Abstract
The HTLV-I oncoprotein Tax interferes with DNA double strand break repair. Since non-homologous end joining (NHEJ) is a major pathway used to repair DNA double strand breaks we examined the effect of Tax on this pathway, with particular interest in the expression and function of Ku80, a critical component of the NHEJ pathway. Tax expression decreased Ku80 mRNA and protein levels, and repressed transcription from the Ku80 promoter. Conversely, Ku80 mRNA increased following siRNA knockdown of Tax in HTLV-I infected cells. Tax expression was associated with an elevated number of micronuclei and nucleoplasmic bridges, hallmarks of improper DNA double strand break repair. Our studies identified Tax as a transcriptional repressor of Ku80 that correlates with decreased DNA repair function. The reduction of Ku80 transcription by Tax may deplete the cell of an essential DNA break binding protein, resulting in reduced repair of DNA double strand breaks and accumulation genomic mutations.
Keywords: HTLV-1, Tax, Non-Homologous End Joining, Ku80, micronulei, DNA repair
Introduction
Human T cell leukemia virus type I (HTLV-I) is associated with the development of adult T cell leukemia (ATL), an aggressive malignancy of mature CD4+ T lymphocytes (Yasunaga and Matsuoka, 2007). HTLV-I transformed lymphocytes isolated from patients, and those immortalized in culture, demonstrate a wide range of chromosomal abnormalities, including deletions, translocations, rearrangements, duplications, and aneuploidy (Chieco-Bianchi et al., 1988; Fujimoto et al., 1999; Itoyama et al., 1990; Kamada et al., 1992; Maruyama et al., 1990; Whang-Peng et al., 1985), however no specific type of chromosomal change has been associated with the development of HTLV-I associated malignancy. Based on the complexity and variability of karyotypic abnormalities found in ATL cells, a multistep oncogenic process is thought to drive the development of ATL (Okamoto et al., 1989). Specifically, it has been proposed that five serial genetic events must occur in the immortalization and transformation process of an HTLV-I infected cell (Miyake et al., 1999; Mortreux et al., 2003).
Several studies have demonstrated that the major transforming activity of HTLV-I resides within the Tax protein (Grassmann et al., 1989; Grassmann et al., 1992; Herdegen and Leah, 1998; Nerenberg et al., 1987; Pozzatti et al., 1990; Smith and Greene, 1991; Tanaka et al., 1990; Yamaoka et al., 1992). Tax expression interferes with various processes involved in DNA metabolism, normal progression through the cell cycle, and apoptotic elimination of cells containing dangerous amounts of DNA damage. Tax does not directly induce DNA lesions by nucleolytic activity but rather, appears to establish a cellular environment with a reduced capacity to repair DNA damage.
DNA alterations resulting from exogenous genotoxic factors or normal replication processes are corrected by several repair pathways including nucleotide excision repair (NER), base excision repair (BER), mismatch repair (MMR), homologous recombination (HR), and non-homologous end joining (NHEJ) (Sancar et al., 2004). Suppression of one or more of these pathways by Tax may increase the occurrence of genomic mutations, potentially contributing to cellular transformation. NER was the first DNA repair pathway specifically shown to be suppressed by Tax (Kao and Marriott, 1999). One of the most versatile cellular repair systems, NER plays a major role in maintaining genome stability and defects in NER are responsible for several cancer predisposition syndromes. The first indication that Tax expression may affect BER came with the finding that Tax can repress transcription of DNA polymerase β (pol β), an essential enzyme involved in BER (Jeang et al., 1990). BER removes a wide variety of genomic lesions including hydrolytic DNA depurination, deamination of cytosine and 5-methylcytosine, reaction products of hydroxyl-free radicals and covalent DNA adducts (Wood and Shivji, 1997). Several lines of evidence suggest that Tax may also interfere with the repair of DNA double-strand breaks (DSBs). Tax-expressing cells form micronuclei (MN) containing whole chromosomes, as well as centric and acentric fragments, indicating the presence of unresolved chromosomal breaks (Majone et al., 1993). Secondly, in situ labelling of DNA ends showed that HTLV-I-transformed cells, as well as cells expressing Tax, have more unprotected DNA ends than controls (Majone and Jeang, 2000). Following activation of the DNA double-strand break repair (DDR) pathway, the cell must repair the broken DNA using HR or NHEJ (Kao et al., 2005). NHEJ is the main pathway used to repair DSBs and an important mechanism for preserving genomic integrity (Burma et al., 2006). HR is thought to repair a smaller number of DSBs, and is fully active only when the cell has replicated its genome in S phase of the cell cycle. Thus, NHEJ is the predominant – if not exclusive – mechanism for repairing DSBs during G0, G1, and early S phases, and continues to repair a minority of breaks during late S and G2 phases.
The NHEJ core components include the DNA-dependent protein kinase (DNA-PK) complex, Artemis and the XRCC4/DNA ligase IV complex. DNA-PK is a large, trimeric protein kinase complex consisting of a DNA binding subunit, Ku, and a kinase subunit (DNA-PKcs). Ku is a stable heterodimer of Ku70 and Ku80 that binds to the ends of dsDNA and, in vitro, can translocate along linear DNA in an ATP-independent manner.
Ku80, a critical component of the NHEJ DNA repair pathway, is thought to protect against chromosomal instability. Ku80 null cells show increased radiosensitivity and chromosomal breakage, and introduction of Ku80 siRNA into human tumor cells increases their sensitivity to radiation and chemotherapeutic agents (Nimura et al., 2007). Studies by Majone et al. using enzymatic labelling of DNA ends showed that expression of the HTLV-I Tax oncoprotein rapidly induced cytogenetic damage reflected as an increase in the prevalence of MN containing free DNA ends (Majone and Jeang, 2000). The increase in MN formation induced by Tax expression was dependent on Ku80 protein expression (Majone et al., 2005). Ku80 null cells display a high basal level of MN and Tax expression does not increase MN formation in these cells (Majone et al., 2005). This observation led us to hypothesize that Tax might target Ku80 resulting in clastogenic DNA damage. To investigate this possibility we analyzed the expression of Ku and DNA-PKcs mRNA and protein levels in Tax-expressing cells. We found that Tax represses the expression of Ku80 mRNA, leading to reduced steady state levels of the Ku heterodimer. Consequently, DNA breaks that occur as a result of genotoxic agents or normal cellular metabolism can not be promptly repaired by non-mutagenic mechanisms. This could lead to loss of chromosomal fragments in micronuclei, degradation by endogenous nucleases or activation of mutagenic repair pathways, such as illegitimate recombination or single strand annealing. The inhibition of DNA double strand break repair by Tax appears to create a state of genomic instability that contributes to HTLV-I induced cellular transformation.
Results
Tax represses Ku80 mRNA expression in Jpx-9 cells
Previous work by Majone et al. demonstrated that MN formation in Tax-expressing cells is dependent on Ku80 expression (Majone and Jeang, 2000). To determine if Tax can affect expression of the Ku heterodimer we analyzed Ku70 and Ku80 mRNA levels in the Tax-inducible Jpx-9 cell line. Under normal growth conditions no Tax is expressed in this cell line. However, upon addition of CdCl2 the inducible promoter is activated and Tax protein can be detected by immunoblotting. We titrated the amount of CdCl2 required for optimal induction and obtained robust Tax expression after two days of treatment with 40 μM CdCl2 (Figure 1A). These conditions were used in subsequent experiments to analyze the expression of Ku70 and Ku80 mRNA levels in these cells. We found that induction of Tax in Jpx-9 cells was associated with a concomitant 60% reduction in Ku80 mRNA, while Ku70 mRNA levels were not significantly affected (Figure 1B). In the absence of Tax, CdCl2 treatment did not alter either Ku80 or Ku70 mRNA levels in the parental Jurkat cell line (Figure 1C).
Figure 1. HTLV-I Tax represses Ku80 mRNA expression.
A. Jpx-9 cells were treated with 20 or 40 μM CdCl2 for 1 to 4 days and processed for immunoblot analysis. The membrane was probed with rabbit anti-Tax serum. The Tax band is indicated by an arrow (right). The asterisk indicates a non-specific band.
B. Jpx9 cells were mock treated or treated with 40μM CdCl2 for two days and quantitative RT-PCR was performed from total RNA using Ku70, Ku80 and GAPDH primers. Ku80 and Ku70 values obtained were normalized to their respective GAPDH values. C. Jurkat cells were either mock treated or treated with 40 μM CdCl2 for two days and analyzed by RT PCR as described in panel B.
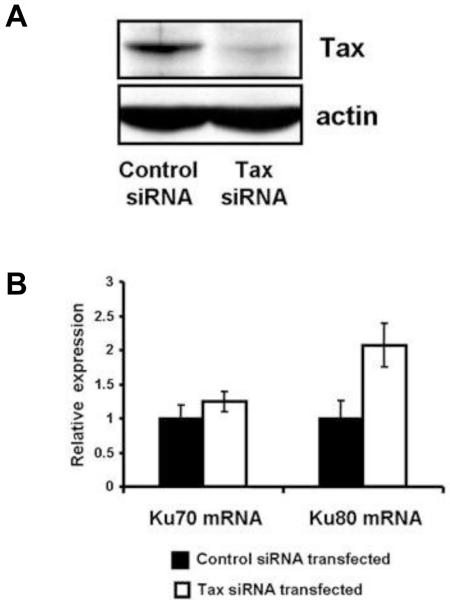
To determine whether Tax expression was specifically responsible for the decreased Ku80 mRNA levels, Tax expression was knocked down using siRNA. We transfected a duplex siRNA directed towards Tax mRNA into C81-66 cells, a human T cell line that expresses abundant amounts of Tax. Immunoblot analysis showed that Tax protein expression was reduced by approximately 80% after siRNA transfection (Figure 2A). This reduction in Tax expression was associated with a 2-fold increase in Ku80 mRNA as analyzed by real time RT-PCR (Figure 2B). Ku70 mRNA levels were similar in cells transfected with a Tax siRNA or a control scrambled siRNA. These results demonstrate that Tax expression is associated with decreased steady state levels of Ku80 mRNA, and that Ku80 mRNA levels can be restored when Tax expression is suppressed.
Figure 2. Down-regulation of Tax induces Ku80 mRNA expression in HTLV-I infected cells.
C81-66 cells were harvested three days after transfection with scrambled siRNA (control) or Tax siRNA. A. Cell lysates were analyzed for Tax and actin expression by immunoblot. B. Total RNA was prepared and analyzed by quantitative RT PCR for Ku70 and Ku80 expression.
Tax represses Ku80 promoter activity
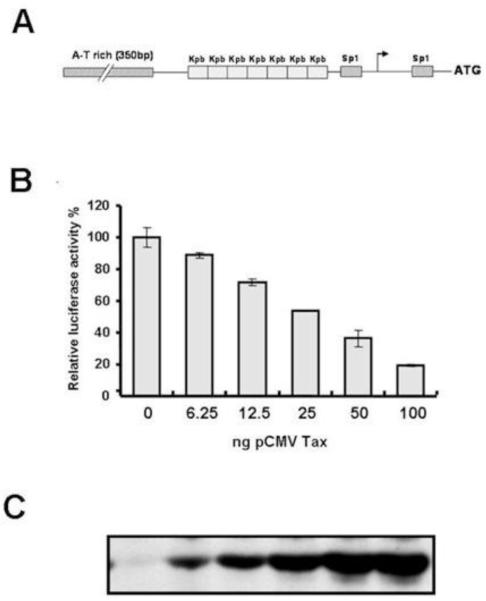
To determine the mechanism responsible for Tax inhibition of Ku80 mRNA expression, we asked if Tax affects transcription of the Ku80 gene. To address this question, we constructed a reporter containing the human Ku80 promoter driving expression of the firefly luciferase gene (Ku80-luc). The promoter construct included regulatory elements previously implicated in the basal transcription of Ku80, including seven copies of a palindromic sequence that has been shown to bind the Ku80 protein itself and two SP1 binding sites that are necessary for basal transcription of Ku (Figure 3A) (Ludwig et al., 1997). To examine the effect of Tax on Ku80 promoter activity, Ku80-luc was transfected into HeLa cells together with pRL-TK as an internal control for transfection efficiency, and increasing amounts of pCMV-Tax. After 48 hours the cells were lysed and luciferase activity was determined. Ku80 promoter activity decreased as the amount of transfected Tax expression vector increased (Figure 3B). Cell extracts were also examined for Tax expression by immunoblot (Figure 3C). These results demonstrate that Tax represses the activity of the Ku80 promoter in HeLa cells.
Figure 3. HTLV-I Tax represses the Ku80 promoter.
A. Schematic representation of the regulatory elements in the Ku80 promoter. B. HeLa cells were co-transfected with pCMV-Tax, pRL-TK and Ku80-luc. Cells were harvested 48 hours post-transfection and extracts were analyzed by luciferase assays. The data were normalized to the renilla luciferase values for each sample. Each condition was performed in duplicate and the experiment was repeated three times. C. Western blot analysis of Tax expression in cell extracts prepared for the experiment in panel B.
Tax expression reduces Ku80 protein expression
To determine whether Ku80 protein expression also decreased upon Tax expression, a Tax expression plasmid was transfected into HeLa cells. At 48 hours post transfection, Tax and Ku80 expression were analyzed by immunofluorescence (Figure 4). DAPI staining to identify the nucleus is shown in panel 4A. Co-staining of transfected cells with antibodies against Tax (panel 4C, red) or Ku80 (panel 4B, green) showed that cells expressing Tax (identified by white arrows in panels 4A, B, and C) had reduced Ku80 staining (panel 4B). While Tax-expressing cells showed reduced Ku80 staining, Ku80 remained localized in the nucleus. Of the cells expressing Tax, only 19% showed normal robust nuclear Ku80 expression. In contrast, 86% of the cells that did not express Tax showed normal nuclear Ku80 expression (Figure 4D). Together, these results suggest that Tax represses Ku80 protein expression in HeLa cells. It has previously been shown that a 50% reduction in Ku80 levels can severely impact NHEJ, and increase radiosensitivity and chromosomal instability (Li and Comai, 2002). Thus, the reduction in Ku80 protein levels in Tax-expressing cells could significantly impair its function in DNA repair, telomere protection or DNA replication.
Figure 4. Tax represses Ku80 protein expression in HeLa cells.
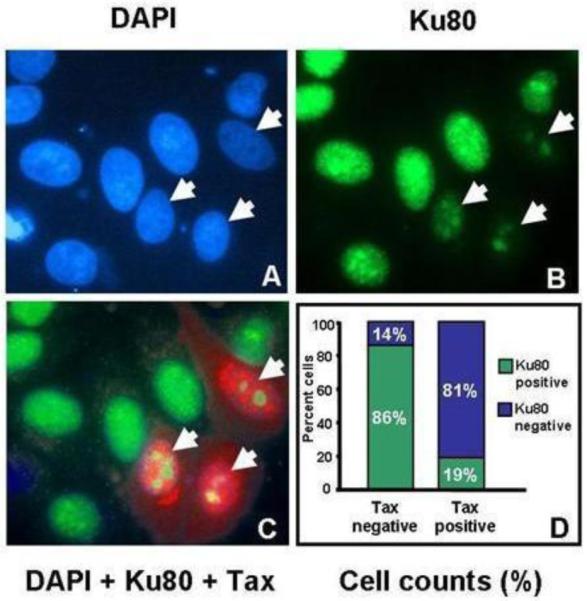
HeLa cells were transfected with pCMV-Tax and 48 hours later processed for immunofluorescence analysis using antibodies against Tax (red, panel C), Ku80 (green, panels B and C) and counter-stained with DAPI (blue, panel A) to visualize nuclei. Arrows point to cells that stain positively for Tax. This experiment was repeated three times and cell counts from one representative experiment are shown in panel D. Cells that showed reduced Ku80 staining, such as those indicated by arrows in panel B were scored as Ku80 negative. Cells that showed intense diffuse nuclear staining were scored as Ku80 positive. Tax immunostaining was used to score cells as Tax positive and Tax negative.
Tax-expressing T lymphocytes show reduced Ku80 expression
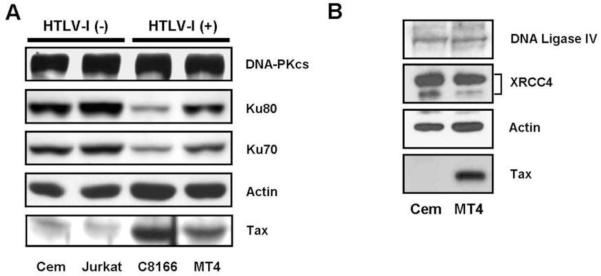
Since epithelial cells are not a typical target for HTLV-I infection, we asked whether T-lymphocytes expressing Tax or infected with HTLV-I have altered Ku80 protein expression. Tax expression was induced in the human T cell line, Jpx-9, and cell lysates were analyzed by western blot for Tax and Ku80. As shown in Figure 5, Ku80 expression was reduced in extracts from Tax expressing cells. CdCl2 treatment of the parental Jurkat cells did not affect Ku80 expression, suggesting that reduced Ku80 expression in induced Jpx-9 cells was due to Tax, and not to a non-specific effect of cadmium on the cells. To determine whether HTLV-I infection alters the expression of DNA repair proteins other than Ku80, whole cell extracts prepared from HTLV-I infected T cell lines (C81-66 and MT4), and uninfected T cell lines (Jurkat and CEM) were examined by western blot (Figure 6A). Both C81-66 and MT4 cell lines express Tax. Consistent with results in Jpx-9 cells, HTLV-I infected T cells displayed lower levels of Ku80 than did uninfected lymphocytes. Ku70 protein expression was also reduced in the HTLV-I infected lymphocytes, which was somewhat surprising since Ku70 mRNA expression was not affected in Tax expressing (Figure 1) or HTLV-I infected (Figure 2) cells. The reduction in Ku70 and Ku80 expression correlated with the amount of Tax expression, as C81-66 cells, which express higher levels of Tax than MT4 cells, showed the greatest reduction in Ku80 and Ku70 expression. The inhibitory effect of Tax appears to be specific for the Ku proteins, as expression of DNA-PKcs was similar in all the cell lines tested. Additionally, the ligase subunits DNA Ligase IV and XRCC4 were expressed at similar levels in uninfected CEM and in HTLV infected MT4 cells (Figure 6B), suggesting that HTLV-I infection does not affect cellular levels of the DNA ligase involved in NHEJ. Since Ku70 mRNA levels were not affected by Tax expression (Figures 1 and 2), we believe that the reduced Ku70 protein expression in HTLV-I infected cells (Figure 6A) is a result of increased Ku70 protein degradation. This effect is consistent with a previous report that Ku80 null cells and cells expressing reduced levels of Ku80, displayed decreased stability of Ku monomers (Gullo et al., 2006)
Figure 5. Tax represses Ku80 protein expression in Jpx-9 cells.
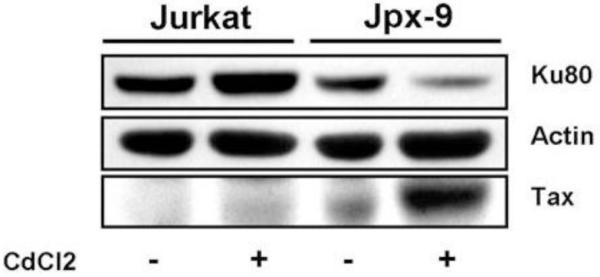
Jpx-9 cells were either untreated or treated with CdCl2 for 48 hours and processed for immunoblotting analysis of Ku80 and Tax expression. Actin expression was used as a loading control.
Figure 6. HTLV-I infected cell lines show reduced amounts of Ku70 and Ku80.
A. HTLV-I uninfected T cell lines (CEM and Jurkat) and HTLV-I infected cells (C81-66 and MT4) were analyzed for Ku70, Ku80 and DNA-PKcs expression by western blot. Tax expression in the HTLV-I infected cells is shown. B. CEM and MT4 cell extracts were analyzed by immunoblot using antibodies against DNA Ligase IV and its associated cofactor XRCC4. In both panels an actin blot is used as the loading control.
Tax-expressing cells have defects in DSB repair
Cells harboring null mutations in the Ku80 gene and cells transfected with Ku80 siRNA show defects in DSB repair that are manifested by an increased frequency of chromosomal fusions and translocations, loss of chromosomal fragments and increased numbers of micronuclei (Gullo et al., 2006). To determine if reduced Ku80 expression induced by Tax is associated with chromosomal instability, we assessed the effect of Tax on DNA end joining in HeLa cells that stably expresses Tax (HeLa-Tax). HeLa-Tax cells displayed abnormal nuclear phenotypes, such as multinucleated cells, giant lobulated nuclei (resembling the “flower cells” described in ATL patients), multipolar mitoses and micronuclei (Figure 7 and data not shown). In situ labelling of DNA ends was performed to determine if some of these features could be attributed to impaired DSB repair.
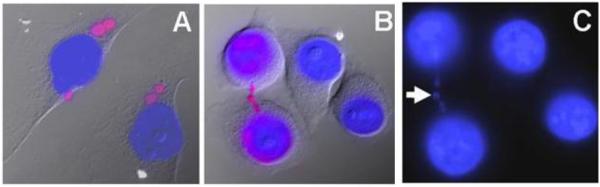
Figure 7. HeLa-Tax cells display broken DNA-containing micronuclei and nucleoplasmic bridges.
HeLa-Tax cells were stained for DNA end labelling (red) and counterstained with DAPI (blue). The bright field image is overlaid in panels A and B. A. HeLa-Tax cells show multiple micronuclei (round pink structures), sometimes 3-4 per cell that stain positive for DNA ends. B. A nucleoplasmic bridge is shown stretching between the nuclei of two daughter cells after cell division. The extracellular part of the bridge stains positive for free DNA ends. C. DAPI signal is shown for the image in panel B. The arrow indicates a broken chromosome segment from the nucleoplasmic bridge. All images were taken at 40× magnification.
HeLa-Tax cells showed a large number of micronuclei, as many as 3 to 4 per cell, and these micronuclei displayed increased incorporation of biotin-dUMP (Figure 7A), suggesting that most of the micronuclei contain chromosomal fragments. Additionally, a large proportion of the HeLa-Tax cells showed nucleoplasmic bridges, which appeared as thin DAPI stained material stretching between the nuclei of two adjacent cells that had undergone mitosis (Figure 7B and C). Nucleoplasmic bridges are thought to contain dicentric chromosomes that form as a result of chromosome end-to-end fusions (due to telomere defects) or interstitial chromosome fusions, which result from improperly repaired DNA breaks. During cytokinesis these chromosomes are pulled to separate daughter cell nuclei, resulting in the formation of a thin bridge containing DNA and nuclear matter between the two cells. Interestingly, some of the nucleoplasmic bridges appeared discontinuous, and stained intensely with biotin-dUMP, suggesting that the tension between the two separating nuclei had exceeded the resistance of the bridge, causing fragmentation of the dicentric chromosome (Figure 7C). This mechanism has been implicated in the formation of some micronuclei (Fenech, 2006).
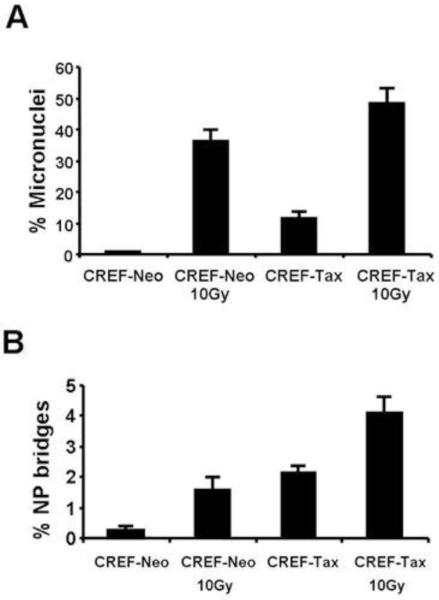
Our attempt to quantitate the micronuclei observed in HeLa-Tax cells was unsuccessful due to the high prevalence of multinucleated cells. We therefore decided to analyze micronuclei formation in CREF-Tax cells, which are more stable and phenotypically resemble their Tax null counterparts. CREF-Tax cells had higher numbers of micronuclei and nucleoplasmic bridges than CREF-Neo cells in both un-stressed conditions and after exposure to gamma irradiation (Figure 8). These data suggest that naïve CREF-Tax cells have a defect in repairing DSBs and that some of these breaks are preferentially repaired by mutagenic mechanisms that can lead to chromosome fusions and formation of dicentric chromosomes. Treatment with gamma irradiation amplifies this effect by introducing additional DNA damage. Collectively, our results show that HeLa and CREF cells that express Tax display abundant micronuclei and nucleoplasmic bridges, which are hallmarks of defective DSB repair.
Figure 8. Tax induces formation of micronuclei and nucleoplasmic bridges.
CREF-Neo and CREF-Tax cells were either mock treated or treated with 10 Gy of gamma irradiation. Cells were fixed 24 hours later and stained with DAPI. Micronuclei and nucleoplasmic bridges were counted for 500 cells per slide. Cells containing micronuclei (A) or nucleoplasmic bridges (B) were scored positive and the percentage of positive cells in each cell population is shown.
Discussion
To maintain genome stability, cells have developed regulatory mechanisms that ensure the order and fidelity of cell cycle events, including DNA replication and cell division. These mechanisms become activated upon exposure to genotoxic agents or other adverse conditions such as viral infection. Failure to establish an appropriate DNA damage response results in genomic instability, which is a known causal factor in tumorigenesis. Previous studies have shown that HTLV-I infected lymphocytes in vivo, and primary ATL cells cultured in vitro, display numerous chromosomal abnormalities, such as deletions, translocations and amplifications of certain chromosomal regions. Such dramatic changes are thought to result from constant genomic injuries that cells encounter during their lifespan, such as single strand and double strand breaks.
In this study we showed that HTLV-I Tax inhibits the expression of Ku80, a central component of the NHEJ pathway. In doing so, Tax potentially interferes with several mechanisms in which Ku80 participates, such as DSB repair, telomere maintenance, regulation of specific gene transcription and apoptosis, regulation of heat shock-induced responses, as well as regulation of the G2 and M phases of the cell cycle (Gullo et al., 2006). A previous microarray study also showed that the XRCC5 gene (encoding Ku80 autoantigen) was repressed 50% upon Tax induction in Jpx-9 cells (Ng et al., 2001). Since this study used matched cell lines that have very similar genetic backgrounds, this effect appears to be due to Tax protein expression.
Using mRNA expression analysis, promoter reporter assays and protein expression we showed that Tax inhibits Ku80 transcription, mRNA, and protein expression. This repressive effect appears to be specific for Ku80 expression, as other components of the NHEJ pathway were not significantly affected by Tax expression. Although Tax expression in lymphocyte cell lines did not completely obliterate Ku80 expression, it was responsible for an approximately 50% reduction in Ku80 protein levels. This level of reduction in Ku80 expression, obtained by knocking out one Ku allele, has previously been shown to cause significant defects in DSB repair, radiosensitivity and chromosomal instability (Li et al., 2001).
Notably, the DNA repair defects encountered in Ku80 deficient cells are remarkably similar to the phenotype displayed by Tax expressing cells. This led us to hypothesize that by reducing the cellular levels of Ku80 protein, Tax disrupts normal mechanisms of DSB repair, allowing for an accumulation of free DNA ends. Indeed, a previous study by Majone and Jeang suggested that Tax targets Ku80 to promote the accumulation of clastogenic DNA damage in mammalian cells manifested by an accumulation of micronuclei (Majone et al., 2005). These authors found that both Tax expressing cells and Ku80 null rodent cells show increased numbers of micronuclei, however, addition of Tax to Ku80 deficient cells did not further enhance the number of micronuclei formed. Therefore the effect of Tax on micronuclei formation is mediated by Ku80. Consistent with these results, our studies show that human cells that express Tax form more micronuclei then their non-expressing counterparts. This difference was further enhanced by treatment with DNA damaging agents, such as ionizing radiation. Our results extend those of Majone and Jeang by showing increased formation of nucleoplasmic bridges in human cells, another aberrant DNA structure induced by Tax expression.
Additionally, we noted a significant increase in the formation of nucleoplasmic bridges in the presence of Tax, which are thought to arise through the fusion of unprotected DNA ends. During cell division, some of the nucleoplasmic bridges break leaving behind DNA material that can be incorporated into micronuclei. Repeated cycles of end to end fusion and breakage of nucleoplasmic bridges during mitosis have been implicated in gene amplification, a phenomenon that can lead to over-expression of oncogenes and other factors promoting tumor growth and survival (Myllykangas and Knuutila, 2006). Interestingly, a study published by our lab has shown that CREF-Tax cells display five fold higher levels of gene amplification compared to CREF-Neo cells (Lemoine and Marriott, 2002). Future studies will determine whether this effect is also mediated by Ku80 depletion, as appears to be the case with micronuclei expression.
Our results show that Tax specifically represses the expression of Ku80; however, it is unclear if HTLV acquired this function to benefit viral infection. Ku80, as a part of the DNA-PK complex, plays important and complex roles in retroviral infection. It has been shown that retroviral infection of NHEJ deficient cells induces cell killing (Daniel et al., 1999; Daniel et al., 2004). Both the presence of unintegrated DNA viral genomes and integration of viral DNA into host chromosomes are perceived by the cell as DNA breaks and can induce cell death if not repaired promptly by the NHEJ machinery (Jeanson et al., 2002; Li et al., 2001). Therefore NHEJ is thought to facilitate retroviral infection. Conversely, it has been shown that Ku80 participates in the targeting of retroviral insertions into silent areas of chromatin, decreasing viral gene expression and therefore productive infection (Masson et al., 2007). While the role of NHEJ in the HTLV-I replicative cycle has not yet been investigated, the repression of Ku80 by Tax could impact post-integration steps, when Tax protein is expressed. A possible role of Ku80 in HTLV-I infection emerged when it was shown that Ku80 can specifically bind to a repressive DNA element in the HTLV-I promoter (Okumura et al., 1996). While the role of Ku80 in regulating the activity of this element has not been established, it is possible that it functions to inhibit viral transcription. This effect would not be unprecedented, since Ku80 has been shown to repress human immunodeficiency virus 1 (HIV-1) transcription (Jeanson et al., 2002). Therefore, by repressing Ku80 expression, Tax might relieve an inhibitory effect of Ku on HTLV-I transcription, allowing for maximal viral gene expression.
Our results showed that the repression of Ku80 by HTLV-I Tax leads to inefficient or delayed repair of DSBs by NHEJ, allowing for the accumulation of multiple DNA ends within a cell nucleus at the same time. HeLa-Tax cells and CREF Tax cells showed increased numbers of micronuclei and nucleoplasmic bridges, which are indicators of improperly repaired DNA breaks. This could allow for loss of chromosomal fragments by segregation in micronuclei and stimulate alternative mechanisms of repair, such as single strand annealing and micro-homology mediated repair, resulting in chromosomal translocations and fusions. While these mechanisms provide a way for the cell to eliminate DNA ends and therefore avoid apoptosis, they cause a drastic increase in the rate of genomic mutations. Inhibition of DNA repair pathways by HTLV-I Tax, in addition to growth stimulation and abrogation of cell cycle checkpoints may thus contribute to genomic instability and accumulation of mutations.
Materials and methods
Cell culture and treatments
CREF-Neo and CREF-Tax cells (Kao et al., 2001) were maintained in Dulbecco’s Modified Eagle Media (DMEM) supplemented with 10% fetal bovine serum (FBS). HeLa cells were grown in DMEM supplemented with 10% FBS and were transfected using Fugene 6 (Roche Applied Science, Indianapolis, IN) following manufacturer’s instructions. HeLa-Tax cells were generated by co-transfecting a Tax-expression plasmid with a plasmid encoding the neomycin resistance gene at a ratio 10:1. Stable clones were selected by culturing transfected cells in growth media containing G418 (Gibco - Invitrogen, Carlsbad, CA) for 4 weeks. Individual colonies were transferred to 60 mm plates and expanded for an additional 2-3 weeks. Tax expression in G418 resistant colonies was analyzed by western blot. HTLV-I negative (CEM, Jurkat, Jpx-9) and HTLV-I-positive (MT4, C81-66) human T cell lines were maintained in RPMI media containing 10% FBS. Jpx-9 T cells were derived from Jurkat cells by stable transfection of a Tax expression plasmid under the control of the metallothionine promoter (Nagata et al., 1989). Where indicated, CdCl2was added directly to the culture media to a final concentration of 40 μM. All cells were grown in a humidified atmosphere at 37°C in 5% CO2. Exponentially growing cells were either mock-treated or treated with ionizing radiation (IR) at the indicated doses.
Immunoblotting
Cells were lysed in Laemmli sample buffer and boiled for 15 min. Collected supernatants were resolved on SDS-PAGE gels, transferred to nitrocellulose membranes and subjected to immunoblotting. Anti-Tax monoclonal antibody Tab170 was obtained from the AIDS Research and Reference Reagent Program, (Germantown, MD) and rabbit anti-Tax serum 586 was obtained from John Brady (NIH). Anti-Ku80 (B-1 and H-300), anti-Ku70 (H-308), and anti-DNA-PKcs antibodies (H-163) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-XRCC4 and anti-DNA ligase IV antibodies were purchased from Novus Biologicals (Littleton, CO). Horseradish peroxidase (HRP) conjugated secondary antibody and anti-actin antibody (A5060) were purchased from Sigma-Aldrich (St. Louis, MO).
Luciferase assays
Cells were harvested 48 h post-transfection, washed once in phosphate-buffered saline and lysed in passive lysis buffer (Promega, Madison, WI) for 20 minutes. Relative luciferase activity was determined by adding 50 μl of firefly luciferase substrate (Promega, Madison, WI) to 10 μl of lysate, and luminescence was detected using a Sirius luminometer (Berthold Detection Systems, Pforzheim, Germany). Relative promoter activity was determined by averaging duplicate results in each experiment and normalizing them to the basal activity of their respective reporter. Results are displayed as the average n-fold activation from three independent experiments.
RNA isolation and cDNA synthesis
RNA was harvested using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Briefly, 5 × 106 cells were pelleted and resuspended in 1 ml of TRIzol. RNA recovered by chloroform extraction and ethanol precipitation was treated with RNase free DNase (Invitrogen, Carlsbad, CA). cDNA was synthesized from 1 μg of RNA using avian myeloblastosis virus reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo-dT primers.
Real-time PCR
Real-time PCR for cDNA analysis was performed using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA). Reaction mixtures containing 1× PCR master mix buffer, specific primers, and 0.1 μg of cDNA were amplified using a Rotor-Gene real-time PCR machine (Corbett Research). All quantitative PCR reactions were performed in duplicate, and each experiment was repeated three times. Primer sequences for quantitative PCR were: Ku70: TGCTCATGGGTTTCAAGCCGTT, TGCACAATGCTGCAACCTCCTT; Ku80: ACCAAAGAGGAAGCCTCTGGAAGT, TGAATGGCCGCATCCAACTTGT; DNA-PKcs: ACAGCAAATGCACCGTTGTGGT, TTGAAACCTATGCTTGCGGGCT. All oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). Relative mRNA expression was calculated by normalizing the values obtained for target mRNA to GAPDH mRNA and then dividing by the relative mRNA expression in mock treated cells. Raw data analysis was performed using the Rotor Gene software.
Tax siRNA design and transfections
siRNA oligonucleotides used for Tax knock down were designed using the dicer substrate siRNA design tool available at www.idtdna.com. Oligonucleotide sequences were: 5′-rGrUrGrArArArUrArUrArArArGrGrArGrGrArGrGrArCrUrGrUrArG-3′ and 5′-/5Phos/rArCrArGrUrCrCrUrCrCrUrCrCrUrUrUrArUrArUrUrUrCAC-3′. Oligonucleotides were mixed and suspended in buffer provided by the manufacturer. C81-66 cells were transfected with Tax siRNA or scrambled siRNA duplexes (Dharmacon, Chicago, IL) using an Amaxa Nucleofector machine (Amaxa Inc., Gaithersburg, MD) according to the manufacturer’s instructions. Briefly, 2×106 cells were suspended in 100̣ul buffer included in the Amaxa Nucleofector Kit V and mixed with siRNA solution to a final concentration of 200μM. The cell suspension was added to the cuvettes included in the kit and subjected to electroporation using the Nucleofector machine program C-16. After transfection, cells were immediately seeded into 12-well plates containing growth media and grown for three days.
Cloning of the Ku80 promoter
A fragment of 1500 nucleotides upstream of the translation start site of the human Ku80 was cloned into pGL3-Basic (Promega, Madison, WI) using a PCR cloning strategy. The fragment was amplified using genomic DNA extracted from Jpx-9 cells as template. Primer sequences were: 5′-TTACTCGAGCTGACAACCTCACAGATC-3′, and 5′-CACATGGTGCCGGTCCTCAGG-3′. The PCR product was directly cloned into pGEM-T Easy vector (Promega, Madison, WI). The promoter fragment was recovered by digesting the vector with Bgl-ll and Nco-I restriction enzymes and was directionally cloned into pGL3-Basic vector. The resulting Ku80-luc promoter construct was verified by sequencing using generic vector primers.
Immunofluorescent Staining
HeLa cells were transfected with pCMV-Tax and 24 hours later, were seeded onto ethanol-washed cover slips and grown to approximately 50% confluence. The cells were washed once with PBS, and fixed in 4% formaldehyde diluted in PEM buffer (80mM potassium PIPES pH 6.8, 5mM EGTA pH 7.0, 2mM MgCl2) for 30 minutes at 4°C. Cells were then permeabilized by incubating in PEM buffer containing 0.5% Triton X-100 for 30 minutes at room temperature. Immunofluorescent staining was performed by incubating with primary antibody diluted in 5% BSA containing TBS + 0.1% Tween 20 (TBS-T) overnight at 4°C. Excess antibody was removed by washing cells three times in TBS-T. Cells were incubated in the dark with a fluorophore-conjugated secondary antibody diluted in TBS-T for 40 minutes at room temperature. Excess antibody was removed by washing the cover slips three times with TBS-T. The cells were stained with DAPI (Sigma-Aldrich, St. Louis, MO) to visualize the nucleus, and mounted on slides using Slow-Fade Anti-fade mounting media (Molecular Probes, Eugene, OR). Cells were visualized on a Zeiss AxioPlan2 microscope using a CoolSnap HQ CCD camera.
Micronucleus assay
The micronucleus assay was performed as previously described (Majone et al., 1993). Briefly, CREF-Neo and CREF-Tax cells were seeded on glass microscope coverslips in 12 well plates. 24 hours later the cells were either mock treated or exposed to 10 Gy gamma irradiation and grown for an additional 24 hrs. At the time of harvest, cells were fixed in 4% formaldehyde diluted in PEM buffer (80mM potassium PIPES pH 6.8, 5mM EGTA pH 7.0, 2mM MgCl2) for 30 minutes at 4°C and permeabilized in PEM buffer containing 0.5% Triton X-100 for 30 minutes at room temperature. The cover slips were stained with DAPI (Sigma-Aldrich, St. Louis, MO) and mounted onto microscopic glass slides. Images were taken using a Zeiss AxioPlan2 Microscope using a CoolSnap HQ CCD camera. Five hundred cells were counted for each slide and scored for the presence of micronuclei and nucleoplasmic bridges.
Enzymatic labelling of DNA ends
In situ labelling of free DNA ends was performed as previously described (Majone and Jeang, 2000) with minor modifications. Briefly, HeLa-Tax cells grown on microscopic cover slips were fixed by immersion in 4% formaldehyde for 30 minutes and permeabilized in PEM buffer containing 0.5% Triton X-100. Cells were washed in PEM buffer and incubated in labelling buffer (1 M potassium cacodylate, 125 mM Tris- HCl, pH 6.6, 1.25 mg/ml bovine serum albumin, 10 mM CoCl2). Terminal deoxy-nucleotidyl transferase (Roche Applied Science, Indianapolis, IN) was added to a final concentration of 200U/ml and biotin-dUTP (Sigma-Aldrich, St. Louis, MO) was added to a final concentration of 20 uM. The reactions were incubated in a moist environment for 1 hour at 37°C, after which the slides were washed three times in PBS. Biotin incorporation was detected using a Streptavidin tyramide signal amplification kit (Invitrogen - Molecular Probes Carlsbad, CA), according to the manufacturer’s instructions and the nuclei were stained with DAPI (Sigma-Aldrich, St. Louis, MO).
Acknowledgements
This study was supported, in part, by the United States Public Service Grant CA-77371 from National Cancer Institute, National Institutes of Health, awarded to S.J.M. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank members of the Marriott laboratory for insight and expertise, as well as for helpful suggestions and editorial comments. We acknowledge the NIH AIDS Reference Reagent Program for providing anti-Tax antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burma S, Chen BP, Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst. ) 2006;5:1042–1048. doi: 10.1016/j.dnarep.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Chieco-Bianchi L, Saggioro D, DelMistro A, Montaldi A, Majone F, Levis AG. Chromosome damage induced in cord blood T-lymphocytes infected in vitro by HTLV-I. Leukemia. 1988;2:223s–232s. [PubMed] [Google Scholar]
- Daniel R, Greger JG, Katz RA, Taganov KD, Wu X, Kappes JC, Skalka AM. Evidence that stable retroviral transduction and cell survival following DNA integration depend on components of the nonhomologous end joining repair pathway. J Virol. 2004;78:8573–8581. doi: 10.1128/JVI.78.16.8573-8581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R, Katz RA, Skalka AM. A role for DNA-PK in retroviral DNA integration. Science. 1999;284:644–647. doi: 10.1126/science.284.5414.644. [DOI] [PubMed] [Google Scholar]
- Fenech M. Cytokinesis-block micronucleus assay evolves into a “cytome” assay of chromosomal instability, mitotic dysfunction and cell death. Mutat. Res. 2006;600:58–66. doi: 10.1016/j.mrfmmm.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Hata T, Itoyama T, Nakamura H, Tsukasaki K, Yamada Y, Ikeda S, Sadamori N, Tomonaga M. High rate of chromosomal abnormalities in HTLV-I-infected T-cell colonies derived from prodromal phase of adult T-cell leukemia: a study of IL-2-stimulated colony formation in methylcellulose. Cancer Genet Cytogenet. 1999;109:1–13. doi: 10.1016/s0165-4608(98)00141-1. [DOI] [PubMed] [Google Scholar]
- Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski JG, Haseltine WA, Ramstedt U. Role of human T-cell leukemia virus type I X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmann R, Dengler C, Muller-Fleckenstein I, McGuire K, Dokhelar MC, Sodroski JG, Haseltine WA. Transformation to continuous growth of primary human T lymphocytes by human T cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3551–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullo C, Au M, Feng G, Teoh G. The biology of Ku and its potential oncogenic role in cancer. Biochim Biophys Acta. 2006;1765:223–234. doi: 10.1016/j.bbcan.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Itoyama T, Sadamori N, Tokunaga S, Sasagawa I, Nakamura H, Yao E, Jubashi T, Yamada Y, Ikeda S, Ichimura M. Cytogenetic studies of human T-cell leukemia virus type I carriers. A family study. Cancer Genet. Cytogenet. 1990;49:157–163. doi: 10.1016/0165-4608(90)90137-y. [DOI] [PubMed] [Google Scholar]
- Jeang KT, Widen SG, Semmes OJ, Wilson SH. HTLV-I trans-activator protein, Tax, is a trans-repressor of the human á-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- Jeanson L, Subra F, Vaganay S, Hervy M, Marangoni E, Bourhis J, Mouscadet JF. Effect of Ku80 depletion on the preintegrative steps of HIV-1 replication in human cells. Virology. 2002;300:100–108. doi: 10.1006/viro.2002.1515. [DOI] [PubMed] [Google Scholar]
- Kamada N, Sakurai M, Miyamoto K, Sancar A, Sadamori N, Fukuhara S, Abe S, Shiraishi Y, Abe T, Kaneko Y, Shimoyama M. Chromosome abnormalities in adult T-cell leukemia/lymphoma: a karyotype review committee report. Cancer Res. 1992;52:1482–1493. [PubMed] [Google Scholar]
- Kao J, Rosenstein BS, Peters S, Milano MT, Kron SJ. Cellular response to DNA damage. Ann N Y Acad Sci. 2005;1066:243–258. doi: 10.1196/annals.1363.012. [DOI] [PubMed] [Google Scholar]
- Kao SY, Lemoine FJ, Marriott SJ. p53-independent induction of apoptosis by the human T cell leukemia virus type I Tax protein following UV irradiation. Virology. 2001;291:292–298. doi: 10.1006/viro.2001.1200. [DOI] [PubMed] [Google Scholar]
- Kao SY, Marriott SJ. Disruption of nucleotide excision repair by the human T-cell leukemia virus type 1 Tax protein. J. Virol. 1999;73:4299–4304. doi: 10.1128/jvi.73.5.4299-4304.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine FJ, Marriott SJ. Genomic instability driven by the human T-cell leukemia virus type 1 (HTLV-I) oncoprotein, Tax. Oncogene. 2002;21:7230–7234. doi: 10.1038/sj.onc.1205898. [DOI] [PubMed] [Google Scholar]
- Li B, Comai L. Displacement of DNA-PKcs from DNA ends by the Werner syndrome protein. Nucleic Acids Res. 2002;30:3653–3661. doi: 10.1093/nar/gkf488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Olvera JM, Yoder KE, Mitchell RS, Butler SL, Lieber M, Martin SL, Bushman FD. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 2001;20:3272–3281. doi: 10.1093/emboj/20.12.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DL, Chen F, Peterson SR, Nussenzweig A, Li GC, Chen DJ. Ku80 gene expression is Sp1-dependent and sensitive to CpG methylation within a novel cis element. Gene. 1997;199:181–194. doi: 10.1016/s0378-1119(97)00366-1. [DOI] [PubMed] [Google Scholar]
- Majone F, Jeang KT. Clastogenic effect of the human T-cell leukemia virus type I tax oncoprotein correlates with unstabilized DNA breaks. J Biol Chem. 2000;275:32906–32910. doi: 10.1074/jbc.C000538200. [DOI] [PubMed] [Google Scholar]
- Majone F, Luisetto R, Zamboni D, Iwanaga Y, Jeang KT. Ku protein as a potential human T-cell leukemia virus type 1 (HTLV-1) Tax target in clastogenic chromosomal instability of mammalian cells. Retrovirology. 2005 doi: 10.1186/1742-4690-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majone F, Semmes OJ, Jeang KT. Induction of micronuclei by HTLV-I Tax: a cellular assay for function. Virology. 1993;193:456–459. doi: 10.1006/viro.1993.1145. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Fukushima T, Kawamura K, Mochizuki S. Chromosome and gene rearrangements in immortalized human lymphocytes infected with human T-lymphotropic virus type I. Cancer Res. 1990;50:5697s–5702s. [PubMed] [Google Scholar]
- Masson C, Bury-Mone S, Guiot E, Saez-Cirion A, Schoevaert-Brossault D, Brachet-Ducos C, Delelis O, Subra F, Jeanson-Leh L, Mouscadet JF. Ku80 participates in the targeting of retroviral transgenes to the chromatin of CHO cells. J Virol. 2007;81:7924–7932. doi: 10.1128/JVI.02015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake H, Suzuki T, Hirai H, Yoshida M. Trans-activator Tax of human T-cell leukemia virus type 1 enhances mutation frequency of the cellular genome. Virology. 1999;253:155–161. doi: 10.1006/viro.1998.9500. [DOI] [PubMed] [Google Scholar]
- Mortreux F, Gabet AS, Wattel E. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia. 2003;17:26–38. doi: 10.1038/sj.leu.2402777. [DOI] [PubMed] [Google Scholar]
- Myllykangas S, Knuutila S. Manifestation, mechanisms and mysteries of gene amplifications. Cancer Lett. 2006;232:79–89. doi: 10.1016/j.canlet.2005.07.045. [DOI] [PubMed] [Google Scholar]
- Nagata K, Ohtani M, Nakamura M, Sugimura K. Activation of endogenous c- fos proto-oncogene expression by human T-cell leukemia virus type I-encoded by p40 tax protein in the human T-cell line, Jurkat. J Virol. 1989;63:3220–3226. doi: 10.1128/jvi.63.8.3220-3226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerenberg M, Hinrichs SH, Reynolds RK, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type I induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Ng PWP, Iha H, Iwanaga Y, Bittner M, Chen YD, Jiang Y, Gooden G, Trent JM, Meltzer P, Jeang KT, Zeichner SL. Genome-wide expression changes induced by HTLV-1 Tax: evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-kappa B activation. Oncogene. 2001;20:4484–4496. doi: 10.1038/sj.onc.1204513. [DOI] [PubMed] [Google Scholar]
- Nimura Y, Kawata T, Uzawa K, Okamura J, Liu C, Saito M, Shimada H, Seki N, Nakagawara A, Ito H, Ochiai T, Tanzawa H. Silencing Ku80 using small interfering RNA enhanced radiation sensitivity in vitro and in vivo. Int J Oncol. 2007;30:1477–1484. [PubMed] [Google Scholar]
- Okamoto T, Ohno Y, Tsugane S, Watanabe S, Shimoyama M, Tajima K, Miwa M, Shimotohno K. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn. J. Can. Res. 1989;80:191–195. doi: 10.1111/j.1349-7006.1989.tb02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K, Sakaguchi G, Takagi S, Naito K, Mimori T, Igarashi H. Sp1 family proteins recognize the U5 repressive element of the long terminal repeat of human T cell leukemia virus type I through binding to the CACCC coremotif. J. Biol. Chem. 1996;271:12944–12950. doi: 10.1074/jbc.271.22.12944. [DOI] [PubMed] [Google Scholar]
- Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kaccmaz K, Linn S. Molecular Mechanisms of Mammalian DNA Repair and the DNA Damage Checkpoints. Annu. Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Smith MR, Greene WC. Type I human T cell leukemia virus Tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J. Clin. Invest. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Takahashi G, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang-Peng J, Bunn PA, Knutsen T, Kao-Shan CS, Broder S, Jaffe ES, Gelman E, Blattner W, Lofters W, Young RC, Gallo RC. Cytogenetic studies in human T-cell lymphoma virus (HTLV)-positive leukemia-lymphoma in the United States. J. Natl. Cancer Inst. 1985;74:357–369. [PubMed] [Google Scholar]
- Wood RD, Shivji MK. Which DNA polymerases are used for DNA-repair in eukaryotes? Carcinogenesis. 1997;18:605–610. doi: 10.1093/carcin/18.4.605. [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Tobe T, Hatanaka M. Tax protein of human T-cell leukemia virus type I is required for maintenance of the transformed phenotype. Oncogene. 1992;7:433–437. [PubMed] [Google Scholar]
- Yasunaga J, Matsuoka M. Human T-cell leukemia virus type I induces adult T-cell leukemia: from clinical aspects to molecular mechanisms. Cancer Control. 2007;14:133–140. doi: 10.1177/107327480701400206. [DOI] [PubMed] [Google Scholar]