Abstract
IRBIT (IP3Rs binding protein released with IP3) is a protein originally identified by the Mikoshiba group as an inhibitor of IP3 receptors function. Subsequently it was found to have multiple functions and regulate the activity of diverse proteins, including regulation of HCO2− transporters to coordinate epithelial HCO3− secretion and to determine localization of the Fip1 subunit of the CPSF complex to regulate mRNA processing. This review highlights the remarkably divers functions of IRBIT that are likely only a fraction of all the potential functions of this protein.
Keywords: IRBIT, IP3 receptors, NBCe1-B, CFTR, NHE3, Fip1
Introduction
IRBIT was discovered multiple times in different contexts but it was not until its re-discovery by the Mikoshiba group [4] that we begun to understand its function and appreciate its potential in regulating a remarkably divers physiological systems that do not have any component in common besides their regulation by IRBIT.
IRBIT was identified as a protein induced during antigen-presenting Dendritic cells (DCs) differentiation [16] and as a gene that is down-regulated in response to treatment with DNA damaging agents [54]. The first function of IRBIT found is interaction with the IP3 receptors (IP3Rs) that is regulated by IP3 and was thus renamed IRBIT (for IP3Rs binding protein released with IP3) [4]. Interaction of IRBIT with IP3Rs turned to play important role in Ca2+ signaling and through this interaction IRBIT regulates the function of the IP3Rs [3, 18]. The Mikoshiba group then discovered that IRBIT regulates the activity of the ubiquitous Na+-HCO3− co-transporter NBCe1-B [52], that has important roles in epithelial HCO3− secretion [9, 41, 53, 59]. More recently, the Mikoshiba group reported an important role for IRBIT in mRNA polyadenylation to regulate gene expression [28]. These finding probably describe only a fraction of the functions regulated by IRBIT that are suggested by its known domains. Discovering these functions and the relationship between them will be a challenge for the future.
The IRBIT Domains
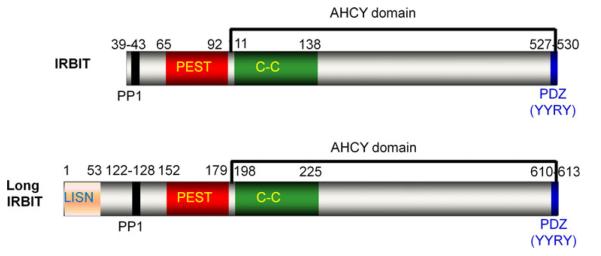
Two IRBIT isoforms have been identified; a short IRBIT and a long IRBIT with high homology to short IRBIT but with an N terminal extension [5]. In this review we refer to short IRBIT as IRBIT and specify when referring to long IRBIT. Fig. 1 depicts the IRBIT and long IRBIT domains identified so far. The IRBITs form the AHCYL subfamily of the adenosyl homocysteinase (AHCY) supperfamily by virtue of their C terminus AHCY domain. The AHCY members have S-adenosyl-l-homocystein hydrolase activity that catalyzes the reversible hydrolysis of S-adenosyl-l-homocystein to adenosine and L-homocysteine to maintain low cellular activity of S-adenosyl-l-homocystein [55]. Accumulation of S-adenosyl-l-homocystein is cytotoxic due to inhibition of DNA and RNA methylation [50]. However, the IRBITs AHCY domain does not have s-adenosyl-l-homocystein hydrolase activity, and thus its role in IRBIT function is not known at present.
Fig. 1.
The IRBIT domains. The figure shows the boundaries of the domain identified so far in short (upper) and long (lower) IRBITs
The two important domains within the AHCY domain are a coiled-coil domain and a PDZ ligand [19]. At the N terminus the IRBITs have a PP1 anchoring site that is followed by a PEST domain [17, 19]. The PEST domain has multiple phosphorylation sites [3] that have a permissive role in all known functions of IRBIT, regulation of IP3 receptors function [3, 18], activation of NBCe1-B [52, 59] and of the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) [59], and mRNA polyadenylation [28]. Long IRBIT has an N terminal extension rich in proline and alanine and was termed LISN, for Long IRBIT Specific N terminal domain [5], which plays a critical role in its function (see below).
IRBITs Expression Pattern
IRBIT is ubiquitous with high levels in the cerebrum and the cerebellum [4], site of high expression levels of IP3 receptors [42, 44]. Relatively high IRBIT expression levels are also found in epithelial tissues such as testis, ovaries, lung, kidney, and spleen [4]. However, IRBIT expression levels appear dynamic and vary with the cellular physiological state. For example, freshly isolated Dendritic and Langerhans cells express low, while activated cells express high IRBIT mRNA levels. Furthermore, the high IRBIT mRNA level is mainly in dermal Dendritic cells [16]. IRBIT levels also change during development. IRBIT mRNA level increases during zebrafish embryogenesis and interference with IRBIT expression level changes the zebrafish morphogenesis [7].
Long IRBIT is expressed in most tissues and, as with IRBIT, the highest levels are found in the cerebrum and cerebellum with high levels in epithelia like the kidney, testis and ovary, and lower levels in the lung and liver [5]. However, long IRBIT is expressed at a much lower levels than IRBIT in all tissues in which they are co-expressed [5], suggesting that their roles are not complementary but rather unique. This is further emphasized in their cell-specific expression in the brain. Long IRBIT is expressed at high levels mainly in cerebellar molecular layer interneurones, while IRBIT is mainly expressed in glial cells [5].
Another level of differential expression is by localization of the IRBITs in cellular domains. When expressed in HeLa cells IRBIT is found in the cytosol and in the ER, most likely associated with IP3 receptors [4, 18]. IRBIT can undergo cleavage within the PEST domain at residue 72 [18]. N terminally cleaved IRBIT translocates to the nucleus [4], where it may regulate gene expression [19]. In addition, IRBIT phosphorylation appears to determine its cellular localization. IRBIT and long IRBIT have multiple phosphorylation sites located in the PEST domain [3, 5]. Phosphorylation of long IRBIT increases during neuronal differentiation and, notably, IRBIT phosphorylation enhances its interaction with the plasma membrane [5]. This may account in part for the accumulation of IRBIT at the apical pole of the polarized pancreatic duct [59]. Like most epithelia [29], the ducts express high level of IP3Rs at the apical pole [34]. The combination of high level of IP3Rs at the apical pole, together with IRBIT phosphorylation may lead to the constitutive high level of IRBIT at the apical pole of the duct. The physiological significance of such localization is discussed bellow.
The IRBITs and the Function of the IP3 Receptors
IRBIT importance was highlighted by the seminal discovery by the Mikoshiba group that IRBIT interacts with the IP3 receptors and can thus regulates Ca2+ signaling [4]. The receptor-evoked Ca2+ signal is initiated by receptor-mediated activation of PLC, hydrolysis of PIP2 and release of IP3. IP3 activates the ER-resident Ca2+ channels, IP3Rs to rapidly release Ca2+ stored in the ER. This event initiates all forms of Ca2+ signaling, including Ca2+ oscillations and Ca2+ waves [29, 44]. ER Ca2+ release by the IP3Rs is followed by activation of Ca2+ influx channels at the plasma membrane that are mediated by the STIM1-regulated Orai and TRPC channels [32, 56]. Ca2+ is then cleared from the cytosol partially by its extrusion through the PMCA type plasma membrane Ca2+ pumps and partially by its reuptake into the ER by the SERCA type ER Ca2+ pumps [29]. The Ca2+ signal is usually initiated at a defined microdomain and spreads to other parts of the cell in the form of propagated Ca2+ waves. Periodical repeat of the Ca2+ release and Ca2+ uptake cycle results in Ca2+ oscillations [10, 29].
Since the discovery of the neuronal IP3R by Mikoshiba et al. [23], this group has been exploring the regulation and physiological roles of the channels [43, 45]. There are three IP3 receptors that are expressed in a tissue specific manner, with IP3R1 expressed at high levels in neuronal tissues and IP3R2 and IP3R3 expressed at high levels in epithelial tissues [44, 61]. The IP3Rs have several well defined domains, including the C terminus channel pore domain and the N terminus IP3 binding domain (residues 224–604 in IP3R1) [42, 44, 61], which consists of a β domain (residues 224–436) and an α domain (residues 437–604) [43]. Mutation analysis and resolving the structure of the IP3 binding domain [12, 60] identified 12 amino acids in the IP3 binding domain of IP3R1 that coordinate IP3 binding.
In a proteomic approach the Mikoshiba group isolated IRBIT as a protein that directly interacts with the N terminus of the IP3R1. The key finding of the initial study was that IP3 dissociated IRBIT from the IP3 binding domain of the IP3Rs with an apparent affinity of about 0.5 μM, thus the name IRBIT: IP3R Binding protein released with inositol 1,4,5 Trisphosphate [4]. IRBIT binds to the full-length IP3Rs and to the IP3 binding domain of the receptor and the binding requires phosphorylation of IRBIT [4, 18]. Further analysis revealed that expressed IRBIT is found in the cytosol and the ER in HeLa cells. Deletion of the first 104 residues resulted in the complete translocation of IRBIT to the nucleus [4]. This may have physiological significance since it was reported that native IRBIT can be cleaved in vivo at residue 72 located within the PEST domain to translocate the truncated IRBIT to the nucleus [18]. However, the functional significance of nuclear IRBIT has not been directly examined, a topic worth careful and extensive examination.
In a careful follow-up study the Mikoshiba group showed that IRBIT and IP3 compete for binding to the same site on IP3Rs, with IRBIT right shifting the dose response for: (a) binding of IP3 to the IP3Rs, (b) activation of the IP3Rs channel activity by IP3 and c) in Ca2+ release from ER stores [3]. This was confirmed in independent study by Devogelaere et al., with measurement of IP3 binding to purified IP3R1 and IP3-mediated Ca2 release in permeabilized cells [18, 19]. These effects required phosphorylation of IRBIT at multiple sites at the PEST domain. Thus, mutations of S68, S70, S71, S72, S74 and S77 to Alanine inhibited interaction of IRBIT with the IP3Rs and its effect on IP3-mediated Ca2+ release [3]. Among all the sites, phosphorylation of S68 appears to be a key phosphorylation site. Preventing phosphorylation of S68 is sufficient to prevent the effect of IRBIT on all its known targets [3, 17, 24, 28, 59]. Phosphorylation of S68 is necessary for phosphorylation of S71 and S74 and phosphor-ylation of S71 and S74 appears to be sufficient for interaction and inhibition of IRBIT by the IP3Rs [17, 18]. S68 is the target of Protein Phosphatase 1 (PP1) [17].
To date the kinases that phosphorylate the native IRBIT in vivo are not known with certainty. IRBIT is predicted to be phosphorylated by the Ca2+-dependent kinases PKD, AMPK, CamK-II-IV and CK1 [19]. Analysis of synaptic phosphoproteins suggested that IRBIT is constitutively phosphorylated at T82, S84 and S85 [14], all of which are different from the major phosphorylation sites identified as the sites regulating interaction of IRBIT with the IP3Rs [3], and thus their significance remain unknown. In addition to the need to determine the kinases that phosphorylate IRBIT in vivo, it is necessary to determine the physiological conditions leading to IRBIT phosphorylation and dephosphorylation.
The study of Devogelaere et al. revealed novel aspects of the regulation by IRBIT. First, they identified a PDZ ligand at the C terminus of IRBIT that was essential for its interaction with the IP3Rs [18]. The PDZ ligand was found to be required for regulation of NBCe1-B and CFTR by IRBIT [59]. These findings indicate that IRBIT is present in complexes with its targets that are assembled by PDZ domains-containing scaffold proteins. Formation of the complexes likely facilitates the direct interaction of IRBIT with its targets to regulate their function. The identity of the scaffolding proteins and whether the same or different scaffolds mediate the various IRBIT complexes is not known at present. IP3Rs interact directly or indirectly with several scaffold proteins that have PDZ domains, like protein 4.1 [22, 62] and Shank [51] and CFTR interact with multiple PDZ domains-containing scaffolds [35]. Second, Devogelaere et al. identified the PP1 binding ligand KQIQF in IRBIT. Binding of PP1 to this site specifically dephosphorylates S68 [17].
The IP3Rs-PDZ scaffold-IRBIT-PP1/kinase complex allows for multiple regulatory events and for flexibility in IRBIT action. In the resting state the majority of IRBIT is likely bound to the IP3Rs to reduce the spontaneous activity of the receptor and Ca2+ leak. Recent studies pointed to the physiological importance of IP3Rs-mediated Ca2+ leak in energy metabolism [13]. In this manner IP3Rs serve to buffer the level of IRBIT available for regulation of other cellular processes and associate these processes with Ca2+ signaling. Cell stimulation that leads to generation of IP3 can lead to shuttle of IRBIT between its targets, depending on the levels of IP3 and the PDZ domain scaffold with which IRBIT and the target proteins interact. Future studies are likely to explore in more detail the role of the scaffolds and the IRBIT shuttle in determining the specificity of IRBIT action and the relationship between the activities of the various IRBIT targets.
IRBIT and Na+-HCO3− Co-Transport
Other transporters regulated by IRBIT with particular importance in epithelial electrolyte, fluid and HCO3− secretion are the Na+-HCO3− co-transporters (NBC) [52]. The NBCs are members of the supperfamily of Na+-coupled HCO3− transporters (NCBT) [11]. The NCBT supperfamily includes the electrogenic NBCe1-A-C and NBCe2a,c and electroneutral NBCn1-A-H and NBCn2-A-D Na+-HCO33 co-transporters and the electroneutral Na+-dependent Cl−/HCO3− exchangers NDCBE-A-D. The domain structure, homology, conservation and specific function of the NCBTs were extensively discussed in [11, 49] and will not be discussed here. The importance for our discussion is the most N terminal domain of the NCBTs comprising between 45–62 residues. This variable domain is found in several, but not all, members of the supperfamily and also varies among isoforms of the same subfamily, including the NBCe1 subfamily [11]. The N terminal extension forms an inhibitory domain and when deleted results in marked activation of the relevant NCBTs [39].
As their name indicates, the NCBTs use the energy in the Na+ gradient to transport HCO−3. Notable members are the three NBCe1 co-transporters. NBCe1-A is expressed almost exclusively in the kidney (also known as kNBC1) and functions as an electrogenic 1Na+/3HCO3− co-transporter at the basolateral membrane to mediate Na+-HCO3− efflux [11, 49]. NBCe1-B is nearly ubiquitous and is found at high levels at the basolateral membrane of all epithelia [49]. It was discovered as the Na+-HCO3− co-transporter in the pancreas (also known as pNBC1) [1]. Na+-HCO3− co-transport was described as the major HCO3− transport mechanism at the basolateral membrane of the pancreatic duct [63] and was later shown to mediate the majority of basolateral HCO3− influx during stimulated ductal HCO3− secretion [26, 53]. Na+-HCO3− co-transport is also important in other epithelial HCO3− secretion, such as salivary glands [36, 64], intestine [8, 9] and the airway [31]. There is little information on the function of NBCe1-C except that it functions as a 1Na+/2HCO3− co-transporter [39], and it is brain specific [11] (also known as bNBC1). The NBCe1-C stoichiometry was determined in Xenopus oocytes and it is not clear whether this stoichiometry is maintained in native cells, although a Na+-HCO3− co-transport activity with 1:2 stoichiometry was reported in glia [15] and astrocytes [46].
While searching for IRBIT-interacting proteins by identifying proteins that co-immunoprecipitate with IRBIT in a membranous brain extract, the Mikoshiba group isolated NBCe1-B as an IRBIT-interacting protein [52]. Structure-function analysis revealed that the unique N terminal domain of NBCe1-B (residues 1-62) interacts with IRBIT. The interaction required phosphorylation of the PEST domain at S68, S71, S74 and S77 [52]. Indeed, deletion of the PEST domain eliminated interaction of NBCe1-B with IRBIT, while deletion of the coiled-coil domain and the PDZ ligand weakened the interaction [59]. Most importantly, when expressed in Xenopus oocytes NBCe1-B had low basal activity and IRBIT markedly increased its activity [52]. Moreover, NBCe1-A, which lacks the N terminal extension of NBCe1-B, had high basal activity and was not further activated by IRBIT [52].
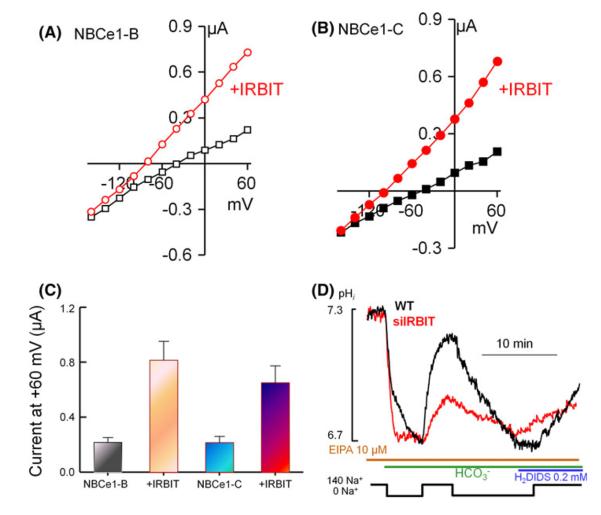
Activation of NBCe1-B by IRBIT when expressed in Xenopus oocytes is illustrated in Fig. 2a, c. NBCe1-C has the same N terminal extension as NBCe1-B with only a single residue not conserved. Interestingly, deletion of the NBCe1-B [52] and NBCe1-C [39] N terminal domain results in activation of both transporters, suggesting that both are regulated by IRBIT. Fig. 2 shows that this is indeed the case. Fig. 2b, c shows that the activity of NBCe1-C in Xenopus oocytes is low and it is markedly activated by IRBIT. Finally, Fig. 2d shows that knockdown of IRBIT in the pancreatic duct inhibits the native NBCe1-B activity, indicating that IRBIT regulates the native NBCe1-B. Ductal NBCe1-B activity was evaluated by measuring the Na+-mediated recovery from intracellular acidification due to incubation in HCO3−-buffered medium in the absence of external Na+. The contribution of the Na+/H+ exchangers was inhibited with EIPA and the sensitivity of the alkalinization to H2DIDS indicated that it is mediated by NBCe1-B. We note that other members of the Na+-driver HCO3− transporters have an N terminal domain similar to that of NBCe1-B [11], which predicts that their activity should also be regulated by IRBIT. This was suggested for NBCn1 and NDCBE [11], although it is not clear which isoform is activated by IRBIT. The N terminal extension of NBCn1-A-H and NDCBE-A-D differ substantially.
Fig. 2.
Activation of NBCe1 by IRBIT. Xenopus oocytes were injected with the cRNA for NBCe1-B (a) or NBCe1-C (b) and with (red) or without IRBIT (black) and the Na+-dependent HCO3− current was measured. c is the summary. In (d) native NBCe1-B activity was isolated by measuring pHi in sealed pancreatic ducts treated with scrambled (black trace) or IRBIT siRNA (red trace). NBCe1-B was isolated by inhibiting NHE with Ethyl-Isopropyl-Amiloride (EIPA) and following the Na+-dependent and di-isothiocyanostilbene disulfonate (DIDS)-sensitive recovery from an acid load. Panel (d) was reproduced from [59]
Structure-function analysis indicates that the N terminal domain of several Na+-coupled HCO33 transporters (NCBTs), including NBCe1-B and NBCe1-C, serves as an inhibitory domain to reduce the activity of the transporters [39]. Binding of IRBIT phosphorylated in positions S68, S71, S74 and S77 to this domain relieve the inhibition, resulting in activation of the transporters. How might IRBIT interact with the NCBTs to activate them? This is not known at present, although a clue is provided by the effect of Mg2+ and polycations [58] and of PIP2 [57] on NBCe1-B activity. The N terminus of NBCe1-B is highly charged with a cluster of negatively charges residues at region 2-24 and a cluster of positively charges residues at region 37-59 [19]. Interaction of IRBIT with NBCe1-B is mediated by the negatively charged IRBIT PEST domain [3, 59]. Thus, it is possible that the PEST domain interacts with NBCe1-B residues 37-59 by an electrostatic interaction.
Regulation by electrostatic interaction is supported by the effect of Mg2+, neomysin and PIP2 on NBCe1-B activity. Mg2+ strongly inhibits NBCe1-B with a Ki of 0.01 mM. Deletion of the IRBIT-binding N terminal domain of NBCe1-B increased the Ki for Mg2+ inhibition by 30 folds [58]. Thus, Mg2+ has two inhibitory effects on NBCe1-B, a high affinity inhibitory effect that requires the IRBIT binding domain and a low affinity inhibitory effect that is independent of the IRBIT-binding domain. The two inhibitory effects were not specific for Mg2+, but they are mimicked by the polycation neomycin [58]. It is possible that at low concentrations Mg2+ and neomycin interact with the negative charges at residues 2–24 of NBCe1-B to shield it and prevent interaction of IRBIT with the NBCe1-B N terminus. PIP2 also has dual effect on NBCe1-B [57]. In excised patches PIP2 activates NBCe1-A and prevents its rundown. However, in intact oocytes PIP2 does not activate NBCe1-A but it does activate NBCe1-B and NBCe1-C [57]. The combined findings suggest that PIP2 is required for the activity of the NBCe1. In addition, PIP2 interacts with the N terminal domain of NBCe1-B and NBCe1-C to prevent their auto-inhibition. PIP2 may do so by interacting with the positive charges at residues 37–59 of NBCe1-B auto-inhibitory domain.
IRBIT and Epithelial HCO3− Secretion
The critical finding of activation of NBCe1-B by IRBIT opened the way in understanding the mechanism and regulation of epithelial HCO3− secretion. Epithelial HCO3− secretion involves NBCe1-B-mediated HCO3− entry across the basolateral membrane. HCO3− is then secreted to the luminal side by the CFTR-SLC26 transporters complex in which the solute carrier family 26 (SLC26) Cl−/HCO3− exchangers secrete HCO3− in exchange for Cl−, while CFTR mainly re-circulates the Cl− to maintain the HCO3− secretory process [21, 30]. In some tissues, like the airway [20] and the distal pancreatic duct [47] CFTR can also secrete some of the HCO3−. It was then not unexpected that IRBIT has a prominent role in epithelial HCO3− secretion. Knockdown of IRBIT markedly inhibited pancreatic duct fluid and HCO3− secretion [59].
Inhibition of ductal fluid and HCO3− secretion by knockdown of IRBIT can be due to inhibition of NBCe1-B (see Fig. 2d) and also due to inhibition of luminal HCO3− transporters. To examine whether IRBIT regulates both basolateral HCO3− entry and luminal HCO3− exit we tested the effect of IRBIT on the luminal transporters. IRBIT activates native CFTR in the pancreatic duct and the recombinant CFTR by increasing CFTR open probability due to a reduction in CFTR interburst duration [59]. Interestingly, although activation of CFTR by IRBIT required phosphorylation of S68 [59], the mode of interaction of IRBIT with CFTR is different from that of NBCe1-B. Thus, deletion of any single IRBIT domain, the PEST, coiled-coil or the PDZ ligand, did not prevent interaction with CFTR. Preventing the interaction required deletion of the combined PDZ ligand and the PEST or PDZ ligand and the coiled-coil domains. However, deletion of any of the IRBIT domains prevented activation of CFTR by IRBIT [59], suggesting that another unknown protein is required for activation of CFTR by IRBIT. The findings in the pancreatic duct and with expressed NBCe1-B and CFTR lead to the model in Fig. 3 for coordination of epithelial fluid and HCO3− secretion by IRBIT.
Fig. 3.
IRBIT coordinates pancreatic duct fluid and HCO3− secretion. The model shows the key transporters involved in pancreatic duct fluid and HCO3− salvage and secretion. In the resting state, IRBIT forms complexes with the luminal NHE3 and NBCn1 to salvage leaked fluid and HCO3−. Cell stimulation results in formation of complexes between IRBIT and the basolateral NBCe1-B and the luminal CFTR that are assembled by PDZ domains-containing scaffolding proteins. The interaction is mediated by the IRBIT PEST domain (red circles) and requires the IRBIT coiled-coil domain (green circles). In the case of CFTR, an additional unknown protein (marked as protein X) is needed for activation of CFTR by IRBIT. Activation of NBCe1-B and CFTR by IRBIT coordinates HCO3− entry at the basolateral membrane and HCO3− exit at the luminal membrane to ensure the fidelity of epithelial fluid and HCO3− secretion
Another effect of IRBIT on ion transport reported recently is modest activation of the Na+/H+ exchanger isoform 3 (NHE3) [24]. The activation appears to be Ca2+ dependent [25], requires phosphorylation of S68, S71 and S74 and may mediate activation of NHE3 by Angiotensin II [24]. NHE3 is prominently expressed in the proximal tubule luminal membrane, where it mediates the bulk of renal Na+ absorption and H+ secretion [6]. NHE3 is also expressed in the luminal membrane of the pancreatic [33] and salivary glands duct [36], in which it was suggested to mediate HCO3− salvage at the resting state. Another Na+-dependent HCO3− salvage mechanism in the duct is NBCn1 (also known as NBC3) [48], which can also be activated by IRBIT (see above). NHE3 can salvage HCO3− by secretion of H+ to the duct lumen that then interacts with luminal HCO3− to generate CO2 that diffuses into the duct to be converted to HCO3−. HCO3− salvage by NBCn1 is by direct absorption of the leaked HCO3− from the duct lumen. Interestingly, NHE3 [2] and NBCn1 [48] are inhibited by CFTR. Hence, it is possible that IRBIT coordinates HCO3− salvage at the resting state and HCO3− secretion at the stimulated state by shifting between NHE3/NBCn1 and CFTR as a result of duct stimulation. This is modeled in Fig. 3.
IRBIT and Other Cellular Functions
Other, less understood functions of IRBIT are the control of dendritic cells (DCs) maturation [16] and mRNA processing [28]. DCs are major cells in the immune system with central role in the inflammatory response. In response to foreign pathogens or to inflammation DCs undergo maturation to secret cytokines, migrate to lymph nodes and present the major histocompetability complexes to T cells [40]. IRBIT mRNA is markedly increased during DCs maturation [16]. However, the role of IRBIT in DCs maturation is not understood at any level.
Recently, the Mikoshiba group reported that IRBIT regulates pre-mRNA processing and thus gene regulation [28]. Processing of pre-mRNA at the 3' end is mediated by cleavage and polyadenylation [37]. Cleavage is mediated by the cleavage and polyadenylation specific factor (CPSF) complex [38]. The CPSF complex contains the Fip1 (factor interacting with PAP1) subunit [27] that interacts with IRBIT [28]. Polyadenylation is catalyzed by poly(A) polymerase (PAP) [37]. IRBIT interaction with Fip1 requires phosphorylation of the serines in the PEST domain, including S68, and resulted in translocation of Fip1 from the nucleus to the cytoplasm [28]. Through the interaction with Fip1, IRBIT is recruited to PAP to reduce its activity and thus pre-mRNA polyadenylation. Regulation of pre-mRNA polyadenylation by IRBIT may be part of the cellular stress response since cell stress increases IRBIT phosphorylation and its interaction with CPSF [28].
Conclusions
The many diverse roles of IRBIT discussed here highlight this remarkable protein as a multifaceted cellular regulator. Undoubtedly, this is due to the multiple and versatile interactions mediated by its PEST domain that is required or mediate all the functions of IRBIT known to date. The PEST domain mediates interaction of IRBIT with IP3 receptors, the NDBTs, CFTR, NHE3 and Fip1 which do not have any domain in common. This would suggest that the PEST domain interacts with conformation that may have a similar fold in all the targets. Nevertheless, these diverse interactions highlight the versatility of the IRBIT PEST domain. The three additional IRBIT domains uncovered so far, the PP1 and PDZ binding ligands and the coiled-coil domain, most probably recruit additional regulators and factors to the IRBIT targets. There are likely additional functional/interacting domains within the IRBIT AHCY domain that increase the spectrum of IRBIT interactions and the physiological processed it regulates. It is quite clear that so far only a small fraction of the cellular functions mediated by IRBIT have been discovered and that we are only at the beginning of our understanding how IRBIT regulates all of these cellular functions and how the function of IRBIT is regulated in resting and stimulated states.
References
- 1.Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- 2.Ahn W, Kim KH, Lee JA, Kim JY, Choi JY, Moe OW, Milgram SL, Muallem S, Lee MG. Regulatory interaction between the cystic fibrosis transmembrane conductance regulator and HCO3− salvage mechanisms in model systems and the mouse pancreatic duct. J Biol Chem. 2001;276:17236–17243. doi: 10.1074/jbc.M011763200. [DOI] [PubMed] [Google Scholar]
- 3.Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006;22:795–806. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1, 4, 5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem. 2003;278:10602–10612. doi: 10.1074/jbc.M210119200. [DOI] [PubMed] [Google Scholar]
- 5.Ando H, Mizutani A, Mikoshiba K. An IRBIT homologue lacks binding activity to inositol 1, 4, 5-trisphosphate receptor due to the unique N-terminal appendage. J Neurochem. 2009;109:539–550. doi: 10.1111/j.1471-4159.2009.05979.x. [DOI] [PubMed] [Google Scholar]
- 6.Aronson PS. Ion exchangers mediating Na+, HCO3− and Cl− transport in the renal proximal tubule. J Nephrol. 2006;19(Suppl 9):S3–S10. [PubMed] [Google Scholar]
- 7.Ashworth R, Devogelaere B, Fabes J, Tunwell RE, Koh KR, De Smedt H, Patel S. Molecular and functional characterization of inositol trisphosphate receptors during early zebrafish development. J Biol Chem. 2007;282:13984–13993. doi: 10.1074/jbc.M700940200. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann O, Reichelt D, Tuo B, Manns MP, Seidler U. Carbachol increases Na+-HCO3− cotransport activity in murine colonic crypts in a M3−, Ca2+/calmodulin-, and PKC-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2006;291:G650–G657. doi: 10.1152/ajpgi.00376.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3− cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol. 2003;284:G37–G45. doi: 10.1152/ajpgi.00209.2002. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol. 2009;212:1697–1706. doi: 10.1242/jeb.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Ikura M. Structure of the inositol 1, 4, 5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 13.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins MO, Yu L, Coba MP, Husi H, Campuzano I, Blackstock WP, Choudhary JS, Grant SG. Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem. 2005;280:5972–5982. doi: 10.1074/jbc.M411220200. [DOI] [PubMed] [Google Scholar]
- 15.Deitmer JW, Schlue WR. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker JW, Budhia S, Angel NZ, Cooper BJ, Clark GJ, Hart DN, Kato M. Identification of an S-adenosylhomocysteine hydrolase-like transcript induced during dendritic cell differentiation. Immunogenetics. 2002;53:993–1001. doi: 10.1007/s00251-001-0402-z. [DOI] [PubMed] [Google Scholar]
- 17.Devogelaere B, Beullens M, Sammels E, Derua R, Waelkens E, van Lint J, Parys JB, Missiaen L, Bollen M, De Smedt H. Protein phosphatase-1 is a novel regulator of the interaction between IRBIT and the inositol 1, 4, 5-trisphosphate receptor. Biochem J. 2007;407:303–311. doi: 10.1042/BJ20070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devogelaere B, Nadif Kasri N, Derua R, Waelkens E, Callewaert G, Missiaen L, Parys JB, De Smedt H. Binding of IRBIT to the IP3 receptor: determinants and functional effects. Biochem Biophys Res Commun. 2006;343:49–56. doi: 10.1016/j.bbrc.2006.02.119. [DOI] [PubMed] [Google Scholar]
- 19.Devogelaere B, Sammels E, De Smedt H. The IRBIT domain adds new functions to the AHCY family. Bioessays. 2008;30:642–652. doi: 10.1002/bies.20772. [DOI] [PubMed] [Google Scholar]
- 20.Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 2008;23:104–114. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- 22.Fukatsu K, Bannai H, Inoue T, Mikoshiba K. 4.1N binding regions of inositol 1, 4, 5-trisphosphate receptor type 1. Biochem Biophys Res Commun. 2006;342:573–576. doi: 10.1016/j.bbrc.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1, 4, 5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 24.He P, Klein J, Yun CC. Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1, 4, 5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2010;285:27869–27878. doi: 10.1074/jbc.M110.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He P, Zhang H, Yun CC. IRBIT, inositol 1, 4, 5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem. 2008;283:33544–33553. doi: 10.1074/jbc.M805534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3− by Na(+)-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495(Pt 1):169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann I, Martin G, Friedlein A, Langen H, Keller W. Human Fip1 is a subunit of CPSF that binds to U-rich RNA elements and stimulates poly(A) polymerase. EMBO J. 2004;23:616–626. doi: 10.1038/sj.emboj.7600070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiefer H, Mizutani A, Iemura S, Natsume T, Ando H, Kuroda Y, Mikoshiba K. Inositol 1, 4, 5-triphosphate receptor-binding protein released with inositol 1, 4, 5-triphosphate (IRBIT) associates with components of the mRNA 3′ processing machinery in a phosphorylation-dependent manner and inhibits polyadenylation. J Biol Chem. 2009;284:10694–10705. doi: 10.1074/jbc.M807136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–459. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreindler JL, Peters KW, Frizzell RA, Bridges RJ. Identification and membrane localization of electrogenic sodium bicarbonate cotransporters in Calu-3 cells. Biochim Biophys Acta. 2006;1762:704–710. doi: 10.1016/j.bbadis.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010;584:2022–2027. doi: 10.1016/j.febslet.2009.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MG, Ahn W, Choi JY, Luo X, Seo JT, Schultheis PJ, Shull GE, Kim KH, Muallem S. Na(+)-dependent transporters mediate HCO(3)(−) salvage across the luminal membrane of the main pancreatic duct. J Clin Invest. 2000;105:1651–1658. doi: 10.1172/JCI9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Naren AP. CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners. Integr Biol (Camb) 2010;2:161–177. doi: 10.1039/b924455g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo X, Choi JY, Ko SB, Pushkin A, Kurtz I, Ahn W, Lee MG, Muallem S. HCO3− salvage mechanisms in the submandibular gland acinar and duct cells. J Biol Chem. 2001;276:9808–9816. doi: 10.1074/jbc.M008548200. [DOI] [PubMed] [Google Scholar]
- 37.Mandel CR, Bai Y, Tong L. Protein factors in pre-mRNA 3′-end processing. Cell Mol Life Sci. 2008;65:1099–1122. doi: 10.1007/s00018-007-7474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandel CR, Kaneko S, Zhang H, Gebauer D, Vethantham V, Manley JL, Tong L. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature. 2006;444:953–956. doi: 10.1038/nature05363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol. 2006;127:639–658. doi: 10.1085/jgp.200609520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 41.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 42.Mikoshiba K. Inositol 1, 4, 5-trisphosphate IP(3) receptors and their role in neuronal cell function. J Neurochem. 2006;97:1627–1633. doi: 10.1111/j.1471-4159.2006.03985.x. [DOI] [PubMed] [Google Scholar]
- 43.Mikoshiba K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp. 2007a:9–22. doi: 10.1042/BSS0740009. [DOI] [PubMed] [Google Scholar]
- 44.Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 45.Mikoshiba K, Hisatsune C, Futatsugi A, Mizutani A, Nakamura T, Miyachi K. The role of Ca2+ signaling in cell function with special reference to exocrine secretion. Cornea. 2008;27(Suppl 1):S3–8. doi: 10.1097/ICO.0b013e31817f246e. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor ER, Sontheimer H, Ransom BR. Rat hippocampal astrocytes exhibit electrogenic sodium-bicarbonate co-transport. J Neurophysiol. 1994;72:2580–2589. doi: 10.1152/jn.1994.72.6.2580. [DOI] [PubMed] [Google Scholar]
- 47.Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139:620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, Kurtz I. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3− salvage transporter human Na+-HCO3− cotransport isoform 3. J Biol Chem. 2002;277:50503–50509. doi: 10.1074/jbc.M201862200. [DOI] [PubMed] [Google Scholar]
- 49.Pushkin A, Kurtz I. SLC4 base (HCO3−, CO3 2−) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol. 2006;290:F580–F599. doi: 10.1152/ajprenal.00252.2005. [DOI] [PubMed] [Google Scholar]
- 50.Rocha PS, Sheikh M, Melchiorre R, Fagard M, Boutet S, Loach R, Moffatt B, Wagner C, Vaucheret H, Furner I. The Arabidopsis HOMOLOGY-DEPENDENT GENE SILENCING1 gene codes for an S-adenosyl-L-homocysteine hydrolase required for DNA methylation-dependent gene silencing. Plant Cell. 2005;17:404–417. doi: 10.1105/tpc.104.028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sala C, Roussignol G, Meldolesi J, Fagni L. Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J Neurosci. 2005;25:4587–4592. doi: 10.1523/JNEUROSCI.4822-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1, 4, 5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotrans-porter 1 (pNBC1) Proc Natl Acad Sci USA. 2006;103:9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 54.Wittig R, Nessling M, Will RD, Mollenhauer J, Salowsky R, Munstermann E, Schick M, Helmbach H, Gschwendt B, Korn B, Kioschis P, Lichter P, Schadendorf D, Poustka A. Candidate genes for cross-resistance against DNA-damaging drugs. Cancer Res. 2002;62:6698–6705. [PubMed] [Google Scholar]
- 55.Wnuk SF. Targeting “hydrolytic” activity of the S-adenosyl-L-homocysteine hydrolase. Mini Rev Med Chem. 2001;1:307–316. doi: 10.2174/1389557013406918. [DOI] [PubMed] [Google Scholar]
- 56.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu J, McNicholas CM, Bevensee MO. Phosphatidylinositol 4, 5-bisphosphate (PIP2) stimulates the electrogenic Na/HCO3 cotransporter NBCe1-A expressed in Xenopus oocytes. Proc Natl Acad Sci USA. 2009;106:14150–14155. doi: 10.1073/pnas.0906303106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi S, Ishikawa T. The electrogenic Na+-HCO3-cotransporter NBCe1-B is regulated by intracellular Mg2+ Biochem Biophys Res Commun. 2008;376:100–104. doi: 10.1016/j.bbrc.2008.08.104. [DOI] [PubMed] [Google Scholar]
- 59.Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009;119:193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshikawa F, Morita M, Monkawa T, Michikawa T, Furuichi T, Mikoshiba K. Mutational analysis of the ligand binding site of the inositol 1, 4, 5-trisphosphate receptor. J Biol Chem. 1996;271:18277–18284. doi: 10.1074/jbc.271.30.18277. [DOI] [PubMed] [Google Scholar]
- 61.Yule DI, Betzenhauser MJ, Joseph SK. Linking structure to function: recent lessons from inositol 1, 4, 5-trisphosphate receptor mutagenesis. Cell Calcium. 2010;47:469–479. doi: 10.1016/j.ceca.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S, Mizutani A, Hisatsune C, Higo T, Bannai H, Nakayama T, Hattori M, Mikoshiba K. Protein 4.1N is required for translocation of inositol 1, 4, 5-trisphosphate receptor type 1 to the basolateral membrane domain in polarized Madin-Darby canine kidney cells. J Biol Chem. 2003;278:4048–4056. doi: 10.1074/jbc.M209960200. [DOI] [PubMed] [Google Scholar]
- 63.Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3− transporters in the rat pancreatic duct. J Gen Physiol. 1994;104:57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao H, Xu X, Diaz J, Muallem S. Na+, K+, and H+/HCO3− transport in submandibular salivary ducts. Membrane localization of transporters. J Biol Chem. 1995;270:19599–19605. [PubMed] [Google Scholar]