SUMMARY
Midbrain dopamine (DA) neurons, which play critical roles in learning and motivated behaviors, are not homogeneous but differ in their molecular properties and responses to external stimuli. Here, we examined whether the modulation of excitatory synapses on DA neurons by rewarding or aversive stimuli depends on the respective brain area to which these DA neurons project. We identified specific DA neuron subpopulations in slices following in vivo injection of “Retrobeads” into single target areas of adult mice and found differences in basal excitatory synaptic properties. In vivo administration of cocaine selectively modified excitatory synapses (assayed by AMPAR/NMDAR ratios) on DA cells projecting to nucleus accumbens (NAc) medial shell while an aversive stimulus selectively modified synapses on DA cells projecting to medial prefrontal cortex. In contrast, synapses on DA neurons projecting to NAc lateral shell were modified by both rewarding and aversive stimuli, which presumably reflects saliency. These results suggest that the mesocorticolimbic DA system may be comprised of three anatomically distinct circuits each modified by distinct aspects of motivationally relevant stimuli.
Keywords: mesolimbic, prefrontal, ventral tegmental area, nucleus accumbens, reward, aversive, plasticity
INTRODUCTION
It is well accepted that midbrain dopamine (DA) neurons and their target structures are critically involved in the neural circuit modifications that underlie a variety of adaptive and pathological behaviors including the development and maintenance of addiction (Wise, 2004; Kalivas and Volkow, 2005; Everitt and Robbins, 2005; Hyman et al., 2006; Schultz, 2007; Wolf, 2010). Until fairly recently, midbrain DA neurons in the ventral tegmental area (VTA) and substantia nigra (SN) were thought to be homogeneous in their properties and behavioral functions. In particular, it has been demonstrated that they express characteristic phasic excitatory responses to rewards and cues that predict rewards while being inhibited by omission of rewards (Schultz, 1998). These findings led to the influential hypothesis that phasic DA cell activity encodes a reward prediction error, which is critical for reinforcement-dependent learning (Schultz, 1998, 2007, 2010; D’Ardenne et al., 2008; Dayan and Niv, 2008). In contrast, studies that monitored behaviorally-relevant in vivo dopamine release often found target selectivity such that, for example, unconditioned rewarding stimuli caused DA release primarily in the nucleus accumbens (NAc) medial shell but not in other regions of the ventral or dorsal striatum (Bassareo et al., 2002; Stuber et al., 2005; Di Chiara and Bassareo, 2007; Goto et al., 2007; Aragona et al., 2008). Furthermore, aversive stimuli can cause DA release in a target-specific manner (Abercrombie et al., 1989; Bassareo et al., 2002; Young et al., 2004). Indeed, a number of in vivo studies in both rodents and primates demonstrated a diversity of firing patterns exhibited by DA cells in response to behaviorally relevant stimuli (Ungless et al., 2010; Bromberg-Martin et al., 2010). In rodents, for example, some VTA DA neurons are phasically excited by aversive stimuli (Mantz et al., 1989; Brischoux et al., 2009). In nonhuman primates, DA neurons in the VTA and dorsolateral substantia nigra pars compacta (SNc) also can encode aversive events and cues predicting such events as well as other features of stimuli including their motivational salience (Matsumuto and Hikosaka, 2009; Bromberg-Martin et al., 2010). These findings have led to the proposal that DA neurons play a variety of critical roles in motivational control in addition to their importance for encoding reward prediction errors (Berridge et al., 2009: Bromberg-Martin et al., 2010; Ungless et al., 2010).
Consistent with the view that midbrain DA cells are not homogeneous are recent findings that the specific molecular and physiological properties of midbrain DA cells are associated with the target structures to which they project (Lammel et al., 2008; Margolis et al., 2008). A subgroup of “unconventional” DA neurons with high frequency firing (>10 Hz) and low DA reuptake capacity (i.e. low DAT/TH expression ratio) is located in the medial posterior VTA and projects to the medial prefrontal cortex (mPFC), nucleus accumbens (NAc) core or NAc medial shell (Lammel et al., 2008). In contrast, "conventional" DA neurons with low frequency firing (<10 Hz) are located in the lateral VTA and SNc and project to NAc lateral shell and dorsal striatum, respectively (Lammel et al., 2008). These findings raise the important question of whether the in vivo synaptic modulation and functional responses of DA cells to different stimuli may be associated with the distinct anatomical target sites to which they project.
Addressing this question experimentally is challenging because it requires unequivocal identification of the specific target area to which an identified DA cell projects. To begin to address this issue, we took advantage of the well-established increase in excitatory synaptic strength on VTA DA neurons caused by passive administration or self-administration of drugs of abuse (Ungless et al., 2001; Saal et al., 2003; Borgland et al., 2004; Dong et al., 2004; Faleiro et al., 2004; Liu et al., 2005; Bellone and Luscher, 2006; Argilli et al., 2008; Engblom et al., 2008; Stuber et al., 2008; Chen et al., 2008; Heikkinen et al., 2009). Specifically, we visually identified and recorded from subpopulations of VTA and SNc DA neurons projecting to different target structures in acute midbrain slices by injecting fluorescent “Retrobeads” into the mPFC, the NAc medial shell, the NAc lateral shell or the dorsolateral striatum of 3-month old adult C57Bl/6 mice (Lammel et al., 2008). We predicted that the excitatory synapses on distinct DA subpopulations would be differently modulated by a rewarding stimulus, specifically the administration of cocaine. We also examined whether an aversive stimulus affected these same sets of synapses in a similar manner. Our results suggest that the long-lasting modulation of synapses on DA cells caused in vivo by rewarding and aversive stimuli is not uniform but rather differs dramatically depending on the respective target structures to which DA neurons project.
RESULTS
Retrogradely Labeled Neurons Are Predominantly Dopaminergic
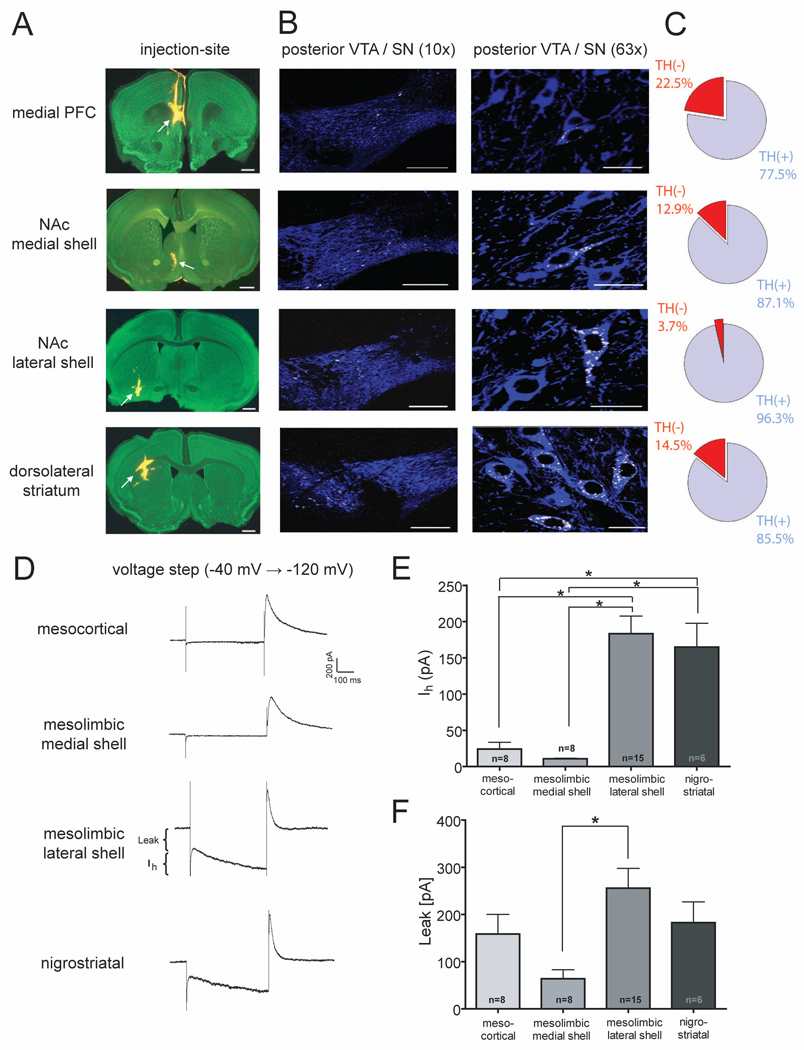
Most previous in vitro electrophysiological studies of midbrain DA neurons appear to have targeted DA neurons in the anterior lateral VTA, predominantly the parabrachial pigmented nucleus (PBP) (Brischoux et al., 2009; Ungless et al., 2010). In addition, putative DA cells were commonly identified by the presence of a large hyperpolarization-activated current (Ih), while cells that lacked this current were considered non-dopaminergic (Ungless et al., 2001; Gutlerner et al., 2002; Saal et al., 2003; Borgland et al., 2004; Faleiro et al., 2004; Bellone and Luscher, 2006; Margolis et al., 2006; Hommel et al., 2006; Argilli et al., 2008; Stuber et al., 2008; Zweifel et al., 2008) even though this criterion does not unequivocally identify DA neurons (Johnson and North, 1992; Ford et al., 2006; Margolis et al., 2006, 2008; Zhang et al., 2010a). Therefore, one major goal of this study was to identify and record from DA cell subpopulations that have largely been neglected because they are located in the posterior medial VTA and/or lack a prominent Ih. Using in vivo Retrobead injections to identify the projection target of individual DA neurons, we first determined the percentage of retrogradely labeled neurons in the posterior VTA that are dopaminergic as defined by immunoreactivity for tyrosine hydroxylase (TH). Injections were made in the mPFC, NAc medial shell, and NAc lateral shell to label VTA DA neurons as well as the dorsolateral striatum for labeling of nigrostriatal DA cells (Figure 1A). In agreement with previous results (Lammel et al., 2008) we found that retrogradely labeled neurons that project to the mPFC and medial shell of the NAc are mainly located in the medial posterior VTA, medial paranigral (PN) nucleus and adjacent medial aspects of the parabrachial pigmented (PBP) nucleus (Figure 1B). In contrast, neurons that project to the lateral shell of the NAc were located in the lateral VTA, mainly in the lateral PBP nucleus. As expected, nigrostriatal neurons were almost exclusively found in the SNc. Approximately 80–95% of the retrogradely labeled cells also were immunopositive for TH indicating that they were dopaminergic (Figure 1C; n=49–140 cells for each group).
Figure 1. Retrograde Labeling of Midbrain DA Neurons.
(A) Injection sites (arrows) in green nissl (488 nm) counterstained sections (100 µm) showing typical locations of Retrobeads (546 nm, yellow). From top to bottom: mPFC (bregma +1.70 mm), NAc medial shell (bregma +1.10 mm), NAc lateral shell (bregma +0.74 mm), dorsolateral striatum (bregma +0.98 mm). Scale bars 500 µm.
(B) Confocal images showing the anatomical distribution of retogradely transported Retrobeads (white) in the posterior VTA and SN following TH-immunohistochemistry (blue) at low magnification (10X, left panels) and high magnification (63X, right panels). Note that cells projecting to the mPFC are located in medial aspects of the posterior VTA and cells projecting to the NAc medial shell are located in the ventromedial areas of the posterior VTA. In contrast, neurons projecting to the NAc lateral shell are located in the dorsolateral region of the posterior VTA and cells projecting to the dorsolateral striatum are completely located in the posterior SN. Scale bars 200 µm (left panel) and 20 µm (right panel).
(C) Pie charts showing the relative number of retrogradely labeled TH-immunopositive and TH-immunonegative cells that were located in the posterior VTA or posterior SN (Bregma −3.80 to −3.28 mm) (mesocortical n=49 cells; mesolimbic medial shell n=101 cells; mesolimbic lateral shell n=107 cells; nigrostriatal n=140 cells).
(D) Representative current traces in response to a voltage step from −40 to −120 mV. Measurements of IRK+Leak currents and Ih are indicated in the mesolimbic lateral shell trace.
(E) Magnitude of Ih for each cell population. Number of cells are indicated (* p<0.05).
(F) Magnitude of IRK + leak currents for each cell population with number of cells indicated (* p<0.05).
Recordings from retrogradely labeled neurons revealed significant differences in the magnitude of Ih depending on the neurons’ projection targets. Cells projecting to the mPFC or NAc medial shell exhibited an Ih that was dramatically smaller than those recorded from neurons projecting to the NAc lateral shell or dorsal striatum (Figures 1D and 1E: mesocortical neurons: 24.2 ± 9.4 pA, n=8; mesolimbic medial shell neurons: 10.7 ± 0.9 pA, n=8; mesolimbic lateral shell neurons: 183.4 ± 24.3 pA, n=15; nigrostriatal neurons: 164.8 ± 32.9 pA, n=6). Time-independent inward leak currents mediated by background conductances were significantly smaller only in the cells projecting to NAc medial shell (Figures 1D and 1F: mesocortical neurons: 158.7 ± 41.6 pA, n=8; mesolimbic medial shell neurons: 63.8 ± 19.1 pA, n=8; mesolimbic lateral shell neurons: 255.7 ± 42.0 pA, n=15; nigrostriatal neurons: 182.7 ± 43.8 pA, n=6). All of the recorded neurons in Figure 1D–F were filled with 0.1% neurobiotin and were confirmed to be TH-positive (i.e. dopaminergic) by immunocytochemistry (Figure S1). Taken together these results demonstrate that, on average, over 80% of retrogradely labeled cells in the posterior VTA are dopaminergic independent of their projection targets. Furthermore, since DA neurons projecting to the mPFC and medial shell of the NAc are primarily located in the medial posterior VTA and lack a prominent Ih, it is likely that these neurons have been neglected in most previous in vitro studies.
Differences in Basal Properties of Excitatory Synapses on DA Neuron Subpopulations
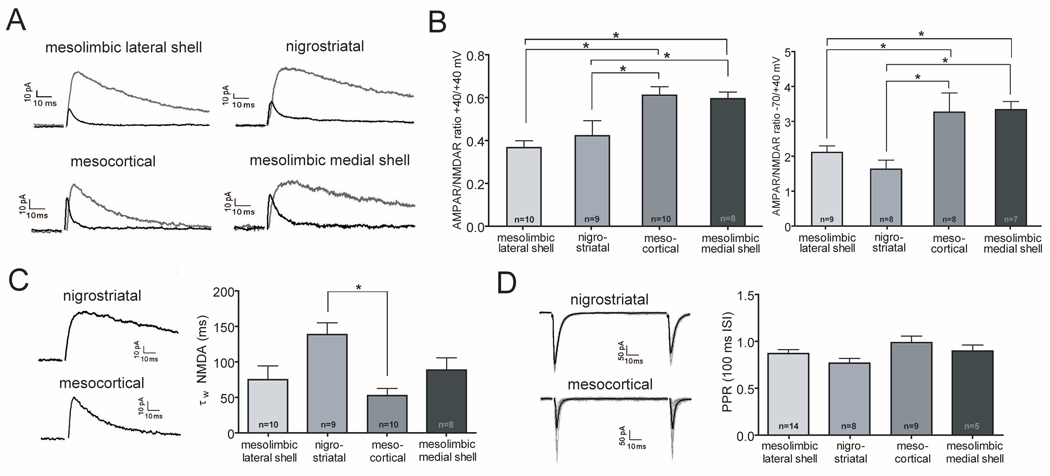
We next examined the basal properties of excitatory synapses on the different DA neuron subpopulations in adult (3 month old) C57Bl/6 mice. Because quantitative estimates of basal evoked synaptic strength are very difficult to obtain in slice preparations in which the magnitude of the activated afferent input cannot be measured, we calculated the ratio of AMPA receptor (AMPAR)-mediated to NMDA receptor (NMDAR)-mediated excitatory postsynaptic currents (EPSCs), a commonly used measure of basal synaptic properties (Kauer and Malenka, 2007). When measured at +40 mV, the AMPAR/NMDAR ratios in cells that express a large Ih and project to the NAc lateral shell and dorsal striatum were very similar to those reported in previous studies where the presence of an Ih was used to identify DA neurons (Figures 2A and 2B: mesolimbic lateral shell neurons: 0.37 ± 0.03, n=10; nigrostriatal neurons: 0.42 ± 0.07, n=9) (Ungless et al., 2001; Saal et al., 2003; Dong et al., 2004; Faleiro et al., 2004; Liu et al., 2005; Bellone and Luscher, 2006; Argilli et al., 2008; Engblom et al., 2008; Stuber et al., 2008; Heikkinen et al., 2009). In contrast, the AMPAR/NMDAR ratios at +40 mV in cells that possess a small Ih and project to mPFC or NAc medial shell were, on average, significantly higher (Figures 2A and 2B: mesocortical neurons: 0.61 ± 0.04, n=10; mesolimbic medial shell neurons: 0.60 ± 0.03, n=8). Because the presence of inwardly rectifying, GluA2-lacking AMPARs can influence the AMPAR/NMDAR ratios when measured at +40 mV (Isaac et al., 2007), we also calculated the AMPAR/NMDAR ratios based on recording AMPAR EPSCs at −70 mV and NMDAR EPSCs at +40 mV. Again, the ratios were significantly higher in cells projecting to the mPFC or NAc medial shell (Figure 2B, right panel: mesolimbic lateral shell neurons, 2.11 ± 0.19, n=9; nigrostriatal neurons, 1.63 ± 0.26, n=8; mesocortical neurons, 3.26 ± 0.55, n=8; mesolimbic medial shell neurons, 3.34 ± 0.23, n=7). We also examined the weighted decay time constant (τW) of the NMDAR EPSCs recorded at +40 mV and found that it tended to be larger in nigrostriatal neurons when compared to the other neuronal subpopulations although this difference reached statistical significance only when compared to the decay time constant of neurons projecting to mPFC (Figure 2C: mesolimbic lateral shell neurons, 75.0 ± 19.4 ms, n=10; nigrostriatal neurons, 138.5 ± 16.5 ms, n=9; mesocortical neurons, 52.5 ± 10.0 ms, n=10; mesolimbic medial shell neurons, 88.5 ± 17.2 ms, n=8). Finally, we measured paired-pulse ratios at 50 ms (data not shown) and 100 ms interstimulus intervals (Figure 2D) but found no differences between the subpopulations of neurons in this estimate of the average presynaptic probability of transmitter release. The larger AMPAR/NMDAR ratios in mesocortical and mesolimbic medial shell neurons are consistent with our suggestion that these neurons have not previously been studied and suggest that the basal properties of their excitatory synapses are different from synapses on mesolimbic lateral shell neurons and nigrostriatal neurons.
Figure 2. Excitatory Synapses on DA Neurons Subpopulations Have Distinct Properties.
(A) Sample AMPAR- and NMDAR EPSCs at +40 mV from different subpopulations of DA neurons.
(B) AMPAR/NMDAR ratios at +40 mV (left panel) and at −70 mV/+40 mV (right panel) in the different DA neuron subpopulations. Number of cells are indicated (* p<0.05).
(C) Sample NMDAR EPSCs at +40 mV for nigrostriatal and mesocortical DA neurons (left). Weighted decay time constants (τW) of NMDAR EPSCs recorded at +40 mV for all DA neuron subpopulations (right). Number of cells are indicated (*p<0.05).
(D) Sample AMPAR EPSCs at −70 mV in response to paired afferent stimuli (100 ms interstimulus interval, ISI) for nigrostriatal and mesocortical DA neurons (left). Calculated paired-pulse ratios (PPR) at 100 ms ISI for all DA neuron subpopulations were not significantly different (right). Number of cells are indicated.
Differences in Cocaine-Induced Synaptic Plasticity in DA Neuron Subpopulations
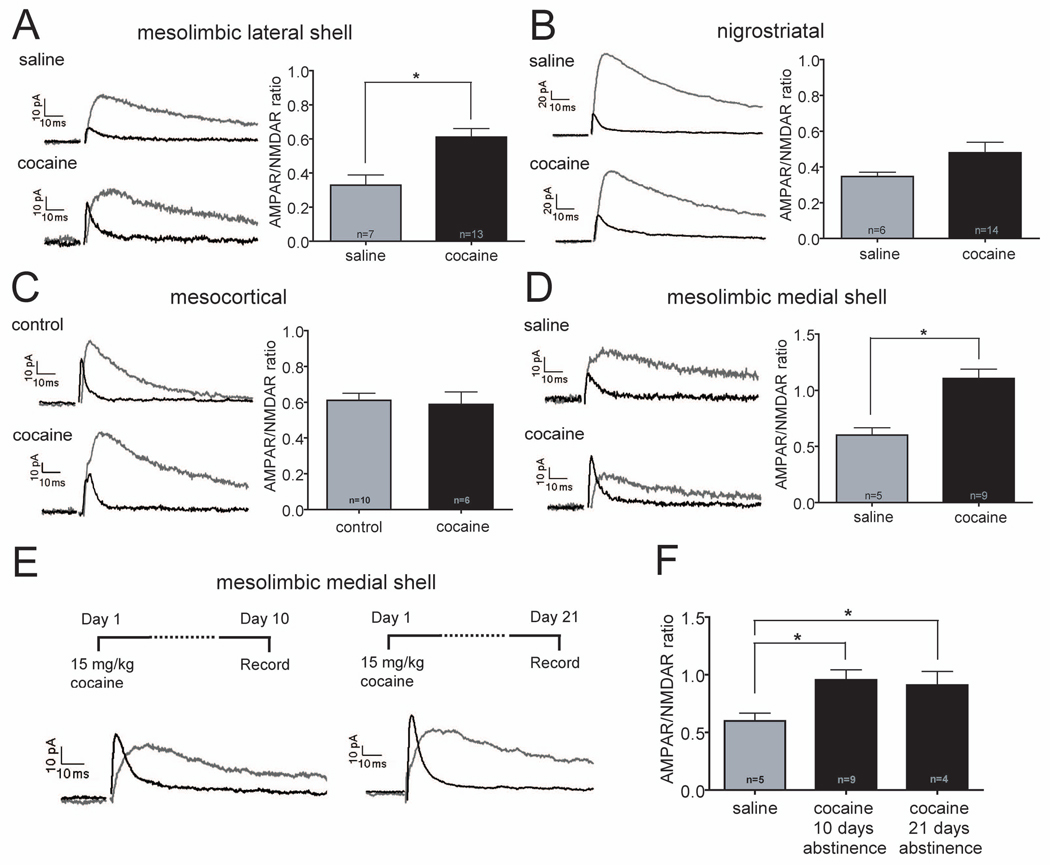
Given that some of the basic properties of DA neurons differ depending on the brain regions to which they project, a critical question is whether these neuronal subpopulations are all modulated in the same manner by a “rewarding” experience. As an initial attempt to address this issue, we took advantage of the well-established modification of excitatory synapses on VTA DA neurons caused by in vivo administration of drugs of abuse; an increase in the AMPAR/NMDAR ratio (Ungless et al., 2001; Saal et al., 2003; Borgland et al., 2004; Dong et al., 2004; Faleiro et al., 2004; Liu et al., 2005; Bellone and Luscher, 2006; Argilli et al., 2008; Chen et al., 2008; Engblom et al., 2008; Heikkinen et al., 2009). Twenty-four hours prior to slice preparation, cocaine (15 mg/kg, i.p.) or, in most experiments, saline (0.9%, i.p., volume matched for experimental injections) was administered to animals that 1–3 weeks previously had been injected with Retrobeads. Consistent with previous results, neurons projecting to NAc lateral shell and which express a large Ih exhibited a clear increase in their AMPAR/NMDAR ratios following cocaine administration (Figure 3A: saline, 0.33 ± 0.06, n=7; cocaine, 0.61 ± 0.05, n=13; p=0.003). Surprisingly, however, cocaine did not significantly increase AMPAR/NMDAR ratios in either nigrostriatal cells (Figure 3B: saline, 0.34 ± 0.02, n=6; cocaine, 0.48 ± 0.06, n=14; p=0.169) or in VTA cells projecting to mPFC (Figure 3C: control, 0.61 ± 0.04, n=10; cocaine, 0.59 ± 0.07, n=6; p=0.765). In marked contrast, even though the basal AMPAR/NMDAR ratios were high, a very large increase occurred in VTA DA neurons projecting to NAc medial shell (Figure 3D: saline, 0.60 ± 0.07, n=5; cocaine, 1.1 ± 0.08, n=9; p=0.002). Cocaine administration did not affect the paired-pulse ratios (50 ms and 100 ms interpulse intervals) in any of these DA neuron subpopulations (data not shown). These results demonstrate that one prominent form of synaptic plasticity in midbrain DA neurons elicited by a “rewarding” experience, the administration of a single dose of cocaine, is associated with the brain area to which the DA neuron projects. Furthermore, the increase in the AMPAR/NMDAR ratio elicited by cocaine does not require a low basal value and is not restricted to neurons with a large Ih.
Figure 3. Cocaine Administration Increases AMPAR/NMDAR Ratios Only in DA Cells Projecting to NAc.
(A–D) Sample AMPAR- and NMDAR EPSCs (left panels) and magnitude of AMPAR/NMDAR ratios (right panels) in different DA neuron subpopulations in animals that received saline or cocaine injections 24 hours prior to slice preparation. Number of cells are indicated (*p<0.05). Note that DA cells projecting to NAc lateral shell (A) and NAc medial shell (D) showed large increases in AMPAR/NMDAR ratios but nigrostriatal cells (B) and cells projecting to mPFC (C) did not. (The control cells in C are the same as those shown in Figure 2B.)
(E) Sample AMPAR- and NMDAR EPSCs recorded from DA cells projecting to NAc medial shell 10 or 21 days after cocaine administration
(F) Magnitude of AMPAR/NMDAR ratios at these time points compared to saline injected animals. Number of cells are indicated (*p<0.05).
Previous studies found that in VTA neurons with a large Ih, the increase in the AMPAR/NMDAR ratio elicited by non-contingent administration of cocaine lasted 5 but not 10 days (Ungless et al., 2001), even following 7 consecutive days of cocaine injections (Borgland et al., 2004). In contrast, self-administration of cocaine caused an increase in this measure lasting 3 months (Chen et al., 2008). These findings raise the question of whether the large cocaine-elicited increase in the AMPAR/NMDAR ratio in DA neurons projecting to NAc medial shell (Figure 3D), cells that have not been studied previously, is long-lasting or not. We first prepared slices 10 days following a single dose of cocaine and found that the AMPAR/NMDAR ratio was still increased (Figures 3E and F: saline, 0.60 ± 0.07, n=5; after 10 days: 0.96 ± 0.09, n=9; p=0.018). Surprisingly, the ratio remained increased even 21 days after cocaine administration (Figures 3E and F: after 21 days: 0.91 ± 0.12, n=4; p=0.047). We also examined whether the lack of increase in the AMPAR/NMDAR ratio in mesocortical and nigrostriatal DA neurons following a single in vivo dose of cocaine could be overcome using a chronic administration protocol. However, repetitive daily administration of cocaine for 5 days had no significant effect in either of these DA cell types (Figure S2A–C, mesocortical: 5 days of cocaine: 0.70 ± 0.14, n=5; 5 days of saline: 0.58 ± 0.06, n=3; p=0.467; nigrostriatal: 5 days of cocaine: 0.41 ± 0.05, n=6 ; 5 days of saline: 0.44 ± 0.06, n=7; p=0.646). These results demonstrate that the modulation of excitatory synaptic function in midbrain DA neurons by in vivo administration of cocaine is not uniform but is strongly associated with the brain area to which the DA neuron projects. Long-lasting changes occur in neurons that project to the NAc medial shell while detectable changes do not occur in neurons projecting to PFC and in nigrostriatal cells.
Differences in Synaptic Plasticity Induced by an Aversive Experience in DA Neuron Subpopulations
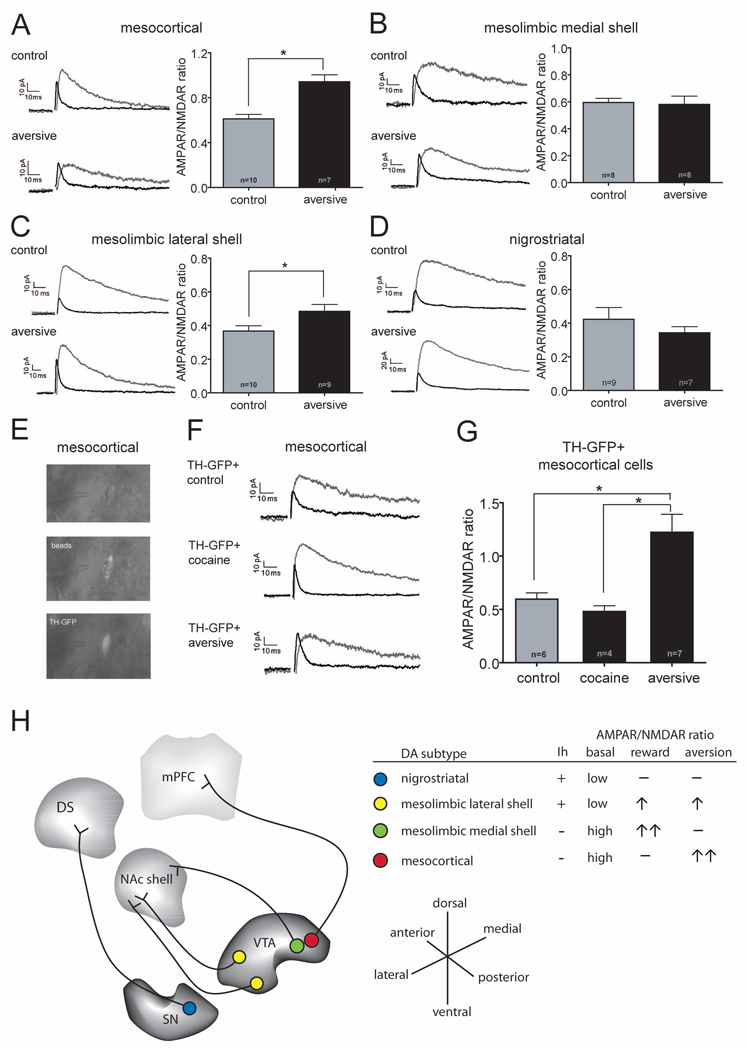
Although in vivo single unit recordings primarily in non-human primates as well as rodents have revealed that many midbrain DA neurons are excited by rewarding stimuli or cues that predict rewards (Schultz, 2010) there are subpopulations of putative DA neurons that are excited by aversive stimuli (Mirenowicz and Schultz, 1996; Brischoux et al., 2009; Matsumuto and Hikosaka, 2009; Bromberg-Martin et al., 2010, Ungless et al., 2010). This raises the possibility that the DA neuron subpopulations that did not exhibit an increase in the AMPAR/NMDAR ratios in response to cocaine might exhibit such a change in response to an aversive experience. To test this hypothesis, animals received a small subcutaneous injection of formalin in the plantar surface of a single hind paw, a stimulus commonly used to generate irritation (Dubuisson and Dennis, 1977) and one that has been reported to increase c-fos immunoreactivity in VTA DA neurons (Ma et al., 1993). In contrast to a cocaine experience, this aversive experience caused a clear increase in the AMPAR/NMDAR ratio in DA neurons projecting to mPFC (Figure 4A: control, 0.61 ± 0.04, n=10; aversive: 0.94 ± 0.06, n=7; p=0.0003). On the other hand, formalin injections did not cause an increase in this synaptic measure in DA neurons projecting to the NAc medial shell (Figure 4B: control, 0.60 ± 0.03, n=8; aversive: 0.58 ± 0.06, n=8; p=0.831) but did cause an increase in the neurons projecting to NAc lateral shell (Figure 4C: control, 0.37 ± 0.03, n=10; aversive: 0.48 ± 0.04, n=9; p=0.035). Finally, the AMPAR/NMDAR ratios in nigrostriatal cells were unaffected by this aversive experience (Figure 4D: control, 0.42 ± 0.07, n=9; aversive, 0.34 ± 0.04, n=7; p=0.372).
Figure 4. An Aversive Stimulus Increases AMPAR/NMDAR Ratio in DA Cells Projecting to the mPFC.
(A–D) Sample AMPAR- and NMDAR EPSCs (left panels) and magnitude of AMPAR/NMDAR ratios (right panels) in different DA neuron subpopulations in animals that received hindpaw formalin injections 24 hours prior to slice preparation. Number of cells are indicated (*p<0.05). Note that DA cells projecting to the mPFC (A) and NAc lateral shell (C) showed increases in AMPAR/NMDAR ratios but nigrostriatal cells (D) and cells projecting to NAc medial shell (B) did not. (The control cells in there panels are the same as those shown in Figure 2B.)
(E) Sample GFP+ cell containing Retrobeads that were injected into the mPFC of a TH-GFP mouse 21 days earlier. Images obtained from a living brain slice using infrared videomicroscopy and epifluorescence.
(F) Sample AMPAR- and NMDAR EPSCs recorded from GFP+ mesocortical cells in slices prepared from TH-GFP mice that had received cocaine or hindpaw formalin injections 24 hours earlier.
(G) Magnitude of AMPAR/NMDAR ratios in GFP+ cells from control TH-GFP mice or mice that had received cocaine or the aversive formalin injections 24 hours earlier. Number of cells are indicated (*p<0.05).
(H) Summary of some of the basal properties of the DA cell subpopulations and the modulation of their excitatory synapses by rewarding and aversive stimuli. Note that DA neurons projecting to the NAc lateral shell can be found in the anterior and posterior VTA (yellow dots), while DA neurons projecting to the mPFC and NAc medial shell are mainly located in the posterior VTA (red and green dots).
In our initial experiments, we found that approximately 20% of the neurons projecting to the mPFC in the posterior VTA did not stain for TH and therefore were likely non-dopaminergic (Figure 1C). Because the mesocortical neurons exhibited the most unusual behavior among the neuronal subpopulations we studied, specifically, a large increase in the AMPAR/NMDAR ratio following an aversive stimulus but not following cocaine, we wanted to confirm that these synaptic changes were in fact occurring in DA neurons. We therefore obtained transgenic mice that expressed GFP under control of the TH promoter (Sawamoto et al., 2001), confirmed that the GFP-expressing mesocortical cells stained for TH (Figure S3), and recorded from GFP-positive cells which were also labeled with Retrobeads that were injected into the mPFC (Figure 4E). Similar to C57Bl/6 mice, in the TH-GFP mice, DA neurons projecting to the mPFC exhibited a high basal AMPAR/NMDAR ratio, no increase in this ratio 24 hours following cocaine administration and a large increase 24 hours following the aversive experience (Figures 4F and G: control, 0.60 ± 0.06, n=6; cocaine, 0.48 ± 0.05, n=4; p=0.2167 aversive: 1.22 ± 0.17, n=7; p=0.0076). Ten days after the aversive experience, however, the AMPAR/NMDAR ratio was no longer significantly increased (0.73 ± 0.15, n=3; p=0.328). Thus, the unusual synaptic modulation observed in mesocortical DA neurons in response to rewarding and aversive stimuli was replicated in a second mouse line in which DA neurons could be visually identified.
DISCUSSION
A major goal of modern neuroscience research is to elucidate how specific modifications in defined neural circuits mediate particular types of experience-dependent behavioral plasticity. Over the last decade, a number of important new approaches have become available to facilitate this effort ranging from genetically modified mice in which transgenes are expressed in specific cell types (Malenka, 2002) to optogenetics (Zhang et al., 2010b). Despite these advances, when cell types are not yet genetically identifiable based on their specific connectivity, other somewhat more traditional approaches remain valuable. Here, we have attempted to define differences in the experience-dependent modulation of subpopulations of midbrain DA neurons that are categorized based on their projections to different target areas as defined by the presence of retrogradely transported fluorescent beads (Koebbert et al., 2000; Lammel et al., 2008). These target areas, which include the mPFC, different subregions of the NAc and the dorsal striatum, are key components of anatomically and functionally related circuits that are involved in a wide range of adaptive and pathological motivated behaviors (Wise, 2004; Everitt and Robbins, 2005; Ikemoto, 2007; Everitt et al., 2008; Berridge et al., 2009; Schultz, 2010; Bromberg-Martin et al., 2010; Ungless et al., 2010; Wolf, 2010). In particular, because DA cell activity and the consequent release of DA in target structures are associated not only with rewards and reinforcement-dependent learning (Schultz, 2010) but also appear to play an important role in the motivational responses to aversive as well as other salient stimuli (Berridge et al., 2009; Bromberg-Martin et al., 2010; Ungless et al., 2010), we wanted to compare the effects of a simple “rewarding” versus “aversive” experience on these different DA subpopulations.
Recording from slices prepared from animals that had received in vivo Retrobead injections in single brain areas (Figure 1A) allowed unequivocal identification of the brain area to which the identified DA neuron sent axons (Lammel et al., 2008). We assayed changes in excitatory synaptic properties by measuring AMPAR/NMDAR ratios because increases in this ratio robustly occur following administration of drugs of abuse as well as acute stressful stimuli and these changes are thought to have important functional consequences for DA neuron activity during adaptive and pathological behaviors (Luscher and Ungless, 2006; Kauer and Malenka, 2007; Kalivas, 2009; Chen et al., 2010; Wolf, 2010). Because this project required injecting Retrobeads in over 300 adult mice, we intentionally kept the assays of synaptic function simple so that adequate numbers of cells from each subpopulation could be recorded and compared. Furthermore, most previous studies that used similar ex-vivo approaches identified putative DA neurons by the presence of a large Ih and thus several of the DA neuron subpopulations we studied likely have not been examined previously.
The major finding of this study was that excitatory synapses on the subpopulations of DA neurons with different axonal projection targets were modified distinctly following a “rewarding” cocaine experience versus an aversive experience (Figure 4H). Synapses on DA neurons projecting to NAc medial shell were selectively modified by the rewarding stimulus while synapses on DA neurons projecting to mPFC were modified only by the aversive stimulus. In contrast, synapses on DA cells projecting to NAc lateral shell were modified by both rewarding and aversive stimuli suggesting that this modulation may encode the occurrence of a salient stimulus independent of its valence. These findings are consistent with the idea that mesocorticolimbic DA circuitry may comprise multiple parallel circuits that encode distinct aspects of a motivational stimulus; its valence in terms of its rewarding or aversive properties as well as its salience (Bromberg-Martin et al., 2010). The strategy of parallel processing and representation of the distinct features of a motivational stimulus in different circuits can be viewed as analogous to the neural circuit mechanisms by which many sensory systems encode complex sensory stimuli. In the context of this hypothesis, an important topic for future research will be to elucidate the mechanisms by which stress and drugs of abuse interact and cross-sensitize, both in terms of their behavioral consequences and the changes they elicit in extracellular dopamine.
The much larger and longer-lasting increase in the AMPAR/NMDAR ratio in DA neurons projecting to the NAc medial shell compared to those projecting to NAc lateral shell is consistent with studies reporting that cocaine administration elicits the largest increase in extracellular DA concentration within the NAc medial shell (Stuber et al., 2005; Di Chiara and Bassareo, 2007; Aragona et al., 2008). This conclusion is based on the assumption that the increase in the AMPAR/NMDAR ratio correlates with a net increase in synaptic strength (Ungless et al., 2001; Kauer and Malenka, 2007) and that this drives increased spiking activity in the DA cell subpopulation in vivo. The long-lasting synaptic changes in the mesolimbic medial shell DA neurons following cocaine administration may also contribute to the delayed yet persistent synaptic adaptations observed at excitatory synapses in the NAc (Kauer and Malenka, 2007; Conrad et al., 2008; Kalivas, 2009; Chen et al., 2010; Wolf, 2010), changes that have been shown to be dependent on the initial synaptic adaptations in midbrain DA neurons (Mameli et al., 2009).
The most surprising results were that excitatory synapses on DA neurons projecting to the mPFC did not appear to be modified by the cocaine experience yet were profoundly changed by an aversive experience. It must be acknowledged that a lack of change in the AMPAR/NMDAR ratio does not prove that no changes in excitatory synaptic properties have occurred. However, in all previous ex vivo studies of putative DA neurons, this measure has been found to be increased by drugs of abuse (Ungless et al., 2001; Saal et al., 2003; Borgland et al., 2004; Dong et al., 2004; Faleiro et al., 2004; Liu et al., 2005; Bellone and Luscher, 2006; Argilli et al., 2008; Chen et al., 2008; Engblom et al., 2008; Heikkinen et al., 2009) as well as reward-dependent learning (Stuber et al., 2008). Thus, it seems unlikely that somehow cocaine administration modified excitatory synapses on mesocortical DA neurons in a manner that did not affect the AMPAR/NMDAR ratio, especially since the aversive experience did increase this ratio in this same neuronal population. In future work, it will be important be determine whether specific sets of synapses are modified and if so, whether synapses made by different inputs are differentially modified by rewarding versus aversive stimuli.
Accepting the assumption that the experience-dependent synaptic adaptations we have identified translate into differences in the synaptic drive onto DA cells and therefore their activity in vivo, there are several implications of our results. They suggest that the DA cells that have been found to be excited by aversive stimuli in vivo (Mirenowicz and Schultz, 1996; Brischoux et al., 2009; Matsumuto and Hikosaka, 2009) may primarily be DA cells that specifically project to the mPFC. Consistent with this possibility are reports that tail shock stress increased extracellular DA levels in the medial frontal cortex to a much greater degree than in dorsal striatum or NAc (Abercrombie et al., 1989), that a noxious tail pinch excites mesocortical but not mesolimbic DA neurons (Mantz et al., 1989) and that aversive taste stimuli rapidly increased DA in the PFC (Bassareo et al., 2002) but not in the NAc medial shell (Bassareo et al., 2002; Roitman et al., 2008). Furthermore, the putative DA cells in rats that were excited by noxious stimuli were located in the ventromedial aspect of the posterior VTA (Brischoux et al., 2009), the same area of the VTA in which we found most mesocortical DA neurons (Figure 1). Our results also suggest that the modulation of circuitry within the brain areas targeted by DA cells will be different for “rewarding” versus aversive stimuli. This makes sense because the behavioral responses to a “rewarding” versus an aversive experience will be different (e.g. approach versus avoidance) and therefore, by definition, will involve different, although perhaps overlapping, neural circuit modifications.
Drug addiction can be conceptualized as the endpoint of a series of behavioral transitions beginning with voluntary drug use because the drug has reinforcing, often hedonic, effects and ending with loss of control over behavior, such that drug intake becomes habitual and ultimately compulsive (Kalivas and Volkow, 2005; Hyman et al., 2006; Everitt and Robbins, 2005; Everitt et al., 2008). At the neural circuit level, these behavioral transitions may correspond to a transition from limbic and prefrontal cortical control over goal-directed behavior to dorsostriatal control as drug intake becomes compulsive (Haber et al., 2000; Kalivas and Volkow, 2005; Everitt and Robbins, 2005; Everitt et al., 2008; Hyman et al., 2006; Ikemoto, 2007). The clear and immediate (within one day) modification of synapses on mesoaccumbens DA neurons by cocaine administration versus the lack of such changes at synapses on nigrostriatal DA neurons can be viewed as consistent with this proposal and suggest that more prolonged exposure to cocaine may be required for changes in nigrostriatal cells to occur. Our results can also be viewed as consistent with a hierarchical organization of drug-evoked plasticity in these circuits (Kalivas and O’Brien, 2008) such that DA neurons projecting to the NAc underlie the initial reinforcing effects of drugs of abuse whereas DA neurons projecting to the mPFC and dorsolateral striatum are possibly engaged later during the transition to addiction.
In summary, the results of this study provide evidence in support of the hypothesis that midbrain DA neurons are not homogenous but instead subserve a variety of functions in support of the control over motivated behaviors (Berridge et al., 2009; Bromberg-Martin et al., 2010; Ungless et al., 2010). They suggest that the long-lasting modulation of individual DA neuron activity by salient stimuli is associated with the specific target brain areas they influence, a conclusion that is not surprising but nevertheless is important because its corollary is that the pathological behaviors involving mesocorticolimbic DA circuitry involve modulation of distinct DA neuron subpopulations. Clearly, the ex vivo approach taken here cannot be used to define the behavioral roles of the DA neuron subpopulations. However, the differences we have demonstrated provide motivation to develop molecular tools that will allow precise in vivo control over the activity of these subpopulations so that their behavioral functions can be clearly elucidated.
EXPERIMENTAL PROCEDURES
Recordings from retrogradely labeled DA neurons were performed essentially as previously described (Lammel et al., 2008). All experimental procedures are described in detail in Supplemental Information.
Supplementary Material
ACKOWLEDGEMENTS
This work was supported by grants from NIDA and by a fellowship from the German Academy of Sciences Leopoldina (to S.L.). Retrograde tracing and immunohistochemistry were by S.L. and D.I.I. Electrophysiology was by S.L. The study was designed and results were analyzed and interpreted by S.L., J.R. and R.C.M. The manuscript was written by S.L., J.R. and R.C.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J. Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J. Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J. Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat. Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr. Opin. Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J. Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA. 2009;106 doi: 10.1073/pnas.0811507106. 894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann. N. Y. Acad. Sci. 2010;1187:129–139. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dayan P, Niv Y. Reinforcement learning: the good, the bad and the ugly. Curr. Opin. Neurobiol. 2008;18:185–196. doi: 10.1016/j.conb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr. Opin. Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(−/−) mice. Proc. Natl. Acad. Sci. USA. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Faleiro LJ, Jones S, Kauer JA. Rapid synaptic plasticity of glutamatergic synapses on dopamine neurons in the ventral tegmental area in response to acute amphetamine injection. Neuropsychopharmacol. 2004;29:2115–2125. doi: 10.1038/sj.npp.1300495. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacol. 2007;53:583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutlerner JL, Penick EC, Snyder EM, Kauer JA. Novel protein kinase A-dependent long-term depression of excitatory synapses. Neuron. 2002;36:921–931. doi: 10.1016/s0896-6273(02)01051-6. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen AE, Moykkynen TP, Korpi ER. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacol. 2009;34:290–298. doi: 10.1038/npp.2008.89. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Ann. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J. Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacol. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog. Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS Med. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Zhou Y, Han JS. Noxious stimulation accelerated the expression of c-fos protooncogene in cholecystokininergic and dopaminergic neurons in the ventral tegmental area. Peptides. 1993;14:561–566. doi: 10.1016/0196-9781(93)90145-7. [DOI] [PubMed] [Google Scholar]
- Malenka R. NIH workshop report: taming the brain's complexity. Neuron. 2002;36:29–30. doi: 10.1016/s0896-6273(02)00938-8. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mantz J, Thierry AM, Glowinski J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res. 1989;476:377–381. doi: 10.1016/0006-8993(89)91263-8. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J. Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J. Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J. Neurosci. 2004;24:547–553. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann. N. Y. Acad. Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat. Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, Yamamoto A, Yoshizaki T, Terashima T, Murakami F, et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc. Natl. Acad. Sci. USA. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav. Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Argilli E, Bonci A. Effects of stress and aversion on dopamine neurons: Implications for addiction. Neurosci. Biobehav. Rev. 2010;35:151–156. doi: 10.1016/j.neubiorev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM. Increased extracellular dopamine in nucleus accumbens in response to unconditioned and conditioned aversive stimuli: studies using 1 min microdialysis in rats. J. Neurosci. Meth. 2004;138:57–63. doi: 10.1016/j.jneumeth.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang TA, Placzek AN, Dani JA. In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacol. 2010a;59:431–436. doi: 10.1016/j.neuropharm.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 2010b;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl. Acad. Sci. USA. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.