’s 80. As shown below, it was not until the 1980’s that the broader use of topical capsaicin appeared in earnest in the literature as a therapy for difficult to manage pain syndromes - especially for the treatment of post-herpetic neuralgia.
Capsaicin mediated analgesia
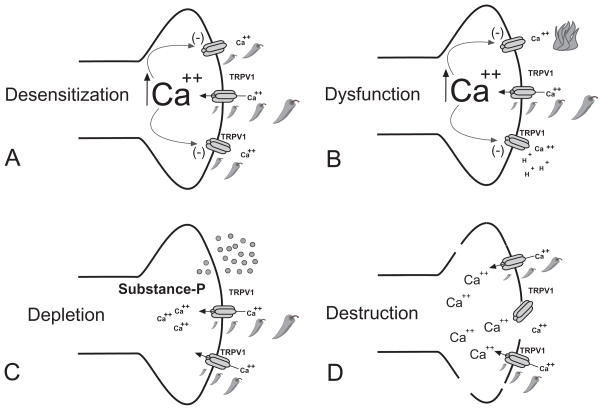
It has long been appreciated that initial applications of capsaicin are painful; but, paradoxically, repeated application produces a topical analgesic effect. Although a combination of mechanisms (Figure 4) including desensitization, nociceptor dysfunction, neuropeptide depletion 81, 82 and nociceptive terminal destruction 83, 84 have been proposed as critical analgesic drivers, it is most likely that the destruction of nociceptor terminals that plays the greatest role in the subsequent analgesic/therapeutic effect.
Figure 4. Mechanisms of topical capsaicin-mediated analgesia.
Repeat application of capsaicin or other vanilloid – like compounds can produce a number of local effects on TRPV1 – expressing nociceptor terminals: (A) Desensitization is a calcium-dependent phenomenon where application of capsaicin leads to a decrease in inward current response during continued capsaicin application. When capsaicin is applied at repeated intervals, each subsequent response becomes smaller and is often referred to as tachyphylaxis. It is proposed that under these conditions, TRPV1 may also be refractory to the effect of inflammatory mediators and intracellular secondary messengers. (B) Repeated or prolonged application of capsaicin can produce nociceptor dysfunction. Under this condition, which may be secondary to an influx and/or excess of store-released calcium, other pain transducing receptor – channels may be inactivated. This could explain analgesic effects that are beyond the scope of TRPV1 function. (C) Depletion of neuropeptides (Substance –P, CGRP) from nociceptive terminal is evoked by capsaicin and high dose or repeat applications have been shown to deplete both central and peripheral terminals. Although the activity of Substance –P has been show to play a key role in facilitating nociceptive neurotransmission in the dorsal horn of the spinal cord, blockade of the Substance –P receptor (NK1R) has failed to show analgesia in humans. (D) Destruction of TRPV1-expressing nociceptive terminals has been the most reliable marker correlating the application of vanilloid –like compounds and analgesia. Although a number of mechanisms have been proposed, vanilloid – induced apoptosis appears to be the likely mechanism.
Several large double-blind, vehicle-controlled studies of patients with chronic postherpetic neuralgia (PHN) were performed to evaluate the efficacy of topically applied capsaicin 0.075% cream. The authors concluded that it was not only effective, but should be considered for the initial management of PHN 71,85. Given the apparent initial success in the treatment of PHN, a number of other painful conditions were considered for topical capsaicin therapy. Topical capsaicin has shown promise in the treatment pain of neuropathic character including patients with complex regional pain syndrome CRPS 86. By extension, capsaicin–based topical applications have been focused on post-surgical neuropathic pain in cancer patients using 0.075 % cream applied four times daily. Impressively, 53% vs. 17% (placebo control) of patients experienced a significantly greater pain relief while using the topical capsaicin 87. Although an early metaanalysis that included patients suffering from diabetic neuropathy and osteoarthritis concluded that topical capsaicin improved pain when compared with a placebo, 88 the analysis includes a number of uncontrolled and/or under-powered trials, a concern that has weakened their impact over time. Moreover, if one applies a more ‘rigorous’ standard for clinical trials (as exists presently) on trial data prior to 2004, topical capsaicin (0.025% or 0.075 %) showed poor to moderate efficacy in the treatment of either musculoskeletal or neuropathic symptoms. 89. Coupled with one third of these study patients experiencing adverse effects, enthusiasm for widespread use of these agents in the absence of concurrent local anesthetic pretreatment appeared to plateau and such treatments were considered for so-called, “nonresponders” rather than as a first-line treatment option.
Several aspects of topical capsaicin treatment appear to limit its overall effectiveness and application in clinical practice. The first is the requirement for repeated capsaicin application (up to 4–5 times daily) to establish and maintain an adequate degree of analgesia. Repeated use of capsaicin containing topical creams leads to the loss of epidermal nerve fibers and can be detected as soon as three days following repeated application. In fact, after three weeks of capsaicin treatment on the volar forearm four times daily, there was an approximately 80% reduction in epidermal nerve processes. Loss of the epidermal fibers was concordant with a reduction in painful sensation to noxious and heat and mechanical stimuli 90. Similar findings were observed when capsaicin was injected subcutaneously in volunteers 84.
Capsaicin and the skin
Although the loss of epidermal nerve fibers are also associated with localized redness and edema, there is surprisingly little damage to the keratinocytes within the epidermal layer of normal skin. Few studies have specifically reported on capsaicin-induced keratinocyte toxicity despite the reported expression of TRPV1 91–93. This could be the result of several factors including a lower overall level of TRPV1 expression in keratinocytes when compared with those found in dorsal root ganglia coupled with the co-expression of an inhibitory TRPV1 splice variant subunit 94. Although there is a reduction of keratinocyte and fibroblast growth in the presence of capsaicin (0.025%), in vitro, at concentrations found in commercial creams 95, capsaicin may have a much greater toxic effect on keratinocytes under pathophysiologic conditions. For example, cultured cells from human squamous cell carcinoma are sensitive to capsaicin-induced apoptosis through inhibition of mitochondrial respiration 96. In fact, certain skin disorders such as prurigo nodularis can be effectively treated with topical capsaicin 97. Moreover, there is an increase in capsaicin receptor (TRPV1) expression in the epidermal keratinocytes and associated nerve fibers in prurigo nodularis lesions that is normalized following capsaicin treatment. This suggests that TRPV1 itself may play a critical role in both the pathology and the treatment of inflammatory disorders of the skin 98. Therefore, it is tempting to speculate that topical capsaicin treatment would afford a selective advantage in the management of pain arising from certain cutaneous manifestations of malignant melanoma and/or squamous cell carcinoma 96, 99.
Combining capsaicin with local anesthetics
To obtain improved patient acceptance and analgesic efficacy using capsaicin based creams, therapeutic trials have progressively shifted to a combination of local anesthetic pretreatment followed by a single application of high-dose capsaicin. In a preliminary trial of ten patients suffering from intractable lower extremity pain with neuropathic features, application of capsaicin (5–10%) under regional anesthesia resulted in a wide range of post treatment pain relief 83. Therefore, this report suggested an alternative approach, a single application period of a high dose capsaicin rather than the onerous task of repeat daily applications of low dose formulations that are associated with a high drop out rate 100.
Later, a high concentration capsaicin patch (8%) was devised and its application to the skin for a period of 1–2 hours produced longer term changes in epidermal nerve fibers that included loss of PGP-9.5 staining and reduction of heat sensitization. This illustrated that a short term application of a high concentration of capsaicin can mimic those changes previously seen under repeat application (3–5 times/day × 1 week) of lower concentration capsaicin cream 101. Subsequently, a randomized double-blinded study, for the treatment of post-herpetic neuralgia using a one hour application of a high dose capsaicin (8%) patch was found to provide significant pain relief between study weeks 2–12 102. Application of the high dose capsaicin patch in this case was generally well-tolerated as it was preceded with the one hour application of 4% lidocaine jelly. Despite this, high dose capsaicin patch treatment was commonly associated with localized pain and erythema 102. A similar study using a capsaicin (8%) patch with lidocaine pretreatment was undertaken for the treatment of painful HIV neuropathy of the lower extremities (feet) that showed modest pain relief (one third of treated patients had > 30% relief) during study weeks 2–12, without a detectable change in the perception of warmth, cold, sharp pain or vibration sensation 103. Interestingly, there was no apparent relationship between the duration of patch application and the degree of analgesia achieved. Adverse events included short-term site swelling and burning sensation with 44% of patients requesting oxycodone/acetaminophen following capsaicin patch placement. A small number of patients also experienced itching or coughing 103.
Is capsaicin safe?
The favorable safety profile of topically applied capsaicin relies on several complementary factors. Although capsaicin can be systemically absorbed through the skin, it does so as a function of its applied concentration and duration of exposure. When the kinetics of systemic capsaicin absorption was investigated in patients receiving a high dose capsaicin (8%) patch for pain arising from either PHN, HIV associated neuropathy (HIV-AN) or from diabetes mellitus, patch application to the trunk (PHN) directed the greatest plasma levels with the highest value observed at 17.8 ng/ml. Capsaicin is rapidly eliminated by the CYP hepatic enzyme system 104, with a population elimination half-life of 1.64 hours 105. Significantly lower plasma concentrations were detected when the patch was applied to the feet (DN, HIV-AN). As application time was increased, (from 60 to 90 minutes) the hourly plasma concentration doubled 105.
Given this low profile of toxicity, the use of purified capsaicin solutions is being investigated for its potential to provide long-term post-operative pain relief and reduction of opioid-based analgesics following intra-operative instillation into surgical wounds 106. Nevertheless, high doses of capsaicin inadvertently administered into the systemic circulation may produce a wide range of effects such in the pulmonary (apnea), cardiovascular (bradycardia) and thermoregulatory (hypothermia) systems 18. The perineural infiltration of capsaicin may be another way in which to selectively target painful conditions. In fact, the idea of applying capsaicin plus a local anesthetic capable of selectively entering nociceptive fibers has been proposed and demonstrated in animal models 107, 108.
Resiniferatoxin (RTX) mediated analgesia
In comparison to capsaicin, it is surprising to find a relative paucity of preclinical and clinical trials using resiniferatoxin (RTX) as an analgesic therapy. Although RTX appears to engender many of the same benefits that were described for capsaicin, its overall structure (phorbol ester) and profile of TRPV1 activation (irreversible) likely have impacted on its translation from the bench to bedside 28, 109. Nevertheless, a resurgence of interest in RTX has shown that intrathecal delivery in animals results in long-term analgesia – likely due to the loss of vanilloid-sensitive sensory neurons 31, 110. Perineural application of RTX has also been shown to produce dose-dependent long-lasting analgesia 111, 112. Importantly, analgesia was achieved in the absence of changes in proprioception or motor control 113, 114. RTX treatment has been shown to improve nociceptive behaviors in animals with tumors and is undergoing Phase 1 and Phase 2 clinical trials to examine the safety and effectiveness of intrathecal RTX for the treatment of advanced cancer pain refractory to other treatments 113.
TRPV1 Antagonist mediated analgesia
Whereas new methodologies have been developed to apply capsaicin or RTX to appropriate target tissues, a completely different effort has been underway to develop high affinity TRPV1 antagonists with the goal of achieving analgesia by systemic administration. Ultimately, the question has become: Will blockade of TRPV1 activation reverse inflammatory pain and hyperalgesia? As previously described in detail, TRPV1 functions of an integrator of multiple noxious stimuli serving as a cellular sensory transducer for the detection of tissue injury. It is therefore plausible that blockade of TRPV1 activation in response to the cornucopia of inflammatory mediators (protons, bradykinin, ATP, products of the lipoxygenase pathway, fatty acid metabolites, growth factors) should provide pain relief and reduce hyperalgesia. Early studies attempted to demonstrate blockade or reversal of experimental hyperalgesia in animal models but were hampered by either their lack of specificity (ruthenium red) or their low affinity (capsazepine) 115–118.
Following the isolation of TRPV1 19, efforts to identify a high affinity antagonist were enabled with high throughput screening techniques and resulted in several promising candidates. Excitedly, an oral compound SB-705498 was shown to be effective in a human trial to reduce the area of experimental capsaicin-evoked flare when compared with placebo 119. In addition, another orally bio-available antagonist of TRPV1 activation- AMG 517 was shown to reverse inflammation-induced pain behavior in rats. AMG 517 was later predicted to have a long half-life in humans that may be amenable to once-a-week dosing. Moreover, it was observed that blockade of TRPV1 centrally was anticipated to help provide a global analgesic effect 120. However, during a double-blind, placebo-controlled, randomized, parallel-group, multi-center study for the management of pain following molar extraction, a test subject experienced prolonged (days) hyperthermia (> 40 °C) after taking a single 2mg dose of the high affinity TRPV1 antagonist 121. Although this event resulted in the suspension of further Phase 1 testing of these compounds, it dramatically revealed the importance of TRPV1 in central core temperature regulation. It is uncertain whether modification of these compounds will result in a more favorable therapeutic window 122.
TRPA1: A TRP channel activated by cold, environmental irritants and cellular products of oxidative stress
Despite the central role TRPV1 plays in the transduction of multiple noxious stimuli, it has yet to be shown that TRPV1 is activated by noxious cold or high threshold mechanical stimuli. In the search for additional TRP channels capable of transducing noxious stimuli, TRPA1 (ANKTM1) was more recently isolated and characterized from sensory ganglion and found to be activated by noxious cold and irritants such as mustard oil and wasabi and certain volatile anesthetics 123–126. Its properties of activation in response to a wide range of irritant chemicals revealed a common mechanism of activation: electrophilic-mediated covalent binding to nucleophilic cysteine 127–129.
TRPA1 is co-expressed in a subset of TRPV1 expressing nociceptors in trigeminal and DRG neurons 130 and functions to detect products of tissue injury, inflammation and oxidative stress, such as 4-Hydroxynonenal, an endogenous aldehyde that causes pain and neurogenic inflammation 128, 131 as well as prostaglandins 132. Under conditions of inflammation/nerve injury, expression of TRPA1 is persistently increased concurrent to TRPV1 133. Given that TRPA1 was activated by endogenous inflammatory/oxidative stress products and implicated in mechanical hyperalgesia 134, it rapidly became a promising therapeutic target for the treatment of pain. High affinity TRPA1 antagonists are now under development and, thus far, have shown promising results in rodent models with reductions in inflammation and nerve injury-induced mechanical hypersensitivity135, 136.
Conclusion
TRP channels TRPV1 and TRPA1 are expressed in overlapping populations of nociceptors and together function to detect noxious stimuli ranging from plant derivatives and environmental irritants to endogenous products of inflammation or oxidative stress. In the case of TRPV1, sensitization by inflammatory mediators acts to lower the thermal threshold of activation, resulting in nociceptor activation at physiologic temperatures. Together with other members of the TRPV family plus TRPA1, these channels have the capacity to warn the body of impending tissue injury over a wide range of temperatures. Both TRPV1 and TRPA1 represent plausible therapeutic targets for novel analgesics. Since TRPV1 is expressed on polymodal nociceptors, administration of capsaicin or RTX can inactivate and/or destroy the entire nociceptive terminal. Consequently, both thermal and mechanically induced pain may be reduced. Given that many of the conditions driving tissue injury result in an increase in TRPV1 and/or TRPA1 in the nociceptors, there may be an additional therapeutic advantage. The observation that TRPV1 and TRPA1 are activated by endogenous products of inflammation established the rationale for development of high affinity antagonists. Although an ‘unusual’ side effect (hyperthermia) 121 has been observed in the trial of an oral TRPV1 antagonists, such agents may still be of therapeutic value if administered locally or regionally. This may be of particular importance with TRPV1, as its role in other physiologic processes from diabetes to obesity becomes clear 137, 138. As the development of additional TRP channel agonists/antagonists advance, the goal of selectively blocking pain at its origin, at the primary afferent nociceptor, appears within reach.
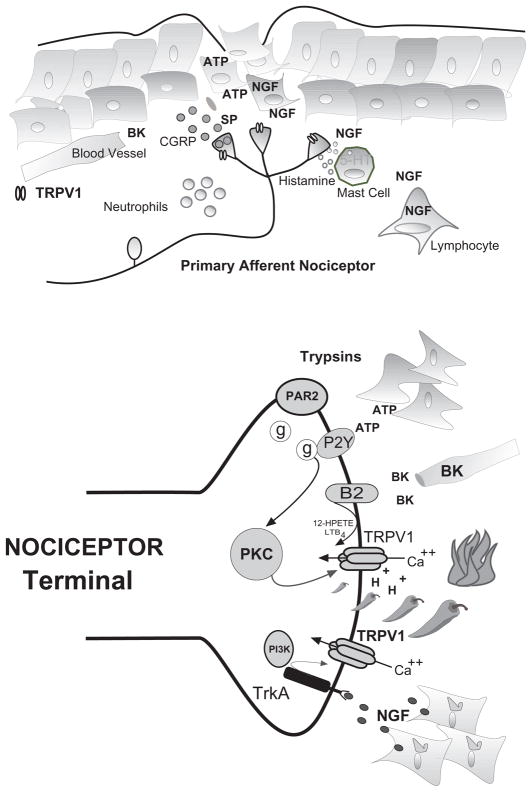
Figure 3. Mechanisms of TRPV1 mediated inflammatory hyperalgesia and pain transduction (top).
Following tissue injury, local tissue responds with increased production and accumulation of inflammatory compounds that activate/sensitize TRPV1. Nociceptive terminals derived from C- and A-delta fibers are interposed with skin fibroblasts, mast cells and the microvasculature. Following injury or inflammation terminals depolarize releasing neuropeptides substance-P (SP) and calcitonin gene related peptide (CGRP) which produces vascular leak and edema. Bradykinin (BK) cleaved from circulating kallikreins and nerve growth factor (NGF) produced by fibroblasts an infiltrating PBMs both activate and sensitize nociceptor terminals. NGF produces additional sensitization through the degranulation of mast cells containing serotonin (5-HT) and histamine. NGF and cytokines are associated with the accumulation of neutrophils and lymphocytes that participate in the maintenance of sensitization -hyperalgesia. (Bottom) Hypothetical nociceptor terminal expressing TRPV1 activated by capsaicin (peppers), noxious heat (fire) and extracellular protons (H+). The resulting inward calcium current depolarizes terminal initiating action potentials that signal higher centers (not shown). Inflammatory mediators such as bradykinin (BK), ATP, trypsins, and NGF act through various secondary messenger systems to activate or sensitize TRPV1, resulting in pain and hyperalgesia.
Acknowledgments
The author wishes to thank Helge Eilers and Kathryn Zavala for helpful suggestions and critical comments. Supported in part by NIH NS038737.
References
- 1.Appendino G, Szallasi A. Euphorbium: modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997;60:681–696. doi: 10.1016/s0024-3205(96)00567-x. [DOI] [PubMed] [Google Scholar]
- 2.Fields HL. Pain syndromes in neurology. London, Boston: Butterworths-Heinemann Ltd; 1990. [Google Scholar]
- 3.Carpenter MB. Core text of neuroanatomy. 3. Baltimore: Williams & Wilkins; 1985. [Google Scholar]
- 4.Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- 5.Janig W, Khasar SG, Levine JD, Miao FJ. The role of vagal visceral afferents in the control of nociception. Prog Brain Res. 2000;122:273–287. doi: 10.1016/s0079-6123(08)62145-7. [DOI] [PubMed] [Google Scholar]
- 6.Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 7.Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Nociceptor discharges and sensations due to prolonged noxious mechanical stimulation-a paradox. Hum Neurobiol. 1984;3:53–58. [PubMed] [Google Scholar]
- 8.McMahon SB, Koltzenburg M. Novel classes of nociceptors: beyond Sherrington. Trends Neurosci. 1990;13:199–201. doi: 10.1016/0166-2236(90)90159-8. [DOI] [PubMed] [Google Scholar]
- 9.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichling DB, Levine JD. Heat transduction in rat sensory neurons by calcium-dependent activation of a cation channel. Proc Natl Acad Sci U S A. 1997;94:7006–7011. doi: 10.1073/pnas.94.13.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cesare P, Moriondo A, Vellani V, McNaughton PA. Ion channels gated by heat. Proc Natl Acad Sci U S A. 1999;96:7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reichling DB, Levine JD. In hot pursuit of the elusive heat transducers. Neuron. 2000;26:555–558. doi: 10.1016/s0896-6273(00)81191-5. [DOI] [PubMed] [Google Scholar]
- 13.McCarter GC, Reichling DB, Levine JD. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci Lett. 1999;273:179–182. doi: 10.1016/s0304-3940(99)00665-5. [DOI] [PubMed] [Google Scholar]
- 14.McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol. 1999;61:835–856. doi: 10.1146/annurev.physiol.61.1.835. [DOI] [PubMed] [Google Scholar]
- 15.Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13:2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh U, Hwang SW, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J Neurosci. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 18.Buck SH, Burks TF. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986;38:179–226. [PubMed] [Google Scholar]
- 19.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 20.Kedei N, Szabo T, Lile JD, et al. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- 21.Kuzhikandathil EV, Wang H, Szabo T, Morozova N, Blumberg PM, Oxford GS. Functional analysis of capsaicin receptor (vanilloid receptor subtype 1) multimerization and agonist responsiveness using a dominant negative mutation. J Neurosci. 2001;21:8697–8706. doi: 10.1523/JNEUROSCI.21-22-08697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eilers H, Lee SY, Hau CW, Logvinova A, Schumacher MA. The rat vanilloid receptor splice variant VR.5’sv blocks TRPV1 activation. Neuroreport. 2007;18:969–973. doi: 10.1097/WNR.0b013e328165d1a2. [DOI] [PubMed] [Google Scholar]
- 23.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 24.Davis JB, Gray J, Gunthorpe MJ, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 25.Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- 26.Jung J, Hwang SW, Kwak J, et al. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szallasi A, Blumberg PM. Specific binding of resiniferatoxin, an ultrapotent capsaicin analog, by dorsal root ganglion membranes. Brain Res. 1990;524:106–111. doi: 10.1016/0006-8993(90)90498-z. [DOI] [PubMed] [Google Scholar]
- 28.Acs G, Biro T, Acs P, Modarres S, Blumberg PM. Differential activation and desensitization of sensory neurons by resiniferatoxin. J Neurosci. 1997;17:5622–5628. doi: 10.1523/JNEUROSCI.17-14-05622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou MZ, Mtui T, Gao YD, Kohler M, Middleton RE. Resiniferatoxin binds to the capsaicin receptor (TRPV1) near the extracellular side of the S4 transmembrane domain. Biochemistry. 2004;43:2501–2511. doi: 10.1021/bi035981h. [DOI] [PubMed] [Google Scholar]
- 30.Raisinghani M, Pabbidi RM, Premkumar LS. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J Physiol. 2005;567:771–786. doi: 10.1113/jphysiol.2005.087874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One. 2009;4:e7021. doi: 10.1371/journal.pone.0007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 33.Vlachova V, Teisinger J, Susankova K, Lyfenko A, Ettrich R, Vyklicky L. Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J Neurosci. 2003;23:1340–1350. doi: 10.1523/JNEUROSCI.23-04-01340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brauchi S, Orta G, Mascayano C, et al. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci U S A. 2007;104:10246–10251. doi: 10.1073/pnas.0703420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhaka A, Uzzell V, Dubin AE, et al. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153–158. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 38.Smart D, Gunthorpe MJ, Jerman JC, et al. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Marzo V, Bisogno T, De Petrocellis L. Anandamide: some like it hot. Trends Pharmacol Sci. 2001;22:346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- 40.van der Stelt M, Trevisani M, Vellani V, et al. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. Embo J. 2005;24:3026–3037. doi: 10.1038/sj.emboj.7600784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang SW, Cho H, Kwak J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin J, Cho H, Hwang SW, et al. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizumura K, Sugiura T, Katanosaka K, Banik RK, Kozaki Y. Excitation and sensitization of nociceptors by bradykinin: what do we know? Exp Brain Res. 2009;196:53–65. doi: 10.1007/s00221-009-1814-5. [DOI] [PubMed] [Google Scholar]
- 44.Moriyama T, Iida T, Kobayashi K, et al. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lakshmi S, Joshi PG. Co-activation of P2Y2 receptor and TRPV channel by ATP:implications for ATP induced pain. Cell Mol Neurobiol. 2005;25:819–832. doi: 10.1007/s10571-005-4936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138:484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lerner DJ, Chen M, Tram T, Coughlin SR. Agonist recognition by proteinase-activated receptor 2 and thrombin receptor. Importance of extracellular loop interactions for receptor function. J Biol Chem. 1996;271:13943–13947. [PubMed] [Google Scholar]
- 50.Amadesi S, Cottrell GS, Divino L, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and\ A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welch JM, Simon SA, Reinhart PH. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc Natl Acad Sci U S A. 2000;97:13889–13894. doi: 10.1073/pnas.230146497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 53.Burgess GM, Mullaney I, McNeill M, Dunn PM, Rang HP. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J Neurosci. 1989;9:3314–3325. doi: 10.1523/JNEUROSCI.09-09-03314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rupniak NM, Boyce S, Webb JK, Williams AR, Carlson EJ, Hill RG, Borkowski JA, Hess JF. Effects of the bradykinin B1 receptor antagonist des-Arg9[Leu8]bradykinin and genetic disruption of the B2 receptor on nociception in rats and mice. Pain. 1997;71:89–97. doi: 10.1016/s0304-3959(97)03343-5. [DOI] [PubMed] [Google Scholar]
- 55.Huganir RL, Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990;5:555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- 56.Khasar SG, Lin YH, Martin A, et al. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PubMed] [Google Scholar]
- 57.Seabrook GR, Bowery BJ, Heavens R, et al. Expression of B1 and B2 bradykinin receptor mRNA and their functional roles in sympathetic ganglia and sensory dorsal root ganglia neurones from wild-type and B2 receptor knockout mice. Neuropharmacology. 1997;36:1009–1017. doi: 10.1016/s0028-3908(97)00065-8. [DOI] [PubMed] [Google Scholar]
- 58.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 59.Holton FA, Holton P. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. J Physiol (London) 1954;126:124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brake A, Schumacher M, Julius D. ATP receptors in sickness, pain and death. Chem Biol. 1996;3:229–232. doi: 10.1016/s1074-5521(96)90101-5. [DOI] [PubMed] [Google Scholar]
- 61.Paukert M, Osteroth R, Geisler HS, Brandle U, Glowatzki E, Ruppersberg JP, Grunder S. Inflammatory Mediators Potentiate ATP-gated Channels through the P2X3 Subunit. J Biol Chem. 2001;276:21077–21082. doi: 10.1074/jbc.M101465200. [DOI] [PubMed] [Google Scholar]
- 62.Chu CJ, Huang SM, De Petrocellis L, et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- 63.De Petrocellis L, Chu CJ, Moriello AS, Kellner JC, Walker JM, Di Marzo V. Actions of two naturally occurring saturated N-acyldopamines on transient\ receptor potential vanilloid 1 (TRPV1) channels. Br J Pharmacol. 2004;143:251–256. doi: 10.1038/sj.bjp.0705924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koltzenburg M. The changing sensitivity in the life of the nociceptor. Pain. 1999;(Suppl 6):S93–102. doi: 10.1016/S0304-3959(99)00142-6. [DOI] [PubMed] [Google Scholar]
- 65.McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nature Med. 1995;1:774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- 66.Lewin GR, Mendell LM. Regulation of cutaneous C-fiber heat nociceptors by nerve growth factor in the developing rat. J Neurophysiol. 1994;71:941–949. doi: 10.1152/jn.1994.71.3.941. [DOI] [PubMed] [Google Scholar]
- 67.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 68.Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–135. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 69.Aloe L, Tuveri MA, Carcassi U, Levi-Montalcini R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum. 1992;35:351–355. doi: 10.1002/art.1780350315. [DOI] [PubMed] [Google Scholar]
- 70.Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nature Genet. 1996;13:485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- 71.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 72.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xue Q, Jong B, Chen T, Schumacher MA. Transcription of rat TRPV1 utilizes a dual promoter system that is positively regulated by nerve growth factor. J Neurochem. 2007;101:212–222. doi: 10.1111/j.1471-4159.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 74.Amaya F, Oh-hashi K, Naruse Y, et al. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- 75.Amaya F, Shimosato G, Nagano M, et al. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20:2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- 76.Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winter J, Forbes CA, Sternberg J, Lindsay RM. Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to the excitotoxin capsaicin. Neuron. 1988;1:973–981. doi: 10.1016/0896-6273(88)90154-7. [DOI] [PubMed] [Google Scholar]
- 78.Petruska JC, Mendell LM. The many functions of nerve growth factor: multiple actions on nociceptors. Neurosci Lett. 2004;361:168–171. doi: 10.1016/j.neulet.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 79.Sherrington CS. The integrative action of the nervous system. New York: C. Scribner’s Sons; 1906. [Google Scholar]
- 80.Szolcsanyi J, Jancso-Gabor A, Joo F. Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1975;287:157–169. doi: 10.1007/BF00510447. [DOI] [PubMed] [Google Scholar]
- 81.Yaksh TL, Farb DH, Leeman SE, Jessell TM. Intrathecal capsaicin depletes substance P in the rat spinal cord and produces prolonged thermal analgesia. Science. 1979;206:481–483. doi: 10.1126/science.228392. [DOI] [PubMed] [Google Scholar]
- 82.Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 83.Robbins WR, Staats PS, Levine J, Fields HL, Allen RW, Campbell JN, Pappagallo M. Treatment of intractable pain with topical large-dose capsaicin: preliminary report. Anesth Analg. 1998;86:579–583. doi: 10.1097/00000539-199803000-00027. [DOI] [PubMed] [Google Scholar]
- 84.Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent\ reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18:8947–8959. doi: 10.1523/JNEUROSCI.18-21-08947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watson CP, Tyler KL, Bickers DR, Millikan LE, Smith S, Coleman E. A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin Ther. 1993;15:510–526. [PubMed] [Google Scholar]
- 86.Kingery WS. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1997;73:123–139. doi: 10.1016/S0304-3959(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 87.Ellison N, Loprinzi CL, Kugler J, et al. Phase III placebo-controlled trial of capsaicin cream in the management of\ surgical neuropathic pain in cancer patients. J Clin Oncol. 1997;15:2974–2980. doi: 10.1200/JCO.1997.15.8.2974. [DOI] [PubMed] [Google Scholar]
- 88.Zhang WY, Li Wan Po A. The effectiveness of topically applied capsaicin. A meta-analysis. Eur J Clin Pharmacol. 1994;46:517–522. doi: 10.1007/BF00196108. [DOI] [PubMed] [Google Scholar]
- 89.Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–145. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 91.Denda M, Fuziwara S, Inoue K, et al. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun. 2001;285:1250–1252. doi: 10.1006/bbrc.2001.5299. [DOI] [PubMed] [Google Scholar]
- 92.Inoue K, Koizumi S, Fuziwara S, Denda S, Denda M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem Biophys Res Commun. 2002;291:124–129. doi: 10.1006/bbrc.2002.6393. [DOI] [PubMed] [Google Scholar]
- 93.Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther. 2003;304:217–222. doi: 10.1124/jpet.102.040675. [DOI] [PubMed] [Google Scholar]
- 94.Pecze L, Szabo K, Szell M, et al. Human keratinocytes are vanilloid resistant. PLoS One. 2008;3:e3419. doi: 10.1371/journal.pone.0003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ko F, Diaz M, Smith P, et al. Toxic effects of capsaicin on keratinocytes and fibroblasts. J Burn Care Rehabil. 1998;19:409–413. doi: 10.1097/00004630-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 96.Hail N, Jr, Lotan R. Examining the role of mitochondrial respiration in vanilloid- induced apoptosis. J Natl Cancer Inst. 2002;94:1281–1292. doi: 10.1093/jnci/94.17.1281. [DOI] [PubMed] [Google Scholar]
- 97.Stander S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471–478. doi: 10.1067/mjd.2001.110059. [DOI] [PubMed] [Google Scholar]
- 98.Stander S, Moormann C, Schumacher M, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 99.Wist E, Risberg T. Topical capsaicin in treatment of hyperalgesia, allodynia and dysesthetic pain caused by malignant tumour infiltration of the skin. Acta Oncol. 1993;32:343. doi: 10.3109/02841869309093606. [DOI] [PubMed] [Google Scholar]
- 100.Paice JA, Ferrans CE, Lashley FR, Shott S, Vizgirda V, Pitrak D. Topical capsaicin in the management of HIV-associated peripheral neuropathy. J Pain Symptom Manage. 2000;19:45–52. doi: 10.1016/s0885-3924(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 101.Malmberg AB, Mizisin AP, Calcutt NA, von Stein T, Robbins WR, Bley KR. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain. 2004;111:360–367. doi: 10.1016/j.pain.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 102.Backonja M, Wallace MS, Blonsky ER, et al. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008;7:1106–1112. doi: 10.1016/S1474-4422(08)70228-X. [DOI] [PubMed] [Google Scholar]
- 103.Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70:2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- 104.Chanda S, Bashir M, Babbar S, Koganti A, Bley K. In vitro hepatic and skin metabolism of capsaicin. Drug Metab Dispos. 2008;36:670–675. doi: 10.1124/dmd.107.019240. [DOI] [PubMed] [Google Scholar]
- 105.Babbar S, Marier JF, Mouksassi MS, Beliveau M, Vanhove GF, Chanda S, Bley K. Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic pain. Ther Drug Monit. 2009;31:502–510. doi: 10.1097/FTD.0b013e3181a8b200. [DOI] [PubMed] [Google Scholar]
- 106.Aasvang EK, Hansen JB, Malmstrom J, Asmussen T, Gennevois D, Struys MM, Kehlet H. The effect of wound instillation of a novel purified capsaicin formulation on postherniotomy pain: a double-blind, randomized, placebo-controlled study. Anesth Analg. 2008;107:282–291. doi: 10.1213/ane.0b013e31816b94c9. [DOI] [PubMed] [Google Scholar]
- 107.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 108.Binshtok AM, Gerner P, Oh SB, et al. Coapplication of lidocaine and the permanently charged sodium channel blocker\ QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111:127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Szallasi A, Blumberg PM. Vanilloid receptors: new insights enhance potential as a therapeutic target. Pain. 1996;68:195–208. doi: 10.1016/s0304-3959(96)03202-2. [DOI] [PubMed] [Google Scholar]
- 110.Mishra SK, Hoon MA. Mol Cell Neurosci. 2009. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation; p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Neubert JK, Karai L, Jun JH, Kim HS, Olah Z, Iadarola MJ. Peripherally induced resiniferatoxin analgesia. Pain. 2003;104:219–228. doi: 10.1016/s0304-3959(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 112.Kissin I. Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity. Anesth Analg. 2008;107:271–281. doi: 10.1213/ane.0b013e318162cfa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karai L, Brown DC, Mannes AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neubert JK, Mannes AJ, Karai LJ, et al. Perineural resiniferatoxin selectively inhibits inflammatory hyperalgesia. Mol Pain. 2008;4:3. doi: 10.1186/1744-8069-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dray A, Forbes CA, Burgess GM. Ruthenium red blocks the capsaicin-induced increase in intracellular calcium and activation of membrane currents in sensory neurones as well as the activation of peripheral nociceptors in vitro. Neurosci Lett. 1990;110:52–59. doi: 10.1016/0304-3940(90)90786-9. [DOI] [PubMed] [Google Scholar]
- 116.Bevan S, Hothi S, Hughes G, et al. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kwak JY, Jung JY, Hwang SW, Lee WT, Oh U. A capsaicin-receptor antagonist, capsazepine, reduces inflammation-induced hyperalgesic responses in the rat: evidence for an endogenous capsaicin-like\ substance. Neuroscience. 1998;86:619–626. doi: 10.1016/s0306-4522(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 118.Walker KM, Urban L, Medhurst SJ, et al. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- 119.Chizh BA, O’Donnell MB, Napolitano A, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 120.Cui M, Honore P, Zhong C, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gavva NR, Tamir R, Qu Y, et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther. 2005;313:474–484. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- 122.Honore P, Wismer CT, Mikusa J, et al. A-425619 [1-Isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a Novel Transient Receptor Potential Type V1 Receptor Antagonist, Relieves Pathophysiological Pain Associated with Inflammation and Tissue Injury in Rats. J Pharmacol Exp Ther. 2005;314:410–421. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- 123.Story GM, Peier AM, Reeve AJ, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 124.Jordt SE, Bautista DM, Chuang HH, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 125.Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A. 2008;105:8784–8789. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eilers H. Anesthetic Activation of Nociceptors: Adding Insult to Injury? Mol Interv. 2008:8. doi: 10.1124/mi.8.5.6. [DOI] [PubMed] [Google Scholar]
- 127.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Macpherson LJ, Dubin AE, Evans MJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 129.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kobayashi K, Fukuoka T, Obata K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 131.Trevisani M, Siemens J, Materazzi S, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- 133.Obata K, Katsura H, Mizushima T, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kwan KY, Allchorne AJ, Vollrath MA, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 135.Petrus M, Peier AM, Bandell M, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eid SR, Crown ED, Moore EL, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and\ neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Razavi R, Chan Y, Afifiyan FN, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 138.Zhang LL, Yan Liu D, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]