Abstract
The FMS-like receptor tyrosine kinase 3 (FLT3) plays an important role in controlling differentiation and proliferation of hematopoietic cells. Activating mutations in FLT3 occur in patients with acute myeloid leukemia (15-35%) resulting in abnormal cell proliferation. Furthermore, both adult and pediatric patients with acute myeloid leukemia (AML) harboring the FLT3 internal tandem duplication (ITD) mutation have a poor prognosis. Several inhibitors have been developed to target mutant FLT3 for the treatment of AML, yet the molecular pathways affected by drug inhibition of the mutated FLT3 receptor alone have not yet been characterized. Linifanib (ABT-869) is a multi-targeted tyrosine kinase receptor inhibitor that suppresses FLT3 signaling. In this paper, we demonstrate that treatment with Linifanib inhibits proliferation and induces apoptosis in ITD mutant cells in vitro and in vivo. We show that treatment with Linifanib reduces phosphorylation of AKT and glycogen synthase kinase 3β (GSK3β). In addition, we show that inhibition of GSK3β decreases Linifanib-induced apoptosis. This study demonstrates the importance of GSK3 as a potential target for AML therapy, particularly in patients with FLT3 ITD mutations.
Keywords: AML, FLT3 Inhibitor
Introduction
FLT3 plays an important role in controlling the differentiation and proliferation of hematopoietic cells. Somatic mutations in the FMS-like tyrosine kinase 3 receptor (FLT3) have been frequently identified in AML (1-3). Mutations in FLT3 primarily consist of internal tandem duplications (ITD) in the juxtamembrane domain affecting 15-34% AML patients, or point mutations in the tyrosine kinase domain in 8-12% of patients (1, 2). These mutations are associated with a poor prognosis in both adult and pediatric AML patients (1, 2). Mutations result in autophosphorylation of the FLT3 kinase domain and as a consequence, there is up-regulation and activation of downstream signaling pathways such as the Ras/Raf/MEK/ERK pathway, the phosphoinositide-3 (PI3K) kinase pathway (PI3K/PTEN/AKT/mTOR), and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways (3, 4). Consequently, there is uncontrolled proliferation, arrest of myeloid cell differentiation, and increased resistance to apoptosis.
AML patients receiving conventional chemotherapy experience significant toxicity and relapse due to drug resistance (5). As a result, inhibitors targeting FLT3, with lower toxicity and higher potency than conventional chemotherapy, have emerged and are currently being investigated (5). Pre-clinical studies using these inhibitors have shown an effect at inhibiting proliferation and inducing apoptosis in human FLT3 mutant cell lines (5). In addition, in vitro studies on the effects of FLT3 inhibitors on human leukemia cell lines with FLT3 mutations have shown inhibition of downstream members of the PI3K pathway such as AKT, members of the Ras/Raf/MEK/ERK pathway such as ERK1/2 and MEK1/2, members of the Jak/STAT pathway such as STAT5, cell cycle regulators as Cyclin D, cyclin E, p p21waf1/cip and p27kip1 (6-10). FLT3 inhibitors have also been shown to affect members of the Bcl-2 family of apoptotic proteins as the pro-apoptotic proteins BAD and Bim and anti-apoptotic proteins Bcl-xl and Mcl-1 (6, 10-13).
Linifanib (ABT-869) is an ATP-competitive tyrosine kinase inhibitor effective against constitutively active FLT3 and other members of the platelet derived growth factor receptor (PDGF) and vascular endothelial growth factor receptor (VEGF) families (14). Linifanib has been shown in vivo to be effective against acute myeloid leukemia cells harboring FLT3 mutations (MV4-11), highly angiogenic fibrosarcoma, small-lung cell carcinoma, epidermoid carcinoma, breast carcinoma, and colon adenocarcinoma (14, 15). Treatment of AML cells with Linifanib in combination with other FLT3 inhibitors as CEP-701 or chemotherapy as cytosine arabinoside (Ara-C) and Doxorubicin (Dox) have demonstrated synergistic effects (15). Pre-clinical studies have shown that Linifanib inhibits proliferation in FLT3 ITD positive human leukemia cell lines MV4-11 and Molm-14 at an IC50 of less than 10nM (7, 10). Linifanib also causes cell cycle arrest and apoptosis through decreased expression of Cyclins D and E and increased expression in cyclin dependent inhibitors p21waf1/cip and p27kip1 (10). Additionally, increased expression of pro-apoptotic BAD, BAK and BID, and decreases in expression of anti-apoptotic protein Bcl-xl are also observed (7, 10, 15). In addition to inhibiting phosphorylation of the FLT3 receptor, Linifanib has been shown to have an inhibitory effect on downstream kinases including AKT, ERK, STAT5 and Pim-1 (7, 10).
Many of the previous studies were performed with human FLT3 ITD leukemia cell lines that may contain other mutations or aberrant signaling pathways. The molecular pathways inhibited by Linifanib downstream of FLT3-ITD alone in the absence of other potential molecular abnormalities have not yet been studied. To characterize the effects of Linifanib specifically on FLT-3 dependent pathways, we used the Ba/F3 pro-B cell line as the model system (16, 17). Modifications to Ba/F3 cells rendering FLT3 receptor constitutively active have shown induction of leukemia-like syndrome in syngeneic mice (18, 19). Ba/F3 pro-B cells require the presence of Interleukin-3 (IL-3) to grow and without it, undergo rapid apoptosis (20, 21). Ba/F3 cells containing the human FLT3 ITD mutation, however, are able to survive independently of IL-3 (18). Phosphatidylinositol (PI) 3-kinase and its downstream target, the protein kinase AKT, have an important role in suppressing apoptosis and regulating this growth factor dependent survival (22-24). In addition, Glycogen Synthase Kinase 3, a serine threonine kinase, has been shown to play a role in growth factor withdrawal induced apoptosis (24, 25). It has been reported that the IL-3 withdrawal mechanism of apoptosis was dependent on GSK3 driving mitochondrial outer membrane permeabilization (24). GSK3 has also recently been implicated in sustaining proliferation of acute leukemia caused by MLL (mixed-lineage leukemia) (26).
Here, we show that Ba/F3 Pro B cells with the human FLT3 ITD mutation and treated with Linifanib undergo apoptosis and inhibition of proliferation. We show that treatment with Linifanib causes reduced phosphorylation of GSK3β at Ser9. This finding is significant as GSK3β has not been previously characterized to play a role in FLT3 ITD mutant signaling in AML cells and thus, may play an important role in combining targeted therapies.
Experimental Procedures
Cell Culture
Ba/f3 human FLT3 WT and FLT3 ITD mutant cell lines were generated by site directed mutagenesis by the laboratory of Dr. Michael Heinrich (16). Cells were tested and authenticated by Sanger sequencing of genomic DNA using pLXSN sequencing primers 5′-CCCTTGAACCTCCTCGTTCGACC–3′ and 5′-GAGCCTGGGGACTTTCCACACCC–3′ in 2007. Ba/F3 WT cells were purchased from ATCC. Ba/F3 Wild type and Ba/F3 hFLT3 wild type cells were cultured in RPMI 1640 (Gibco/Invitrogen Grand Island, NY) with 10% Fetal bovine serum (Omega Scientific Inc. Tarzana, CA), penicillin (10,000Units/ml), Streptomycin (10,000 μg/ml) and L-glutamine (29.2mg/ml) (Gibco/Invitrogen Grand Island, NY), and 10% conditioned Wehi-3 media, as a source of IL-3. Ba/f3 FLT3 ITD were maintained in RPMI 1640 (Gibco/Invitrogen Grand Island, NY) with 10% Fetal Bovine Serum (Omega Scientific, Inc Tarzana, CA) and penicillin (10,000Units/ml), Streptomycin (10,000 μg/ml) and L-glutamine (29.2mg/ml) (Gibco/Invitrogen Grand Island, NY). Cells were cultured at 37° and 5% CO2 in a humidified atmosphere. For experiments involving WT cells, grown in human FLT3 ligand, a concentration of 100ng/ml was used (Sigma St. Louis, MO) instead of Wehi-3 supernatant. MV-411 cells were obtained from ATCC and maintained in Iscove's Complete Medium (Gibco/Invitrogen Grand Island, NY) 10% Fetal bovine serum (Omega Scientific Inc. Tarzana, CA), penicillin (10,000Units/ml), Streptomycin (10,000 μg/ml) and L-glutamine (29.2mg/ml) (Gibco/Invitrogen Grand Island, NY).
Linifanib (ABT-869)
Linifanib lyophilized powder was provided by Abbott Pharmaceutical, Inc (North Chicago, IL). The compound was dissolved in DMSO (Sigma, St.Louis, MO) to a concentration of 10mM and stored in aliquots at -80°C for use for in vitro experiments. Linifanib was dissolved in corn oil for in vivo experiments at a concentration of 4mg/ml and stored in aliquots at -80°C. Structure of linifanib is shown in Supplemental Figure 1.
Proliferation Assays
To assess cell proliferation, we measured the innate metabolic activity of Ba/F3 FLT3 ITD and Ba/F3 FLT3 WT cells with alamarBlue® aqueous dye (Biosource Inc, Europe, Nivelle Belgium). We plated 1×104 (in 100μL media) cells at 97% confluency overnight in a 96-well plate (Corning Incorporated, Corning, NY) and treated the next day with either 100pM, 1nM, 10nM, 100nM, 1μM, and 10μM of Linifanib, vehicle control (DMSO) or left untreated. Cell/drug mixtures were allowed to incubate for 24 hours. At 24 hours, 10% alamarBlue® dye was added and plates were incubated for 4 hours. alamarBlue® reduction was measured spectrophotometrically at wavelengths 540nm and 620nm to calculate %reduction of alamarBlue® dye as a measure of reduced cell metabolic activity. To assess total cell number, we plated 5×104 (in 0.5mL of media) cells at 97% confluency overnight in a 24-well plate (Corning Incorporated, Corning NY) and treated the next day with either 100pM, 1nM, 10nM, 100nM, 1μM, and 10μM of Linifanib, vehicle control (DMSO) or left untreated. Cell/drug mixtures were allowed to incubate for 24 hours. Cells were counted using Trypan Blue exclusion and Vi-CELL® Cell counter (Beckman Coulter, Miami FL).
Apoptosis assays
Ba/F3 FLT3 ITD mutant cell lines and Ba/F3 FLT3 WT control cell lines (1×106 in 10mL media) were plated overnight in 25cm2 cell culture flasks (Corning Inc., Corning, NY). Cells were either left untreated, treated with vehicle control (DMSO), or 1nM, 10nM, 100nM, 1μM, or 10μM concentration of Linifanib for 24 hours. After 24 hours, Ba/F3 FLT3 ITD and WT cells (1×106) were first washed in cold 1× PBS and then incubated with a 1/400 dilution of Annexin V (Roche Indianapolis, IN) and Propidium Iodide (Roche Indianapolis, IN) in incubation HEPES buffer (Roche, Indianapolis, IN). Data was acquired at the UCLA Johnsson Comprehensive Cancer Center Flow Cytometry Core Facility on the BD LSR II and analyzed using flojo software (www.flojo.com).
Statistical analysis
For statistical analysis of apoptosis assays, we used AnalystSoft Inc., StatPlus:mac - statistical analysis program for Mac OS. Version 2009. See www.analystsoft.com/en/. Data are presented as the mean ± standard deviation of percent Annexin V/Propidium Iodide (early and late apoptosis) positive cells. Groups were compared using one-way ANOVA followed by Tukey's HSD test for differences between means. A P-value < 0.05 was considered to be statistically significant.
IL-3 Rescue and Depletion
Ba/F3 FLT3 ITD mutant cell lines (1×106 in 10mL media) were plated overnight. Cells were then treated with Linifanib 10nM alone, and with or without recombinant mouse IL-3 (1μg/ml) (R&D systems, Minneapolis, MN) for 24 hours. Cells (1×106) were then stained with Annexin-V and Propidium Iodide and analyzed by flow cytometry for apoptosis as described above. 1×106 cells were then lyzed with RIPA buffer. Whole cell lysates were run on SDS-Page gels and western blots were probed with anti-phospho GSK3β (Cell signaling technology, Beverly, MA).
Immunoprecipitation and Western Blot analysis
Ba/F3 FLT3 ITD cells (3×106 in 30mL media) were plated on 75cm2 cell culture flasks at a concentration of 1×105cells/ml and left overnight. For PARP blots, after overnight incubation, cells were treated for 0, 2, 4, and 6 hours with 1nM, 10nM or 100nM of Linifanib. Cell lysates were run on SDS-Page gels and western blots were probed with anti-uncleaved and cleaved PARP antibody (Cell Signaling Technology, Beverly, MA).
For FLT3 and AKT blots, cells were treated after overnight incubation for 15, 30, 60, and 120 minutes with Linifanib (10nM) or vehicle control (DMSO). Cell lysates were immunoprecipitated (IP) with 1μg/ml of anti-FLT3 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-AKT (Cell signaling technology Beverly, MA) antibodies and Protein A/G sepharose beads (Santa Cruz Biotechnology, Santa Cruz, CA). Western blots were probed with (a) anti-phospho-FLT3 antibody Tyr591 (Cell Signaling technology, Beverly, MA) or (b) anti-phospho-AKT (Ser473) antibodies (Cell Signaling Technology, Beverly, MA)
For GSK3 immunoblots, cells were treated for 15, 30, 60, and 120 minutes with 10nM of Linifanib or with vehicle control, DMSO. Cells were then lyzed with RIPA buffer. Whole cell lysates were run on SDS-Page gels and western blots were probed with anti-phospho GSK3β (Cell signaling technology, Beverly, MA), or anti-GSK3 (α/β) (Cell signaling technology, Beverly, MA). MV-411 cells were treated with 10nM of linifanib for 1 hours. Cells were then lysed with RIPA buffer. Whole cell lysates were run on SDS-Page gels and Western blots were probed with anti-phospho GSK3β (Cell signaling technology, Beverly, MA), or anti-GSK3 (α/β) (Cell signaling technology, Beverly, MA).
Generation of GFP-luciferase Ba/F3 cell lines for in vivo studies
GFP-luciferase retrovirus (#74 RRL.sin.cPPT.fLuc.IRES.emdGFP) was obtained from the virus vector core laboratory at UCLA. One day prior to infection, Ba/F3 WT and Ba/F3 FLT3 ITD mutant cells were diluted to 0.5×106 cells/ml. Concentrated virus was diluted 1:4 to a concentration of 20μg/ml. Cells (0.5×106) were centrifuged, and 1mL of diluted virus was added in addition to 1mg/ml of protamine sulfate (UCLA). Cell/Virus suspension was transferred to a 6-well plate and incubated at 5% CO2 and 37°C overnight. A day after transduction, cells were washed twice with 2mL of RPMI media and 2mL of Fresh media was added to cell wells. Three days after transduction, cells were sorted for GFP positive cell population at the UCLA flow cytometry core.
NOD/SCID mice (Jackson laboratories Sacramento, CA) received Ba/F3 FLT3 WT, FLT3 ITD GFP-luciferase mutant cell lines (1×106) (5 mice per group) by tail vein injection. Mice were then treated by oral gavage daily with 0.2mL per 20grm with Linifanib or with vehicle control (corn oil). Mice were monitored for disease progression by weight loss and bioluminescence imaging. Bioluminescent images were acquired using Xenogen IVIS hardware (Xenogen Hudson, NY) and Living Image software (Caliper Life Sciences). Prior to acquisition, mice were anesthetized with isoflourane and subsequently given 126 mg per mouse weight of D-luciferin by Intraperitoneal injection for detection of luciferase. Animals were sacrificed after showing symptoms of illness as ruffled fur, labored breathing, and hunched back.
Statistical analysis
Survival data were analyzed using the SAS program (SAS Institute, www.sas.com) and a Kaplan-Meier survival model. The log-rank test was used for comparing survival curves.
Results
Linifanib inhibits proliferation and induces apoptosis of ITD mutant cells in vitro and in vivo
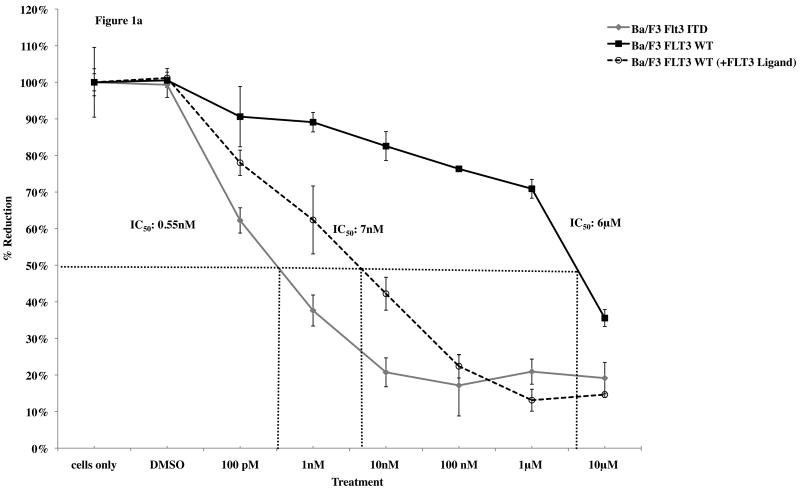
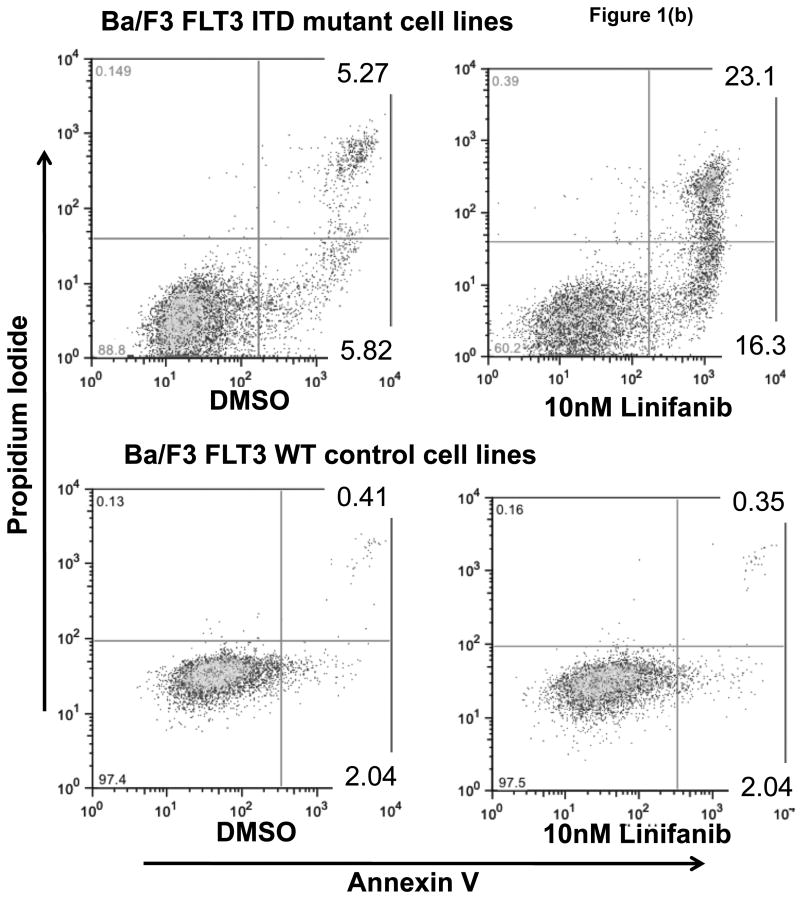
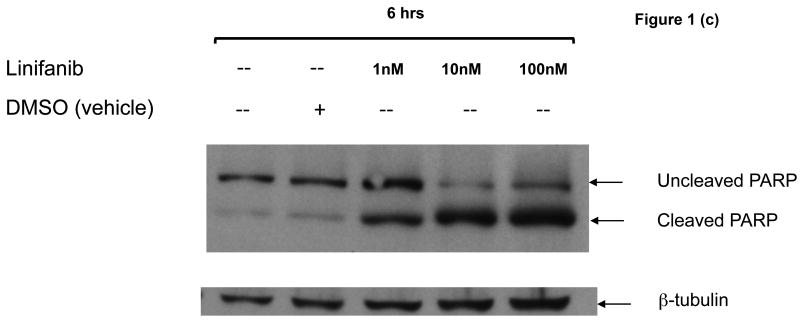
To determine whether Linifanib had anti-proliferative and apoptotic effects in vitro on ITD mutant cell lines, we performed dose response alamarBlue® assays and apoptotic assays on both Ba/F3 FLT3 ITD mutant and WT cells. AlamarBlue® assays show that after 24 hours, Linifanib is more effective at inhibiting cell growth in ITD mutant cells compared to WT cells (Fig. 1a). The half maximal inhibitory concentration (IC50) of Linifanib on ITD cells was 0.55nM whereas the IC50 for WT cells was 6μM (Fig. 1a). Growing WT cells with FLT3 ligand, however, demonstrated similar inhibition of cell growth as ITD mutant cells, minor differences can be accounted for by differences in rate of cell growth (Fig. 1a and Supplemental Fig.2). This demonstrated that the effects of FLT3 inhibitor were specific to FLT3. Viable cell counts were also measured (Supplemental Fig.2). In addition, treatment with 10nM of Linifanib induced apoptosis in ITD mutant cells, whereas no effect was observed on WT cells (Fig. 1b). Linifanib treatment did not show any differences at reducing cell viability or inhibiting proliferation between WT and FLT3 mutant cells containing the D835V point mutation (data not shown). To ascertain the time frame for induction of apoptosis, we treated ITD mutant cells with Linifanib in a time course from 0 to 24 hours. PARP cleavage was detected as early as 6 hours of treatment (Fig. 1c).
Figure 1. Linifanib inhibits proliferation and induces apoptosis of Ba/F3 hFLT3 ITD mutant cells in vitro.
(a) Ba/F3 FLT3 ITD and Ba/F3 FLT3 WT cells (grown with IL-3), and Ba/F3 FLT3 WT cells (grown with FLT3 ligand) were treated with varying concentrations of Linifanib. AlamarBlue® dye reduction is used as a measure of proliferation after treatment for 24 hours. (b) Ba/F3 FLT3 ITD mutant cell lines and Ba/F3 FLT3 WT control cell lines were treated with 100pM to 100nM concentration of Linifanib for 24 hours. Ba/F3 FLT3 ITD and WT cells (1×106) were then stained with Annexin V and Propidium Iodide (PI) and analyzed by flow cytometry. The 10nM Linifanib treatment is shown and is representative of multiple experiments. (c) Ba/F3 hFLT3 ITD cells were treated for 0, 2, 4 or 6 hours with 1, 10nM and 100nM Linifanib, vehicle control (DMSO), or untreated. Whole cell lysates were run on SDS-Page gels and Western blot analysis with anti-cleaved and uncleaved PARP antisera was performed. Here, the 6 hour time point is shown.
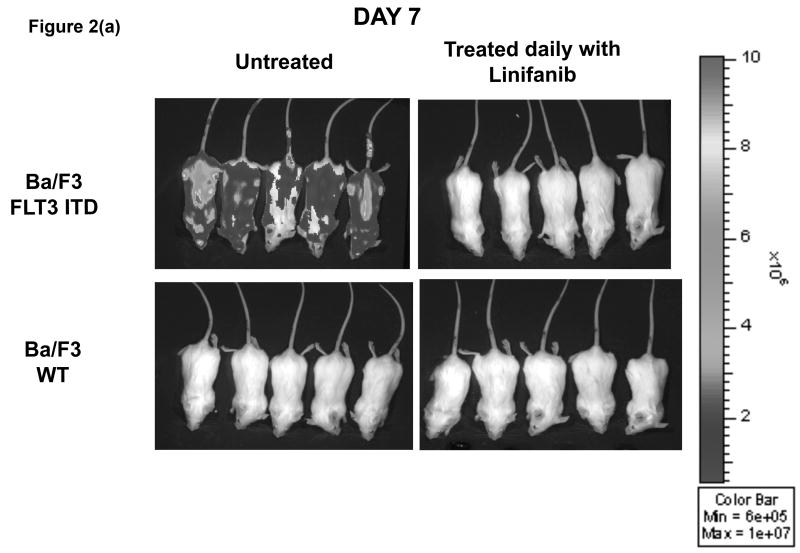
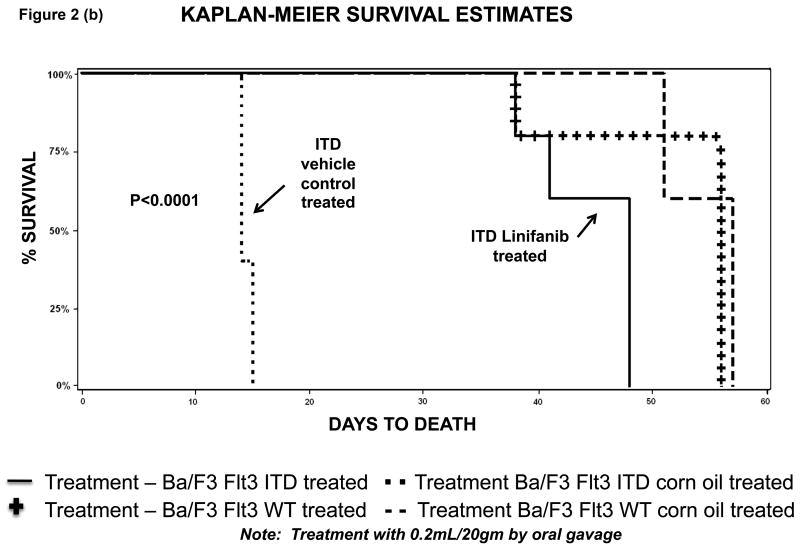
In vivo, xenograft experiments with NOD/SCID mice showed that mice injected with ITD mutant cells and treated daily orally by gavage with Linifanib had a decreased rate of leukemia progression compared to untreated mice (Fig. 2). At day 7, untreated mice showed rapid progression of ITD mutant cells, whereas mice treated with Linifanib had no detectable disease by bioluminescence (Fig. 2a). Additionally, survival for untreated mice receiving ITD mutant cells was significantly shorter (p<0.01) than for those receiving daily treatment with Linifanib or injected with WT cells (Fig. 2b).
Figure 2. Linifanib prolongs survival of Ba/F3 FLT3 ITD injected mice in vivo.
(a) SCID mice were injected with 1×106 of Ba/F3 FLT3 WT, FLT3 ITD GFP+/ luciferase+ mutant cell lines. Mice were treated daily by oral gavage with 0.2ml per 20 gms mouse weight of Linifanib or vehicle control (corn oil). Mice were monitored for disease progression by weight loss and bioluminescence imaging. Animals were sacrificed after developing symptoms of illness. (b) Demonstrates the distribution of survival times using Kaplan-Meier analysis.
As Linifanib showed anti-proliferative and apoptotic effects on ITD mutant cells both in vitro and in vivo, we next sought to examine the mechanism by which this occurred.
IL-3 rescues apoptotic effects of Linifanib
Since treatment with Linifanib has been shown to induce apoptosis rapidly (within 6 hours), we hypothesized that apoptosis induced by Linifanib results from Ba/F3 FLT3 ITD mutant cells defaulting to an IL-3 deficient state and thereby undergoing apoptosis. We therefore hypothesized, that adding IL-3 would reverse Linifanib induced apoptotic effects.
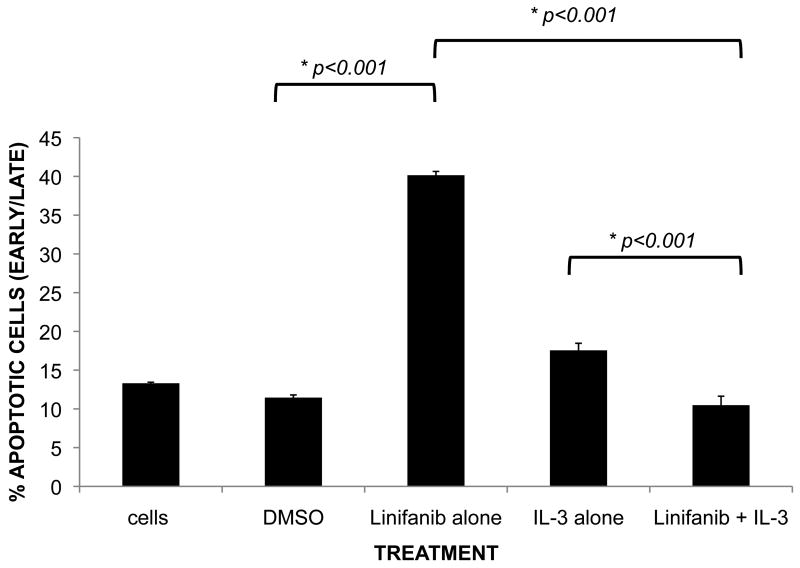
To test this hypothesis, recombinant IL-3 was simultaneously added to cells in combination with 10nM Linifanib (Fig. 3). Our data revealed that adding recombinant IL-3 reversed the apoptotic effects of Linifanib alone with a reduction from 40.2% overall apoptosis with Linifanib treatment alone down to control levels (p<0.001) (Fig.3).
Figure 3. Recombinant IL-3 rescues the apoptotic effects of Linifanib treatment on Ba/F3 ITD mutant cell lines.
Ba/F3 FLT3 ITD mutant cells were treated with 10nM Linifanib and grown without and in the presence of recombinant IL-3 (1μg/ml) for 24 hours. Ba/F3 ITD mutant cells (1×106) were then stained with Annexin-V (early apoptosis) and Propidium Iodide (PI) (late apoptosis) and analyzed by flow cytometry. The mean ± standard deviation of percent Annexin-V/PI positive cells were analyzed with a one-way ANOVA followed by Tukey (post-hoc) test. Results are representative of triplicate experiments. * Significant differences between combinations were observed (P < 0.05).
IL-3 withdrawal induced apoptosis has been shown to occur through the PI3K/AKT/GSK3β pathway (24). Since ITD mutant cells were rescued with IL-3, we hypothesized that Linifanib is working through the same pathway. To test this possibility, we next sought to determine if PI3K, AKT and GSK3 are downstream kinase targets affected by treatment with Linifanib.
Linifanib inhibits phosphorylation of AKT, and GSK3β in Ba/F3 FLT3 ITD mutant cells and IL-3 rescues phosphorylation of GSK3β
It has been established that in the IL-3 dependent cells, removal of IL-3 induces apoptosis by inhibiting AKT and GSK3 phosphorylation (22-24). Since IL-3 rescues Linifanib induced apoptosis, we hypothesized that treatment with Linifanib reduces phosphorylation of AKT and GSK3 in the Ba/F3 FLT3 ITD mutant cell line.
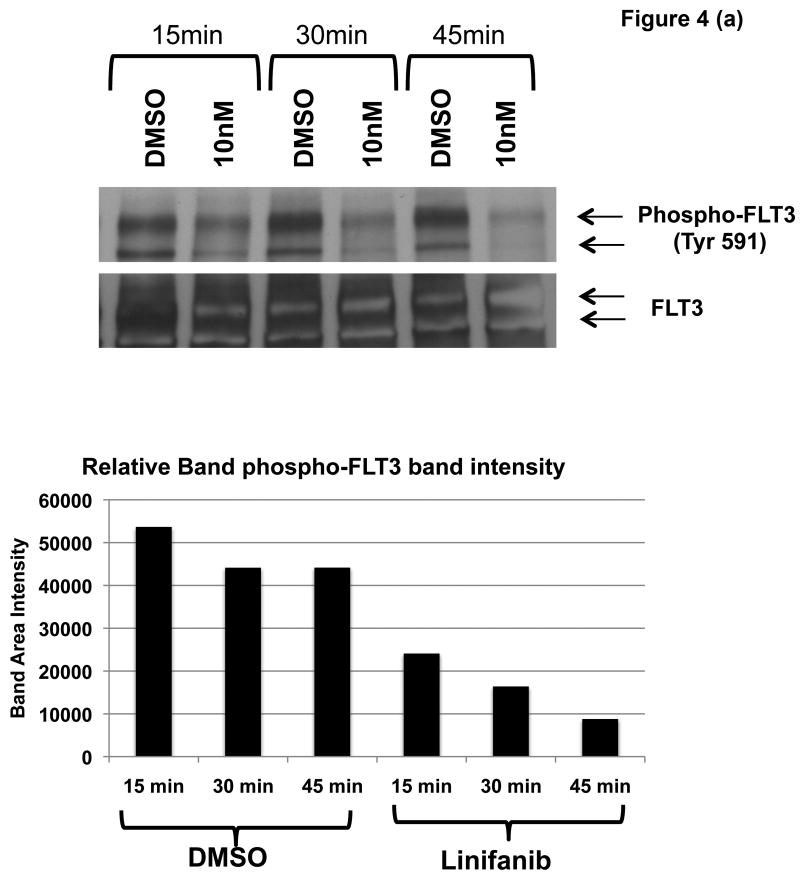
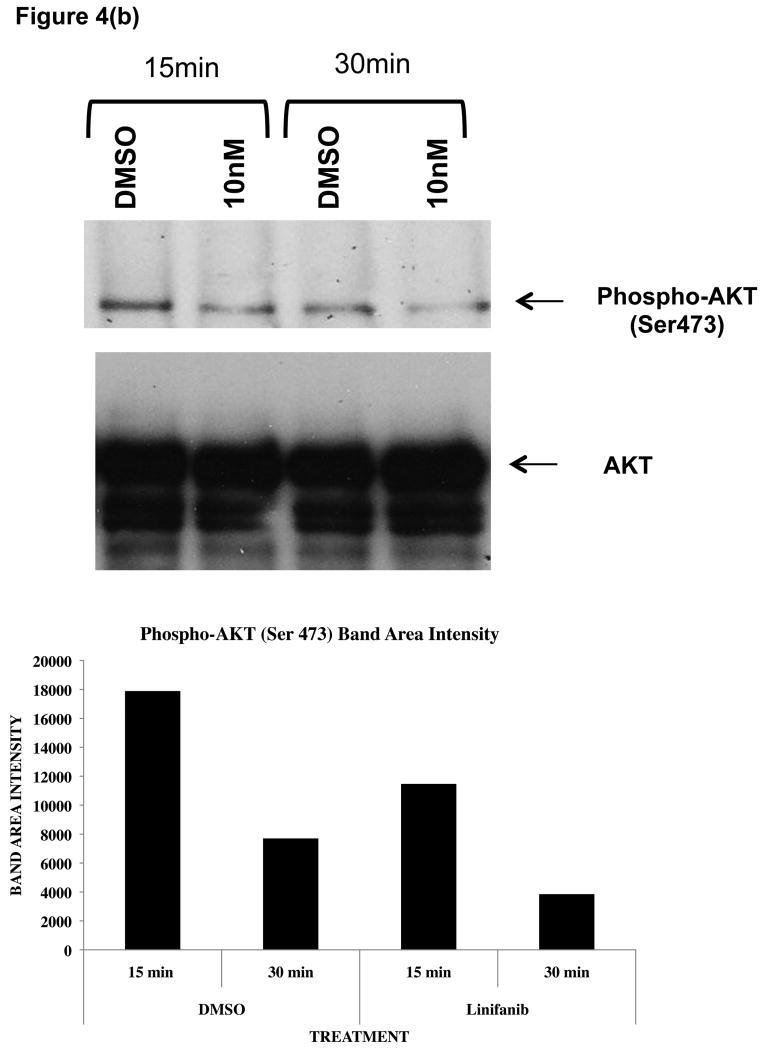
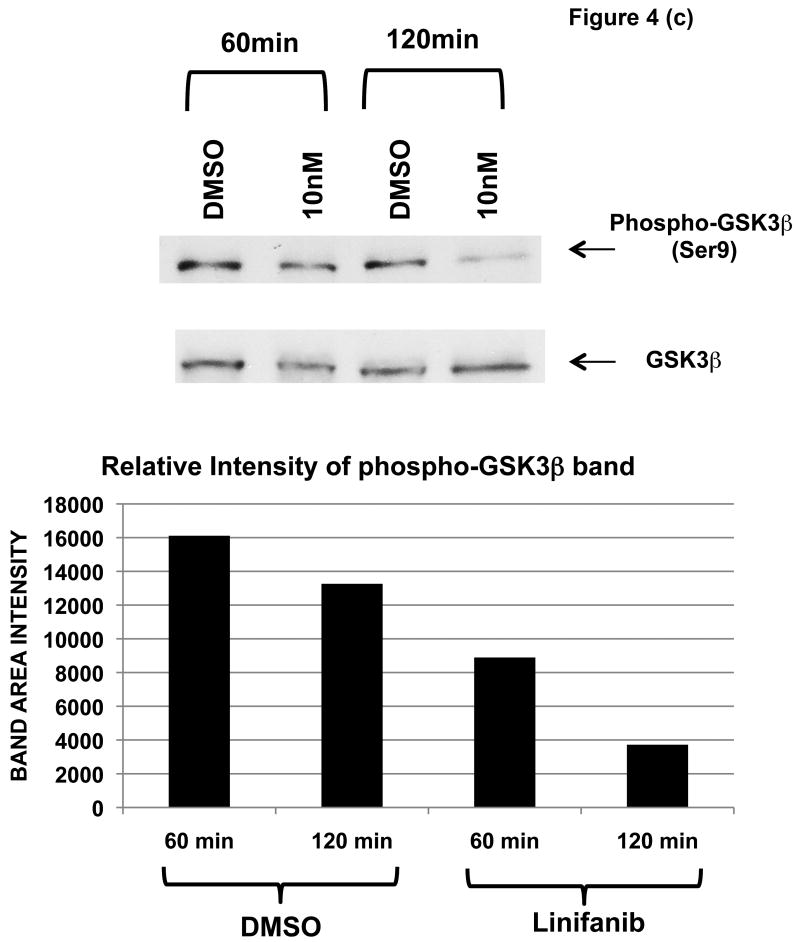
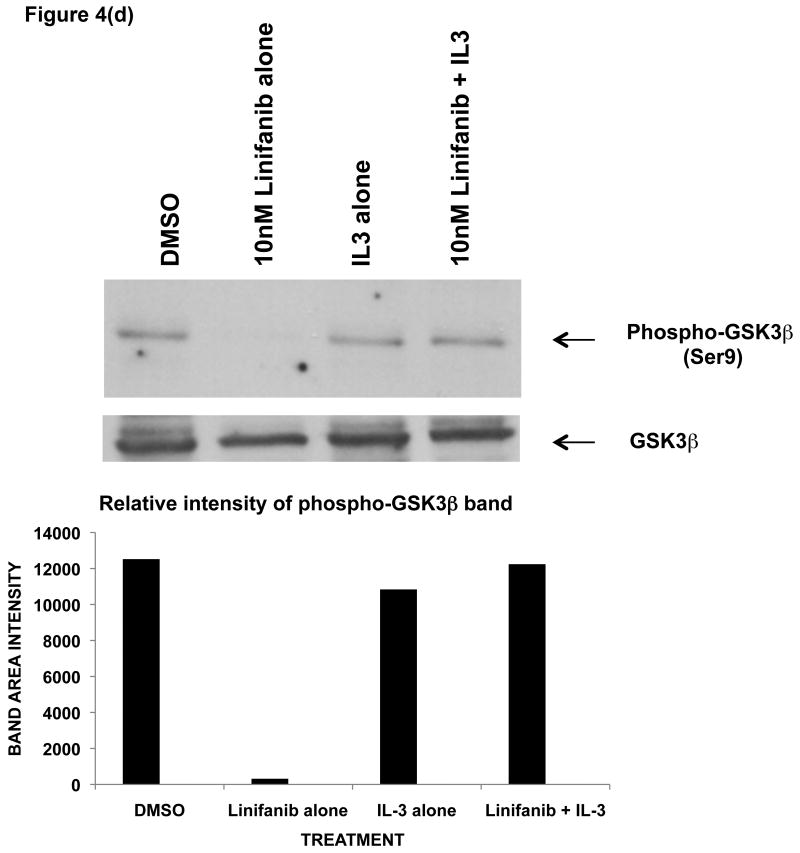
To test this possibility, ITD mutant cell lines were examined for phosphorylation of AKT and GSK3β by immunoprecipitation, SDS-Page, and western blot analysis (Fig. 4). We show that Linifanib is effective at inhibiting phosphorylation of FLT3 in Ba/F3 FLT3 ITD cell lines at a concentration of 10nM (Fig. 4a). In addition, Linifanib reduced phosphorylation of AKT at Ser473 after treatment with 10nM of Linifanib (Fig. 4b). To test whether GSK3β phosphorylation was affected after treatment with Linifanib, we treated the ITD mutant cells with 10nM Linifanib and examined phosphorylation of GSK 3β at Ser9 (Fig. 4c) or GSK 3α at Ser21 (data not shown). Treatment with 10nM Linifanib resulted in decreased phosphorylation of GSK3β Ser 9 as early as 60 minutes (Fig. 4c). GSK3α at Ser21 only demonstrated reduced phosphorylation after 8 hours (data not shown). To test whether GSK3β phosphorylation is rescued with recombinant IL-3, we treated the ITD mutant cells with a combination of 10nM Linifanib and recombinant IL-3 and examined phosphorylation of GSK3β at 24 hours. Treatment with a combination of Linifanib and IL-3 resulted in rescue of GSK3β phosphorylation (Fig. 4d). To test whether the same GSK3β phosphorylation is observed in human AML FLT3 ITD mutant cells, the MV-411 cell line was treated with linifanib. It was found that treatment with 10nM of linifanib reduced GSK3β phosphorylation as well (Supplemental Fig. 3). This emphasizes the importance of GSK3β in not only mouse cells, but also human cells.
Figure 4. Linifanib inhibits phosphorylation of FLT3, AKT, and GSK3β in Ba/F3 hFLT3 ITD mutant cell lines and IL-3 rescues phosphorylation of GSK3β.
(a) Ba/F3 FLT3 ITD cells were treated for 15, 30 or 60 minutes with 10nM Linifanib or vehicle control (DMSO). Cell lysates were immunoprecipitated (IP) with anti-FLT3 polyclonal antisera or anti-AKT(Pan) monoclonal antisera and sepharose beads. Western blots were probed with (a) anti-phospho-FLT3 (tyr591) polyclonal antisera (b) anti-phospho-AKT (Ser473) monoclonal antisera. Cell lysates of Ba/F3 FLT3 ITD cells treated from 15,30, 60, or 120minutes with 10nM Linifanib or vehicle control (DMSO) were run on SDS-Page gels. Western blots were probed with (c) phospho-GSK3β (Ser9) anti sera. (d) Ba/F3 FLT3 ITD cells were treated for 24 hours with vehicle control (DMSO), 10nM Linifanib, 1μg/ml recombinant IL-3 alone, or in combination with 10nM Linifanib. Cell lysates of Ba/F3 FLT3 ITD cells were run on SDS-Page gels. Western blots were probed with anti-phospho-GSK3β (Ser9). Band area intensity was quantified using NIH Image J (34).
Our results therefore suggest that one of the possible mechanisms by which Linifanib induces apoptosis is through modulation of AKT and GSK3β phosphorylation.
Combination treatment with GSK3 inhibitor Lithium Chloride reduces Linifanib induced apoptotic effects
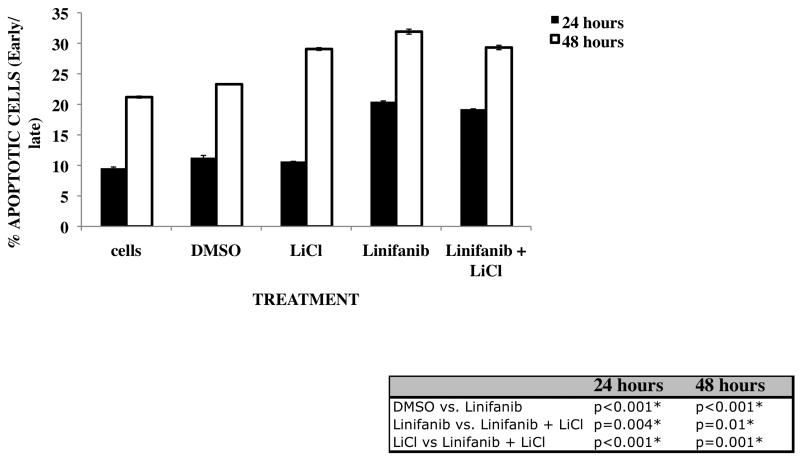
To determine whether GSK3 has a major role in inducing apoptosis upon treatment with Linifanib, we treated ITD mutant cells with a combination of 10nM Linifanib and 10mM Lithium Chloride, a known GSK3 inhibitor (Fig. 5). We hypothesized that since GSK3β phosphorylation is reduced as a result of Linifanib treatment, that it may have a major role to play in induction of apoptosis in ITD mutant cells. Although not as large as we expected, we have shown that combination treatment with Lithium Chloride causes a reduction in apoptosis at 24 and 48 hours (p<0.01) (Fig. 5). These results suggest that modulation of GSK3β phosphorylation may be at least a contributing factor for Linifanib-induced apoptosis.
Figure 5. Inactivation of GSK3β with Lithium Chloride decreases Linifanib induced apoptotic effects.
Ba/F3 hFLT3 ITD mutant cell lines were treated with 10nM Linifanib alone or in combination with GSK3β inhibitor Lithium Chloride at a concentration of 10mM for 24 hours and 48 hours. 1×106 cells were stained with AnnexinV/Propidium Iodide and analyzed by flow cytometry. From multiple experiments, the mean ± standard deviation of percent Annexin-V/PI positive cells were analyzed with a one-way ANOVA followed by Tukey (post-hoc) test. * Significant differences between combinations were observed (P < 0.05)
Discussion
In this paper, we have characterized a new downstream target of Linifanib induced FLT3 inhibition. We have shown that FLT3 inhibition by Linifanib in ITD mutant cells results in reduced GSK3β phosphorylation.
Initially, we showed that Linifanib induces apoptosis rapidly in ITD mutant cell lines. Due to this, we hypothesized that Linifanib is inducing apoptosis in ITD mutant cells by mimicking IL-3 withdrawal induced apoptosis. We therefore speculated that IL-3 would rescue any Linifanib induced apoptotic effects. Our data have shown that IL-3 is able to reverse the effects of Linifanib induced apoptosis.
We also hypothesized that since IL-3 rescues the effects of Linifanib induced apoptosis, that apoptosis in ITD mutant cell lines is occurring through the same pathway as IL-3 withdrawal induced apoptosis by inhibiting PI3K activation, reducing AKT phosphorylation, and reducing phosphorylation of GSK3β. Our data has shown that treatment with Linifanib reduces AKT phosphorylation and GSK3β phosphorylation.
Other studies with FLT3 inhibitors have demonstrated that inhibiting FLT3 phosphorylation leads to suppression of downstream targets such as STAT5, members of the PI3K pathway, MAPK pathway, and the BCL-2 family of proteins, and cell cycle regulators (6-10, 13). As seen in previous studies, we have observed similar downstream targets of Linifanib in ITD mutant cells as AKT, ERK1, Bcl-xl, and BAD (data not shown). However, GSK3β as a target of Linifanib has not yet been characterized.
GSK3 is a serine threonine protein kinase that regulates cell differentiation and apoptosis, the canonical wnt signaling pathway, and is also a regulator of glycogen synthesis (27). GSK3 has been demonstrated to phosphorylate substrates as cytoskeletal proteins, affect cell cycle regulation by targeting β-catenin, MYC, cyclin D1, cyclin E and Bcl-3, transcription factors as c-Jun, c-myc, c-myb, and CREB, and other metabolic regulators (24, 28, 29). Although increased activity of GSK3 has been observed in chronic metabolic disorders as type-II diabetes, mood disorders, Alzheimer's disease, and in acute leukemia caused by MLL (mixed-lineage leukemia), its role has not yet been characterized in AML with FLT3 ITD mutations (25, 26, 28).
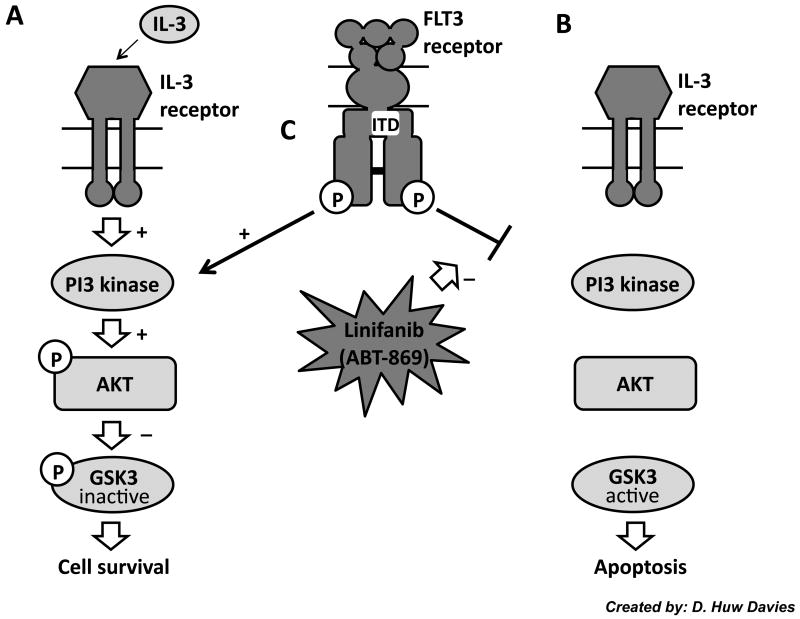
In growth factor dependent hematopoietic cells, it has been shown that one of the pathways responsible for survival is the PI3 kinase and AKT pathway (30). In addition, dominant negative forms of AKT were able to accelerate IL-3 induced apoptosis (30). Recent studies have also shown that growth factor induced apoptosis occurs by reducing phosphorylation of GSK3β (reducing its kinase activity) (24). In addition, it has been shown that inhibiting GSK3β activity through a variety of small molecule inhibitors prevented apoptosis from occurring (24). We propose that Ba/F3 FLT3 ITD mutant cell lines are able to survive in an IL-3 independent manner because the FLT3 ITD constitutive mutation renders these cells alive through PI3K/AKT signaling, which is the same pathway as IL-3 survival (Fig. 6A) (24). Inhibiting FLT3 with Linifanib, however, we propose prevents PI3K activation, reduces AKT and GSK3β phosphorylation and therefore ITD mutant cell lines default to a mechanism mimicking IL-3 withdrawal induced apoptosis (Fig. 6B and C). Studies with one other FLT3 inhibitor, AG1296, also saw similar rescue of apoptosis by IL-3, but the role of GSK3β was not characterized in this study (31).
Figure 6. Proposed Model.
It is proposed that one pathway by which ABT-869 induces apoptosis and inhibits proliferation is through the PI3K/AKT pathway. This is the same pathway through which growth factor withdrawal induces apoptosis (24). Previous research has shown that growth factor dependent hematopoietic cells are able to survive by activating PI3K, phosphorylating AKT, and inhibitory phosphorylation of GSK3β, which prevents apoptosis (A). (24) Removal of IL-3, however, prevents this activation from occurring and therefore prevents AKT from phosphorylating GSK3β and allows apoptosis to occur (B). (24) We hypothesize that Linifanib inhibits FLT3 (C), reduces AKT and GSK3β phosphorylation, and induces apoptosis in a manner mimicking IL-3 withdrawal induced apoptosis (B).
Further studies are required to understand the precise role of GSK signaling in the pathogenesis of AML cells. Commercially available GSK3 inhibitors could be used to characterize these pathways. Our preliminary studies using Lithium Chloride inhibitor found a slight reduction in overall apoptosis when combined with Linifanib (Fig. 5). This is evidence that GSK3 does have a role in Linifanib induced apoptosis, yet may not be the only factor involved in inducing apoptosis in the ITD cells since there may be crosstalk between other pathways downstream of FLT3 activation that can also affect apoptosis. Signaling targets as GSK3β, however, may help to elucidate the mechanism by which Linifanib induces apoptosis. Combination studies of FLT3 inhibitors with other inhibitors have been successful at inhibiting the progression of AML by enhancing apoptosis and anti-proliferative effects (32, 33). GSK3 inhibitors may be alternative viable candidates in these combination studies.
In conclusion, the development of FLT3 inhibitors for treatment of AML has been successful to an extent. Previous studies have found that the use of FLT3 inhibitors in conjunction with other inhibitors or with conventional chemotherapy drugs may prove to be more successful in effectively treating AML. The development of drug resistance in human AML cell lines after initial therapy provides an avenue for testing combinations of new inhibitors that target different pathways. The use of FLT3 inhibitors in combination with GSK3 inhibitors or chemotherapy may be a more optimal approach to treat AML.
Supplementary Material
Acknowledgments
K.M.S. is supported by National Institutes of Health (NIH) HL75826 and HL83077, William Lawrence and Blanche Hughes Foundation, and the St. Baldrick's Foundation. K.M.S. is also a Scholar of the Leukemia and Lymphoma Society. M.H. is supported in part by a Merit Review Grant from the Department of Veterans Affairs and a SCOR grant from the Leukemia and Lymphoma Society. Thank you to Jenny Chang and Salemiz Sandoval for advice and assistance with animal work. Thank you also to Dr. Linda Baum and Dr. D.H. Davies for critical review and advice in the preparation of this manuscript.
Supported by National Institutes of Health (NIH) HL075826, AI075565 and RR022259-06.
Supported by National Institutes of Health, NIAID
References
- 1.Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006 Dec 1;108(12):3654–3661. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Small D. Targeting FLT3 for the treatment of leukemia. Semin Hematol. 2008 Jul;45(3 Suppl 2):S17–21. doi: 10.1053/j.seminhematol.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Basecke J, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008 Apr;22(4):708–722. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 4.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003 Sep;3(9):650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD. FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat. 2009 Jun;12(3):81–89. doi: 10.1016/j.drup.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordigarden A, Kraft M, Eliasson P, Labi V, Lam EW, Villunger A, et al. BH3-only protein Bim more critical than Puma in tyrosine kinase inhibitor-induced apoptosis of human leukemic cells and transduced hematopoietic progenitors carrying oncogenic FLT3. Blood. 2009 Mar 5;113(10):2302–2311. doi: 10.1182/blood-2008-07-167023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar DB, Li J, Tapang P, Owen McCall J, Pease LJ, Dai Y, et al. ABT-869, a multitargeted receptor tyrosine kinase inhibitor: inhibition of FLT3 phosphorylation and signaling in acute myeloid leukemia. Blood. 2007 Apr 15;109(8):3400–3408. doi: 10.1182/blood-2006-06-029579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007 Mar;21(3):439–445. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 9.Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002 Jun 1;99(11):3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Pan M, Xie Z, Loh SL, Bi C, Tai YC, et al. Synergistic antileukemic effects between ABT-869 and chemotherapy involve downregulation of cell cycle-regulated genes and c-Mos-mediated MAPK pathway. Leukemia. 2008 Jan;22(1):138–146. doi: 10.1038/sj.leu.2404960. [DOI] [PubMed] [Google Scholar]
- 11.Kim KT, Levis M, Small D. Constitutively activated FLT3 phosphorylates BAD partially through pim-1. Br J Haematol. 2006 Sep;134(5):500–509. doi: 10.1111/j.1365-2141.2006.06225.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008 Apr;22(4):808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 13.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005 Oct 20;24(46):6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 14.Albert DH, Tapang P, Magoc TJ, Pease LJ, Reuter DR, Wei RQ, et al. Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2006 Apr;5(4):995–1006. doi: 10.1158/1535-7163.MCT-05-0410. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Goh BC, Albert DH, Chen CS. ABT-869, a promising multi-targeted tyrosine kinase inhibitor: from bench to bedside. J Hematol Oncol. 2009;2:33. doi: 10.1186/1756-8722-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schittenhelm MM, Yee KW, Tyner JW, McGreevey L, Haley AD, Town A, et al. FLT3 K663Q is a novel AML-associated oncogenic kinase: Determination of biochemical properties and sensitivity to Sunitinib (SU11248) Leukemia. 2006 Nov;20(11):2008–2014. doi: 10.1038/sj.leu.2404374. [DOI] [PubMed] [Google Scholar]
- 17.Yee KW, Schittenhelm M, O'Farrell AM, Town AR, McGreevey L, Bainbridge T, et al. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood. 2004 Dec 15;104(13):4202–4209. doi: 10.1182/blood-2003-10-3381. [DOI] [PubMed] [Google Scholar]
- 18.Tse KF, Mukherjee G, Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia. 2000 Oct;14(10):1766–1776. doi: 10.1038/sj.leu.2401905. [DOI] [PubMed] [Google Scholar]
- 19.Gilliland DG, Griffin JD. Role of FLT3 in leukemia. Curr Opin Hematol. 2002 Jul;9(4):274–281. doi: 10.1097/00062752-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Cornelis S, Bruynooghe Y, Van Loo G, Saelens X, Vandenabeele P, Beyaert R. Apoptosis of hematopoietic cells induced by growth factor withdrawal is associated with caspase-9 mediated cleavage of Raf-1. Oncogene. 2005 Feb 24;24(9):1552–1562. doi: 10.1038/sj.onc.1208401. [DOI] [PubMed] [Google Scholar]
- 21.Warmuth M, Kim S, Gu XJ, Xia G, Adrian F. Ba/F3 cells and their use in kinase drug discovery. Curr Opin Oncol. 2007 Jan;19(1):55–60. doi: 10.1097/CCO.0b013e328011a25f. [DOI] [PubMed] [Google Scholar]
- 22.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998 Aug 7;273(32):19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 23.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997 Oct 17;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 24.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006 Mar 17;21(6):749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008 Oct 30;455(7217):1205–1209. doi: 10.1038/nature07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Iwasaki M, Ficara F, Lin C, Matheny C, Wong SH, et al. GSK-3 promotes conditional association of CREB and its coactivators with MEIS1 to facilitate HOX-mediated transcription and oncogenesis. Cancer Cell. Jun 15;17(6):597–608. doi: 10.1016/j.ccr.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong H, Zou H, Semenov MV, Moshinsky D, He X, Huang H, et al. Characterization and development of novel small-molecules inhibiting GSK3 and activating Wnt signaling. Mol Biosyst. 2009 Nov;5(11):1356–1360. doi: 10.1039/b905752h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Sintes R, Hernandez F, Bortolozzi A, Artigas F, Avila J, Zaratin P, et al. Neuronal apoptosis and reversible motor deficit in dominant-negative GSK-3 conditional transgenic mice. EMBO J. 2007 Jun 6;26(11):2743–2754. doi: 10.1038/sj.emboj.7601725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welsh GI, Wilson C, Proud CG. GSK3: a SHAGGY frog story. Trends Cell Biol. 1996 Jul;6(7):274–279. doi: 10.1016/0962-8924(96)10023-4. [DOI] [PubMed] [Google Scholar]
- 30.Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tse KF, Novelli E, Civin CI, Bohmer FD, Small D. Inhibition of FLT3-mediated transformation by use of a tyrosine kinase inhibitor. Leukemia. 2001 Jul;15(7):1001–1010. doi: 10.1038/sj.leu.2402199. [DOI] [PubMed] [Google Scholar]
- 32.Schittenhelm MM, Kampa KM, Yee KW, Heinrich MC. The FLT3 inhibitor tandutinib (formerly MLN518) has sequence-independent synergistic effects with cytarabine and daunorubicin. Cell Cycle. 2009 Aug 15;8(16):2621–2630. doi: 10.4161/cc.8.16.9355. [DOI] [PubMed] [Google Scholar]
- 33.Kojima K, Konopleva M, Tsao T, Andreeff M, Ishida H, Shiotsu Y, et al. Selective FLT3 inhibitor FI-700 neutralizes Mcl-1 and enhances p53-mediated apoptosis in AML cells with activating mutations of FLT3 through Mcl-1/Noxa axis. Leukemia. Jan;24(1):33–43. doi: 10.1038/leu.2009.212. [DOI] [PubMed] [Google Scholar]
- 34.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. July;11(7):36–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.