Non-technical summary
The dorsal horn of the spinal cord is the first site in the central nervous system where painful sensory information is processed before transmission to the brain. In vitro recordings in spinal slices established that this processing relies on both plasticity of synaptic connections and intrinsic electrical properties of dorsal horn neurones (DHNs). DHNs may generate plateau potentials, which underlie intense discharges and long-lasting after-discharges in response to a brief stimulation, and represent a putative endogenous mechanism for amplification of painful sensory inputs. Using patch-clamp recordings in the anaesthetized adult rat, we show that DHNs do generate plateau potentials in vivo, which shape their responses to natural sensory stimulation. Moreover, we give direct evidence for the involvement of these amplification properties in both short-term (windup) and long-term sensitisation associated with neuropathic pain, raising the possibility that plateau potentials could be putative therapeutic targets to control spinal component of neuropathic pain.
Abstract
Abstract
The dorsal horn of the spinal cord is the first central relay where nociceptive inputs are processed. Based on the expression and modulation of intrinsic electrophysiological properties in in vitro slice preparations, dorsal horn neurones (DHNs) display different discharge patterns (tonic, plateau or rhythmic), which shape the neurone's response to sensory inputs. However, it is unclear whether intrinsic properties play any role in sensory processing in vivo. Using in vivo patch clamp recordings in the adult rat, we here examine whether these intrinsic properties are present, and to what extent they determine the DHN response to natural stimulation. We focused primarily on wide dynamic range neurones in deep laminae. These cells displayed a multicomponent peripheral receptive field, comprising an excitatory firing zone, a low-probability firing fringe, and adjacent inhibitory zones. Deep DHNs presented similar intrinsic properties to those observed in vitro, including plateau potentials. These plateaus, underlying high frequency accelerating discharges and after-discharges, were triggered by mechanical stimulation of the excitatory receptive field. Persistent activities induced by activation of plateau potentials were interrupted by stimulation of peripheral inhibitory zones. Moreover, we show that plateau activation is necessary for the expression of windup in response to repetitive, nociceptive stimulation. Finally, using the spinal nerve ligation model of neuropathy, we demonstrate a significant increase in the proportion of plateau neurones in deep dorsal laminae. Our data, therefore, establish that intrinsic amplification properties are expressed within intact spinal circuits and suggest their involvement in neuropathy-induced hyperexcitability of deep DHNs.
Introduction
The dorsal horn of the spinal cord is the first integration site in the pain pathways, where primary sensory fibres activate relay neurones, which are primarily located in the superficial lamina I and in the deep lamina V. These connections are monosynaptic, or polysynaptic through a complex network of interneurones. In vitro recordings in spinal slices established that nociceptive input integration in the dorsal horn relies not only on synaptic plasticity but also on the intrinsic electrical properties of dorsal horn neurones (DHNs) and their modulation by neurotransmitters (Sandkuhler, 2009). DHNs can display a complex repertoire of membrane conductances, which specify their integrative and firing properties. In response to a sustained depolarisation, they may produce tonic firing, initial bursting, delayed firing, or single spike discharge (Yoshimura & Jessell, 1989; Grudt & Perl, 2002; Ruscheweyh & Sandkuhler, 2002). In addition, DHNs in deep laminae in vitro may produce voltage-dependent plateau potentials (Russo & Hounsgaard, 1996; Morisset & Nagy, 1998): an endogenous mechanism for input–output amplification that could profoundly change the response properties of DHNs to sensory inputs (Russo & Hounsgaard, 1996; Morisset & Nagy, 1998; Reali & Russo, 2005). Plateaus can also be generated repetitively, leading to rhythmic bursting (Derjean et al. 2003).
Strong, although indirect, arguments indicate that DHN amplification properties are determinant for spinal sensitisation to pain. Both L-type calcium currents (IL) and a calcium-activated, non-selective cationic current (Ican) underlie plateau potentials, as demonstrated in in vitro rat spinal cord slices (Morisset & Nagy, 1999). We previously showed that windup of DHN discharge, a form of short-term sensitisation to pain, depended strictly on the expression of both IL and ICANin vitro (Morisset & Nagy, 2000) as well as in vivo (Fossat et al. 2007). Moreover, in the spinal nerve ligation (SNL) model of neuropathic rats (Kim & Chung, 1992), long-term mechanical allodynia is reversed in vivo after the expression of the CaV1.2 channel was blocked (Fossat et al. 2010). CaV1.2 is one of the two subtypes of L-type calcium channels expressed by DHNs in the lumbar spinal cord.
However, it remained to directly demonstrate the expression of amplification properties in deep DHNs in vivo, and to assess the functional implication of these intrinsic properties in shaping the responses to natural sensory stimulation, and in contributing to pain-related central sensitisation. We used an in vivo preparation for patch-clamp recordings of deep DHNs in the adult rat spinal cord to address the following questions. (1) Are the different firing patterns of DHNs previously observed in vitro, specifically plateau potentials, also present in vivo? (2) What is the effect of these properties on the response to physiological stimulation of the ipsilateral hind limb? (3) Are the intrinsic properties of DHNs modified (presumably enhanced) in the SNL model of neuropathy?
Methods
Ethical approval
All surgical and experimental procedures were performed in accordance with the ethical guidelines of the local ethics committee (agreement number AP 1/04/2005) and of the International Association for the Study of Pain (IASP). Every precaution was taken to minimise animal stress and the number of animals used.
Animal preparation
Data were obtained from 43 adult male Wistar rats (250–300 g). Animals were anaesthetised intraperitoneally (i.p.) with urethane (1.1 mg kg−1) and a tracheotomy was performed. Rats were placed in a stereotaxic frame and the body temperature was maintained with a heating blanket. A laminectomy was performed on lumbar vertebrae L1–L3 to expose L4–L5 spinal cord segments. The dura was removed, and the dorsal aspect of the cord was superfused with artificial cerebrospinal fluid (ACSF; in mm: 124 NaCl, 3.6 KCl, 2.4 CaCl2, 1.3 MgCl2, 26 NaHCO3 1.25 Hepes and 10 glucose) throughout the experiment. A small hole was then made in the pia to allow proper penetration of the patch electrode. At the end of each experiment, rats were killed with pentobarbital (2.2 mg kg−1, i.p.).
In vivo patch-clamp recordings
Whole cell patch-clamp recordings were made with glass capillaries (9–12 MΩ, Harvard Apparatus, Holliston, MA, USA) and filled with the following patch solution (concentrations in mm): 122 potassium gluconate, 5 Na-ATP, 2.5 MgCl2, 0.0003 CaCl2, 5.6 magnesium gluconate, 5 K-Hepes and 5 H-Hepes. Electrodes were positioned with a microdrive (Luigs & Neumann, Ratingen, Germany) and lowered into the spinal cord. Location of the recorded cells was done primarily by measuring the depth of the electrode tip from the dorsal surface of the cord, and was confirmed in some experiments by adding biocytin (0.2%) to the patch solution. Recordings were performed with a multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA) driven by pCLAMP software (pCLAMP 10, Molecular Devices). The passive and active properties of DHNs were characterised by applying current pulses lasting between 500 ms and 5 s at different levels of holding current. After electrophysiological characterisation of the recorded cells, responses to various natural stimuli to the dorsal surface of the ipsilateral hindlimb were analysed. Innocuous mechanical stimuli were produced by means of a fine pencil (touch), a cotton tip (brush), or Von Frey hairs (10 g). Von Frey hairs of 60 g or the tip of fine surgical forceps were used to produce noxious stimuli (pinprick). Von Frey hairs (60 g) attached to the cone of a loudspeaker driven by a programmable stimulator (Master-8; AMPI, Jerusalem, Israel) were used to produce reproducible stimuli.
Animal model of neuropathy
We used the spinal nerve ligation (SNL) model of neuropathy adapted from Kim & Chung (1992). In 10 adult male rats, the right L4 and L5 spinal branches of the sciatic nerve were tightly ligatured with a 7.0 silk thread. After complete haemostasis, the incision was sutured. For all surgeries, rats were under gaseous anaesthesia with a mixture of isoflurane (5% for induction and 2% for maintenance) and a 1:1 air–O2 flow ratio. Rats resumed normal activity within 30 min of termination of the gaseous anaesthesia.
Mechanical response thresholds were monitored 1 day prior to surgery (reference value for each animal), and 7 days after surgery. For habituation, rats were placed in the testing cage 1 h prior to the test. The withdrawal threshold was determined for the leg ipsilateral to the operated side in response to mechanical stimuli applied to the plantar surface of the foot. Limb withdrawal thresholds were measured using an electronic device (Bioseb, France) derived from Von Frey filaments. Only SNL animals showing a decreased in noxious mechanical threshold ≤30% of their control response as measured before surgery were retained. Non-operated rats were used as controls.
Statistical analysis
Figures were generated using SigmaPlot software (version 3.5, Systat Software, California). All values are mean ± SEM, and n indicates the number of tested cells. Statistical analyses were performed with SigmaStat software (Systat Software Inc., San Jose, CA, USA). Student's paired t-test was used to compare animal withdrawal threshold before and after SNL. Fisher's exact test was used to compare the proportion of plateau cells between normal and neuropathic rats. The criterion for significance was P < 0.05.
Results
Forty-two DHNs were recorded at depths between 200 to 1000 μm from the dorsal surface of the spinal cord (Fig. 1A), including 32 in control and 10 in neuropathic rats (Table 1). Moreover, a majority of them (31/42) were recorded in deep laminae, i.e. deeper than 350 μm. We focused on wide dynamic range (WDR) neurones, which integrate both innocuous (brush, Von Frey hairs delivering a force of 10 g) and noxious mechanical stimuli (pinprick, Von Frey hairs delivering a force of 60 g) (Fig. 1B), because these cells recorded in vitro show the highest probability for expression of plateau potentials (Morisset & Nagy, 1998).
Figure 1. Different components of deep DHN receptive field.
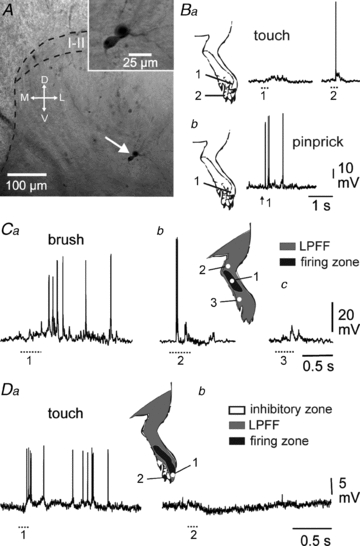
A, cell body location of biocytin-labelled, patch-clamp recorded deep DHNs. In this particular case, two neurones appeared dye-coupled raising the possibility of gap junction coupling (inset). I–II, laminae I–II; M, medial; L, lateral; D, dorsal; V, ventral. B, selected cells are wide dynamic range (WDR) neurones, responding to both innocuous (touch) and noxious (pinprick) stimulations (Ba and b, respectively). Part of the receptive field may be subthreshold for touch (Ba, 1), and suprathreshold for pinprick (Bb, 1). C, DHNs present a firing zone (inset, black area) in which innocuous stimulation leads to a burst of spikes (Ca, 1) surrounded by a low probability firing fringe (inset, grey area). D, most DHNs have both a suprathreshold excitatory receptive field (Da, 1) and inhibitory zones (Db, 2). At resting potential, individual IPSPs are barely visible. B and C, same deep DHN. D, superficial DHN.
Table 1.
In neuropathic animals, a larger proportion of DHNs generate plateau potentials
| Mechanical threshold | Plateau neurones in superficial laminae | Plateau neurones in deep laminae | ||||
|---|---|---|---|---|---|---|
| Force (g) | % | n | % | n | % | |
| Control | 67.1 ± 3.9 | 100 ± 6.6† | 0/7 | 0 | 7/25 | 28.0* |
| SNL | 31.9 ± 1.8 | 47.6 ± 2.6† | 0/4 | 0 | 5/6 | 83.3* |
| †P < 0.0001 | *P = 0.02 | |||||
P = 0.02 versus naive rats.
The expression of plateaus appears specific to DHNs in deep laminae (deeper than 350 μm of the spinal cord surface). Recordings were obtained from allodynic rats presenting a clear decrease in the threshold force required to trigger withdrawal of the paw
P < 0.0001.
The neurone shown in Fig. 1C responded to mechanical stimulation of the paw (zone 1) with a strong burst of postsynaptic potentials (PSPs) inducing several spikes. When zone 2 was stimulated, the same neurone produced only a few spikes, if any, while stimulation of zone 3 yielded only subthreshold PSPs. Zone 1 belongs to the excitatory receptive field of the neurone, whereas zones 2 and 3 belong to the surrounding low probability firing fringe (Woolf & King, 1989). Finally, when stimulation was delivered to an adjacent territory, most of the recorded neurones were inhibited (Fig. 1D).
Tonic and adapting cells
Overall, 8 of 32 DHNs maintained a tonic discharge during a current pulse of several seconds (Fig. 2A), with a linear relationship between firing frequency and current intensity. Among these, 4 DHNs persisted in discharging during a stimulation lasting several seconds, but with marked adaptation (Fig. 2Bb). Below a given threshold of current intensity, the duration of the discharge decreased with the current (Fig. 2Ba).
Figure 2. Tonic or phasic-tonic firing patterns of deep DHNs.
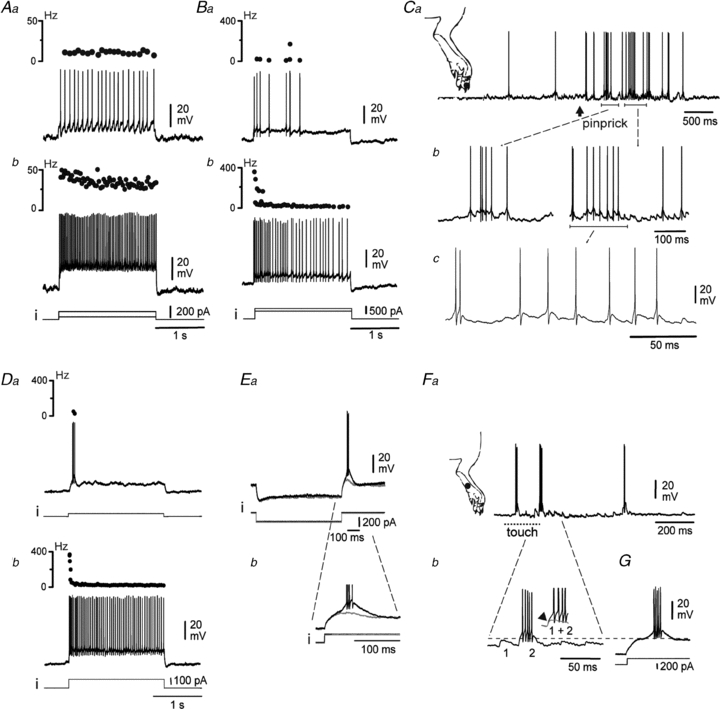
A and B, tonic (A) and/or slightly adapting firing (B) of DHNs in response to low (Aa and Ba) or high (Ab and Bb) intensities of injected current (i). Upper trace in each panel indicates instantaneous firing frequency. Noxious stimulation of the peripheral excitatory receptive field triggers a barrage of EPSPs eliciting spikes (Ca–c). A and B–C, deep and superficial neurones, respectively. D–G, phasic–tonic DHNs. A depolarising current pulse triggers a high frequency burst of spikes at the onset of stimulation (Da). Stronger stimulation elicits a subsequent tonic discharge (Db). Postinhibitory rebound following a hyperpolarising current pulse (Ea). Note the barrage of spikes in the rebound once the T-spike threshold is crossed (Eb). Once EPSPs cross the T-spike threshold (Fa and b, dashed line), peripheral stimulations evoke a high frequency burst of spikes, very similar to the one observed on top of a rebound (G). Da and b and E–G show two deep DHNs.
Noxious stimulation of the paw provoked a barrage of excitatory PSPs (EPSPs). In tonic and adapting DHNs, these EPSPs usually induced single spikes, and discharges in bursts, if any, resulted primarily from underlying bursts of EPSPs (Fig. 2C).
Phasic–tonic cells
Another category of DHNs (16/32) responded to sustained depolarising currents with either a phasic or a phasic–tonic discharge, depending on the stimulus intensity. When depolarised with a mild current pulse from a hyperpolarised potential (Fig. 2Da, –59 mV), these neurones responded with a brief, high frequency burst of action potentials on top of a 100 ms regenerative depolarisation. This depolarisation may develop upon release from hyperpolarisation and had the main characteristics of a low threshold Ca2+ T-spike (Fig. 2E, see Discussion). With a stronger current pulse, the brief, initial high frequency burst of spikes was followed by a tonic discharge (Fig. 2Db). The rebound depolarisation is the critical distinction between phasic–tonic cells and adapting neurones.
A DHN with these phasic–tonic firing properties responded to mechanical stimulation of the paw with a barrage of EPSPs (Fig. 2Fa), which may individually support a burst of high-frequency Na+ spikes (Fig. 2Fb) very similar to those supported by the T-spike in the same cell on release from hyperpolarisation (Fig. 2G). Thus, these bursts most likely resulted from a T-spike-mediated amplification of the EPSP amplitude (Fig. 2Fb inset, arrow).
Plateau potentials
In deep laminae exclusively, a third category of neurones (7/25 in control animals, see Table 1) displayed more complex patterns of discharge. During a sustained current pulse (2 s in Fig. 3A), they generated an accelerating discharge, leading to high frequency firing, followed by an after-discharge after termination of the stimulation. Depending on the neurone, the after-discharge spontaneously repolarised after a few hundred milliseconds or lasted for several minutes (not shown). It was possible to interrupt the after-discharge with a pulse of hyperpolarising current (Fig. 3Bb), demonstrating bistability. The phenomenon was voltage dependent. Holding the neurone at a slightly more negative potential (–59.3 mV in Fig. 3Baversus–56.3 mV) unravelled a slow, active depolarisation that resulted in spiking only after a substantial delay, and greatly shortened the after-discharge (Fig. 3Ba). These features are characteristics of plateau potentials and have been previously described for deep DHNs in in vitro slice preparations (see Discussion). Lastly, one deep WDR neurone generated rhythmical bursts of high frequency firing (not shown).
Figure 3. Plateau properties of deep DHNs.
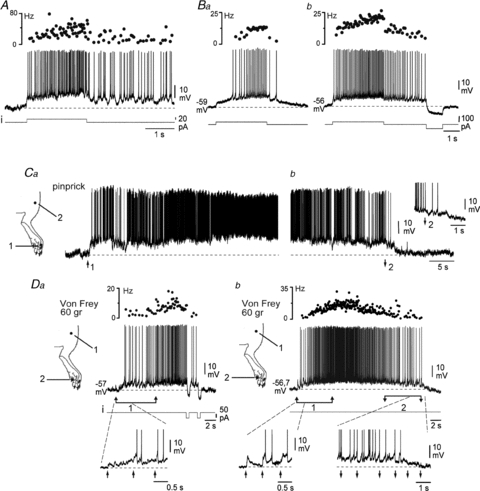
A, plateau potentials support an accelerating discharge during current injection (i) and a sustained after-discharge. Upper trace indicates instantaneous firing frequency, and dashed line is the mean pre-pulse resting potential. B, firing frequency and duration of the after-discharge are voltage-dependent (Ba and b, same neurone; note the different holding potentials). The after-discharge can be prematurely switched off by a brief hyperpolarising current pulse (Bb). C, noxious pinprick of a DHN excitatory receptive field (Ca, 1) can trigger a long-lasting plateau potential, which can be switched off by subsequent stimulation of an inhibitory zone of the receptive field (Cb, 2). Ca and Cb show different after-discharges of the same neurone. D, repetitive noxious stimulations of the receptive field with a 60 g Von Frey filament (Da and b, 1), evoke a strong after-discharge, which is efficiently switched off by hyperpolarising current steps (Da) or by repetitive mechanical stimulation of an inhibitory zone of the receptive field (Db, 2). Da and b, same neurone as Ba and b.
We next aimed to determine whether the plateau properties could interact with synaptic inputs induced by natural stimulation. A single noxious pinprick in the excitatory receptive field (zone 1, Fig. 3Ca) of a lamina V DHN may trigger an intense and prolonged discharge, which was interrupted by a noxious pinprick in an adjacent inhibitory receptive field (zone 2, Fig. 3Cb). Therefore, the transition between the two meta-stable states (active or silent) of the membrane potential may be triggered by sensory inputs from different receptive fields of a DH plateau neurone.
Generally, when neurones were at their resting potential, the triggering of high frequency accelerating firing required repetitive stimulation (Fig. 3D, upward arrows; noxious mechanical stimuli with a 60 g Von Frey hair). Individual stimuli elicited EPSPs and a cumulative depolarisation, which was responsible for the accelerating discharge (insets Fig. 3Da 1 and Db 1, upward arrows). Moreover, the after-discharge was strongly decreased and then interrupted by two consecutive short pulses of hyperpolarising current (Fig. 3Da), indicating it was mediated by activation of an intrinsic plateau potential. Moreover, in the same cell, the plateau potential and associated intense firing were also interrupted by similar repetitive noxious mechanical stimuli delivered to the inhibitory receptive field of the neurone (Fig. 3Db, inset 2, downward arrows).
Short-term, activity-dependent plasticity
The higher propensity of repetitive natural mechanical stimuli to elicit a plateau potential, as compared to a single stimulus, is reminiscent of the ability of deep DHNs to display windup (see Discussion). Thus, we next considered the involvement of plateau potentials in this form of short-term, activity-dependent plasticity. In deep DHNs recorded in vitro, windup can be mimicked by repetitive injection of brief depolarising current pulses inducing a cumulative activation of the plateau potential (Russo & Hounsgaard, 1994; Morisset & Nagy, 2000). In our in vivo experiments, the same phenomenon was induced in every tested neurone expressing plateau properties (n = 12/12). In the example illustrated in Fig. 4A, the number of action potentials gradually increased during the sequence of depolarising current pulses (500 ms, 0.67 Hz), leading to persistent firing between stimuli. The long-lasting after-discharge following stimulation was interrupted by a brief pulse of hyperpolarising current (Fig. 4B). This short-term plasticity was also induced by repetitive, noxious mechanical stimulation of the neurone's peripheral receptive field (Fig. 4C, zone 1). Individual stimulations consisted of a burst of nine pinpricks (100 Hz during 100 ms) with a 60 g Von Frey hair. The successive stimulations, delivered at 1 Hz, induced both a cumulative depolarisation and a progressive increase in the response of the DHN, followed by a high frequency after-discharge. The after-depolarisation and after-discharge were interrupted by a single hyperpolarising current pulse, indicating the involvement of an intrinsic regenerative depolarisation.
Figure 4. Windup specific to plateau-generating neurones.
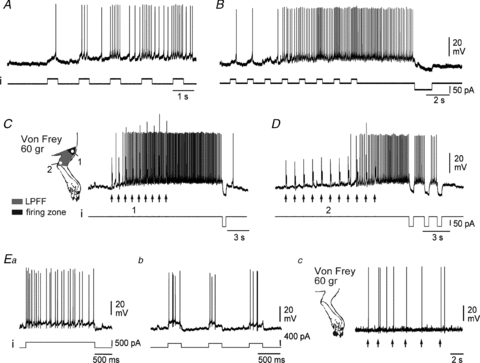
A and B, low frequency (0.6 Hz) repetitive stimulation by intracellular current steps elicits a progressive increase in the firing frequency, so-called windup (A), and an after-discharge (B). C and D, windup can also be triggered by repetitive noxious stimulations of the excitatory receptive field, both in the firing zones (C, 1, arrows) and in the low probability firing fringe (D, 2, arrows). The after-discharge is switched off by hyperpolarising current pulses, indicative of a plateau potential (B, C and D). E, DHNs that do not show plateau potentials (Ea) never display a windup of their discharge in response to either depolarising current pulses (Eb) or to noxious stimulation of the receptive field (Ec). A–D, same deep DHN. Ea–c, same superficial DHN.
Delivering a similar 100 ms single stimulation to the adjacent, low-probability firing fringe of the receptive field of the same plateau-generating neurones (Fig. 4C inset, zone 2) never induced firing. Interestingly, repeating the same stimulation at 1 Hz (Fig. 4D) progressively induced a cumulative depolarisation and an intense discharge, which was interrupted by brief pulses of hyperpolarising current. These findings indicate that the development of a plateau potential may transform a subthreshold portion of the peripheral receptive field into a suprathreshold zone.
Finally, neurones that did not display plateau potentials (Fig. 4Ea) never demonstrated windup of their discharge, either during repetitive injections of depolarising current pulses (Fig. 4Eb, n = 4/4) or during a sequence of noxious pinpricks delivered to their excitatory receptive field (Fig. 4Ec).
Expression of plateau potentials in neuropathic rats
Our findings are of particular importance because they raise the possibility that over-expression of plateau potentials could participate in the expansion of excitatory receptive fields reported in situations of pain sensitisation (see Discussion). In this context, we sought to determine whether the proportion of deep DHNs generating plateau potentials would change in a chronic pain state.
To test this possibility, we chose the SNL model of neuropathy, which is characterised by the induction of a long-lasting mechanical allodynia. We selected a group of 10 animals showing a decreased noxious mechanical threshold (≤30% of their control response) as measured before surgery (Table 1).
The proportion of deep DHNs expressing plateau potentials significantly increased from 28.0% in control animals to 83.3% in SNL animals. By contrast, no plateau potential was ever recorded in superficial laminae, either in control or SNL rats. These data indicate an over-expression of plateau potentials in the presence of chronic neuropathic pain, specifically in the nociceptive relay zone of the deep dorsal horn.
Discussion
Using in vivo patch-clamp recordings, we show that firing patterns of deep DHNs in the lumbar spinal cord of adult rats display the same diversity as those previously described in vitro in juvenile animals. In particular, DHNs generate plateau potentials, an intrinsic mechanism for input/output amplification and of major importance in spinal processing of peripheral sensory information. Moreover, we show direct evidence for the involvement of these amplification properties in both short-term windup, and long-term sensitisation associated with neuropathic pain.
in vivo firing patterns of deep DHNs
In the present in vivo patch clamp study, we focused on WDR neurones. In line with previous in vitro studies (Schneider, 2005), these neurones preferentially displayed tonic or phasic-tonic firing. When depolarised from rest, 50% of this population generated an initial, brief, high frequency burst of spikes on top of a regenerative depolarisation presenting the main characteristics of a low threshold Ca2+ T-spike (Jahnsen & Llinas, 1984). Our data suggest that the T-spike contributes to input-output amplification that may enhance the signal to noise ratio by transforming a short barrage of EPSPs elicited by a mechanical stimulation of the paw into a burst of high-frequency firing.
In vitro, a proportion of DHNs generate plateau potentials (Russo & Hounsgaard, 1996; Morisset & Nagy, 1998; Dougherty & Hochman, 2008). Plateau-generating DHNs respond with intense and prolonged discharges to brief electrical stimulations of nociceptive primary afferent fibres, exhibiting non-linear input–output relationships (Morisset & Nagy, 1998; Reali & Russo, 2005).
In slice preparations, the ability to generate plateaus is a latent property which depends on a dynamic balance between antagonistic neuromodulatory controls (Russo et al. 1998; Derjean et al. 2003), whereby deep DHNs may individually present three different firing modes: a slowly adapting tonic discharge, single plateau potentials, or repetitively generated plateaus and rhythmic bursting. Each of these modes is associated with different capabilities for sensory information transfer (Derjean et al. 2003). The relative incidence of the three modes in single DHNs is significantly associated with the neurone's state of modulation, with about 90% of the neurones displaying tonic firing in control slices, and 90% displaying plateaus or rhythmic bursting when properly modulated (Derjean et al. 2003).
We show that, in vivo, deep DHNs present the same three patterns as observed in in vitro slices, i.e. tonic or phasic–tonic (72%), plateaus (28%), and rhythmic bursting (1 neurone). In contrast, no plateaus were recorded in superficial laminae. The relatively low proportion of plateau neurones indicates that in the intact animal, the inhibitory control of deep DHN amplification properties predominates over the excitatory modulation. The scarcity of the rhythmic bursting firing mode is also consistent with a previous in vivo study (Jiang et al. 1995) and with in vitro recordings made in slices without plateau-promoting neuromodulators (Derjean et al. 2003).
Windup
Low-frequency repetitive stimulation of nociceptive afferent fibres induces a progressive build-up of DHN excitability referred to as action potential windup. This form of short-term sensitisation is one criterion commonly used to assess early-onset plasticity of spinal pain processing in animal models (see reviews of Baranauskas & Nistri, 1998; Herrero et al. 2000) and is considered to be a good predictor of clinical pain intensity in several pain syndromes (Staud et al. 2004).
Windup includes a synaptic component, involving N-methyl-d-aspartate (NMDA) and neurokinin receptors (Baranauskas & Nistri, 1998; Herrero et al. 2000), and a critical cellular component, which is the expression of plateau potentials by post-synaptic DHNs. This was demonstrated in vitro (Russo & Hounsgaard, 1994; Morisset & Nagy, 2000) and reported to be very likely in humans following spinal cord injury (Hornby et al. 2003). In a previous study (Fossat et al. 2007), we showed that windup of the DHN discharge in vivo was suppressed by blockers of IL and ICAN, the two main depolarising conductances of plateau potentials (Morisset & Nagy, 1999). However, extracellular electrophysiology precluded a direct assessment of the participation of the DHN intrinsic properties in windup.
Here, for the first time in vivo, we demonstrate a strict correlation (all or none) between windup expression and the ability of DHNs to generate plateau potentials. We also show that windup, elicited by natural nociceptive inputs from their excitatory receptive field, was mimicked by repetitive intracellular current injection. Moreover we show that the resulting after-discharge in both forms of windup was interrupted by a brief hyperpolarising current, demonstrating that it relied on intrinsic DHN amplification properties. Our results provide in vivo validation of previous in vitro experiments and highlight the important role played by these intrinsic DHN properties in short-term central sensitisation to pain.
Plateau potentials and dynamics of peripheral receptive fields
The cutaneous mechanoreceptive field of lumbar DHNs has a complex organisation. It is composed of a firing zone, where skin stimulation has a high probability to elicit action potentials, and a surrounding low-probability firing fringe, where the response to the same stimulus primarily generates a subthreshold depolarisation and thus no output (Woolf & King, 1989). The spatial extent of the fringe is extremely variable depending on the neurone and may even cover an entire limb (Woolf & King, 1989). Moreover, expansion of the firing zone together with increased responsiveness may occur under diverse noxious conditions (see references in Woolf & King, 1990). The expansion of the firing zone is due to the incorporation of portions of the formerly low-probability firing fringe, when previously subthreshold EPSPs become suprathreshold. Changes in DHN membrane properties have been proposed as a potential mechanism for modulation of the receptive field (Woolf & King, 1990). Our present findings indicate that the presence of the plateau potential is a crucial factor for this intrinsic plasticity. We show that in plateau-generating neurons, repetitive stimulation within the low-probability firing fringe of the receptive field produced a slow cumulative depolarisation mediated by activation of the plateau that enabled previously subthreshold EPSPs to generate spike firing (see Fig. 4D). These properties thereby expand the firing zone and contract the low-probability firing fringe of the receptive field. Together, our results represent the first direct evidence of the roles of DHN intrinsic amplification properties in short-term, dynamic plasticity of their receptive fields.
Intrinsic amplification properties and central sensitisation to pain
Expansion of receptive fields, increased excitability, and implication in windup strongly suggest that expression of DHN plateau potentials participate in central sensitisation to pain. One of the two main depolarising conductances of plateau potentials is carried by L-type calcium channels (LTCs), and an antisense-based knockdown of the CaV1.2 LTC gene induced a strong anti-allodynic effect in the rat SNL model and a reversal of DHN hyperexcitability (Fossat et al. 2010). These results indicate that LTC-dependent intrinsic properties of deep DHNs are crucial factors for the maintenance of chronic neuropathic pain. We show here that in the deep dorsal horn of SNL rats, the number of plateau-generating neurones significantly increased from 28% to 83%.
A larger number of plateau neurones may change the nociceptive information transfer within the spinal cord. As shown in vitro, a DHN operating in a tonic firing mode faithfully transmits sensory inputs in terms of duration and intensity, whereas plateau-expressing neurones strongly increase this transfer capability by producing long lasting after-discharges (Derjean et al. 2003). In in vivo experimental models of persistent pain, after-discharges are exaggerated (Sotgiu et al. 1995) and are produced by a larger number of spinothalamic tract cells (Palecek et al. 1992). These observations are consistent with the increased number of plateau-generating neurones demonstrated in the present paper.
With respect to the mechanisms that promote expression of amplification properties of deep DHNs in conditions of chronic pain, one possibility is the reported up-regulation of CaV1.2 LTCs in models of neuropathic pain (Dobremez et al. 2005; Fossat et al. 2010). Another putative mechanism is a reduction of inhibitory modulatory control in the dorsal horn. In vitro, the level of plateau potential expression is negatively controlled by activation of GABAB receptors (Russo et al. 1998; Derjean et al. 2003). Recent data indicate that chronic pain conditions result in a loss of GABAB receptor functionality (Wang et al. 2007; Goudet et al. 2009). It is worth noting that in the SNL model of neuropathy, 83% of deep WDR DHNs demonstrate intrinsic active properties (present paper), a proportion very similar to the 90% of neurones observed in slices following GABAb receptor blockade and group 1 mGluR activation (Derjean et al. 2003). The detailed molecular mechanisms of plateau potential enhancement reported here during neuropathic pain remains to be elucidated.
In conclusion, our data highlight the importance of intrinsic amplification properties in shaping the DHN response to physiologically relevant inputs. These data also demonstrate the involvement of DHN plateau properties in short-term windup and their increased presence in neuropathic animals, thereby raising the possibility that plateau potentials could be considered as putative therapeutic targets to control spinal sensitisation in neuropathic pain conditions.
Acknowledgments
This work was supported by an ‘ECOS-Sud’ program (U03S01) to F.N. and R.E.R., by grants from the Conseil Régional d'Aquitaine (20010301213, and 2008/30/023), and from the ANR (ANR-07-NEURO-015-01) to F.N. We thank Dr Marie-Christine Lombard for valuable technical advice.
Glossary
Abbreviations
- DHN
dorsal horn neurone
- GABAB
γ-amino-butyric-acid B
- ICAN
calcium-activated non-selective cationic current
- IL
L-type calcium current
- LTC
L-type calcium channel
- mGluR
metabotropic glutamate receptor
- NMDA
N-methyl-d-aspartate
- SNL
spinal nerve ligation
- WDR
wide dynamic range neurone
Author contributions
The five authors have contributed to conception and design, or analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content. All authors gave final approval of the version to be published.
References
- Baranauskas G, Nistri A. Sensitization of pain pathways in the spinal cord: Cellular mechanisms. Prog Neurobiol. 1998;54:349–365. doi: 10.1016/s0301-0082(97)00067-1. [DOI] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Le Masson G, Landry M, Morisset V, Nagy F. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat Neurosci. 2003;6:274–281. doi: 10.1038/nn1016. [DOI] [PubMed] [Google Scholar]
- Dobremez E, Bouali-Benazzouz R, Fossat P, Monteils L, Dulluc J, Nagy F, Landry M. Distribution and regulation of L-type calcium channels in deep dorsal horn neurons after sciatic nerve injury in rats. Eur J Neurosci. 2005;21:3321–3333. doi: 10.1111/j.1460-9568.2005.04177.x. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Hochman S. Spinal cord injury causes plasticity in a subpopulation of lamina I GABAergic interneurons. J Neurophysiol. 2008;100:212–223. doi: 10.1152/jn.01104.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat P, Dobremez E, Bouali-Benazzouz R, Favereaux A, Bertrand SS, Kilk K, Leger C, Cazalets JR, Langel U, Landry M, Nagy F. Knockdown of L calcium channel subtypes: differential effects in neuropathic pain. J Neurosci. 2010;30:1073–1085. doi: 10.1523/JNEUROSCI.3145-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat P, Sibon I, Le Masson G, Landry M, Nagy F. L-type calcium channels and NMDA receptors: a determinant duo for short-term nociceptive plasticity. Eur J Neurosci. 2007;25:127–135. doi: 10.1111/j.1460-9568.2006.05256.x. [DOI] [PubMed] [Google Scholar]
- Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RWT, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009;60:43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Rymer WZ, Benz EN, Schmit BD. Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol. 2003;89:416–426. doi: 10.1152/jn.00979.2001. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MC, Cleland CL, Gebhart GF. Intrinsic properties of deep dorsal horn neurons in the L6-S1 spinal cord of the intact rat. J Neurophysiol. 1995;74:1819–1827. doi: 10.1152/jn.1995.74.5.1819. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Nociceptive integration in the rat spinal cord: Role of nonlinear membrane properties of deep dorsal horn neurons. Eur J Neurosci. 1998;10:3642–3652. doi: 10.1046/j.1460-9568.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Plateau potential-dependent windup of the response to primary afferent stimuli in rat dorsal horn neurons. Eur J Neurosci. 2000;12:3087–3095. doi: 10.1046/j.1460-9568.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- Palecek J, Paleckova V, Dougherty PM, Carlton SM, Willis WD. Responses of spinothalamic tract cells to mechanical and thermal stimulation of skin in rats with an experimental peripheral neuropathy. J Neurophysiol. 1992;67:1562–1573. doi: 10.1152/jn.1992.67.6.1562. [DOI] [PubMed] [Google Scholar]
- Reali C, Russo RE. An integrated spinal cord-hindlimbs preparation for studying the role of intrinsic properties in somatosensory information processing. J Neurosci Methods. 2005;142:317–326. doi: 10.1016/j.jneumeth.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkuhler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol. 2002;541:231–244. doi: 10.1113/jphysiol.2002.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Short-term plasticity in turtle dorsal horn neurons mediated by L-type Ca2+ channels. Neuroscience. 1994;61:191–197. doi: 10.1016/0306-4522(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Plateau-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol. 1996;493:39–54. doi: 10.1113/jphysiol.1996.sp021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RE, Nagy F, Hounsgaard J. Inhibitory control of plateau properties in dorsal horn neurones in the turtle spinal cord in vitro. J Physiol. 1998;506:795–808. doi: 10.1111/j.1469-7793.1998.795bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Schneider SP. Mechanosensory afferent input and neuronal firing properties in rodent spinal laminae III-V: re-examination of relationships with analysis of responses to static and time-varying stimuli. Brain Res. 2005;1034:71–89. doi: 10.1016/j.brainres.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Sotgiu ML, Biella G, Riva L. Poststimulus afterdischarges of spinal WDR and NS units in rats with chronic nerve constriction. Neuroreport. 1995;6:1021–1024. doi: 10.1097/00001756-199505090-00018. [DOI] [PubMed] [Google Scholar]
- Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–696. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol. 2007;579:849–861. doi: 10.1113/jphysiol.2006.126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Subthreshold components of the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat lumbar spinal cord. J Neurophysiol. 1989;62:907–916. doi: 10.1152/jn.1989.62.4.907. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci. 1990;10:2717–2726. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]


