Abstract
Locomotor activity of single, freely walking flies in small tubes is analyzed in the time domain of several hours. To assess the influence of the mushroom bodies on walking activity, three independent noninvasive methods interfering with mushroom body function are applied: chemical ablation of the mushroom body precursor cells; a mutant affecting Kenyon cell differentiation (mushroom body miniature1); and the targeted expression of the catalytic subunit of tetanus toxin in subsets of Kenyon cells. All groups of flies with mushroom body defects show an elevated level of total walking activity. This increase is attributable to the slower and less complete attenuation of activity during the experiment. Walking activity in normal and mushroom body-deficient flies is clustered in active phases (bouts) and rest periods (pauses). Neither the initiation nor the internal structure, but solely the termination of bouts seems to be affected by the mushroom body defects. How this finding relates to the well-documented role of the mushroom bodies in olfactory learning and memory remains to be understood.
Attempts at structure/function mapping in the insect brain have implicated the mushroom bodies (MBs) in a variety of complex behavioral functions. Most conclusively, the MBs have been shown to be required for olfactory learning and memory (Menzel et al. 1974; Heisenberg et al. 1985; de Belle and Heisenberg 1994; Connolly et al. 1996). They were also invoked as centers for courtship behavior in different insects. Electrical stimulation in brain areas close to or in the MBs elicited courtship behavior in freely moving crickets and grasshoppers (Huber 1960; Otto 1971; Wahdepuhl and Huber 1979; Wahdepuhl 1983). In Drosophila melanogaster gynandromorphs, Hall (1979) showed that the MBs (or tissue in their vicinity) had to be male for flies to display male courtship behavior. More recently, with the new P[GAL4] enhancer-trap technique (Brand and Perrimon 1993) a feminizing transgene (transformer1) was expressed specifically in parts of the MBs (besides other tissues). This intervention caused the males to court both sexes indiscriminately (Ferveur et al. 1995; O’Dell et al. 1995).
Much less is known about MB function in motor control. Unilateral MB ablation caused aberrant cocoon spinning in Cecropia larvae (van der Kloot and Williams 1953), and MB lesions in adult male crickets led to an elevated general motor activity (Huber 1955). Locusts and bees with MB lesions also showed a general increase of behavioral activity. In addition, many of the animals displayed rivaling motor patterns (Howse 1974). Increased locomotor activity was noted for mushroom body miniature1 (mbm1) mutant flies (Heisenberg et al. 1985). Recently, in search of an implication of MBs in the circadian clock, J.S. de Belle, J. Wulf, and C.H. Förster (pers. comm.) also found flies with chemically ablated MBs to be more active than their respective controls. All these results suggest that MBs might play a role in the generation, regulation, or coordination of motor patterns.
Locomotor activity in Drosophila has been recorded by many methods and in different contexts. To mention just a few, in an early study, spontaneous walking activity in an open field apparatus was used to select for active/inactive Drosophila strains (Connolly 1966, 1967) and later for single gene mutants with aberrant locomotor activity (Meehan and Wilson 1987; Burnet et al. 1988; O’Dell and Burnet 1988). In an object fixation task (Buridan’s paradigm) developed by Götz (1980), single flies with clipped wings are allowed to walk freely on a platform between two opposing inaccessible landmarks (vertical black stripes). Different parameters, such as walking speed, straightness of walk, walking activity, and time course of activity are extracted from a video record (Götz 1989; Strauss et al. 1992; Strauss and Heisenberg 1993). Locomotor activity of flies has also been extensively analyzed with respect to circadian rhythms. Walking activity in these recordings is monitored in the range of hours to days and is evaluated for circadian and ultradian oscillations (Konopka and Benzer 1971; for review, see Hall 1995). Foraging is another example of locomotion. In this case, the distance away from a feeding site a larva or adult fly reaches in a certain amount of time is the parameter of interest (Sokolowski 1980; de Belle and Sokolowski 1987; Pereira and Sokolowski 1993). All these tests consider walking either in the time domain of minutes or, as in the case of the biological clock, days. Here, we present an analysis of the pattern of walking activity in the time window of several hours and investigate the influence of the MBs on this aspect of behavior.
At present, only Drosophila provides several independent noninvasive techniques of blocking the MB pathway. We use three approaches: chemical ablation of the MB precursor cells; a single gene mutant affecting MB development; and targeted gene expression in the MBs. This latter technique already mentioned above, takes advantage of enhancer-trap lines expressing the yeast transcription factor GAL4 (Brand and Perrimon 1993) in groups of Kenyon cells. In our case, GAL4 drives expression of a transgene for the catalytic subunit of tetanus toxin (Cnt-E; Sweeney et al. 1995), which is known to block synaptic transmission in the respective neurons by cleaving synaptobrevin, a docking factor for synaptic vesicles (Niemann et al. 1994). We show that blocking the MB pathway invariably causes an increase in walking activity. Spontaneously walking, MB-deficient flies have difficulty stopping.
Materials and Methods
FLIES
Stocks (D. melanogaster) were maintained at 25°C on a standard cornmeal–molasses medium in a 16-hr light–8-hr dark cycle at 60% humidity. Wild-type strains Canton-S and Berlin (WT-Berlin) and the mutant mbm1 were used. This mutant was isolated by mass histology (Heisenberg and Böhl 1979) following ethylmethane sulfonate mutagenesis and has been back-crossed to WT-Berlin several times. The P[GAL4] enhancer-trap lines generated as described by Brand and Perrimon (1993) were kindly provided by K. Kaiser (P[GAL4]201Y; Yang et al. 1995) and by T. Raabe (P[GAL4]H24 and P[GAL4]17D; H. Pfister and T. Raabe, unpubl.). We used the gene for tetanus toxin light chain inserted on the second chromosome in a Canton-S genetic background as a UASGAL4 reporter (called Cnt-E). The Cnt-E stock was kindly provided by C. O’Kane (Sweeney et al. 1995). The three enhancer-trap lines are homozygous viable. Because GAL4-directed tetanus toxin expression was tested in flies heterozygous for both the P[GAL4] and P[UASGAL4–tetanus toxin] constructs, the corresponding heterozygous P[GAL4]/Canton-S flies were tested as controls. For hydroxyurea (HU) ablation of MB neuroblasts we followed the protocol of de Belle and Heisenberg (1994). The HU ablation was performed on wild-type Canton-S flies; experimental and control animals were treated strictly in parallel.
MEASURING WALKING ACTIVITY
The apparatus was a transparent rectangular chamber (40 × 3 × 3 mm3) with an infrared light gate situated in the middle. A moist filter paper permitted the fly to drink water. Single female flies at the age of 3 days were tested. Recordings lasted for 4.5 hr and were conducted in complete darkness. The light gate was sampled at a rate of 1 Hz. If the fly had passed the light gate once or several times since the last sampling event the computer recorded a count (stored as a value of 1); if the fly had not passed the light gate during that one second interval the computer stored a value of 0 (no count). Experiments were performed at 25°C and 50%–60% humidity always starting at 6:00 pm. The data represent the mean of three recordings performed on three successive days. Because there were no significant differences between recordings on successive days, data were pooled. In addition, all experiments were performed three times at three different periods of the year 1997 (May, September, and November). In all three experiments, MB-disturbed flies showed elevated locomotor activity compared to their controls.The data presented are from the November recording.
PROCESSING DATA
Data analysis was programmed in C++ (Microsoft Visual C++). For statistical analysis Statistica (StatSoft, Inc.) was used. Total activity was approximated as the total number of counts for each fly during the recorded time. For the time course of activity, counts were summed for successive 10-min periods. Time intervals between consecutive counts (intercount intervals; ICIs) were calculated as the sum of the 0 events between counts. To reveal the clustering (bout structure) in the time traces, we calculated for each fly a minimum pause, which is the shortest ICI separating bouts. To obtain the minimum pause we plotted the cumulative ICI frequencies (on a logarithmic scale) for increasing ICI duration (log-survivorship curve; Machlis 1977; Slater and Lester 1982; Sibly et al. 1990). This curve provided an unambiguous criterion for the minimum pause for each fly. To obtain the number and duration of bouts as well as the duration of the pauses between bouts, the onset and end of each bout were determined in the time traces using the individual minimum pauses as a criterion (for a more detailed description of this analysis, see J.-R. Martin, R. Ernst, and M. Heisenberg, in prep.). As is commonly found for events in time series, the parameters extracted were not normally distributed. Therefore, we subjected the data in Figure 5 (B–D) to a log transformation to approximate a normal distribution and then calculate the means and standard errors of the mean, which were weighted to the number of observations for each fly, as a conservative statistical test (Sachs 1992). For a more reliable representation of the bout structure, all bouts consisting of a single count were removed together with the subsequent pause. Also the first bout of each fly was omitted. The results are not qualitatively affected by this processing step.
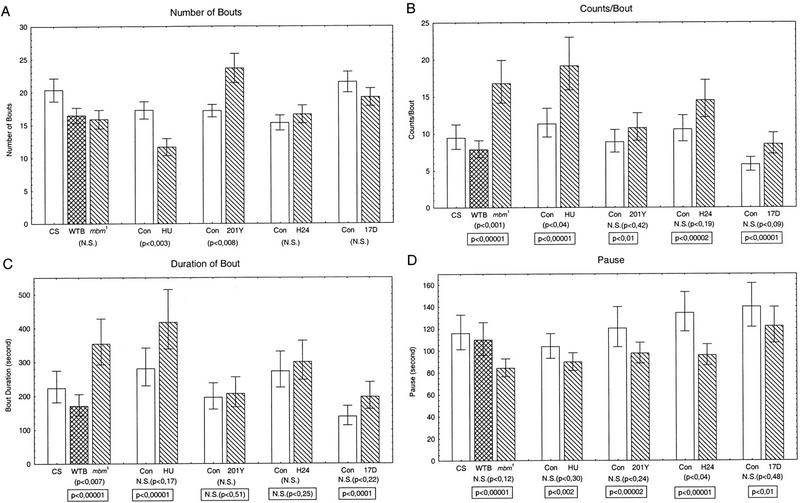
Figure 5.
(A) Mean number of bouts ±s.e.m. for each group of MB-disturbed flies compared to their respective controls. Original data are the same as in Fig. 2. For each group, a one-way ANOVA was performed (P value below each group column). For HU- and P[GAL4]201Y flies, a significant difference is seen compared to their controls. (B–D) Three parameters of the bout structure: counts per bout; duration of bouts; and duration of pauses. For statistical analysis, the data were converted to log scale and the means and standard errors of the means, weighted to the number of observation, were calculated. For each group, a one-way ANOVA was performed (P value below each group column). The additional P values inside the boxes indicate the statistical significance of the differences with the pooled number of bouts as sample sizes (n). For presentation, the data are reconverted to normal values (note that the s.e.m. is not symmetrically spread on both side of the mean). Data are the same as in Fig. 2. The P values below the WTB-mbm1 column are for the WT-Berlin and mbm1 comparison only (CS not included).
IMMUNOHISTOCHEMISTRY
Immunohistochemistry methods of Buchner et al. (1988) were used. Briefly, Drosophila adult heads were fixed for 4 hr in buffered 4% paraformaldehyde and washed overnight in 25% sucrose solution. Frontal sections were cut on a cryostat microtome at 10 μm thickness and incubated overnight at 4°C with a monoclonal anti-tetanus toxin antibody (1:10,000). Antibody and unpublished information were kindly provided by J. Thierer and H. Niemann. Biotin–streptavidin coupled to peroxidase (ABC Kit; Vectastain) with diaminobenzidine as the chromogen was used to visualize the primary antibody.
Results
METHODS OF MB PATHWAY INTERVENTION
Three kinds of intervention were used to generate flies with defective MBs (MB-disturbed flies). One was the use of mutants. We chose the strain mbm1 in the WT-Berlin genetic background. The degree of MB neuropil reduction in females of this stock has been quantitatively determined by de Belle and Heisenberg (1996). The mean volume of the calyx was about 10% of that in the WT-Berlin control. After that study the structural phenotype in the stock started to revert toward wild type. Therefore, the stock was crossed back to WT-Berlin and was reisolated from a single F2 paired mating. In the present line, the mutant phenotype is as pronounced as published previously. Approximately 80% of the animals have no or minute MBs on both sides. In the remaining 20%, the size ranges from small to normal. [Histology was performed on mbm1 females of the same vials from which experimental animals were taken. Mean calyx volume over all mbm1 female flies was about 10% of that in WT-Berlin (data not shown).]
In the second kind of intervention, MB formation during development was suppressed by feeding HU to first instar larvae (Prokop and Technau 1994). This treatment leads to a nearly complete loss of MB Kenyon cells in the adult. All HU flies were subjected to paraffin histology after the behavioral experiment. Of the 34 flies tested, only one had small MBs on both sides. Its performance in the behavioral experiment did not fall outside the range of behavioral phenotypes of the remaining animals and was included in the mean data.
In the third intervention we applied the P[GAL4] enhancer-trap technique (Brand and Perrimon 1993). Three lines expressing the transcription factor GAL4 in subsets of Kenyon cells were used to up-regulate in these cells the expression of the transgene for a tetanus toxin light chain (Cnt-E; Sweeney et al. 1995). One necessary criterion for selecting the lines was that flies had to survive to adulthood in combination with the Cnt-E transgene. In many MB-specific P[GAL4] lines, this was not the case. Even in one of the lines used here (P[GAL4]201Y), introduction of the Cnt-E-transgene led to low viability.
The expression patterns of the three lines are depicted in Figure 1. They differ in the intensity of labeling in the MBs, in the types of Kenyon cells expressing the transgene, and in the staining patterns outside the MBs. The line P[GAL4]201Y, already extensively described and used in other studies (O’Dell et al. 1995; Yang et al. 1995; Connolly et al. 1996), exhibits staining preferentially in the γ-lobes whereas the α- and β-lobes are only weakly stained. This pattern is also apparent in cross-sections of the pedunculus. The strong peripheral staining derives from fibers of the γ-lobe (γ-fibers) and the small dark core belongs to fibers that cause the diffuse staining in the α- and β-lobe (α/β-fibers). Interestingly, the α′-lobe (Ito et al. 1997) which is formed by Kenyon cells of the γ-lobe system is also only weakly stained, suggesting a further subdivision of Kenyon cell types.
Figure 1.
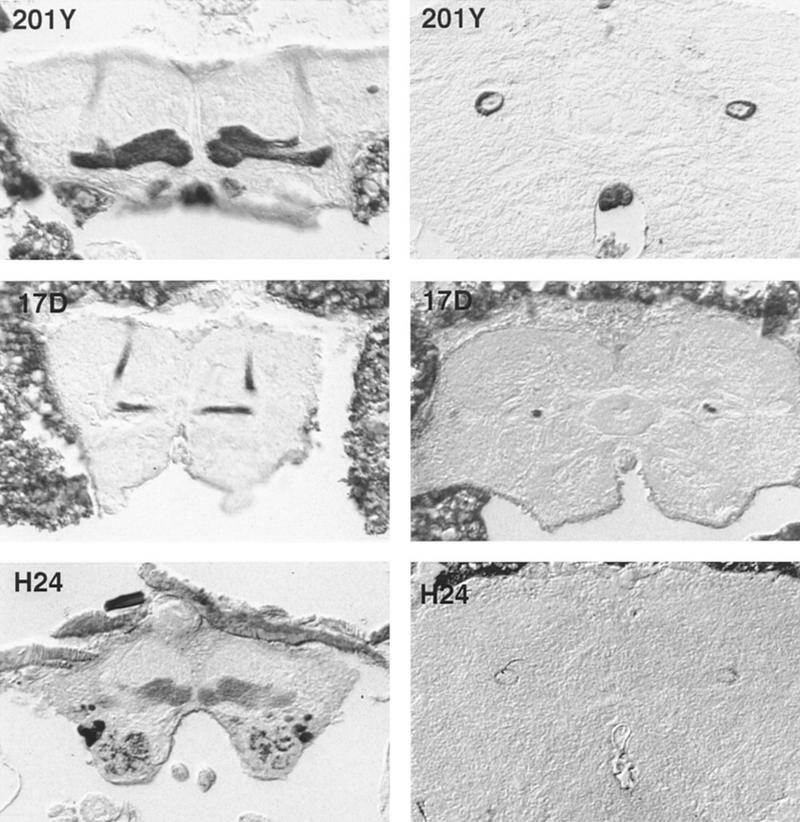
Tetanus toxin light chain staining in adult brains of the three groups of P[GAL4]/Cnt-E flies used in this study. The tetanus toxin expression is revealed by anti-tetanus toxin antibody. The expression pattern correlates well with that of other reporter genes (e.g., β-galactosidase; data not shown). Cryostat frontal sections (10 μm) at the level of the α- and β/γ-lobes (left) and peduncule cross-sections at the level of the CC (right). Line P[GAL4]201Y exhibits an extensive staining in the γ-lobe, whereas only faint staining is observed in the α- and β-lobes. In the peduncule, most of the staining is restricted to an outer ring (γ-fibers). The small dot in the center may correspond to the immunopositive fibers of the α- and β-lobes (α/β-fibers). In line P[GAL4]17D staining is found in the α- and β-lobes but not the γ-lobes. In the peduncule, staining is restricted to the central core, absence of staining in outer ring correlates with absence of γ-lobe staining. Line P[GAL4]H24 shows, in the MBs, a staining only in the γ-lobe. The faint staining restricted to the periphery of the peduncule corresponds to the γ-lobe staining. The difference in the size of the stained patterns between the three P[GAL4] lines is attributable to a difference of head sizes.
In the P[GAL4]17D line (Pfister 1997) expression of Cnt-E is localized in Kenyon cells of the α- and β- but not the γ-lobes (α/β-fibers; no γ-fibers). In the pedunculus, the staining is confined to the central core (Fig. 1). Caudally, this bundle splits into four tracts (not shown), revealing the clonal origin of the respective Kenyon cells as described by Ito et al. (1997). In the MBs, the line P[GAL4]H24 (Pfister 1997) shows an expression pattern restricted only to the γ-lobes. In pedunculus cross-sections, faint staining is seen at the periphery, corresponding to the weak staining in the γ-fibers. Again, no staining of the α′-lobe is observed. In addition, line P[GAL4]H24 exhibits staining in some other parts of the brain. In the central complex (CC) the transgene is expressed in a group of ring-neurons of the ellipsoid-body (EB) (Hanesch et al. 1989). About 40 cell bodies located rostro latero ventrally to the EB can be counted on either side of the brain. They send their fibers toward the EB where they arborize in the periphery of the ring. In addition, patchy staining is observed in the antennal lobes. The expression patterns of the three lines outside the brain and throughout the life cycle have not been studied in detail.
LOCOMOTOR ACTIVITY
Flies continuously walk in the tubes from one end to the other and back for extended periods of time. Their activity is recorded only at the moment when they pass the light gate in the middle of the tube’s long axis. During the interval between consecutive passages at the light gate they normally walk to the end of the tube, walk around there as if trying to escape, and finally return. Turning around before the end of the tube is rare (e.g., Fig. 3F, below). Periods of walking are interrupted by periods of rest for varying lengths of time. The total number of light gate crossings (counts) of each fly, therefore, are taken as its total walking activity (Fig. 2).
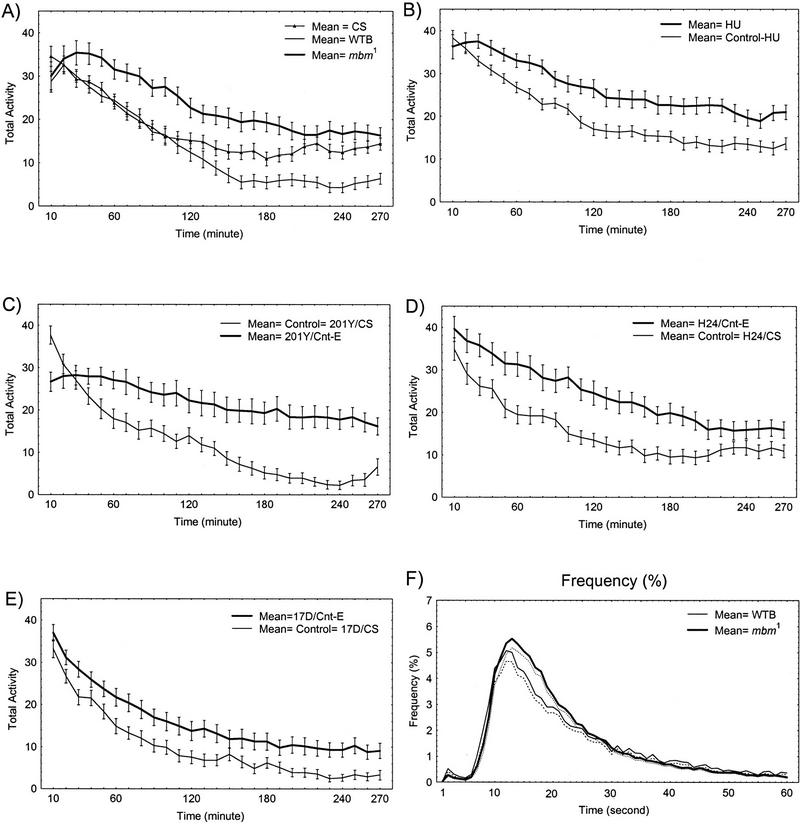
Figure 3.
(A–E) Time course of walking activity for each group of MB-disturbed flies compared to their respective controls. The curves represent the means ±s.e.m. of activity for successive 10-min periods. See legend in each graph. Same data as in Fig. 2. In F as an example, histograms (plotted as a continuous line) of the relative mean frequency [%; ±s.e.m. (dotted curve)] of ICIs over increasing ICI duration between 1 and 60 sec are shown for mbm1 and its controls.
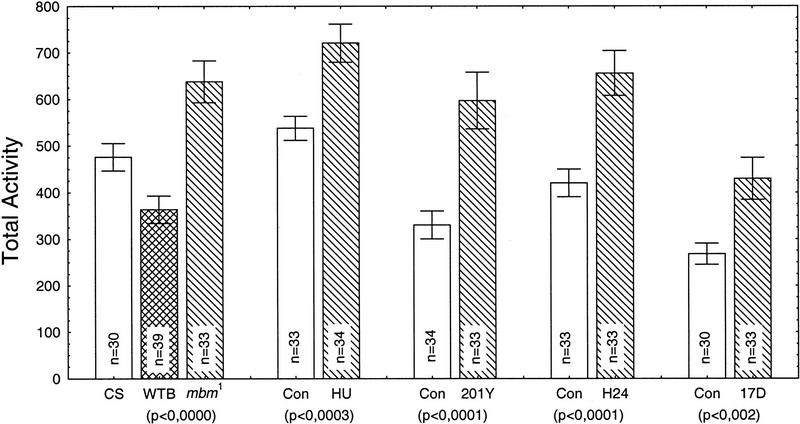
Figure 2.
Total walking activity during 4.5 hr for each group of MB-disturbed flies compared to their respective controls. (CS) Canton-S; (WTB) WT-Berlin; (Con) Control. Total activity is represented as the mean ±s.e.m. over n (number in each column) flies. For each group, a one-way ANOVA of total activity revealed significant difference (P value below each group column). The P value below the WTB-mbm1 column is for the WT-Berlin and mbm1 comparison only (CS not included).
The five groups of MB-disturbed flies (mbm1, HU, and the three types of P[GAL4]/Cnt-E flies) present a common effect: Their total walking activity is significantly increased in comparison to the respective controls. In P[GAL4]201Y/Cnt-E flies activity is nearly doubled. Note that different controls are used for all five groups. In Figure 2 (left), we show the activity of Canton-S to allow a comparison with the P[GAL4] flies, which are all on a Canton-S genetic background. The activity of WT-Berlin, which is the appropriate control for mbm1 is slightly lower than that of Canton-S. In the case of the HU flies (Canton-S), controls and experimental animals were processed strictly in parallel. Only HU was omitted for the controls. For the P[GAL4]/Cnt-E flies, the controls are heterozygous for the P[GAL4] transposon, as are the P[GAL4]/Cnt-E flies. Surprisingly, all three P[GAL4] controls have a significantly lower activity than Canton-S. Apparently, the transposon causes a decrease of locomotor activity. Because the three lines have the transposon inserted at different locations in the genome the effect is likely attributable to the P[GAL4] transposon itself rather than to position effects.
TIME COURSE OF WALKING ACTIVITY
To explore further the increase in total activity, its time course was plotted. During the 4.5 hr experiment activity was determined separately for each successive 10-min period. Figure 3 shows the decay of walking activity as a function of time for each group of flies. Control flies (e.g., Canton-S and HU controls; Fig. 3A–B) exhibit two components in the time course: an early phase lasting 60–120 min in which the decay of activity is fast and a late phase in which it is slow or reaches a plateau. The steep part of the curve represents the fly’s fading response to the new environment or the previous handling, whereas the plateau is attributable to the fly’s spontaneous activity (Connolly 1967; Meehan and Wilson 1987). All five types of MB-disturbed flies show a smaller decrease of activity with time than the corresponding controls.
Some differences can be noted in the time courses of activity in the five kinds of flies. Both mutant mbm1 and HU-flies (Fig. 3A,B), in which MBs are almost completely removed, start their activity at about the same level as the control flies but show very little attenuation of activity during the first hour. In P[GAL4]201Y/Cnt-E flies (Fig. 3C), the early, reactive component seems to be largely missing. P[GAL4]H24/Cnt-E and P[GAL4]17D/Cnt-E flies (Fig. 3D,E) driving the tetanus toxin in small (but different) subsets of Kenyon cells show a general upward shift of the curve over the whole time course.
As an example, histograms of time intervals between successive counts (ICIs) for wild-type Berlin and mbm1 flies are plotted in Figure 3F. Because the total activity of each group varies, frequencies are expressed as relative values to permit a better comparison. As pointed out above, the decay of the frequency curve toward small intervals may be attributable to the special design of the apparatus. The fly needs a certain time to reach the end of the tube and to come back to the light gate. For intervals above 13 sec, the curve shows that short ICIs are frequent and long ones rare.
WALKING ACTIVITY IS CLUSTERED IN BOUTS
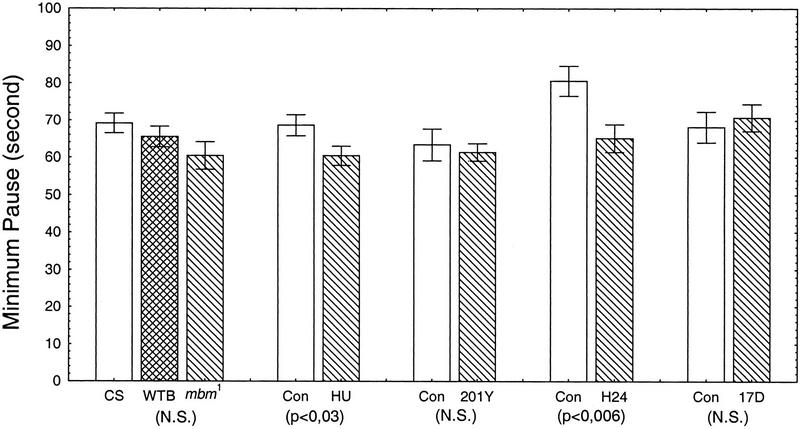
Visual examination of the time traces suggested that the activity was not uniformly or randomly spread but was clustered in bouts of activity, as are many other behaviors (e.g., Slater 1974; Machlis 1977). In behavioral analysis, a bout is characterized by the repeated and temporally clustered occurrences of a behavior. Short intervals between the events occur within bouts; long intervals separate two successive bouts. As will be described in detail elsewhere (J.-R. Martin, R. Ernst, and M. Heisenberg, in prep.), we determined whether walking activity under our experimental conditions, indeed, had a bout structure. For this purpose we calculated for the ICIs of each fly a so-called log-survivorship curve, which is the cumulative frequency of ICIs (on a logarithmic scale) as a function of increasing ICI duration (on a linear scale). The linear part of this function indicates the decreasing probability for ICIs of increasing duration within bouts. At the critical minimum pause defining bouts, the probability abruptly increases above the value extrapolated from the linear part of the curve. In fact, for each single fly this break in the log-survivorship curve is obvious and allows us to determine an individual criterion for the minimum pause. Figure 4 shows the histogram of the mean of this minimum pause for each group of flies. Differences between experimental and control groups are generally small, with the exception of P[GAL4]H24.
Figure 4.
Minimal pause, determining the bout structure, for each group of MB-disturbed flies compared to their respective controls. Minimal pause, in seconds, is represented as the mean ±s.e.m. For each group, a one-way ANOVA was performed (P value below each group column). The P value below the WTB-mbm1 column is for the WT-Berlin and mbm1 comparison only.
The determination of the minimum pause for each fly enabled us to describe walking activity in terms of bout structure. The time traces were characterized by four parameters, which were partially dependent on each other: the number of bouts, the number of counts per bout, the duration of bouts, and, finally, the duration of interbout pauses. In Figure 5A the histogram of the mean number of bouts for each group of flies is depicted. As shown in Figures 2 and 3 all MB-disturbed flies have an increase in total walking activity. This increase must be reflected somewhere in the pattern of activity, either in the number of bouts, or in the number of counts/bout, for example, in the intrinsic bout structure. In mbm1, P[GAL4]H24/Cnt-E and P[GAL4]17D/Cnt-E flies, the number of bouts is not significantly affected compared to the appropriate controls. HU flies show a significant decrease, while P[GAL4]201Y/Cnt-E flies show a significant increase in the number of bouts compared to their respective controls (Fig. 5A).
In all groups of MB-disturbed flies there is a tendency toward an increase in the mean of the number of counts/bout (Fig. 5B). This increase is highly significant for mbm1 and still significant (P < 0.05) for HU-flies (see Materials and Methods for the different estimates of confidence limits). Because a time series recording is a continuous process, the increase in the number of counts/bout has to change the bout structure by one or both of two possible ways: Either the duration of the bouts is increased while the frequency of counts is unchanged, or the frequency of counts is increased without an increase in the duration of bouts. Figure 5C shows that bout duration closely parallels counts/bout. It seems, therefore, that the increase in total activity is represented by an increase in bout duration, leaving the frequency of counts unchanged (data not shown).
In all five groups, the duration of the inactive period between two successive bouts (pause) is inversely affected compared to the duration of the bouts (Fig. 5D) although, by the conservative criterion, the decrease is significant only for P[GAL4]H24/Cnt-E flies. This inverse relationship between the duration of bouts and the duration of pauses implies that the period between consecutive starts of bouts is fixed and that the pause is the dependent variable (i.e., period − bout duration = pause). The result suggests that only bout duration, but neither period nor pause, is the primary target of MB function.
The defects in locomotor activity, both in the overall level and in the time structure are not an unspecific side effect of any brain damage. Expression of the tetanus toxin in other parts of the brain by various P[GAL4] enhancer-trap lines not affecting MBs did not generate such defects. Mutations affecting different substructures of the CC, for example, no-bridge1 (nob1), ellipsoid-body-open (ebo1, ebo2, ebo3, and ebo4, central-body-defect1 (cbd1) did not cause an increase in locomotor activity (but showed different locomotion phenotypes; J.-R. Martin, R. Ernst, and M. Heisenberg, in prep.).
Discussion
Recording locomotion of individual Drosophila flies for several hours, we have shown here that MBs act as inhibitors of walking activity. The three different and independent methods used to ablate or disturb the MBs, all lead to a substantial increase in total locomotor activity. Surprisingly, the degree of this increase seems not to be correlated with the number of Kenyon cells affected by the intervention. In P[GAL4]H24/Cnt-E and P[GAL4]17D/Cnt-E only small fractions of Kenyon cells express the tetanus toxin while the increase in activity over the controls is as large as in mbm1 or HU-flies in which 90% or more of the Kenyon cell fibers are missing. This non-linear relationship might indicate that the effect on overall activity depends on the MB as an integral structure and that a loss of a small number of Kenyon cells already abolishes total MB function. Alternatively, traces of toxin might invade and block the nonstained fibers. However, the interpretation is complicated by the fact that the P[GAL4] transposons by themselves seem to have a suppressive effect on walking activity. In comparison to Canton-S, the P[GAL4]201Y/Cnt-E and P[GAL4]H24/Cnt-E flies have only a small and P[GAL4]17D/Cnt-E flies no increase of total walking activity.
During the first few hours in the small tube walking activity of Drosophila gradually diminishes. As pointed out by Connolly (1967), the declining component of the activity can be interpreted as a response to the previous handling and/or the novel situation of the tube. The increased activity in MB-disturbed flies is, at least in part, attributable to a slower and less complete attenuation of activity. In all five experimental groups, not only the overall attenuation but also the initial (negative) slope of the time course is less steep than in the respective controls. These two parameters, amplitude and initial slope of attenuation, correlate better than overall activity with the assumed degree of impairment afflicted to the MBs. As would be expected from the number of Kenyon cells expressing the toxin, P[GAL4]H24/Cnt-E and P[GAL4]17D/Cnt-E flies are the least affected in these two parameters of the reactive component.
Our analysis of the data shows that the activity is distributed neither randomly nor regularly with respect to time. Rather, time is divided into periods of rest and walking (bouts). The pattern generator for this bout structure is not yet known. We suggest that the MBs are part of, or have a specific influence on, the pattern generator. Although our data set is still too small for drawing definitive conclusions, it provides several interesting observations. The main difference between MB-disturbed flies and controls lies in the duration of the bouts. The number of bouts seems not to be directly affected as shown for mbm1 and two of the P[GAL4]/Cnt-E flies in Figure 5A. A significant increase is observed only for P[GAL4]201Y/Cnt-E. These flies are also exceptional because they show no increase in bout duration. Their activity pattern seems to be influenced by additional defects in the brain. The reduced number of bouts in HU-flies can be explained by the extremely large mean bout duration and variance in these flies.
In the present interpretation, the pattern generator consists of three components, bout initiation, bout termination, and the regulation of the internal structure of the bout. If the number of bouts is not directly affected by the MBs as we have just argued, their initiation must also be MB independent. As a consequence, an increase in bout duration entails shortening of the following pause (Fig. 5, cf. C and D). Also, the MBs seem not to influence the internal bout structure. Counts per bout closely parallel duration of bouts (Fig. 5, cf. B and C) and in four of the five groups, the minimum pause is little changed (Fig. 4). Therefore, the MBs seem to contribute specifically to bout termination. This does not necessarily imply that the MBs are an integral part of the pattern generator. For instance, they might exert their influence by regulating arousal, which in turn would influence bout duration.
Our results corroborate and extend the conclusions drawn by Huber and co-workers (Huber 1955, 1960; for review, see Huber 1963, 1965; Otto 1971; Wahdepuhl 1983) from their ablation and stimulation experiments in crickets and grasshoppers. In their experiments, the animals with MB lesions had an elevated locomotor activity, and some of them sang until completely exhausted. These authors also found that injury to the CC inhibited behavioral activity, and they proposed a simple model in which the MBs suppress and the CC up-regulates (initiates) it. More recently in Drosophila, the behavioral analysis of structural brain mutants in combination with genetic mosaics and deoxyglucose activity staining has confirmed the CC as a pre-motor control center for walking and flight (Strauss et al. 1992; Strauss and Heisenberg 1993; Ilius et al. 1994; Bausenwein et al. 1994). Whether the pattern generator for the bout structure of walking activity described here is specifically affected by the CC, and if so in which way, will have to be investigated.
Recently it has been reported that rats with hippocampal lesions walk more than control rats and these rats also exhibit a perturbation in the pattern of walking activity. Although the hippocampal formation has projections to the striatum, which is known to modulate locomotion, the mechanism by which this lesion-induced change occurs remains enigmatic (Whishaw et al. 1995, 1997). It will be interesting to find out whether similar behavioral modifications are found in mice with genetic lesions in the hippocampus (Mayford et al. 1996; Wilson and Tonegawa 1997).
Although Kenyon cells have many characteristic properties in common and may well be considered an isomorphic set of functionally equivalent neurons, it has long been known that they comprise different morphological types (for review, see Schürmann 1987). Recently, in Drosophila, the P[GAL4] enhancer-trap technique has permitted a convenient way to visualize and manipulate such fiber types (Yang et al. 1995; Ito et al. 1997). In the P[GAL4]H24/Cnt-E flies only a small subset of Kenyon cell fibers of the γ-lobe (γ-fibers), while in the P[GAL4]17D/Cnt-E flies only α/β-fibers and no γ-fibers express the tetanus toxin gene (Fig. 1). One might expect to find behavioral impairments to be defective in only one and not the other of these two groups of flies. However, our data provide no evidence that the suppressive effect of the MBs on walking might be associated with only one and not the other type of Kenyon cells. It is possible that locomotion is similarly affected by the different MB subsystems. Further investigations with additional P[GAL4] enhancer-trap lines and with different behavioral impairments will be needed. Moreover, we did not investigate whether the walking performance of individual mbm1 or HU flies might be correlated with their structural impairments. No such correlation had been observed for olfactory learning with mbm1 (Heisenberg et al. 1985).
The only other MB function demonstrated as unambiguously as the one discussed here is the impairment of olfactory learning and memory. How are these two behavioral effects related? What do they have in common? Obviously, the answer to these questions requires further behavioral experiments. Connolly et al. (1996) have shown that Gs signaling in Kenyon cells is necessary for odor learning by expressing a constitutively active Gsα protein subunit in Kenyon cells. Preliminary experiments with the same effector gene and the three P[GAL4] lines of the present study indicate that Gs signaling is also important for the suppression of walking activity (J.-R. Martin, R. Ernst, and M. Heisenberg, in prep.). In addition to more refined methods of intervention, other kinds of behavioral paradigms need to be exploited.
Acknowledgments
We are indebted to K. Kaiser and T. Raabe for generously providing us the GAL4 enhancer trap-lines and S. Sweeney and C. O’Kane for the UAS-tetanus toxin line (Cnt-E). We are also indebted to J. Thierer and H. Niemann for the tetanus toxin antibody and to K. Oechsner and H. Kaderschabek for excellent technical support. We are especially grateful to Markus Reif for helpful discussion on statistical analysis of the data, and T. Zars for critical reading of the manuscript. J.-R.M. was supported by the Pasteur Institute, Paris, France, and M.H. by grants of the Deutsche Forschungsgemeinschaft (He 986) and Fonds der Chemischen Industrie.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Bausenwein B, Müller NR, Heisenberg M. Behavior-dependent activity labeling in the central complex of Drosophila during controlled visual stimulation. J Comp Neurol. 1994;340:255–268. doi: 10.1002/cne.903400210. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Buchner E, Bader R, Buchner S, Cox J, Emson PC, Flory E, Heizmann CW, Hemm S, Hofbauer A, Oertel WH. Cell-specific immuno-probes for the brain of normal and mutant Drosophila melanogaster. I. Wildtype visual system. Cell Tissue Res. 1988;253:357–370. doi: 10.1007/BF00222292. [DOI] [PubMed] [Google Scholar]
- Burnet B, Burnet L, Connolly K, Williamson N. A genetic analysis of locomotor activity in Drosophila melanogaster. Heredity. 1988;61:111–119. [Google Scholar]
- Connolly JB, Roberts IJH, Armstrong JD, Kaiser K, Forte M, Tully T, O’Kane CJ. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- Connolly K. Locomotor activity in Drosophila II. Selection for active and inactive strains. Anim Behav. 1966;14:444–449. doi: 10.1016/s0003-3472(66)80043-x. [DOI] [PubMed] [Google Scholar]
- ————— Locomotor activity in Drosophila III. A distinction between activity and reactivity. Anim Behav. 1967;15:149–152. doi: 10.1016/s0003-3472(67)80026-5. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- ————— Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: A case study of the mushroom body miniature gene (mbm) Proc Natl Acad Sci. 1996;93:9875–9880. doi: 10.1073/pnas.93.18.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle JS, Sokolowski MB. Heredity of rover/sitter: Alternative foraging strategies of Drosophila melanogaster larvae. Heredity. 1987;59:73–83. [Google Scholar]
- Ferveur JF, Störtkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- Götz KG. Visual guidance in Drosophila. In: Siddiqui O, Babu P, Hall LM, Hall JC, editors. Development and neurobiology of Drosophila. New York, NY: Plenum Press; 1980. pp. 391–407. [Google Scholar]
- ————— . Search and choice in Drosophila. In: Singh RN, Strausfeld NJ, editors. Neurobiology of sensory systems. New York, NY: Plenum Press; 1989. pp. 139–153. [Google Scholar]
- Hall JC. Control of male reproductive behavior by the central nervous system of Drosophila: Dissection of a courtship pathway by genetic mosaics. Genetics. 1979;92:437–457. doi: 10.1093/genetics/92.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Tripping along the trail to the molecular mechanisms of biological clocks. Trends Neurosci. 1995;18:230–240. doi: 10.1016/0166-2236(95)93908-g. [DOI] [PubMed] [Google Scholar]
- Hanesch U, Fischbach K-F, Heisenberg M. Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res. 1989;257:343–366. [Google Scholar]
- Heisenberg M, Böhl K. Isolation of anatomical brain mutants of Drosophila by histological means. Z Naturforsh. 1979;34:143–147. [Google Scholar]
- Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- Howse PE. Design and function in the insect brain. In: Brown LB, editor. Experimental analysis of insect behavior. Berlin, Germany: Springer; 1974. pp. 180–194. [Google Scholar]
- Huber F. Sitz und Bedeutung nervöser Zentren für Instinkthandlungen beim Männchen von Gryllus campestris L. Z Tierpsychol. 1955;12:12–48. [Google Scholar]
- ————— Untersuchungen über die Funktion des Zentralnervensystems und insbesondere des Gehirnes bei der Fortbewegung und der Lauterzeugung der Grillen. Z vergl Physiol. 1960;44:60–132. [Google Scholar]
- ————— . The role of the central nervous system in Orthoptera during the co-ordination and control of stridulation. In: Busnel RG, editor. Acoustic behaviour of animals. Amsterdam, The Netherlands: Elsevier; 1963. pp. 440–488. [Google Scholar]
- ————— . Brain controlled behaviour in Orthopterans. In: Treherne JE, Beament JWL, editors. The physiology of the insect central nervous system. London, UK: Academic Press; 1965. pp. 233–246. [Google Scholar]
- Ilius M, Wolf R, Heisenberg M. The central complex of Drosophila melanogaster is involved in flight control: Studies on mutants and mosaics of the gene ellipsoid-body-open. J Neurogenet. 1994;9:189–206. doi: 10.3109/01677069409167279. [DOI] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlis L. An analysis of temporal patterning of pecking in chicks. Behavior. 1977;63:1–70. [Google Scholar]
- Mayford M, Baranes D, Podsypanina K, Kandel ER. The 3′-untranslated region of CaMKIIα is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc Natl Acad Sci. 1996;93:13250–13255. doi: 10.1073/pnas.93.23.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan MJ, Wilson R. Locomotor activity in the Tyr-1 mutant of Drosophila melanogaster. Behav Genet. 1987;17:503–512. doi: 10.1007/BF01073117. [DOI] [PubMed] [Google Scholar]
- Menzel R, Erber R, Masuhr T. Learning and memory in the honeybee. In: Browne LB, editor. Experimental analysis of insect behavior. Berlin, Germany: Springer; 1974. pp. 195–217. [Google Scholar]
- Niemann H, Blasi J, Jahn R. Clostridial neurotoxins: New tools for dissecting exocytosis. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- O’Dell KMC, Burnet B. The effect on locomotor activity and reactivity of the hypoactive and inactive mutations of Drosophila melanogaster. Heredity. 1988;61:199–207. [Google Scholar]
- O’Dell KMC, Armstrong JD, Yang MY, Kaiser K. Functional dissection of the Drosophila mushroom bodies by selective feminization of genetically defined subcompartiments. Neuron. 1995;15:55–61. doi: 10.1016/0896-6273(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Otto D. Untersuchungen zur zentralnervösen Kontrolle der Lauterzeugung von Grillen. Z vergl Physiol. 1971;74:227–271. [Google Scholar]
- Pereira HS, Sokolowski MB. Mutations in the larval foraging gene affect adult locomotory behavior after feeding in Drosophila melanogaster. Proc Natl Acad Sci. 1993;90:5044–5046. doi: 10.1073/pnas.90.11.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. Etablierung und Charakterisierung Pilzkörper-spezifischer GAL4-enhancer-trap-Linien in Drosophila melanogaster. Diplomarbeit. Würzburg, Germany: University of Würzburg; 1997. [Google Scholar]
- Prokop A, Technau GM. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev Biol. 1994;161:321–337. doi: 10.1006/dbio.1994.1034. [DOI] [PubMed] [Google Scholar]
- Sachs L. Angewandte statistik. 7th ed. Berlin, Germany: Springer-Verlag; 1992. Statistische Entscheidungstechnik; pp. 29–305. [Google Scholar]
- Schürmann FW. The architecture of the mushroom bodies and related neuropils in the insect brain. In: Gupta AP, editor. Arthropod brain. New York, NY: Wiley-Interscience; 1987. pp. 231–264. [Google Scholar]
- Sibly RM, Nott HMR, Fletcher DJ. Splitting behavior into bouts. Anim Behav. 1990;39:63–69. [Google Scholar]
- Slater PJB. The temporal pattern of feeding in the zebra finch. Anim Behav. 1974;22:506–515. [Google Scholar]
- Slater PJB, Lester NP. Minimising errors in splitting behavior into bouts. Behavior. 1982;79:153–161. [Google Scholar]
- Sokolowski MB. Foraging strategies of Drosophila melanogaster: A chromosomal analysis. Behav Genet. 1980;10:291–302. doi: 10.1007/BF01067774. [DOI] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R, Hanesch U, Kinkelin M, Wolf R, Heisenberg M. No-bridge of Drosophila melanogaster: Portrait of a structural brain mutant of the central complex. J Neurogenet. 1992;8:125–155. doi: 10.3109/01677069209083444. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- van der Kloot WG, Williams CM. Cocoon construction by the Cecropia silkworm. Behavior. 1953;5:141–174. [Google Scholar]
- Wahdepuhl M. Control of grasshopper singing behavior by the brain responses to electrical stimulation. Z Tierpsychol. 1983;63:173–200. [Google Scholar]
- Wahdepuhl M, Huber F. Elicitation of singing and courtship movements by electrical stimulation of the brain of the grasshopper. Naturwissenschaften. 1979;66:320–322. [Google Scholar]
- Wilson MA, Tonegawa S. Synaptic plasticity, place cells and spatial memory: study with second generation knockouts. Trends Neurosci. 1997;20:102–106. doi: 10.1016/s0166-2236(96)01023-5. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, McKenna JE, Maaswinkel H. Hippocampal lesions and path integration. Curr Opin Neurobiol. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Cassel J-C, Jarrard LE. Rats with fimbria-fornix lesions display a place response in a swimming pool: A dissociation between getting there and knowing where. J Neurosci. 1995;15:5779–5788. doi: 10.1523/JNEUROSCI.15-08-05779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]