Abstract
Objective. To retrospectively evaluate ANCA testing in a cohort of unselected Greek in- and outpatients. Methods. In 10803 consecutive serum samples, ANCA were tested by indirect immunofluorescence (IIF) and ELISA. ELISA in inpatients was performed only on IIF positive sera. Results. Low prevalence (6.0%) of IIF positive samples was observed. Among these samples, 63.5% presented perinuclear (p-ANCA), 9.3% cytoplasmic (c-ANCA) and 27.2% atypical (x-ANCA) pattern. 16.1% of p-ANCA were antimyeloperoxidase (anti-MPO) positive, whereas 68.3% of c-ANCA were antiproteinase-3 (anti-PR3) positive. Only 17 IIF negative outpatients' samples were ELISA positive. ANCA-associated vasculitides (AAV), connective tissue disorders and gastrointestinal disorders represented 20.5%, 23.9%, and 21.2% of positive results, respectively. AAV patients exhibited higher rates of MPO/PR3 specificity compared to non-AAV (93.8% versus 8%). Conclusions. This first paper on Greek patients supports that screening for ANCA by IIF and confirming positive results by ELISA minimize laboratory charges without sacrificing diagnostic accuracy.
1. Introduction
Antineutrophil cytoplasmic antibodies (ANCA) are autoantibodies directed against constituents of primary granules of neutrophils and monocytes lysosomes [1]. Since the first description of ANCAs in 1982 in patients with necrotizing glomerulonephritis [2], ANCA testing currently plays a critical role in diagnosis and monitoring of a subgroup of vasculitides, referred as ANCA-associated vasculitides (AAV): Wegener's granulomatosis (WG), microscopic polyangiitis (MPA) and Churg-Strauss syndrome (CSS) [1, 3, 4]. Indirect immunofluorescence (IIF) and ELISA remain the most widely used techniques for ANCA detection. Using IIF on ethanol-fixed neutrophils, three patterns are recognized: diffuse cytoplasmic staining (c-ANCA), perinuclear staining (p-ANCA) and atypical staining (x-ANCA) [5]. Among several antigenic targets described, only antibodies targeting proteinase 3 (anti-PR3) and myeloperoxidase (anti-MPO) are of clinical value [6]. c-ANCA mainly target PR3 and are often observed in WG patients, while p-ANCA predominantly bind to MPO and are common in patients with MPA and CSS [1]. However, ANCAs have been reported in a number of other conditions including infections, malignancies, connective-tissue diseases, renal diseases, and other vasculitides and gastrointestinal disorders [1, 5, 6].
It is believed that genetic susceptibility (e.g., polymorphisms of HLA, FcγR, IL-10, a1-antitrypsin genes) and various environmental factors (e.g., infections, medication, silica, and climate) are implicated in the induction of ANCA. Differences in distribution or magnitude of these factors lead to geographical, ethnic, regional and seasonalvariations in the epidemiological characteristics of AAV [7, 8]. In this context, Greek patients with autoimmune diseases and AAV present significant genetic differences as compared to other Caucasians [9–12].
Although, several studies evaluating ANCA testing have been reported [13–21], those that evaluate the clinical value of ANCA under actual routine conditions in unselected patients are rather rare [13–16]. Moreover, few data regarding Greek population are available [12, 22]. Thus, the aim of this large retrospective study was (i) to determine the frequency of ANCA in a cohort of unselected consecutive in- and outpatients of a tertiary care hospital, where patients from all over Greece are referred, (ii) to analyze the results of ANCA testing taking into consideration clinical features, histopathological characteristics and final diagnoses, and finally (iii) to evaluate the two different strategies that have been adopted in our institution, in order to establish an algorithm on ANCA detection that ensures low laboratory charges with maximal diagnostic accuracy.
2. Patients and Methods
This study was approved by the Greek Authority of Personal Data Protection. The study population included 10803 patients of Greek ethnicity, that underwent ANCA testing from September 2003 to August 2006, at “Evangelismos” General Hospitalof Athens. This is the largesttertiary health care center of Greece (863 beds) that serves all geographical locations of the country. Out of 10803 patients, 6342 (58.7%) were female and 4461 (41.3%) were male. The mean age of the tested population was 52.1 (range 10–95) years old.
A total of 6017 serum samplesfrom different departments (nephrology, neurology, gastroenterology, internal medicine, respiratory medicine and others) and 4786 serum samples from the outpatient clinics, were submitted to the Immunology-Histocompatibility Department, with a test request for ANCA. For inpatients, IIF was initially performed and ELISA, for antibodies to PR3 and MPO, was additionally used in all IIF positive sera. On the other hand, a parallel testing that included both IIF and ELISA was applied to all outpatients.
2.1. ANCA Detection Methods
Indirect immunofluorescence (IIF) was performed on commercially prepared neutrophil substrate slides, fixed with ethanol (ANCA kit/substrate slides, The Binding Site). Sera titration ranged from 1/20 to 1/640. Titres ≥ 1/20 were considered positive.For the detection of MPO-ANCA and PR3-ANCA commercially available ELISA kits (QUANTA Lite MPO/PR3, INOVA diagnostics) were used. The results were recorded as positive when MPO/PR3 ANCA levels were ≥20 U.
2.2. Diagnostic Classification
ANCA positive results were correlated with the patients' diagnoses, as established by their treating physician at the time of sampling. Physicians makingthe diagnoses were a diverse group of cliniciansincluding nephrologists, neurologists, gastroenterologists, pneumonologists and oncologists. The medical files of patients were carefully reviewed by two of the authors (K. Tsiveriotis and A. Tsirogianni). In discrepant cases, a consensus with the patients' treating physician took place. The following disease categories were defined: (1) AAV, (2) connective tissue diseases, (3) gastrointestinal disorders, (4) other vasculitides, (5) infections, (6) neurological disorders, (7) malignancies, (8) renal diseases, (9) medication, (10) miscellaneous disorders, and (11) undiagnosed cases. The specific conditions that were included in each disease category and the diagnostic criteria that were used to adjudicate cases are presented in Table 1.
Table 1.
Diseases and diagnostic criteria.
| ANCA-associated vasculitides (AAV) | |
|---|---|
| Microscopic polyangiitis (MPA) | Chapel Hill Consensus Conference nomenclature [23] |
| Wegener's granulomatosis (WG) | ACR Classification Criteria for WG [24] |
| Churg-Strauss syndrome (CSS) | ACR Classification Criteria for CSS [25] |
| Other vasculitides | |
| Polyarteritis nodosa (PAN) | ACR Classification Criteria for PAN [26] |
| Behçet's disease (BD) | International Criteria for BD [27] |
| Henoch-Schönlein purpura (HSP) | ACR Classification Criteria for HSP [28] |
| Cryoglobulinemic vasculitis | Chapel Hill Consensus Conference nomenclature [23] |
| Secondary vasculitis | Diagnostic criteria of underlying disease, histological confirmation |
| Undefined vasculitis | Clinical features without histological confirmation |
| Gastrointestinal disorders (GD) | |
| Inflammatory bowel disease (IBD) | Clinical, endoscopic, radiological and histological criteria [29] |
| Primary sclerosing cholangitis | Clinical, radiological and immunological criteria [30] |
| Autoimmune hepatitis (AIH) | International Criteria for AIH [31] |
| Primary biliary cirrhosis | Clinical, biochemical, immunological and histological criteria [32] |
| Connective tissue diseases (CTD) | |
| Systemic lupus erythematosus (SLE) | Updated revised ACR Criteria for SLE [33] |
| Rheumatoid arthritis (RA) | Revised ARA criteria for RA [34] |
| Felty's syndrome | Clinical, radiological and laboratory criteria [35] |
| Systemic sclerosis (SSc) | ARA preliminary classification Criteria for SSc [36] |
| Dermatomyositis (DM) | Bohan and Peter diagnostic Criteria for DM [37, 38] |
| Sjögren's syndrome (SjS) | Revised International Classification Criteria for SjS [39] |
| Mixed connective tissue disease (MCTD) | Alarcon-Segovia Diagnostic Criteria for MCTD [40] |
| Reiter's syndrome | Clinical, radiological and immunological criteria [41] |
| Ankylosing spondylitis | Clinical, radiological and immunological criteria [42] |
| Juvenile chronic arthritis | Clinical, radiological and immunological criteria [43] |
| Relapsing polychondritis | Clinical, radiological and histological criteria [44] |
| Psoriasis | Clinical features |
| Antiphospholipid syndrome | Clinical and laboratory criteria [45] |
| Rheumatic polymyalgia | Clinical features and laboratory findings |
| Infections | |
| Tuberculosis (TBC) | |
| S. aureus (subacute endocarditis) | |
| Other bacterial infections | Clinical features and laboratory findings |
| AIDS | |
| Hepatitis C | |
| Aspergillosis | |
| Renal diseases (RD) | |
| Poststreptococcal nephropathy (PSGN) | |
| IgA nephropathy | Laboratory and immunological findings, histological confirmation |
| Goodpasture's disease | |
| Membranous nephropathy | |
| Malignancies | |
| Carcinoma | |
| Lymphoma | Clinical features, laboratory findings, histological confirmation |
| Chronic myelocytic leukaemia | |
| Myelodysplasia | |
| Monoclonal gammopathies | |
| Neurological disorders (ND) | |
| Demyelinating disease Stroke |
Neurological symptoms and imaging findings |
| Miscellaneous disorders | |
| Sarcoidosis | Clinical features, laboratory and imaging findings |
| Autoimmune hemolytic anemia (AHA) | Clinical features and laboratory findings |
| Pulmonary fibrosis | Imaging findings, histological confirmation |
| Medication | |
| Antithyroid drugs | |
| Allopurinol | History of medication treatment |
| Phenytoin | |
2.3. Statistical Analysis
For the comparison of IIF titers and ELISA levels between AAV and non-AAV patients, a chi-square test and a t-test were used, respectively. P values <.05 were considered statistically significant.
The positive predictive value (PPV) of ANCA testing for AAV was estimated, defined as the percentage of positive patients who had the disease: (true positives ÷ (true positives + false positives) × 100). The 95% confidence intervals (CI) were determined.
3. Results
From September 2003 to August 2006, 10803 sera samples of Greek patients were screened in Immunology-Histocompatibility Department of “Evangelismos” General Hospital, for the presence of ANCA. The majority of them (10142/10803—93.9%) were found negative by both IIF and ELISA and only 661 (6.1%) were found positive by IIF and/or ELISA. Among positive samples, 288 (43.6%) derived from outpatients, whereas 373 (56.4%) derived from hospitalized patients.Out of 661 ANCA positive patients, 441 (62.0%) were female and 251 (38.0%) were male. The mean age was 60.1 (range 19–95) years old.
The results obtained by IIF and ELISA are presented in Table 2. p-ANCA pattern predominated among IIF-positive patients (409/644—63.5%). The majority of p-ANCA positive sera were testednegative for both anti-MPO and anti-PR3 (339/409—82, 9%). Eighty sera were testedanti-MPO positive, 66 (82.5%) of which were also p-ANCA positive. Sixty sera (9.3%) showed c-ANCA pattern, 41 (68.3%) with specificity for PR3 and none for MPO. Among the 175 (27.2%) x-ANCA, 157 (89.7%) were ELISA negative, whereas 10 were presented anti-MPO and 8 anti-PR3 positive. As all outpatients' sera were screened by both IIF and ELISA, a small number (17) were found IIF (−)/ELISA (+), 13 of them were positive for anti-PR3 and 4 positive for anti-MPO.
Table 2.
Relationship between IIF pattern and ELISA test results.
| IIF results | Anti-MPO positive | Anti-PR3 positive | Anti-MPO/anti-PR3 negative | Total (N) | |
|---|---|---|---|---|---|
| p-ANCA | N | 66 | 4 | 339 | 409 |
| % | 16.14 | 0.98 | 82.89 | ||
| c-ANCA | N | 0 | 41 | 19 | 60 |
| % | 0.00 | 68.33 | 31.67 | ||
| x-ANCA | N | 10 | 8 | 157 | 175 |
| % | 5.71 | 4.57 | 89.71 | ||
| Negative | N | 4 | 13 | 10142 | 10159 |
| % | 0.04 | 0.13 | 99.83 | ||
| Total | N | 80 | 66 | 10657 | 10803 |
| % | 0.74 | 0.61 | 98.65 | ||
The relationship between the results of ANCA testing and clinical diagnosis is shown in Table 3. This analysis included 552 patients, 109 excluded due to insufficient data. ANCA-associated vasculitides (AAV) represented only 20.5% (113/552) of positive results. This group included microscopic polyangiitis, Wegener's granulomatosis, Churg-Strauss syndrome and the localized forms of these diseases. The majority of AAV patients with ANCA, were found positive by ELISA (106/113—93.8%). In particular, most MPA and CSS patients exhibited p-ANCA/MPO-ANCA positivity (67.2% and 66.7%, resp.), whereas Wegener's granulomatosis was closely associated with c-ANCA/PR3-ANCA (73.9%).
Table 3.
Relationship between the results of ANCA testing and clinical diagnoses.
| Diseases | p-ANCA | c-ANCA | x-ANCA | IIF (−)/ELISA (+) | Anti-MPO (+) | Anti-PR3 (+) | Total (N) | |
|---|---|---|---|---|---|---|---|---|
| MPA | N | 45 | 4 | 6 | 6 | 46 | 10 | 61 |
| % | 73.8 | 6.6 | 9.8 | 9.8 | 75.4 | 16.4 | ||
| WG | N | 6 | 34 | 1 | 5 | 4 | 40 | 46 |
| % | 13.0 | 73.9 | 2.2 | 10.9 | 8.7 | 87 | ||
| CSS | N | 4 | 0 | 2 | 0 | 6 | 0 | 6 |
| % | 66.7 | 0.0 | 33.3 | 0.0 | 100 | 0.0 | ||
| CTD | N | 97 | 2 | 31 | 2 | 9 | 1 | 132 |
| % | 73.5 | 1.5 | 23.5 | 1.5 | 6.8 | 0.7 | ||
| GD | N | 67 | 10 | 40 | 0 | 1 | 8 | 117 |
| % | 57.3 | 8.5 | 34.2 | 0.0 | 17.9 | 7.7 | ||
| Other vasculitides | N | 27 | 1 | 11 | 0 | 7 | 3 | 39 |
| % | 69.2 | 2.6 | 28.2 | 0.0 | 5.6 | 0.0 | ||
| Infections | N | 23 | 1 | 12 | 0 | 2 | 0 | 36 |
| % | 63.9 | 2.8 | 33.3 | 0.0 | 5.6 | 0.0 | ||
| ND | N | 23 | 1 | 9 | 0 | 0 | 0 | 33 |
| % | 69.7 | 3.0 | 27.3 | 0.0 | 0.0 | 0.0 | ||
| Malignancies | N | 21 | 1 | 9 | 0 | 1 | 0 | 31 |
| % | 67.7 | 3.3 | 29.0 | 0.0 | 3.3 | 0.0 | ||
| RD | N | 18 | 0 | 4 | 0 | 2 | 0 | 22 |
| % | 81.8 | 0.0 | 18.2 | 0.0 | 9.0 | 0.0 | ||
| Medication | N | 10 | 0 | 5 | 0 | 0 | 0 | 15 |
| % | 66.7 | 0.0 | 33.3 | 0.0 | 0.0 | 0.0 | ||
| Miscellaneous disorders | N | 5 | 2 | 6 | 1 | 0 | 1 | 14 |
| % | 35.7 | 14.3 | 42.9 | 7.1 | 0.0 | 7.1 | ||
| Undefined diagnosis | N | 63 | 4 | 39 | 3 | 2 | 3 | 109 |
| % | 57.8 | 3.7 | 35.8 | 2.7 | 1.8 | 2.7 | ||
MPA: microscopic polyangiitis, WG: Wegener's granulomatosis, CSS: Churg-Strauss syndrome, CTD: connective tissue diseases, GD: gastrointestinal diseases, ND: neurological diseases, RD: renal diseases.
The majority (79.5%) of positive sera derived from non-AAV patients. The most common disease categories were connective tissue disorders (132/552—23.9%) and gastrointestinal disorders (117/552—21.2%). However, several other conditions were recognized such as other vasculitides (39/552—7.0%), infections (36/552—6.5%), neurological diseases (33/552—6.0%), malignancies (31/552—5.6%), renal disease (22/552—4%), medication (15/552—2.7%), and miscellaneous disorders (14/552—2.6%). p-ANCA, followed by x-ANCA, was the predominant IIF pattern in the non-AAV group, whereas, unlike vasculitic group, the majority (404/439—92.0%) of positive sera were tested negative by ELISA. However, in 35 samples (8%) of this group, anti-MPO or anti-PR3 antibodies were detected. The spectrum of diseases that were associated with anti-MPO antibodies included secondary vasculitides (6/22), SLE (6/22), RA (3/22), TBC (2/22), AIH (1/22), undefined vasculitis (1/22), PSGN (1/22), multiple myeloma (1/22), and Goodpasture's syndrome (1/22). On the other hand, ulcerative colitis (UC) (7/13), Crohn's disease (CD) (1/13), PAN (1/13), Henoch-Schönlein purpura (1/13), undefined vasculitis (1/13), psoriasis (1/13) and AHA (1/13) were associated with anti-PR3 antibodies.
In AAV patients statistically significant higher IIF titers were observed, compared to non-AAV (chi-square test, P < .001). Specifically, 57% of IIF positive sera exceeded 1/80 titer versus 35% in non-AAV patients. Moreover, difference in anti-PR3 levels between AAV and non-AAV patients (58.8 ± 32.4 U versus 35.9 ± 17.2 U) were also statistically significant (one tailed t-test, P = .001). On the other hand, the respective comparison in anti-MPO (52.6 ± 24.5 U versus 43.2 ± 22.5 U) did not achieve statistical significance (one tailed t-test, P = .057).
The positive predictive values of ANCA testing for AAV were calculated, before and after separating inpatients from outpatients. Obtained results are shown in Table 4. It is obvious that the combination of IIF and ELISA improved diagnostic accuracy, compared to either IIF or ELISA alone. The PPV of a combined IIF plus MPO/PR3 ELISA test was estimated 84.5%. On the contrary, when IIF and MPO/PR3 ELISA were considered separately, the respective PPVs were significantly lower (20.6% and 74.1%). Except for the PPV of p-ANCA pattern that was very low both in inpatients and in outpatients (14.6% and 17.8%, resp.), the outpatients' group corresponded to higher rates of true positive results than any other test combination.
Table 4.
Positive predictive values (PPV) of ANCA testing for AAV.
| Immunological marker | All patients PPV% (95%CI) | Inpatients PPV% (95%CI) | Outpatients PPV% (95%CI) |
|---|---|---|---|
| p-ANCA | 13.0 (9.5–16.6) | 14.6 (9.8–19.4) | 17.8 (11.5–24.2) |
| c-ANCA | 67.9 (55.6–80.1) | 57.9 (42.2–73.6) | 88.8 (74.4–100) |
| p-ANCA plus c-ANCA | 20.6 (16.7–24.6) | 21.3 (16.2–26.4) | 25.9 (19.1–32.8) |
| Anti-MPO | 71.7 (61.8–81.8) | 67.4 (53.8–80.9) | 78.1 (63.8–92.4) |
| Anti-PR3 | 76.9 (66.7–87.2) | 69.4 (54.4–84.5) | 86.2 (73.7–98.8) |
| Anti-MPO plus anti-PR3 | 74.1 (66.9–81.3) | 68.3 (58.2–78.4) | 81.9 (72.3–91.6) |
| p-ANCA/MPO | 80.3 (70.4–90.3) | 77.7 (64.2–91.4) | 84.0 (69.6–98.4) |
| c-ANCA/PR3 | 90.5 (81.6–99.4) | 84.6 (70.7–98.5) | 100 |
| p-ANCA/MPO plus c-ANCA/PR3 | 84.5 (77.5–91.5) | 80.6 (70.8–90.5) | 90.2 (81.2–99.3) |
4. Discussion
Several studies underline the diagnostic value of antineutrophil cytoplasmic antibodies, introducing them as a significant serological marker for a subset of patients with primary systemic vasculitis. However, the predictive values of ANCA testing for vasculitides in most reports range widely, depending on the test characteristics of the assays used and also on the patient populations studied. The present work summarizes the experience of a 3-year period from the largest hospital of Athens, providing data for a cohort of 10803 unselected consecutive Greek patients. This is the first large retrospective study assessing ANCA testing in Greek population. Both inpatients (6017) from several different departments and outpatients (4786) were included to avoid selection bias and better reflect ANCA test, requesting in routine clinical practice. Noteworthy, the clinical information was obtained retrospectively, and a number of cases with insufficient data were excluded from the results evaluation.
Several publications reveal differences in the genetic profile of Greek patients with autoimmune diseases [9–11]. Boki et al. in their study of 66 Greek patients with necrotizing vasculitis did not find any significant associations of HLA-class I and II alleles with PAN and CSS. In WG patients, an increased frequency only in HLA-DR1 achieved statistical significance [12]. These findings are inconsistent with previous reports supporting that WG in Germany is associated with HLA-DPB1*0401, whereas CSS in Italy is associated with HLA-DRB3 and HLA-DRB4 [8]. Since genetic and environmental factors are implicated in the production of ANCA, differences in distribution or magnitude of these factors may influence the epidemiology of ANCA-associated disorders in various populations.
Schönermark et al. have reported high rates (13.5%) of IIF positivity in patients with rheumatological disorders [17]. More than half of these patients (58.3%) had a final diagnosis of AAV. On the other hand, significantly lower rates of IIF positivity (4.8–13%) were noted in less selected groups of patients [13, 16, 19]. McLaren et al. found a wide variation in diagnostic yield of ANCA among different departments (2–18%) of UK hospitals [14]. Of these patients, 0–56% had confirmed AAV. Our data showed that the yield of a positive test by IIF was low (6%) and only 20.5% of ANCA positive results were correlated with AAV. It was also shown that the frequency of anti-MPO antibodies among p-ANCA positive sera and the frequency of anti-PR3 antibodies among c-ANCA positive sera was lower (16.1% and 68.3%, resp.), compared to previous reports [14, 17]. These findings mirror the highly heterogeneous population studied, the variety of diseases that may mimic vasculitides and the different clinical practices among the diverse groups of physicians ordering the tests.
Concerning ANCA specificity, present data confirm their high association with AAV (93.8%), especially of c-ANCA/anti-PR3 with WG (73.9%) and to a lesser extent of p-ANCA/anti-MPO with MPA (67.2%). Anti-PR3 antibodies were detected in 87% of WG patients and in 16.4% of MPA patients. Anti-MPO antibodies were found in 75.4% of MPA patients, in 8.7% of WG patients and in 100% of CSS cases (all 5 patients studied). These findings are consistent with previous reports in Europe [46]. On the other hand, reports from China suggest that MPO-ANCA positive WG cases are very common (60.7% [47]. In the present study, MPA predominated among AAV patients. This finding confirms that in Europe, WG appears to be more common at high latitudes, whereas MPA shows the reverse pattern [48].
In the present study, connective tissue diseases and gastrointestinal disorders mainly constituted the non-AAV group that predominated among ANCA positive patients, followed by several other conditions including infections, other vasculitides, medication, neurological diseases, renal diseases and malignancies. Our data demonstrate that low IIF titers and ELISA levels as well as low prevalence of anti-MPO and anti-PR3 antibodies characterized these disorders. Several reports support the high specificity of anti-MPO and anti-PR3 antibodies for AAV, indicating that other constituents of the primary granules of neutrophils might be the target antigens of ANCA in non-AAV conditions [6, 20, 21]. Testing for antibodies to these antigens could be very helpful in the diagnostic approach of these diseases.
Apart from AAV, anti-MPO or anti-PR3 antibodies have been also reported in other disorders [49–55]. These disorders should be carefully ruled out when considering an AAV diagnosis. In the present study, the anti-MPO group included patients with secondary and undefined vasculitis, RA, SLE, AIH, TBC, PSGN, Goodpasture's disease, and multiple myeloma. The anti-PR3 group included patients with IBD (mainly UC), other vasculitides, SLE, psoriasis, and AHA.
How confident can a clinician be that an ANCA positive patient is an AAV patient? The positive predictive value (PPV) of ANCA testing for AAV can answer this question. Our results demonstrated that combined testing with IIF plus PR3/MPO ELISA increased the PPV of ANCA for AAV from 20.6% to 84.5%. This finding confirms that the combination of IIF with ELISA is more helpful than either IIF or ELISA alone in the diagnostic approach of MPA, WG, and CSS patients. However, higher PPVs of antineutrophil cytoplasmic antibodies testing for AAV, especially of IIF results (55–59%), have been reported [13, 14]. Since the predictive value of any laboratory test depends on the prevalence of the disease in the population under study, these differences on PPV imply the low pretest probability of AAV in the present clinical setting.
A significant difference in PPV of ANCA testing for AAV was observed after separating outpatients from inpatients. The group of outpatients yielded lower rates of false-positive results (higher PPVs) when compared to the inpatients' group. This variation in the diagnostic yield was likely based on differences in clinical practice and in the composition of the tested populations. A significant number of AAV patients visit outpatient' clinics for a followup, leading to higher rates of true positive results. On the other hand, a high proportion of inpatients undergo ANCA testing for a wide range of other ANCA-associated conditions.
Several reports have stated that the combination of IIF plus antigen-specific ELISA, provides higher sensitivity and specificity than either assay used separately [14, 56]. Since, parallel IIF/ELISA testing is time consuming and expensive and most ELISA assays have a lower sensitivity than IIF, IIF is recommended as a screening method and ELISA as a confirmatory method [57–59]. However, various alternative strategies on ANCA detection that include IIF, solid phase MPO/PR3 ELISA and capture ELISA assays have been reported to provide diagnostic accuracy [15, 60, 61]. In our department, two different algorithms on ANCA detection have been implemented. All outpatients undergo testing by IIF plus ELISA, whereas all inpatients are initially screened by IIF followed by ELISA in positive results. As it was previously mentioned, ANCA testing is also useful for the diagnosis of a number of non-AAV disorders. Since the majority of these disorders are not associated with anti-MPO or anti-PR3, reported algorithms that use a sensitive ELISA at the initial stage and proceed to IIF for confirmation of positive results cannot be applied.
A substantial decrease in the total cost of ANCA testing would be expected for outpatients if ELISA was applied in only IIF positive sera. By this strategy, among 4786 outpatients, only 17 IIF negative/ELISA positive cases would have been missed. Of these, 3 represented first diagnosed AAV, while 8 had an already established AAV diagnosis, 3 represented non-AAV disorders (SLE, psoriasis, AHA) and in 3 cases the final diagnosis was undefined. Based on these data, it could be suggested that screening by IIF and confirming only IIF positive results by ELISA, in all patients, would lead to substantial labor savings without sacrificing diagnostic accuracy. However, in cases where a high clinical suspicion index for AAV remains, despite a negative IIF result, ELISA test requesting should not be discouraged.
To summarize, the present report that highlighted ANCA testing in a Greek Hospital under actual routine conditions showed that (i) a large number of false positive ANCA test results, especially by IIF, are to be expected in highly heterogeneous groups of patients (ii) the non-AAV group that was mainly represented by connective tissue diseases and gastrointestinal disorders displayed very low rates of MPO/PR3 ELISA positivity, (iii) since AAV are closely associated with MPO/PR3 ANCA, the combination of IIF and MPO/PR3 ELISA maximizes the diagnostic value of ANCA testing, (iv) parallel testing with both IIF and ELISA did not seem to offer any significant diagnostic advantages, (v) screening by IIF and confirming positive results by ELISA, results in substantial laboratory and cost efficiency, (vi) assessment of clinicopathological data and compliance with test-ordering guidelines will facilitate the interpretation of test results and the diagnostic approach of patients with ANCA-associated disorders.
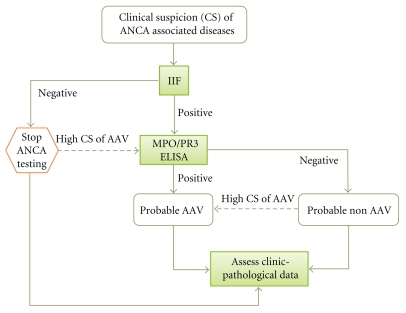
In conclusion, based on our data we suggest that ANCA testing should be restricted in patients with clinical picture consistent with ANCA-associated diseases (e.g., AAV, Good pasture's disease, IBD, AIH and PSC), following the proposed algorithm (Figure 1). This rational use of ANCA testing can reduce the total number of tests performed, minimizing false positive results and laboratory charges and prevent unnecessary and potentially harmful diagnostic and the rapeutic procedures.
Figure 1.
Algorithm on ANCA testing.
References
- 1.Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet. 2006;368(9533):404–418. doi: 10.1016/S0140-6736(06)69114-9. [DOI] [PubMed] [Google Scholar]
- 2.Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? British Medical Journal. 1982;285(6342):p. 606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Der Woude FJ, Rasmussen N, Lobatto S. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1(8426):425–429. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 4.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. New England Journal of Medicine. 1988;318(25):1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt WH, Van Der Woude FJ. Clinical applications of antineutrophil cytoplasmic antibody testing. Current Opinion in Rheumatology. 2004;16(1):9–17. doi: 10.1097/00002281-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Savige J, Davies D, Falk RJ, Jennette JC, Wiik A. Antineutrophil cytoplasmic antibodies and associated diseases: a review of the clinical and laboratory features. Kidney International. 2000;57(3):846–862. doi: 10.1046/j.1523-1755.2000.057003846.x. [DOI] [PubMed] [Google Scholar]
- 7.van Wijngaarden RAFD, van Rijn L, Hagen EC, et al. Hypotheses on the etiology of antineutrophil cytoplasmic autoantibody-associated vasculitis: the cause is hidden, but the result is known. Clinical Journal of the American Society of Nephrology. 2008;3(1):237–252. doi: 10.2215/CJN.03550807. [DOI] [PubMed] [Google Scholar]
- 8.Willcocks LC, Lyons PA, Rees AJ, Smith KGC. The contribution of genetic variation and infection to the pathogenesis of ANCA-associated systemic vasculitis. Arthritis Research and Therapy. 2010;12(1, article 202) doi: 10.1186/ar2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boki KA, Panayi GS, Vaughan RW, Drosos AA, Moutsopoulos HM, Lanchbury JS. HLA class II sequence polymorphisms and susceptibility to rheumatoid arthritis in Greeks: The HLA-DRβ shared-epitope hypothesis accounts for the disease in only a minority of Greek patients. Arthritis and Rheumatism. 1992;35(7):749–755. doi: 10.1002/art.1780350706. [DOI] [PubMed] [Google Scholar]
- 10.Roitberg-Tambur A, Friedmann A, Safirman C, et al. Molecular analysis of HLA class II genes in primary Sjogren’s syndrome: a study of Israeli Jewish and Greek non-Jewish patients. Human Immunology. 1993;36(4):235–242. doi: 10.1016/0198-8859(93)90130-s. [DOI] [PubMed] [Google Scholar]
- 11.Reveille JD, Arnett FC, Olsen ML, Moulds JM, Papasteriades CA, Moutsopoulos HM. HLA-class II alleles and C4 null genes in Greeks with systemic lupus erythematosus. Tissue Antigens. 1995;46(5):417–421. doi: 10.1111/j.1399-0039.1995.tb03140.x. [DOI] [PubMed] [Google Scholar]
- 12.Boki KA, Dafni U, Karpouzas GA, Papasteriades C, Drosos AA, Moutsopoulos HM. Necrotizing vasculitis in Greece: clinical, immunological and immunogenetic aspects, a study of 66 patients. British Journal of Rheumatology. 1997;36(10):1059–1066. doi: 10.1093/rheumatology/36.10.1059. [DOI] [PubMed] [Google Scholar]
- 13.Mandl LA, Solomon DH, Smith EL, Lew RA, Katz JN, Shmerling RH. Using antineutrophil cytoplasmic antibody testing to diagnose vasculitis: can test-ordering guidelines improve diagnostic accuracy? Archives of Internal Medicine. 2002;162(13):1509–1514. doi: 10.1001/archinte.162.13.1509. [DOI] [PubMed] [Google Scholar]
- 14.McLaren JS, Stimson RH, McRorie ER, Coia JE, Luqmani RA. The diagnostic value of anti-neutrophil cytoplasmic antibody testing in a routine clinical setting. The Quarterly Journal of Medicine. 2001;94(11):615–621. doi: 10.1093/qjmed/94.11.615. [DOI] [PubMed] [Google Scholar]
- 15.Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clinical Immunology. 2002;103(2):196–203. doi: 10.1006/clim.2001.5200. [DOI] [PubMed] [Google Scholar]
- 16.Stone JH, Talor M, Stebbing J, et al. Test characteristics of immunofluorescence and ELISA tests in 856 consecutive patients with possible ANCA-associated conditions. Arthritis Care and Research. 2000;13(6):424–434. doi: 10.1002/1529-0131(200012)13:6<424::aid-art14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Schönermarck U, Lamprecht P, Csernok E, Gross WL. Prevalence and spectrum of rheumatic diseases associated with proteinase 3-antineutrophil cytoplasmic antibodies (ANCA) and myeloperoxidase-ANCA. Rheumatology. 2001;40(2):178–184. doi: 10.1093/rheumatology/40.2.178. [DOI] [PubMed] [Google Scholar]
- 18.Franssen C, Gans R, Kallenberg C, Hageluken C, Hoorntje S. Disease spectrum of patients with antineutrophil cytoplasmic autoantibodies of defined specificity: distinct differences between patients with anti-proteinase 3 and anti-myeloperoxidase autoantibodies. Journal of Internal Medicine. 1998;244(3):209–216. doi: 10.1046/j.1365-2796.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 19.Venkatachalam S, Major P, Morgan L, Smith J, Cawley MID. Clinical utility of antineutrophil cytoplasmic antibody (ANCA) test: an audit. Annals of the Rheumatic Diseases. 1999;58:5–6. [Google Scholar]
- 20.Merkel PA, Polisson RP, Chang Y, Skates SJ, Niles JL. Prevalence of antineutrophil cytoplasmic antibodies in a large inception cohort of patients with connective tissue disease. Annals of Internal Medicine. 1997;126(11):866–873. doi: 10.7326/0003-4819-126-11-199706010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Vassilopoulos D, Niles JL, Villa-Forte A, et al. Prevalence of antineutrophil cytoplasmic antibodies in patients with various pulmonary diseases or multiorgan dysfunction. Arthritis Care and Research. 2003;49(2):151–155. doi: 10.1002/art.10997. [DOI] [PubMed] [Google Scholar]
- 22.Koutroubakis IE, Petinaki E, Mouzas IA, et al. Anti-Saccharomyces cerevisiae mannan antibodies and antineutrophil cytoplasmic autoantibodies in Greek patients with inflammatory bowel disease. American Journal of Gastroenterology. 2001;96(2):449–454. doi: 10.1111/j.1572-0241.2001.03524.x. [DOI] [PubMed] [Google Scholar]
- 23.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an international consensus conference. Arthritis and Rheumatism. 1994;37(2):187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 24.Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis and Rheumatism. 1990;33(8):1101–1107. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 25.Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 Criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis and Rheumatism. 1990;33(8):1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 26.Lightfoot RW, Jr., Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis and Rheumatism. 1990;33(8):1088–1093. doi: 10.1002/art.1780330805. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler B, Davatchi F, Mizushima Y, et al. Criteria for diagnosis of Behcet’s disease. Lancet. 1990;335(8697):1078–1080. [PubMed] [Google Scholar]
- 28.Mills JA, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch-Schonlein purpura. Arthritis and Rheumatism. 1990;33(8):1114–1121. doi: 10.1002/art.1780330809. [DOI] [PubMed] [Google Scholar]
- 29.Podolsky DK. Medical progress: inflammatory bowel disease (Second of two parts) New England Journal of Medicine. 1991;325(14):1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- 30.Lee YM, Kaplan MM. Primary sclerosing cholangitis. New England Journal of Medicine. 1995;332(14):924–933. doi: 10.1056/NEJM199504063321406. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. Journal of Hepatology. 1999;31(5):929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan MM. Medical progress: primary biliary cirrhosis. New England Journal of Medicine. 1996;335(21):1570–1580. doi: 10.1056/NEJM199611213352107. [DOI] [PubMed] [Google Scholar]
- 33.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40(9):p. 1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 34.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 35.Balint GP, Balint PV. Felty’s syndrome. Best Practice and Research: Clinical Rheumatology. 2004;18(5):631–645. doi: 10.1016/j.berh.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Masi AT, Rodnan GP, Medsger TA. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis and Rheumatism. 1980;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 37.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) New England Journal of Medicine. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 38.Bohan A, Peter JB. Polymyositis and dermatomyositis (Second of two parts) New England Journal of Medicine. 1975;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 39.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Annals of the Rheumatic Diseases. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alarcon-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. Journal of Rheumatology. 1989;16(3):328–334. [PubMed] [Google Scholar]
- 41.Amor B. Reiter’s syndrome. Diagnosis and clinical features. Rheumatic Disease Clinics of North America. 1998;24(4):677–695. doi: 10.1016/s0889-857x(05)70037-5. [DOI] [PubMed] [Google Scholar]
- 42.Goei The HS, Steven MM, Van Der Linden SM, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a comparison of the Rome, New York and modified New York criteria in patients with a positive clinical history screening test for ankylosing spondylitis. British Journal of Rheumatology. 1985;24(3):242–249. doi: 10.1093/rheumatology/24.3.242. [DOI] [PubMed] [Google Scholar]
- 43.Swann M. Juvenile chronic arthritis. Clinical Orthopaedics and Related Research. 1987;219:38–49. [PubMed] [Google Scholar]
- 44.Freedman SF, Amedee RG. Relapsing polychondritis. The Journal of the Louisiana State Medical Society. 1992;144(4):131–133. [PubMed] [Google Scholar]
- 45.Levine JS, Branch W, Rauch J. The antiphospholipid syndrome. New England Journal of Medicine. 2002;346(10):752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 46.Wiik A. Rational use of ANCA in the diagnosis of vasculitis. Rheumatology. 2002;41(5):481–483. doi: 10.1093/rheumatology/41.5.481. [DOI] [PubMed] [Google Scholar]
- 47.Chen M, Yu F, Zhang Y, Zou WZ, Zhao MH, Wang HY. Characteristics of Chinese patients with Wegener’s granulomatosis with anti-myeloperoxidase autoantibodies. Kidney International. 2005;68(5):2225–2229. doi: 10.1111/j.1523-1755.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- 48.Watts RA, Scott DGI. Epidemiology of the vasculitides. Current Opinion in Rheumatology. 2003;15(1):11–16. doi: 10.1097/00002281-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Flores-Suárez LF, Cabiedes J, Villa AR, van der Woude FJ, Alcocer-Varela J. Prevalence of antineutrophil cytoplasmic autoantibodies in patients with tuberculosis. Rheumatology. 2003;42(2):223–229. doi: 10.1093/rheumatology/keg066. [DOI] [PubMed] [Google Scholar]
- 50.Choi HK, Lamprecht P, Niles JL, Gross WL, Merkel PA. Subacute bacterial endocarditis with positive cytoplasmic antineutrophil cytoplasmic antibodies and anti-proteinase 3 antibodies. Arthritis and Rheumatism. 2000;43(1):226–231. doi: 10.1002/1529-0131(200001)43:1<226::AID-ANR27>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 51.Wu YY, Hsu TC, Chen TY, et al. Proteinase 3 and dihydrolipoamide dehydrogenase (E3) are major autoantigens in hepatitis C virus (HCV) infection. Clinical and Experimental Immunology. 2002;128(2):347–352. doi: 10.1046/j.1365-2249.2002.01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi HK, Merkel PA, Walker AM, Niles JL. Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis and Rheumatism. 2000;43(2):405–413. doi: 10.1002/1529-0131(200002)43:2<405::AID-ANR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 53.Lamprecht P, Gadola S, Schnabel A, Gross WL. ANCA, infectious endocarditis, glomerulonephritis and cryoglobulinemic vasculitis. Clinical Nephrology. 1998;49(6):389–390. [PubMed] [Google Scholar]
- 54.Ruffatti A, Sinico RA, Radice A, et al. Autoantibodies to proteinase 3 and myeloperoxidase in systemic sclerosis. Journal of Rheumatology. 2002;29(5):918–923. [PubMed] [Google Scholar]
- 55.Ardiles LG, Valderrama G, Moya P, Mezzano SA. Incidence and studies on antigenic specificities of antineutrophil-cytoplasmic autoantibodies (ANCA) in poststreptococcal glomerulonephritis. Clinical Nephrology. 1997;47(1):1–5. [PubMed] [Google Scholar]
- 56.Hagen EC, Daha MR, Hermans JO, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. Kidney International. 1998;53(3):743–753. doi: 10.1046/j.1523-1755.1998.00807.x. [DOI] [PubMed] [Google Scholar]
- 57.Csernok E, Ahlquist D, Ullrich S, Gross WL. A critical evaluation of commercial immunoassays for antineutrophil cytoplasmic antibodies directed against proteinase 3 and myeloperoxidase in Wegener’s granulomatosis and microscopic polyangiitis. Rheumatology. 2002;41(11):1313–1317. doi: 10.1093/rheumatology/41.11.1313. [DOI] [PubMed] [Google Scholar]
- 58.Savige J, Gillis D, Benson E, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA) American Journal of Clinical Pathology. 1999;111(4):507–513. doi: 10.1093/ajcp/111.4.507. [DOI] [PubMed] [Google Scholar]
- 59.Savige J, Dimech W, Fritzler M, et al. Addendum to the International Consensus Statement on testing and reporting of antineutrophil cytoplasmic antibodies. Quality control guidelines, comments, and recommendations for testing in other autoimmune diseases. American Journal of Clinical Pathology. 2003;120(3):312–318. doi: 10.1309/WAEP-ADW0-K4LP-UHFN. [DOI] [PubMed] [Google Scholar]
- 60.Csernok E, Holle J, Hellmich B, et al. Evaluation of capture ELISA for detection of antineutrophil cytoplasmic antibodies directed against proteinase 3 in Wegener’s granulomatosis: First results from a multicentre study. Rheumatology. 2004;43(2):174–180. doi: 10.1093/rheumatology/keh028. [DOI] [PubMed] [Google Scholar]
- 61.Vermeersch P, Vervaeke S, Blockmans D, et al. Determination of anti-neutrophil cytoplasmic antibodies in small vessel vasculitis: comparative analysis of different strategies. Clinica Chimica Acta. 2008;397(1-2):77–81. doi: 10.1016/j.cca.2008.07.026. [DOI] [PubMed] [Google Scholar]