Abstract
Background and Aims. Deoxyribonuclease I (DNaseI) is an endonuclease that facilitates chromatin breakdown and promotes susceptibility to autoimmune disorders. The aim of current study was to investigate serum DNase I activity in patients with inflammatory bowel diseases (IBD). Patients and Methods. A cohort of 110 IBD patients was evaluated, aged 35 ± 12 years, 77 with Crohn's disease (CD) and 33 with ulcerative colitis (UC). 50 SLE patients and 50 healthy blood donors were examined as control groups. Results. DNase I activity in IBD patients was significantly lower than in healthy individuals, but higher than in SLE patients (P < .0001). Patients with UC showed higher DNase I activity than CD patients, P = .21. DNase I activity in female patients with IBD was significantly lower than in males, P = .024; however, no differences in DNase I activity were found in relation to gender in healthy individuals. DNase I activity has shown a strong negative correlation with the serum concentration of anti-nucleosomal antibodies in the autoimmune (SLE + IBD) cohort, as well as in the separate IBD cohort. Conclusions. Reduced serum DNase I activity probably has pathogenetic consequences in IBD. Induction of autoantibodies towards nucleosomes could be a reflection of impaired DNase I activity.
1. Introduction
Autoimmune diseases affect more than 5% of the population in developed countries and are an important cause of morbidity and mortality [1]. They are often of multifactorial etiology and are characterized by the presence of autoantibodies and/or autoreactive lymphocytes. Different mechanisms for the pathogenesis of autoimmune diseases have been suggested, including deregulation of the immune system which leads to loss of self-tolerance; molecular mimicry mechanisms; idiotypical cross-reactions; defective apoptosis of antigen-presenting cells; aberrant B-cell receptor-mediated feedback; defective removal of cellular debris after cell death.
The last mentioned removal of cellular debris during cell death is important for preserving autoimmunity to cellular, especially nuclear, antigens. The degradation of DNA into nucleosomal units during cell death is mediated by enzymes called deoxyribonucleases (DNases) [2]. The two main types of DNase found in humans are known as DNase I and DNase II. DNase I is an endonuclease that participates in degradation of DNA that was not efficiently engulfed [3]. It is secreted into most body fluids by a variety of endocrine and exocrine glands [3–5], and its physiological functions are supposed to be degrading the DNA of dietary substances within the intestinal tract (to supply an organism with oligonucleotides) and suppressing anti-DNA autoimmunity by degrading chromatin released from dying cells [6, 7]. Deficiency in DNase I enzyme, and the resulting difficulty of removing DNA from nuclear antigens, promotes susceptibility to autoimmune disorders [7]. Failure of apoptosis leads to the development of a lupus-like syndrome [8]. DNase I deficiency was also found in patients with systemic lupus erythematosus (SLE), correlating with high titers of antibodies against nucleosomal antigens [9–11]. It has been shown that SLE patients with a single nucleotide mutation in the gene for DNase I exhibit 10–40% of the DNase I activity that healthy individuals normally display [11].
We have hypothesized that altered DNase I activity could be related to the immunopathogenesis of autoimmune disorders that are distinct from SLE. We focused, in particular, on inflammatory bowel diseases (IBD). IBD, of which there are two main types—Crohn's disease (CD) and ulcerative colitis (UC)—may affect between 20,000 to 40,000 people in the Czech Republic [12]. The pathogenesis of IBD is only partially understood yet. Various environmental and host factors, some genetic and immunological, are involved. During the last decade, several new pathways have been suggested for the immunopathogenesis of IBD. It is proposed, for example, that in the antigen-rich environment of the gut, lymphocyte activation and expansion must be highly controlled; this is ensured by T-lymphocyte apoptosis. In IBD patients, Fas/FasL activation has been proven to diminish apoptosis [13, 14], and defective apoptosis seems to be a relevant pathogenetic mechanism in chronic IBD.
The aims of our study were to (i) investigate serum DNase I activity in patients with CD and UC; (ii) compare values from IBD patients with SLE patients and healthy individuals; (iii) investigate a possible link between serum DNase I activity and serum levels of antibodies against nuclear autoantigens in IBD patients.
2. Material and Methods
2.1. Patients
We evaluated a cohort of 110 IBD patients, in whom diagnosis of IBD was established in accordance with standardized sets of clinical, endoscopic, and/or radiological and histopathological criteria [15]. Calculated mean age in the cohort was 35 ± 12 years. Seventy-seven patients were diagnosed with CD, and 33 patients had UC. Sixty-nine patients were women and 41 were men. All of the IBD patients examined had severely active disease and have had an inadequate response to previous conventional therapy.
Two control age-matched groups were examined: 50 SLE patients (40 females, 10 males) and 50 healthy blood donors (equal numbers of women and men).
Blood samples were drawn before the initiation of biological treatment by infliximab or adalimumab. In 45 randomly selected IBD patients, an additional blood sample was taken after the induction phase of treatment, that is, six weeks later. All SLE patients fulfilled the diagnostic criteria of the American College of Rheumatology [16]. Nine had an active disease, in 41 clinical remission was achieved. Forty-six (92%) of SLE patients had proven lupus nephritis. Samples from healthy blood donors were taken before blood donation.
2.2. Ethical Aspects
The study was approved by the Institutional Ethical Committee. The purpose and procedures of the study were explained to participants, who signed informed consent forms.
2.3. Laboratory Examinations
Serum was used for DNase I activity and serum IgG antinucleosomal antibodies measurement. In patients with IBD who were repeatedly examined during the anti-inflammatory biological treatment, C-reactive protein (CRP) was measured as an acute phase reactant, to assess the effectiveness of treatment. Blood samples were taken from the cubital vein and centrifuged for 10 minutes at ambient temperature and 1300 g. Separated serum aliquots were frozen at −80°C. The frozen serum samples were thawed once on ice before analysis. All determinations were done in two replicates. Samples were measured together to preclude the possibility of errors derived from interassay variability.
Serum DNase I activity was determined by a novel validated standardized enzyme-linked immunosorbent assay (DNase Activity, Orgentec, Germany). In short, samples were added to a DNA-coated 96 well microplate and incubated for 1 hour at 37°C. Horseradish peroxidase (HRP) conjugated anti-DNase I antibodies colorare added and incubated for 15 minutes at room temperature to react with the remaining uncleaved DNA. The intensity of the color developed during the enzymatic reaction (hydrolysis of HRP-substrate, 3,3′,5,5′-tetramethyl-benzidine (TMB) and acid addition) was measured photometrically at 450 nm using a MRXII (Dynatech, UK) photometer and analyzed it using the software Revelation (Dynatech, UK). A standard series, as well as positive and negative controls, were included in the kit. The amount of color was inversely proportional to the amount of DNase activity. 75% was set as a cutoff value for DNase I activity (which corresponds to the 25% activity reduction).
Antinucleosomal antibodies in the IgG isotype were detected using a validated, standardized, enzyme-linked immunosorbent assay (Anti-Nucleosome, Orgentec, Germany). Values below 20 U/mL were considered negative according to manufacturer's recommendations. CRP was measured using nephelometry (BNII, Siemens Healthcare Diagnostics, Germany). The reference range 0–5 mg/L was used per the manufacturer's recommendations.
2.4. Statistical Analysis
Prior to running experiments, required sample size was calculated by Statistica Power Analysis (StatSoft Inc., USA).
Statistical analysis was performed using the software Statistica CZ 7.0 (StatSoft Inc., USA). Different groups were compared using the Mann-Whitney U-test or a two-sided Kruskal-Wallis nonparametric test. The Spearman rank test was used to identify correlations between variables. The threshold for significance was set at P < .05.
3. Results
3.1. DNase I Activity in IBD
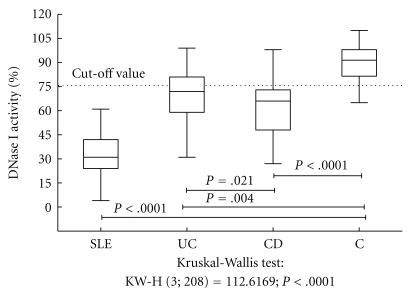
DNase I activity in IBD patients (63 ± 19%) was significantly lower than that of healthy blood donors (92 ± 11%), but higher than that of SLE patients (36 ± 19%), P < .0001. Patients with UC showed higher DNase I activity (74 ± 19%) than CD patients (61 ± 18%); see Figure 1. Only 21 of 110 IBD patients (19%) had DNase I activity above the reference cutoff value of 75%.
Figure 1.
DNase I activity in different groups of patients. DNase I: deoxyribonuclease I; SLE: systemic lupus erythematosus; UC: ulcerative colitis; CD: Crohn's disease; C: healthy controls. The boxplot represents the lower and upper quartiles, the horizontal line represents the median, and the whiskers represent the sample minimum and maximum.
3.2. DNase I Activity and Gender
DNase I activity in female patients with IBD (52 ± 21%) was significantly lower than in male patients (69 ± 21%), P = .024. Similarly, in SLE patients, female DNase I activity (31 ± 14%) was lower than male DNase I activity (40 ± 21%), P = .032. However, in healthy individuals, no differences in DNase I activity were found in relation to gender; see Figure 2.
Figure 2.
DNase I activity and gender. DNase I: deoxyribonuclease I; SLE: systemic lupus erythematosus; IBD: inflammatory bowel diseases; CTRL: healthy controls.
3.3. DNase I Activity and Inflammation
DNase I activity did not oscillate during anti-inflammatory biological treatment with infliximab or adalimumab. In addition, DNase I activity did not correlate with serum CRP (r = 0.128, P = .654). Each patient treated had stable DNase I activity during the entire induction phase of biological therapy (see Table 1).
Table 1.
Dynamics of DNase I activity and serum CRP concentration in IBD patients during the induction phase of biological treatment (n = 45).
| W0 | W6 | P-value* | |
|---|---|---|---|
| DNase I activity (%) | 67 (54,75) | 68 (51,78 ) | .724, NS |
| CRP (g/L) | 31 (24, 39) | 5 (1, 9) | <.001 |
Data are expressed as medians (interquartile range).
W0: week 0 of biological treatment; W6: week 6 of biological treatment; DNase I: deoxyribonuclease I; CRP: C-reactive protein; NS: nonsignificant
*Mann-Whitney U-test.
3.4. DNase I Activity and Antinucleosomal Antibodies
DNase I activity has shown a strong negative correlation with the serum concentration of antinucleosomal antibodies in the autoimmune (SLE + IBD) cohort, as well as in the separate IBD cohort (see Figures 3(a) and 3(b)). However it must be emphasized that concentrations of antinucleosomal autoantibodies in IBD patients did not reach as high values as SLE patients (P = .001).
Figure 3.
Correlation of DNase I activity with the level of antinucleosomal antibodies. DNase I: deoxyribonuclease I; Ab: antibodies.
4. Discussion
To our knowledge, this is the first report on serum DNase I activity in IBD patients. We have found that DNase I activity is significantly decreased in IBD patients, though the reduction was not as great as in SLE patients. CD patients had lower values than UC patients, while female patients had lower values than male patients. DNase I activity was not affected by anti-inflammatory processes during biological treatment of IBD. Similarly to SLE, DNase I activity in IBD patients has shown a negative correlation with the occurrence of antinucleosomal autoantibodies.
A deficiency in DNase I enzyme, as well as the resulting difficulty of removing DNA from nuclear antigens, promotes susceptibility to autoimmune disorders. DNase I activity is often abnormally low in SLE patients. Low DNase I activity has already been proven in different systemic and organ specific autoimmune diseases: SLE [17], Sjögren's disease [18] and thyroid autoimmunity [19].
The immunopathogenesis of IBD is not clear yet. Our results suggest that imbalance of the events that control cleanup of the cell debris may contribute to the pathogenesis of IBD. One might expect lower DNase I activity in IBD patients with active local and/or systemic inflammation. However, we did not find a significant difference between the DNase I activity of our IBD patient groups before and after the induction phase of anti-inflammatory biological treatment. This indicates that reduced DNase I activity could be inherent, independent of the inflammatory processes and cascades. The true reason for reduced endonuclease activity has not been determined. A genetic mutation of the DNASEI gene has been reported in SLE patients [20]. To date, no reports about this mutation in IBD patients were published. We are not able to claim if subjects with reduced DNase I activity may be more susceptbile to IBD compared with the normal activity levels harboring ones. However, genetic heterogeneity in the DNase I gene affecting its in vivo activity has been less demonstrated as yet.
Interestingly, CD patients from our IBD cohort have shown DNase I activity that is lower than that of UC patients. This could be explained by the different etiopathogenesis of these two (CD and UC) inflammatory entities.
Our results did not validate Martinez-Valle's finding that men have lower DNase I activity than women [11]. On the contrary, in our IBD and SLE cohorts, DNase I activity was lower in female patients. We suggest that defective cellular waste disposal in females with lower DNase I activity leads to the release of nuclear components (and prolonged exposure to them) and that lower DNase I activity in women could be an additional feature of sexual dimorphism of the immune system. On the other hand, DNase I activity in healthy females did not differ from the healthy males. This could reflect genetic predisposition in affected females.
As is known, DNA itself is not immunogenic, and only when bound to proteins (i.e., histones) it can induce immune response [21–23]. Therefore, DNA-histone complexes (nucleosomes) are proposed to be the genuine autoantigens in autoimmune diseases. Sallai et al. firstly described a negative correlation between DNase I activity and antinucleosomal antibodies in people afflicted with SLE [21]. In support of an association between antinucleosomal antibody production and DNase I activity, we found that IBD patients with positive antinucleosomal antibodies had significantly lower DNase I activity than their counterparts with low concentrations of these autoantibodies. The balance between levels of circulating nucleosomes, DNase I activity and the formation of antinucleosomal autoantibodies is a dynamic process and, therefore, obtaining clear proof of the association between these three would require simultaneous investigation of DNase I activity and circulating nucleosomes and antinucleosomal antibody levels.
Our study has some limitations. The most important limitation concerns the constitution of our IBD cohort. All of the 110 IBD patients had active severe disease, with the necessity of the biological treatment. Therefore, our cohort does not represent a typical IBD group of patients, but their most serious fraction. Secondly, some of our IBD patients have shown positive antinucleosomal antibodies, which are not common in IBD. We suggest that in such severe inflammation and coincidental low DNase I activity, apoptotic cells are not cleared sufficiently and they deviate from a well-organized apoptotic process into secondary necrosis. This could result in an altered self-form of chromatin unmasking, which is able to provide the same danger signal as infectious nonself structures and could be a target structure for induced antinucleosomal autoantibodies.
In summary, our results indicate that reduced serum DNase I activity is common in severe IBD and that decreased DNase I activity could be one of the pathogenetic factors in IBD development.
Acknowledgments
This work was supported by Grant GAUK 69810 from Charles University in Prague. The authors are grateful to Marcela Jarolímová for excellent help in handling samples and Iva Staščáková for skilled technical assistance.
References
- 1.Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. Journal of Autoimmunity. 2010;34(3):J168–J177. doi: 10.1016/j.jaut.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391(6662):43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 3.Napirei M, Ricken A, Eulitz D, Knoop H, Mannherz HG. Expression pattern of the deoxyribonuclease 1 gene: lesson from the Dnase1 knockout mouse. Biochemical Journal. 2004;380(3):929–937. doi: 10.1042/BJ20040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacks SA. Deoxyribonuclease I in mammalian tissues. Specificity of inhibition by actin. The Journal of Biological Chemistry. 1981;256(6):2644–2648. [PubMed] [Google Scholar]
- 5.Takeshita H, Yasuda T, Nakajima T, Hosomi O, Nakashima Y, Kishi K. Mouse deoxyribonuclease I (DNase I): biochemical and immunological characterization, cDNA structure and tissue distribution. Biochemistry and Molecular Biology International. 1997;42(1):65–75. doi: 10.1080/15216549700202441. [DOI] [PubMed] [Google Scholar]
- 6.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Möröy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nature Genetics. 2000;25(2):177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 7.Napirei M, Wulf S, Mannherz HG. Chromatin breakdown during necrosis by serum Dnase1 and the plasminogen system. Arthritis and Rheumatism. 2004;50(6):1873–1883. doi: 10.1002/art.20267. [DOI] [PubMed] [Google Scholar]
- 8.Gaipl US, Sheriff A, Franz S, et al. Inefficient clearance of dying cells and autoreactivity. Current Topics in Microbiology and Immunology. 2006;305:161–176. doi: 10.1007/3-540-29714-6_8. [DOI] [PubMed] [Google Scholar]
- 9.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134(4):587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez Valle F, Balada E, Ordi-Ros J, Vilardell-Tarres M. DNase 1 and systemic lupus erythematosus. Autoimmunity Reviews. 2008;7(5):359–363. doi: 10.1016/j.autrev.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Valle F, Balada E, Ordi-Ros J, Bujan-Rivas S, Sellas-Fernandez A, Vilardell-Tarres M. DNase 1 activity in patients with systemic lupus erythematosus: relationship with epidemiological, clinical, immunological and therapeutical features. Lupus. 2009;18(5):418–423. doi: 10.1177/0961203308098189. [DOI] [PubMed] [Google Scholar]
- 12.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World Journal of Gastroenterology. 2006;12(38):6102–6108. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ina K, Itoh J, Fukushima K, et al. Resistance of Crohn’s disease T cells to multiple apoptotic signals is associated with a Bcl-2/Bax mucosal imbalance. Journal of Immunology. 1999;163(2):1081–1090. [PubMed] [Google Scholar]
- 14.Itoh J, de la Motte C, Strong SA, Levine AD, Fiocchi C. Decreased Bax expression by mucosal T cells favours resistance to apoptosis in Crohn’s disease. Gut. 2001;49(1):35–41. doi: 10.1136/gut.49.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. The Canadian Journal of Gastroenterology. 2005;19(supplement A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism. 1997;40(9):p. 1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 17.Kim I, Hur NW, Shin HD, Park BL, Cheong HS, Bae SC. Associations of DNase IV polymorphisms with autoantibodies in patients with systemic lupus erythematosus. Rheumatology. 2008;47(7):996–999. doi: 10.1093/rheumatology/ken125. [DOI] [PubMed] [Google Scholar]
- 18.Belguith-Maalej S, Hadj-Kacem H, Kaddour N, Bahloul Z, Ayadi H. DNase1 exon2 analysis in Tunisian patients with rheumatoid arthritis, systemic lupus erythematosus and Sjögren syndrome and healthy subjects. Rheumatology International. 2009;30(1):69–74. doi: 10.1007/s00296-009-0917-4. [DOI] [PubMed] [Google Scholar]
- 19.Dittmar M, Bischofs C, Matheis N, Poppe R, Kahaly GJ. A novel mutation in the DNASE1 gene is related with protein instability and decreased enzyme activity in thyroid autoimmunity. Journal of Autoimmunity. 2009;32(1):7–13. doi: 10.1016/j.jaut.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Yasutomo K, Horiuchi T, Kagami S, et al. Mutation of DNASE1 in people with systemic lupus erythematosus. Nature Genetics. 2001;28(4):313–314. doi: 10.1038/91070. [DOI] [PubMed] [Google Scholar]
- 21.Sallai K, Nagy E, Derfalvy B, Müzes G, Gergely P. Antinucleosome antibodies and decreased deoxyribonuclease activity in sera of patients with systemic lupus erythematosus. Clinical and Diagnostic Laboratory Immunology. 2005;12(1):56–59. doi: 10.1128/CDLI.12.1.56-59.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urowitz MB. Association of antinucleosome antibodies with disease flare in serologically active clinically quiescent patients with SLE. Future Rheumatology. 2007;2(2):139–141. [Google Scholar]
- 23.Amoura Z, Koutouzov S, Chabre H, et al. Presence of antinucleosome autoantibodies in a restricted set of connective tissue diseases: antinucleosome antibodies of the IgG3 subclass are markers of renal pathogenicity in systemic lupus erythematosus. Arthritis and Rheumatism. 2000;43(1):76–84. doi: 10.1002/1529-0131(200001)43:1<76::AID-ANR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]