Abstract
Research on medullary thyroid carcinoma (MTC) over the last 55 years has led to a good understanding of the genetic defects and altered molecular pathways associated with its development. Currently, with the use of genetic testing, patients at high risk for MTC can be identified before the disease develops and offered prophylactic treatment. In cases of localized neck disease, surgery can be curative. However, once MTC has spread beyond the neck, systemic therapy may be necessary. Conventional chemotherapy has been shown to be ineffective; however, multikinase inhibitors have shown promise in stabilizing disease, and this year will probably see the approval of a drug (Vandetanib) for advanced unresectable or metastatic disease, which represents a new chapter in the history of MTC. In this paper, we explore newly understood molecular pathways and the most promising emerging therapies that may change the management of MTC.
1. Introduction
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor derived from parafollicular cells of the thyroid gland [1]. MTC represents less than 3% of thyroid carcinomas in the United States [2]. The first description of its major histological features and characterization as a separate entity was done in 1959 by Hazard et al. [3]. It was then rapidly recognized that this carcinoma had distinctive clinical features, in that MTC was found to be associated with pheochromocytomas and other tumors, an association now known as multiple endocrine neoplasia type 2 (MEN2) [4]. The identification of familial cases led to the conclusion that many MTCs were probably hereditary [5]. In 1966, MTC was found to arise from the calcitonin-secreting parafollicular cells [6]. Subsequently, calcitonin provocation tests with calcium and/or pentagastrin were used to identify individuals susceptible to familial MTC, and those individuals were offered prophylactic thyroidectomy [7].
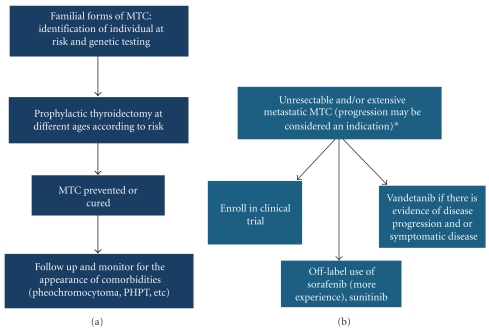
Activating mutations of the Rearranged during Transfection (RET) proto-oncogene were described for the first time in patients with familial forms of MTC in 1993 [8, 9]. Since then, several germline RET proto-oncogene mutations have been found in almost 100% of hereditary MTCs. Additionally, somatic RET proto-oncogene mutations have been found in approximately 40% of patients with sporadic MTC [10, 11]. These discoveries created new paradigms for the management of MTC: (1) the identification of germline RET proto-oncogene mutation carriers would allow the removal of the thyroid cells at risk for transformation early in life (this paradigm is perhaps the most perfect example of primary cancer prevention in humans to date), (2) the identification of several hidden familial medullary thyroid cancers [12], and (3) the abnormally activated RET gene might become a target to treat patients with advanced sporadic and hereditary MTC. Our goal in this paper is to describe the molecular pathways associated with MTC tumorigenesis and emerging therapies against this disease (Figure 1).
Figure 1.
From prevention of MTC to treatment of incurable disease. Ideal approach to familial forms of MTC (a) versus treatment options in unresectable and/or extensive metastatic disease and/or progression. (b) *Every patient should be evaluated in an individual basis, and the decision to treat as well as the indication is not always clear cut as one must take into consideration quality of life issues and adverse events associated with treatment.
2. MTC and the RET Proto-Oncogene
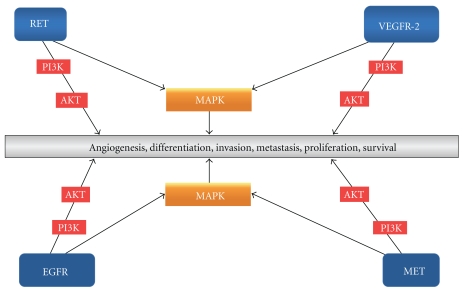
Autonomous cell growth is the defining feature of all benign or malignant tumors. Malignant neoplasms have the capacity to invade the surrounding normal tissue and metastasize to distant sites. Molecules that are responsible for growth and other fundamental cell functions are frequently mutated in cancers. An example of such molecules is the tyrosine kinase (TK) receptors (Figure 2). TK receptors are membrane-spanning proteins with large N-terminal extracellular domains that act as ligand-binding sites and intracellular domains that catalyze the transfer of the γ phosphate of adenosine-5′-triphosphate (ATP) to hydroxyl groups of tyrosines of target proteins. TKs control a wide range of fundamental processes of cells such as the cell cycle, proliferation, angiogenesis, differentiation, motility, apoptosis, and survival.
Figure 2.
Simplified schematic representation of some of the TKs and pathways involved in MTC carcinogenesis as well normal physiology. These TKs represent important targets of TKIs. Written in the gray box are the consequences of the activation of multiple pathways and not of any one in particular.
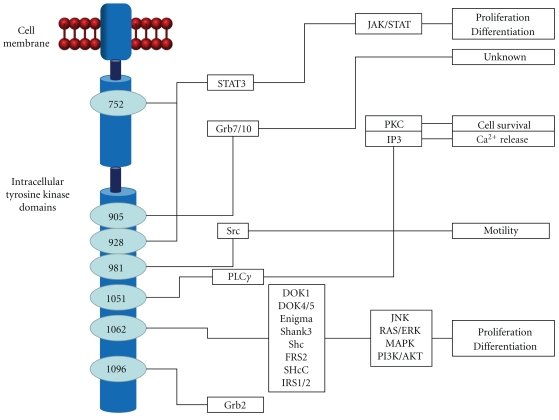
The RET proto-oncogene is located in chromosome 10q11.2 [13]. The gene has 21 exons [14] and codes for a receptor TK [15]. The RET receptor is a transmembrane protein constituted by extracellular, transmembrane, and cytoplasmatic domains. The extracellular domain has a stretch of approximately 100 amino acids that are similar to members of the cadherin family of Ca2+dependent cell adhesion molecules [16]. The binding of calcium to this cadherin-like domain is needed for conformational changes necessary for the interaction with different glial cell line-derived neurotrophic factor ligand family members (GDNF, neurturin, artemin, and persephin) [17]. These ligands in conjunction with a ligand-specific coreceptor (GFRα 1–4) activate RET [18]. These ligands or coreceptors are not always needed for RET activation [19]. Following RET activation, specific tyrosine residues are phosphorylated. These residues serve as docking sites for adaptor proteins that link the receptor to the main signal transduction pathways. Different activated sites trigger the activation of different pathways. For instance, tyrosine 1015 is a binding site for phospholipase C that activates protein kinase C (PKC). Other examples are given by the phosphorylated γ tyrosine 981 which is responsible for Src activation upon RET engagement [20] and the phosphorylation of tyrosine 1062, several adaptor or effector proteins are recruited including Shc, FRS2, Dok family proteins, insulin receptor substrate 2, and Enigma [21]. Then, various pathways that regulate cell survival, differentiation, proliferation, and chemotaxis [20] are activated, including RAS-extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K)-Akt, p58 mitogen-activated protein kinase (MAPK), and Jun N-terminal kinase (JNK) [22] (Figure 3).
Figure 3.
A summary of the signaling pathway mediated by RET.
Mutated RET is expressed in derivatives of neural crest cells, including hereditary and sporadic MTC and pheochromocytoma [23]. These mutations are referred to as gain-of-function, because they lead to either a constitutively active TK or decreased specificity of the TK for its substrate [24].
3. RET Genotype-Phenotype Correlations
3.1. Sporadic MTC
Sporadic MTC constitutes 65% to 75% of MTC cases [25]. The most frequent clinical presentation is that of a thyroid nodule. Up to 75% of patients with palpable MTC have nodal metastases in the central and ipsilateral neck compartments, and 47% of patients with palpable MTC have nodal metastases in the contralateral neck [26]. Distant metastases frequently occur in the liver, lungs, and bones.
Somatic mutations occur in 30% to 40% of cases [10, 11]. Exon 16, codon 918 ATG → ACG mutation is the most common somatic mutation in sporadic MTC [27]. This mutation is associated with larger tumors and a more advanced disease stage at diagnosis [11].
3.2. Hereditary MTC
Hereditary MTC constitutes 25% to 35% of MTC cases [25]. Hereditary MTC is preceded by C-cell hyperplasia and is usually bilateral and multicentric [28]. Hereditary forms of MTC are caused by germline RET proto-oncogene mutations and occurs as part of the MEN2 syndromes. MEN2A is characterized by MTC in almost 100% of gene carriers, pheochromocytomas, and parathyroid tumors. The most common mutations in MEN2A occur in one of six cysteine residues (codons 609, 611, 618, 620, 630, and 634) in the RET extracellular domain. The most frequently mutated residue found in patients with MEN2A is cysteine 634, in which removal of one-half of an intramolecular disulfide bond allows formation of an intermolecular disulfide bond with a second mutant molecule, thus leading to constitutive receptor dimerization [29]. PI3K-Akt and MAPK pathways have been implicated in MEN2A [30].
There are three variants of the syndrome: (1) MEN2A with Hirschsprung disease, (2) MEN2A associated with cutaneous lichen amyloidosis, and (3) familial MTC, in which MTC is the only manifestation. Familial MTC RET-mutation affects the extracellular cysteine-rich region and the TK domain. This variant tends to be the least aggressive form of hereditary MTC.
MEN2B is the most distinctive and aggressive MEN2 syndrome. The most common mutations associated with MEN2B are M918T and A883F. These mutations, unlike MEN2A, are in the TK domain and lead to an activated monomeric form, thus altering substrate specificity [29]. The PI3K/Akt cascade has been shown to be important in the pathogenesis of MEN2B in cell lines [31].
4. TK Receptors Other Than RET Involved in MTC Tumorigenesis
4.1. Epidermal Growth Factor Receptor
The epidermal growth factor receptor (EGFR/HER-1/erbB1) is a TK receptor. It is one of four homologous transmembrane receptors (the others are HER-2/erbB-2, HER-3/erbB-3, and HER-4/erbB-4) that mediate the actions of different growth factors, such as epidermal growth factor, transforming growth factor-α, and neuregulins [32]. The binding of ligands to these receptors induces EGFR homo- and/heterodimer formation, kinase domain activation, and phosphorylation of specific tyrosine residue that serve as docking sites for molecules that lead to the activation of several cascades, including the MAPK and PI3K pathways [33].
EGFR oncogenic activation can occur due to several mechanisms: excess ligand or receptor expression, activating mutations, failure of inactivation, or transactivation through receptor dimerization [34]. To date, two major types of EGFR-targeting agents exists monoclonal antibodies and small-molecule ATP-competitive TK inhibitors (TKIs) [35, 36]. PKI166, a potent EGFR kinase inhibitor, also decreases RET autophosphorylation and signaling in cell extracts despite lacking an effect on RET kinase activity. PKI166 was tested in clinical trial in patients with MTC amongst others. However, due to liver toxicities the development of this drug was halted [37]. AEE788, another EGFR kinase inhibitor, inhibits RET-induced growth at concentrations below its half maximal inhibitory concentration (IC50) [38]. However, AEE788 does not have any active clinical trials in MTC patients. A study of 153 primary and metastatic MTC samples revealed that although EGFR mutations were rare, EGFR expression was higher in metastatic sites than in primary tumor sites [39]. MTC samples associated with RET 883 and 918 mutations had a significantly lower number of EGFR polysomes and a tendency toward less EGFR immunopositivity compared with samples associated with other RET mutations. Therefore, it is speculated that the most aggressive RET mutations are less dependent on EGFR activation, thereby explaining why EGFR inhibitors are less effective in codon 918-mutated cell lines than in codon 634-mutated cell lines.
4.2. Vascular Endothelial Growth Factor
The vascular endothelial growth factor (VEGF) family of growth factors stimulates angiogenesis, endothelial cell proliferation, migration, survival, and vascular permeability by various TK receptors: VEGFR-1, VEGFR-2, and VEGFR-3 [40]. There are several ligands for VEGFRs: VEGF-A (VEGF) binds to both VEGFR-1 and VEGFR-2; VEGF-B and placenta growth factor bind to only VEGFR-1; and VEGF-C and VEGF-D are specific ligands for VEGFR-3 [41].
Angiogenesis is one of the essential alterations in cell physiology that predispose to malignancy in many tumors, and it is fundamental in tumor growth and metastasis. Many molecules have been implicated as positive regulators of angiogenesis, including VEGF, hepatocyte growth factor, interleukin-8, and platelet-derived growth factor (PDGF). The major mediator of tumor angiogenesis is VEGF, which signals mainly through VEGFR-2. Activation of this receptor leads to a cascade of different pathways, including PLC γ-PKC-Raf-MEK-MAPK and PI3K-Akt [42]. Lymphangiogenesis is also involved in tumor biology, and since lymphatic vessels arise from blood vessels, some of the angiogenic mechanisms are also used in this process. VEGF-C and VEGF-D stimulate both angiogenesis and lymphangiogenesis and link both processes [43]. VEGFR-3 is expressed mainly in lymphatic endothelial cells and is thought to be primarily involved in lymphangiogenesis.
MTC has at least twofold expression when compared with normal thyroid tissue of VEGF and VEGF-R2 [44]. There is also an up to 20-fold increased expression of VEGF-C and VEGF-R3 in metastatic MTC [45]. Overexpression and activation of VEGFR-2 in MTC correlate with metastasis [39].
4.3. c-MET
The c-met (MET) proto-oncogene codes for the TK receptor of the hepatocyte growth factor [46]. MET is an important factor in tumorigenesis. Deregulated activation of MET confers unrestricted proliferative, antiapoptotic, cell motility/migration, invasive, metastatic, and angiogenenic properties to cancer cells [47]. Silencing the endogenous MET proto-oncogene, which is overexpressed in tumor cells, has been proven to impair the invasive growth in vitro, to decrease the generation of metastases in vivo, and to promote the regression of already established metastases [48]. MET and hepatocyte growth factor coexpression has been seen in a subset of MTC tumors and is associated with multifocality in MTC [49].
5. Targeted Therapy
Different TKs and pathways are abnormally activated in MTC cells. Inhibiting only one receptor may induce other TKs compensatory activation [50]. Therefore, simultaneous inhibition of different activated TKs may be the best way to approach MTC (Table 1) [51]. To date, systemic targeted therapy for MTC has been administered in the context of clinical trials or has consisted of off-label use of drugs approved for other solid tumors. In this section, we review the most promising TK inhibitors against MTC.
Table 1.
Some of the TKIs currently used for the treatment of MTC in clinical trials and off-label.
| Drug | Oral daily dose | Major targets |
|---|---|---|
| Vandetanib | 100–300 mg | VEGFR-1, VEGFR-2, VEGFR-3, RET, EGFR |
| Sorafenib | 400–800 mg | RET, VEGFR-2, VEGFR-3, Flt-3, PDGFRβ, KIT, RAF-1 |
| Sunitinib | 37.5 mg every day 50 mg daily 4 weeks on 2 weeks off |
VEGFR-2, PDGFRβ, KIT, RET |
| Cabozantinib (XL184) | 125–175 mg/day | MET, VEGFR-2, RET, KIT, Flt-3, Tie-2 |
| E7080 | 24 mg | VEGFR-2, VEGFR-3, VEGFR-1, KIT, FGFR1, PDGFR, EGFR |
5.1. Vandetanib
Vandetanib is a 4-anilinoquinazoline that is available as an oral daily agent. It inhibits VEGFR-2, VEGFR-3, RET, and to a lesser extent EGFR and VEGFR-1 [52]. The 4-anilinoquinazoline docks to the ATP binding pocket of RET kinase, inhibiting it [53].
At pharmacologically relevant doses, vandetanib inhibits tumor cell proliferation, survival, and angiogenesis without leading to direct cytotoxic effects on tumor or endothelial cells [52]. In 2002, vandetanib was shown to inhibit the kinase activity of NIH-RET/C634R (MEN2A) and NIH-RET/M918T (MEN2B) oncoproteins in vitro and to inhibit RET/MEN2B phosphorylation and RET/MEN2B-dependent MAPK activation in vivo in NIH-RET/MEN2B [54]. Two years later, a panel of point mutations targeting the RET kinase domain in MEN2 and sporadic MTC was screened for susceptibility to vandetanib. Most of the mutant oncoproteins (RET/E768D, RET/L790F, RET/Y791F, RET/S891A, and RET/A883F) were sensitive to vandetanib, while mutations substituting valine 804 either to leucine or to methionine (as occur in some cases of MEN2A) rendered the RET kinase significantly resistant. This is probably due to steric hindrance, because the Val804Gly mutation increased the sensitivity of RET to vandetanib [55]. Mice carrying a RET C634R mutation from a sporadic human MTC treated with vandetanib had inhibition of tumor growth [56].
Inhibition of other kinases seems to be very important, too. MTC metastases express more EGFR and VEGFR-2 than primary tumor sites. Both EGFR and VEGFR-2 have been shown to be phosphorylated in TT and MZ-CRC-1 cells and inhibited by vandetanib. Yet, in the presence of active RET, neither plays a prominent role in TT cell proliferation. However, when RET activity is inhibited, overstimulation of EGFR is able to partially replace RET through a partial rescue of the MAPK pathway. In such scenario, the inhibition of EGFR by vandetanib was shown to prevent this rescue of the MAPK pathway. These data support the idea that dual inhibition of RET and EGFR is important, as it may overcome the risk of MTC cells' escaping from RET blockade through compensatory overstimulation of EGFR [50].
In phase I clinical studies of patients with solid tumors (not including MTC) [57], doses of vandetanib up to 300 mg/day were well tolerated, and adverse effects were generally mild and controlled with either dose adjustments or symptomatic therapy. The most common adverse events were rash, diarrhea, fatigue, asymptomatic QTc prolongation, proteinuria, and hypertension. Since QT prolongation was note as an adverse event, patients should have EKG and electrolytes at baseline and at regular intervals during the course of treatment.
In a phase II study, 30 adult patients with unresectable, locally advanced, or metastatic hereditary MTC received 300 mg/day of vandetanib [58]. The primary endpoint was the objective response rate (ORR) according to the 2000 Response Evaluation Criteria in Solid Tumors (RECIST) guidelines [59]. Objective partial responses (PRs) were observed in 20% of patients, and the median duration of PR was 10.2 months. Additionally, 53% of patients had stable disease (SD) for a median of 24 weeks. In another trial of vandetanib at 100 mg/day (or up to 300 mg/day in cases with disease progression), patients with similar disease characteristics achieved similar results (ORR 68%) [60]. Both trials showed a ≥50% reduction in calcitonin and carcinoembryonic antigen levels from baseline. However, the reduction in calcitonin levels did not correlate with the degree of tumor growth inhibition. It seems that RET activity is required for ligand-induced calcitonin gene expression [61]. In that sense, carcinoembryonic antigen levels may be a better marker of tumor response to vandetanib. Of interest, there was no apparent association between specific RET germline mutations and response to treatment (no patients with 804 RET mutation were included). Other phase I and II studies are ongoing to determine the effectiveness of vandetanib in sporadic MTC and its safety and efficacy in children and adolescents. (http://www.ClinicalTrials.gov/).
Data on vandetanib have been presented to the United States Food and Drug Administration (FDA), including results from the largest randomized, double-blind, placebo-controlled trial, which was conducted in 331 patients with advanced unresectable or metastatic MTC, “Study D4200C00058”. This trial showed that median progression-free survival (PFS) was 11 months longer in the group randomly assigned to vandetanib and 45% had an ORR. As the drug seems to be effective in stabilizing symptomatic and/or progressive disease, it will likely become the first FDA-approved drug for MTC.
Nuclear factor κB (NF-κB) activation can block cell-death pathways and contribute to the oncogenic state by driving proliferation, enhancing cell survival, and promoting angiogenesis and metastasis. NF-κB has a high baseline activity in MTC cell lines through RET-induced phosphorylation, ubiquitination, and proteosomal degradation of inhibitors of NF-kB (IkB), which allows NF-κB to enter the nucleus and bind to the DNA [62]. Bortezomib inhibits proteosome-mediated IkB degradation in MTC cells, resulting in its accumulation and thus preventing NF-κB translocation to the nucleus [63], thereby leading to apoptosis. A phase I/II trial of the combination of vandetanib plus bortezomib is currently recruiting patients (http://www.ClinicalTrials.gov/). Patients with MTC will participate in the phase II study.
5.2. Sorafenib
Sorafenib is a small TKI that targets RET, VEGFR-2, VEGFR-3, Flt3, PDGFR-β, KIT, and the RAF family serine/threonine kinases RAF-1 and BRAF. It inhibits the growth of RET-driven tumors by a combination of activities that target RET-dependent thyroid cancer cell proliferation and VEGF-dependent tumor angiogenesis. In vitro, sorafenib inhibits RET signaling and the growth of RET-transfected fibroblasts and human thyroid cancer cells that harbor RET/PTC and RET/MEN2 oncogenes. Sorafenib action is mainly cytostatic, but the drug also exerts a proapoptotic effect. Sorafenib has been shown to significantly reduce tumor growth in nude mice with xenograft tumors derived from MTC cell lines [64]. Sorafenib has been investigated in four phase I trials with different doses and administration schedules. A dose of 400 mg orally twice daily was found to be safe and generally well tolerated, and the most frequently reported drug-related adverse events were fatigue, anorexia, diarrhea, rash/desquamation, and hand-foot syndrome. Hand-foot syndrome is characterized by painful erythematous lesions that affect the palmo-plantar surface. It is the most common reported adverse effect in patients taking the multikinase inhibitors like sorafenib and sunitinib. The lesions are pronounced on the pressure points on the palms and the soles but can also affect the margins of the feet and skin between fingers and toes. These lesions are not life threatening but significantly impair the quality of life requiring dose reduction or even discontinuation of the drug [65].Severe hematological, cardiovascular, hepatic, and renal toxic effects were not reported. Treatment-related hypertension was reported in 5% to 11% of patients in all four phase I trials. Sorafenib demonstrated evidence of antitumor activity by inducing disease stabilization in patients with refractory tumors, a finding that was consistent with the results of preclinical studies [66]. No patients with thyroid cancer were included in the phase I study. Because of the role of RET signaling in MTC and the antitumor activity exhibited by sorafenib in preclinical and in vitro studies, MTC was recognized as a potential target for sorafenib. In a small 2007 pilot study that included five patients with metastatic MTC with excessive calcitonin secretion, calcitonin secretion was decreased by >50% in all patients after 3 months of treatment, and all patients were free of calcitonin-related symptoms. After 6 months of therapy, one patient had a complete response (CR), and patient had a PR [67]. Sorafenib was administered orally at a dose of 400 mg twice daily continuously in a larger, open-label phase II study in patients with histologically confirmed metastatic or locally advanced MTC. Patients were monitored regularly with physical examination and biochemical and radiologic testing. In the event of any significant drug-related adverse event, the drug was withheld and restarted at a lower dose of 400 to 600 mg/day with dose re-escalation as tolerated. The median duration of therapy with sorafenib was 15 months. ORR was assessed using RECIST version 1.0. Of the 15 evaluable patients in this study, all showed some degree of tumor shrinkage. One patient achieved PR; 14 patients had SD, eight of whom had SD ≥15 months; and one patient had clinically progressive disease. Most patients had decreased calcitonin levels 2 months after treatment initiation, but they did not correlate with the degree or duration of response as assessed using RECIST [68]. Sorafenib has been approved by the FDA for treatment of renal cell and hepatocellular carcinoma. Therefore, sorafenib is an option for patients with advanced MTC who are not eligible for clinical trials [69].
5.3. Tipifarnib
Tipifarnib inhibits farnesylation of RAS and other proteins. Farnesylation is a type of lipid modification that is critical for the biological functionality including several signal transduction proteins. Farnesyltransferase inhibitors target multiple pathways, including the RAS pathway, and are among the first systematically investigated drugs in oncogene-targeted therapy. RAS genes encode proteins involved in cell proliferation, differentiation, and adhesion and apoptosis regulation. At least three associated genes (H-RAS, K-RAS, and N-RAS) are present in mammalian cells. Of all human tumors, 30% might have a mutated RAS isoform. Thyroid cancer has mutations in all three RAS genes. In in vitro studies, tipifarnib inhibited the growth of several human tumor cell lines, and in in vivo studies, tipifarnib was shown to inhibit colon and pancreatic cancer xenografts in a dose-dependent manner. The antitumor effects were mainly due to decreased cell proliferation, antiangiogenesis, and apoptosis. A phase I trial of tipifarnib in combination with sorafenib in patients with advanced malignancies included 15 patients with thyroid cancer, eight of whom had MTC. Three of the six patients who reached first restaging had PRs, whereas the others had some minor regressions and hence SD lasting from 12 to 16 months. The most common side effects reported were rash, hyperglycemia, and diarrhea. RET mutational analysis in these six patients revealed RET mutations; thus, it is unclear whether the response to sorafenib and tipifarnib was entirely due to RET inhibition by sorafenib [70]. In a previously reported case, the rate of response rate to combination therapy was higher than that reported for sorafenib alone. It should be noted that the RET pathway is complex and the RET kinase can activate a cascade of signaling pathways. Tipifarnib can also affect various other pathways, including Akt and MAP/ERK, and may have acted synergistically to produce the clinical response [71]. The FDA has not approved tipifarnib because of its inferior outcomes in phase III trials in patients with other malignancies [72]. However, the data from trials of thyroid cancer so far seem encouraging, and studies combining various oncogene-targeted therapies are needed.
Preclinical studies have shown that activating RET mutations in V804 (V804L and V804M) causes resistance to various structural classes, including vandetanib. Mutations in V804 slightly affect RET susceptibility to sorafenib, thus indicating that a structurally different inhibitor may be used to overcome the mutational resistance to a particular TKI [73]. This might be clinically significant as a recent study showed RET V804M (19.6%) is a prevalent cause of hereditary MTC [74].
5.4. Sunitinib
Sunitinib is a derivative of indolinone and inhibits the activity of many TKs, including VEGFR, PDGFR, KIT, and RET. Sunitinib exerts antitumor activity by affecting cell proliferation and survival in cancers in which these receptors are involved [75]. Its inhibitory effect on VEGF and RET makes this drug a rational choice for treating MTC. In a phase II study of sunitinib in patients with progressive thyroid cancer that included six patients with MTC, disease stabilization was seen in five of the six patients (83%) [76]. Results from another phase II study that included only patients with progressive MTC also showed responses. Among the 23 patients evaluated, eight (35%) achieved PR, with a median response duration of 37 weeks, and 13 (57%) had SD, with a median response duration of 32 weeks [77]. A trial using a lower dose of 37.5 mg/day in a continuous manner included six patients with MTC. Three of the six patients had an objective response [78]. The most common drug-related adverse events were fatigue, diarrhea, palmar-plantar erythrodysesthesia, neutropenia, and hypertension. Sunitinib has been approved by the FDA as the treatment of renal cell carcinoma and is therefore available for use in selected patients with MTC not enrolled in a clinical trial [69].
5.5. Cabozantinib (XL184)
Cabozantinib (XL184) is a small molecule that inhibits MET, VEGFR-2, RET, KIT, Flt-3, and Tie-2 [79]. In the context of MTC, preclinical data have demonstrated that XL184 can inhibit the proliferation of cells harboring activated RET. In 2009, results of a phase I trial that included 37 patients with MTC revealed that 44% of patients achieved at least 30% reduction in tumor size, and 29% of patients confirmed PR. There was no correlation between RET mutation status (either germline or somatic) and tumor response [80]. Side effects included fatigue, diarrhea, appetite loss, weight loss, hair hypopigmentation, and hypertension. Other effects, such as elevated aspartate aminotransferase, alanine aminotransferase, lipase elevations, palmar/plantar erythema, and mucositis, were dose dependent. Because of the noted antitumor effects of XL184, a phase III clinical trial called the “Efficacy of XL184 in Advanced Medullary Thyroid Cancer (EXAM)” is recruiting patients (http://www.ClinicalTrials.gov/). The purpose of the study is to evaluate PFS with XL184 compared to PFS with placebo in subjects with unresectable, locally advanced, or metastatic MTC.
Recently, in addition to giving XL184 a generic drug name, FDA had granted XL184 an orphan drug designation for treatment of follicular, medullary, and anaplastic thyroid carcinoma, and metastatic or locally advanced papillary thyroid cancer.
5.6. E7080
E7080 inhibits VEGFR-1, VEGFR-2, VEGFR-3, KIT, FGFR1, PDGFR, and to a lesser extent EGFR. This drug has been shown to be a potent inhibitor of in vitro angiogenesis in human small cell lung cancer via inhibition of VEGF/VEGF-2 and the stem cell factor/KIT signaling pathways. Via dual inhibition of VEGFR-2 and VEGFR-3, E7080 has also been shown to decrease lymphatic vessel density in the primary tumors of VEGFC-overexpressing MDA-MB-231 mammary fat pad xenograft models as well as within the metastatic nodules in the lymph nodes of nude mice [81].
In phase I trials, E7080 caused hypertension and proteinuria, which were the major dose-limiting toxic effects [82]. Other observed adverse events included thrombosis, tachycardia, febrile neutropenia, and thrombocytopenia.
A phase II trial to evaluate the safety and efficacy of oral E7080 in medullary and iodine-131-refractory, unresectable differentiated thyroid cancers is ongoing (http://www.clinicaltrials.gov/). The primary purpose of the trial is to determine the effect of E7080 on the objective tumor response rate according to RECIST.
5.7. Pazopanib
Pazopanib is an oral multikinase inhibitor. In vitro studies have shown that it is a potent inhibitor of VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-α and −β, and KIT [83]. The antineoplastic activity of pazopanib is primarily due to its effect on the angiogenic pathways. Phase II studies of pazopanib for MTC are ongoing [84].
6. Conclusion
Research on MTC over the last 55 years has led to a good understanding of the genetic defects and altered molecular pathways associated with its development. Subsequently, promising targeted therapies have been developed for progressive and advanced MTC. Multikinase inhibitors have shown good results in terms of stabilizing disease, and this year will probably see the approval of a drug for advanced unresectable or metastatic MTC, which would represent a new chapter in the history of this disease. The challenge for the years to come is to discover more effective ways to target multiple key pathological pathways as well as the identification of the individuals who will benefit the most.
Conflict of Interests
The authors do not have any conflict of interest to disclose.
Acknowledgments
This research is supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672. K. Gómez and J. Varghese equally contributed to this work and should be rewarded as joint first authors.
References
- 1.DeLellis RA, Lloyd R, Heitz PU, Eng C. WHO Classification of Tumours, Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Journal of the American Medical Association. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Hazard JB, Hawk WA, Crile G., Jr. Medullary (solid) carcinoma of the thyroid: a clinicopathologic entity. Journal of Clinical Endocrinology & Metabolism. 1959;19(1):152–161. doi: 10.1210/jcem-19-1-152. [DOI] [PubMed] [Google Scholar]
- 4.Sipple JH. The association of pheochromocytoma with carcinoma of the thyroid gland. The American Journal of Medicine. 1961;31(1):163–166. [Google Scholar]
- 5.Williams ED, Brown CL, Doniach I. Pathological and clinical findings in a series of 67 cases of medullary carcinoma of the thyroid. Journal of Clinical Pathology. 1966;19(2):103–113. doi: 10.1136/jcp.19.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams ED. Histogenesis of medullary carcinoma of the thyroid. Journal of Clinical Pathology. 1966;19(2):114–118. doi: 10.1136/jcp.19.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graze K, Spiler IJ, Tashjian AH, Jr., et al. Natural history of familial medullary thyroid carcinoma. Effect of a program for early diagnosis. New England Journal of Medicine. 1978;299(18):980–985. doi: 10.1056/NEJM197811022991804. [DOI] [PubMed] [Google Scholar]
- 8.Donis-Keller H, Dou S, Chi D, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Human Molecular Genetics. 1993;2(7):851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- 9.Mulligan LM, Kwok JBJ, Healey CS, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363(6428):458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 10.Dvorǎká S, Václavíková E, Sýkorová V, et al. New multiple somatic mutations in the RET proto-oncogene associated with a sporadic medullary thyroid carcinoma. Thyroid. 2006;16(3):311–316. doi: 10.1089/thy.2006.16.311. [DOI] [PubMed] [Google Scholar]
- 11.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. Journal of Clinical Endocrinology and Metabolism. 2008;93(3):682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 12.Romei C, Cosci B, Renzini G, et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC) Clinical Endocrinology. 2011;74(2):241–247. doi: 10.1111/j.1365-2265.2010.03900.x. [DOI] [PubMed] [Google Scholar]
- 13.Gardner E, Papi L, Easton DF, et al. Genetic linkage studies map the multiple endocrine neoplasia type 2 loci to a small interval on chromosome 10q11.2. Human Molecular Genetics. 1993;2(3):241–246. doi: 10.1093/hmg/2.3.241. [DOI] [PubMed] [Google Scholar]
- 14.Pasini B, Hofstra RMW, Yin L, et al. The physical map of the human RET proto-oncogene. Oncogene. 1995;11(9):1737–1743. [PubMed] [Google Scholar]
- 15.Takahashi M, Buma Y, Iwamoto T, Inaguma Y, Ikeda H, Hiai H. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene. 1988;3(5):571–578. [PubMed] [Google Scholar]
- 16.Iwamoto T, Taniguchi M, Asai N, Ohkusu K, Nakashima I, Takahashi M. cDNA cloning of mouse ret proto-oncogene and its sequence similarity to the cadherin superfamily. Oncogene. 1993;8(4):1087–1091. [PubMed] [Google Scholar]
- 17.Anders J, Kjær S, Ibáñez CF. Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. Journal of Biological Chemistry. 2001;276(38):35808–35817. doi: 10.1074/jbc.M104968200. [DOI] [PubMed] [Google Scholar]
- 18.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nature Reviews Neuroscience. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 19.Tsui-Pierchala BA, Milbrandt J, Johnson EM., Jr. NGF utilizes c-Ret via a novel GFL-independent, inter-RTK signaling mechanism to maintain the trophic status of mature sympathetic neurons. Neuron. 2002;33(2):261–273. doi: 10.1016/s0896-6273(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 20.De Groot JWB, Links TP, Plukker JTM, Lips CJM, Hofstra RMW. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocrine Reviews. 2006;27(5):535–560. doi: 10.1210/er.2006-0017. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine and Growth Factor Reviews. 2001;12(4):361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 22.Ichihara M, Murakumo Y, Takahashi M. RET and neuroendocrine tumors. Cancer Letters. 2004;204(2):197–211. doi: 10.1016/S0304-3835(03)00456-7. [DOI] [PubMed] [Google Scholar]
- 23.Eng C. Seminars in medicine of the Beth Israel Hospital, Boston: the RET proto- oncogene in multiple endocrine neoplasia type 2 and Hirschsprung's disease. New England Journal of Medicine. 1996;335(13):943–951. doi: 10.1056/NEJM199609263351307. [DOI] [PubMed] [Google Scholar]
- 24.Zbuk KM, Eng C. Cancer phenomics: RET and PTEN as illustrative models. Nature Reviews Cancer. 2007;7(1):35–45. doi: 10.1038/nrc2037. [DOI] [PubMed] [Google Scholar]
- 25.Jiménez C, Hu MI-N, Gagel RF. Management of medullary thyroid carcinoma. Endocrinology and Metabolism Clinics of North America. 2008;37(2):481–496. doi: 10.1016/j.ecl.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Annals of Surgery. 1999;229(6):880–888. doi: 10.1097/00000658-199906000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh DJ, Learoyd DL, Andrew SD, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clinical Endocrinology. 1996;44(3):249–257. doi: 10.1046/j.1365-2265.1996.681503.x. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe HJ, Melvin KE, Cervi-Skinner SJ, et al. C-cell hyperplasia preceding medullary thyroid carcinoma. New England Journal of Medicine. 1973;289(9):437–441. doi: 10.1056/NEJM197308302890901. [DOI] [PubMed] [Google Scholar]
- 29.Drosten M, Pützer BM. Mechanisms of disease: cancer targeting and the impact of oncogenic RET for medullary thyroid carcinoma therapy. Nature Clinical Practice Oncology. 2006;3(10):564–574. doi: 10.1038/ncponc0610. [DOI] [PubMed] [Google Scholar]
- 30.Hennige AM, Lammers R, Arlt D, et al. Ret oncogene signal transduction via a IRS-2/PI 3-kinase/PKB and a SHC/Grb-2 dependent pathway: possible implication for transforming activity in NIH3T3 cells. Molecular and Cellular Endocrinology. 2000;167(1-2):69–76. doi: 10.1016/s0303-7207(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 31.Murakami H, Iwashita T, Asai N, et al. Enhanced phosphatidylinositol 3-kinase activity and high phosphorylation state of its downstream signalling molecules mediated by Ret with the MEN 2B mutation. Biochemical and Biophysical Research Communications. 1999;262(1):68–75. doi: 10.1006/bbrc.1999.1186. [DOI] [PubMed] [Google Scholar]
- 32.Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Experimental Cell Research. 2003;284(1):31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 33.Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Experimental Cell Research. 2003;284(1):99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 34.Vlahovic G, Crawford J. Activation of tyrosine kinases in cancer. Oncologist. 2003;8(6):531–538. doi: 10.1634/theoncologist.8-6-531. [DOI] [PubMed] [Google Scholar]
- 35.Arteaga CL. ErbB-targeted therapeutic approaches in human cancer. Experimental Cell Research. 2003;284(1):122–130. doi: 10.1016/s0014-4827(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 36.Vivanco I, Mellinghoff IK. Epidermal growth factor receptor inhibitors in oncology. Current Opinion in Oncology. 2010;22(6):573–578. doi: 10.1097/CCO.0b013e32833edbdf. [DOI] [PubMed] [Google Scholar]
- 37.Traxler P. Tyrosine kinases as targets in cancer therapy—successes and failures. Expert Opinion on Therapeutic Targets. 2003;7(2):215–234. doi: 10.1517/14728222.7.2.215. [DOI] [PubMed] [Google Scholar]
- 38.Croyle M, Akeno N, Knauf JA, et al. RET/PTC-induced cell growth is mediated in part by epidermal growth factor receptor (EGFR) activation: evidence for molecular and functional interactions between RET and EGFR. Cancer Research. 2008;68(11):4183–4191. doi: 10.1158/0008-5472.CAN-08-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez-Antona C, Pallares J, Montero-Conde C, et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocrine-Related Cancer. 2010;17(1):7–16. doi: 10.1677/ERC-08-0304. [DOI] [PubMed] [Google Scholar]
- 40.Terman BI, Dougher-Vermazen M, Carrion ME, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochemical and Biophysical Research Communications. 1992;187(3):1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 41.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Experimental Cell Research. 2006;312(5):549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Kerbel RS. Tumor angiogenesis. New England Journal of Medicine. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1(3):219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 44.Capp C, Wajner SM, Siqueira DR, Brasil BA, Meurer L, Maia AL. Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid. 2010;20(8):863–871. doi: 10.1089/thy.2009.0417. [DOI] [PubMed] [Google Scholar]
- 45.Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. American Journal of Pathology. 1999;155(6):1967–1976. doi: 10.1016/S0002-9440(10)65515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251(4995):802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 47.Sattler M, Salgia R. The MET axis as a therapeutic target. Update on Cancer Therapeutics. 2009;3(3):109–118. doi: 10.1016/j.uct.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corso S, Migliore C, Ghiso E, De Rosa G, Comoglio PM, Giordano S. Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene. 2008;27(5):684–693. doi: 10.1038/sj.onc.1210697. [DOI] [PubMed] [Google Scholar]
- 49.Papotti M, Olivero M, Volante M, et al. Expression of hepatocyte growth factor (HGF) and its receptor (MET) in medullary carcinoma of the thyroid. Endocrine Pathology. 2000;11(1):19–30. doi: 10.1385/ep:11:1:19. [DOI] [PubMed] [Google Scholar]
- 50.Vitagliano D, De Falco V, Tamburrino A, et al. The tyrosine kinase inhibitor ZD6474 blocks proliferation of RET mutant medullary thyroid carcinoma cells. Endocrine-Related Cancer. 2011;18(1):1–11. doi: 10.1677/ERC-09-0292. [DOI] [PubMed] [Google Scholar]
- 51.Ocana A, Amir E, Seruga B, Pandiella A. Do we have to change the way targeted drugs are developed? Journal of Clinical Oncology. 2010;28(24):e420–e421. doi: 10.1200/JCO.2010.28.9918. [DOI] [PubMed] [Google Scholar]
- 52.Herbst RS, Heymach JV, O’Reilly MS, Onn A, Ryan AJ. Vandetanib (ZD6474): an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis. Expert Opinion on Investigational Drugs. 2007;16(2):239–249. doi: 10.1517/13543784.16.2.239. [DOI] [PubMed] [Google Scholar]
- 53.Knowles PP, Murray-Rust J, Kjær S, et al. Structure and chemical inhibition of the RET tyrosine kinase domain. Journal of Biological Chemistry. 2006;281(44):33577–33587. doi: 10.1074/jbc.M605604200. [DOI] [PubMed] [Google Scholar]
- 54.Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Research. 2002;62(24):7284–7290. [PubMed] [Google Scholar]
- 55.Carlomagno F, Guida T, Anaganti S, et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene. 2004;23(36):6056–6063. doi: 10.1038/sj.onc.1207810. [DOI] [PubMed] [Google Scholar]
- 56.Johanson V, Ahlman H, Bernhardt P, et al. A transplantable human medullary thyroid carcinoma as a model for RET tyrosine kinase-driven tumorigenesis. Endocrine-Related Cancer. 2007;14(2):433–444. doi: 10.1677/ERC-06-0033. [DOI] [PubMed] [Google Scholar]
- 57.Holden SN, Eckhardt SG, Basser R, et al. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Annals of Oncology. 2005;16(8):1391–1397. doi: 10.1093/annonc/mdi247. [DOI] [PubMed] [Google Scholar]
- 58.Wells SA, Jr., Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. Journal of Clinical Oncology. 2010;28(5):767–772. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. Journal of the National Cancer Institute. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 60.Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. Journal of Clinical Endocrinology and Metabolism. 2010;95(6):2664–2671. doi: 10.1210/jc.2009-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akeno-Stuart N, Croyle M, Knauf JA, et al. The RET kinase inhibitor NVP-AST487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. Cancer Research. 2007;67(14):6956–6964. doi: 10.1158/0008-5472.CAN-06-4605. [DOI] [PubMed] [Google Scholar]
- 62.Ludwig L, Kessler H, Wagner M, et al. Nuclear factor-κB is constitutively active in C-cell carcinoma and required for RET-induced transformation. Cancer Research. 2001;61(11):4526–4535. [PubMed] [Google Scholar]
- 63.Mitsiades CS, McMillin D, Kotoula V, et al. Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in vitro. Journal of Clinical Endocrinology and Metabolism. 2006;91(10):4013–4021. doi: 10.1210/jc.2005-2472. [DOI] [PubMed] [Google Scholar]
- 64.Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. Journal of the National Cancer Institute. 2006;98(5):326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 65.Degen A, Alter M, Schenck F, et al. The hand-foot-syndrome associated with medical tumor therapy—classification and management. Journal of the German Society of Dermatology. 2010;8(9):652–662. doi: 10.1111/j.1610-0387.2010.07449.x. [DOI] [PubMed] [Google Scholar]
- 66.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12(4):426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 67.Kober F, Hermann M, Handler A, Krotla G. Effect of sorafenib in symptomatic metastatic medullary thyroid cancer. Journal of Clinical Oncology. 2007;25, abstract 14065 [Google Scholar]
- 68.Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. Journal of Clinical Oncology. 2010;28(14):2323–2330. doi: 10.1200/JCO.2009.25.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sherman SI. NCCN Practice guidelines for thyroid cancer. Version 1.2011. 2011.
- 70.Hong DS, Sebti SM, Newman RA, et al. Phase I trial of a combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in advanced malignancies. Clinical Cancer Research. 2009;15(22):7061–7068. doi: 10.1158/1078-0432.CCR-09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong D, Ye L, Gagel R, et al. Medullary thyroid cancer: targeting the RET kinase pathway with sorafenib/tipifarnib. Molecular Cancer Therapeutics. 2008;7(5):1001–1006. doi: 10.1158/1535-7163.MCT-07-2422. [DOI] [PubMed] [Google Scholar]
- 72.Tsimberidou AM, Chandhasin C, Kurzrock R. Farnesyltransferase inhibitors: where are we now? Expert Opinion on Investigational Drugs. 2010;19(12):1569–1580. doi: 10.1517/13543784.2010.535516. [DOI] [PubMed] [Google Scholar]
- 73.Lanzi C, Cassinelli G, Nicolini V, Zunino F. Targeting RET for thyroid cancer therapy. Biochemical Pharmacology. 2009;77(3):297–309. doi: 10.1016/j.bcp.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 74.Romei C, Mariotti S, Fugazzola L, et al. Multiple endocrine neoplasia type 2 syndromes (MEN 2): results from the ItaMEN network analysis on the prevalence of different genotypes and phenotypes. European Journal of Endocrinology. 2010;163(2):301–308. doi: 10.1530/EJE-10-0333. [DOI] [PubMed] [Google Scholar]
- 75.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clinical Cancer Research. 2003;9(1):327–337. [PubMed] [Google Scholar]
- 76.Cohen EE, Needles BM, Cullen KJ, et al. Phase 2 study of sunitinib in refractory thyroid cancer. Journal of Clinical Oncology. 2008;26, abstract 6025 [Google Scholar]
- 77.De Souza JA, Busaidy N, Zimrin A, et al. Phase II trial of sunitinib in medullary thyroid cancer (MTC) Journal of Clinical Oncology. 2010;28, abstract 5504 [Google Scholar]
- 78.Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clinical Cancer Research. 2010;16(21):5260–5268. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eder JP, Vande Woude GF, Boerner SA, Lorusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clinical Cancer Research. 2009;15(7):2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 80.Sherman SI. Targeted therapy of thyroid cancer. Biochemical Pharmacology. 2010;80(5):592–601. doi: 10.1016/j.bcp.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. International Journal of Cancer. 2008;122(3):664–671. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 82.Keizer RJ, Gupta A, Mac Gillavry MR, et al. A model of hypertension and proteinuria in cancer patients treated with the anti-angiogenic drug E7080. Journal of Pharmacokinetics and Pharmacodynamics. 2010;37(4):347–363. doi: 10.1007/s10928-010-9164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sonpavde G, Hutson TE, Sternberg CN. Pazopanib, a potent orally administered small-molecule multitargeted tyrosine kinase inhibitor for renal cell carcinoma. Expert Opinion on Investigational Drugs. 2008;17(2):253–261. doi: 10.1517/13543784.17.2.253. [DOI] [PubMed] [Google Scholar]
- 84.Bible KC, Suman VJ, Molina JR, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. The Lancet Oncology. 2010;11(10):962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]