Non-technical summary
Obesity is known to result from energy intake in excess of expenditure. What is not known is how individuals are able to eat in excess of their energy needs. We show that after chronic consumption of a high fat diet (which causes obesity), intestinal sensory nerves are less responsive to chemicals released from the gut during a meal (cholecystokinin and 5-hydroxytryptamine) as well as to distension of the gut as might occur during a meal. This appears to be due to the fact that the ability of the nerve cells to be excited is impaired. This suggests that consumption of an unhealthy diet that leads to obesity causes decreased signalling from the intestine, which may lead to increased food intake and contribute to further weight gain, or allow the maintenance of excess weight and obesity.
Abstract
Abstract
Gastrointestinal vagal afferents transmit satiety signals to the brain via both chemical and mechanical mechanisms. There is indirect evidence that these signals may be attenuated in obesity. We hypothesized that responses to satiety mediators and distension of the gut would be attenuated after induction of diet induced obesity. Obesity was induced by feeding a high fat diet (60% kcal from fat). Low fat fed mice (10% kcal from fat) served as a control. High fat fed mice were obese, with increased visceral fat, but were not hyperglycaemic. Recordings from jejunal afferents demonstrated attenuated responses to the satiety mediators cholecystokinin (CCK, 100 nm) and 5-hydroxytryptamine (5-HT, 10 μm), as was the response to low intensity jejunal distension, while responses to higher distension pressures were preserved. We performed whole cell patch clamp recordings on nodose ganglion neurons, both unlabelled, and those labelled by fast blue injection into the wall of the jejunum. The cell membrane of both labelled and unlabelled nodose ganglion neurons was less excitable in HFF mice, with an elevated rheobase and decreased number of action potentials at twice rheobase. Input resistance of HFF neurons was also significantly decreased. Calcium imaging experiments revealed reduced proportion of nodose ganglion neurons responding to CCK and 5-HT in obese mice. These results demonstrate a marked reduction in afferent sensitivity to satiety related stimuli after a chronic high fat diet. A major mechanism underlying this change is reduced excitability of the neuronal cell membrane. This may explain the development of hyperphagia when a high fat diet is consumed. Improving sensitivity of gastrointestinal afferent nerves may prove useful to limit food intake in obesity.
Introduction
Obesity and its resultant health consequences are rapidly becoming the most pressing public health issue of our time. In western societies, the prevalence of obesity is now in excess of 20% (Shields & Tjepkema, 2005) and the number of individuals overweight is nearly half the population. Despite the prevalence of obesity, medical treatments have been disappointing and achieve only modest weight loss (Lean & Finer, 2006). Diet alteration and exercise is the preferred mode of treatment, but fails to achieve a sustained weight loss in the vast majority (Avenell et al. 2006). Surgical treatment is highly effective, but carries a finite risk of morbidity and mortality, and utilizes resources to a degree where its widespread application is impractical. Clearly there is a need for improved treatment of obesity; however our understanding of how it develops is incomplete.
Afferent impulses, carried predominately via the vagus nerve, are the first link in gut–brain signalling and provide a neural pathway conveying information on a minute to minute basis regarding the quantity and quality of ingested nutrients. Indeed vagal afferents have been shown to have tremendous versatility, being able to alter levels of receptor expression in response to different feeding or hormonal conditions (Dockray, 2009; De Lartigue et al. 2010a,b; Burdyga et al. 2010; Dockray & Burdyga, 2011). The chemical constituents of food are detected by specialized secretory cells in the gut epithelium releasing satiety mediators many of which act through the stimulation of vagal afferents including cholecystokinin (CCK), 5-hydroxytryptamine (5-HT), glucagon like peptide-1 (GLP-1), peptide YY (PYY) and others (Moran et al. 1997; Abbott et al. 2005; Talsania et al. 2005; Avenell et al. 2006; Berthoud, 2008; Bucinskaite et al. 2009). In addition the presence of food in the gut results in activation of vagal afferent mechanosensors, also sending satiety signals to the CNS (Fox et al. 2001, 2002; Cummings & Overduin, 2007). Despite the fact that there is good evidence that afferent neural pathways from the gut play a significant role in controlling meal size, the function of these afferents in obesity has to date received relatively little attention. There is, however, indirect evidence for altered function of these afferent pathways in obesity. For example the food intake-suppressive effects of satiety peptides such as CCK, known to act through vagal afferents, are diminished in obesity (Covasa & Ritter, 1999). In addition the satiating effect of intraluminal fat is also attenuated in obesity, and this is accompanied by decreased neural activation in central vagal nuclei such as the nucleus of the solitary tract, the site of termination of vagal afferents (Covasa et al. 2000b). These studies point to a possible role for dysfunction of vagal afferents, but there has been no direct assessment of responses of gut vagal afferents to potential meal related stimuli in an animal model of obesity.
With the above factors in mind, we aimed to study the effects of chronic consumption of a high fat, obesogenic diet on chemo- and mechanosensitive responses of intestinal afferents. Jejunal afferents were chosen for study as these have been shown to respond to CCK and 5-HT (Grundy et al. 1998; Hillsley & Grundy, 1998; Hillsley et al. 1998; Lal et al. 2001). Furthermore, afferent activation and satiation by nutrients within the jejunal lumen have been shown to involve both of these mediators – in a vagus-dependent fashion (Covasa & Ritter, 1999; Zhu et al. 2001; Lal et al. 2001; Savastano et al. 2005). The model of obesity we used is the high fat fed C57Bl6/J mouse, which develops obesity when fed a diet composed of 60% calories from fat (Surwit et al. 1995). This model exploits a genetic predisposition to develop obesity (there are other mouse strains that do not develop obesity on this diet) with an environmental factor (high fat feeding) to cause obesity, similar to most cases of human obesity. As this particular model is reported to be hyperphagic, this also suggests that this model results in impaired short term controls on food intake (Surwit et al. 1995; Lin et al. 2000b; Collins et al. 2004; Gallou-Kabani et al. 2007). With this in mind, we hypothesized that after chronic high fat feeding intestinal afferents would be less sensitive to satiety mediators and mechanical stimuli. Our results demonstrate markedly decreased sensitivity of intestinal afferents to satiety mediators and distension. In addition, through whole cell patch clamp techniques, we show decreased membrane excitability of vagal afferents that may underlie some of the decreased responsiveness to both chemical and mechanical stimulation.
Methods
Animals
All experiments were performed in accordance with the Queen's University Animal Care Committee, under an approved protocol, in accordance with guideline from the Canadian Council for Animal Care. Male C57Bl6/J mice from Charles River Canada (Montreal, QC, Canada) or The Jackson Laboratory (Bar Harbor, ME, USA) were used in all experiments.
Induction of diet induced obesity
Four-week-old male mice were placed on commercially available chow composed of either 10% or 60% total calories from fat (Research Diets Inc., New Brunswick, NJ, USA) and fed ad libitium. Mice were housed under identical conditions with a standard light–dark cycle. Animals were maintained on these diets until the time of experimentation at 12–14 weeks of age. Due to space limitations in our facility, some animals were purchased from The Jackson Laboratory at 11 weeks of age where they had been fed the identical high or low fat diets; these mice were housed in our facility and maintained on the same diets until the time of experimentation (at 12–14 weeks of age). Equal numbers of HFF and LFF mice were purchased from The Jackson Laboratory, avoiding biasing results due to external conditions. Weight gain was similar for both mice fed in our facility and mice from The Jackson Laboratory, and therefore data from both groups were pooled. All mice had free access to food and water up to the time of the experiment.
Measurement of weight gain, visceral fat and blood glucose
Mice were weighed weekly. In a subset of animals, blood was collected at 09.00 h by cardiac puncture immediately following killing and blood glucose levels were measured using an instant read glucometer (Bayer Contour). As an index of visceral fat deposition, the epididymal fat pads were collected and weighed.
Multiunit jejunal afferent nerve recordings
In vitro extracellular recording from jejunal afferent nerves
Mice were killed by isoflurane overdose followed by exsanguination. The abdomen was immediately opened, and the jejunum (small intestine distal to the ligament of Treitz, but proximal to 10 cm from the ileocecal valve) was excised and placed immediately in cold carbogenated Krebs solution (composition in mmol l−1: NaCl, 118.4; NaHCO3, 24.9; CaCl2, 1.9; MgSO4, 1.2; KH2PO4, 1.2; glucose, 11.7). Segments of jejunum 3–3.5 cm in length were prepared, each with a mesenteric neurovascular arcade emanating from the middle of the segment. Preparations were transferred to a purpose built recording chamber and continuously superfused at 3 ml min−1 with carbonated Krebs solution and kept at 35°C. A mesenteric nerve bundle was isolated and placed in a glass suction electrode connected to a Neurolog NL100AK headstage (IBIS Instrumentation, Ottawa, Canada). Signals were amplified with an NL104 amplifier, band pass filtered at 200–3000 Hz with an NL125 filter. The signal was acquired at 20 kHz using a Micro1401 interface and recorded and displayed on a PC running the Spike2 software package (Cambridge Electronic Design, Cambridge, UK). All drugs were applied by bath superfusion for 10 min with a washout period of 30 min between drug applications. Responses were assessed by mean change in afferent activity over a 100 s period (peak) compared to a 100 s baseline period. All data were expressed as means ± SEM. Significant differences between two groups was determined by Student's t test for unpaired data (P < 0.05).
Investigating mechanosensitivity of jejunal afferents
In order to investigate mechanosensitivity, segments of jejunum were cannulated at both ends and connected to an input and an output port. The input port was attached to an infusion pump enabling the continuous perfusion of the lumen with Krebs solution when the output port was open and periodic distension of the jejunum when the output port was closed. A pressure transducer (NL 108, Digitimer, Welwyn Garden City, UK) was attached in parallel to the input port, allowing the intraluminal pressure to be continuously monitored. Preparations were distended at 10 min intervals with Krebs solution at a rate of 200 μl min−1 to a maximal intraluminal pressure of 50 mmHg. The pressure–response relationship was determined using a custom made script (provided by Cambridge Electronic Design, CED) in the Spike2 interface.
The single unit discrimination function of Spike2 was used to identify individual single afferent units based on their spike shape and morphology. Single units were classified into two broad groups depending on their activation thresholds. A unit was considered as responding to a stimulus if the afferent discharge increased by 20% above baseline. Units increasing their discharge rate at pressures <15 mmHg were classified as low threshold and units responding at pressures >15 mmHg were classified as high threshold. Multiple comparisons were made using ANOVA with the Bonferroni post hoc test.
Isolated neuron recordings
Retrograde labelling
To identify nodose ganglion neurons projecting to the GI tract, the fluorescent retrograde tracer fast blue was utilized as previously described (Beyak et al. 2004). Briefly, following general anaesthesia with ketamine–xylazine, a midline laparotomy was performed. The small intestine was exposed, but care was taken not to manipulate it as much as possible. Five to ten injections (1 μl) of fast blue (17 mg ml−1 in water) were made using a Hamilton microlitre syringe, with a 32 gauge needle. The injection sites were carefully inspected for leakage, the abdomen was irrigated with warm saline, and then sutured closed. Nodose ganglia were harvested 7 days later as described below.
Nodose ganglion neuron isolation
The nodose ganglia were located by following the cervical vagi to the base of the skull, rapidly excised and placed in sterile Ca2+ and Mg2+-free Hanks’ balanced salt solution (HBSS; Gibco-BRL, Burlington, ON, Canada) pH 7.4, and adhering connective tissue dissected without desheathing the ganglia. The neurons were then dissociated using the following protocol. Whole ganglia were incubated (10 min, 37°C) in 0.2 mg ml−1 papain (Worthington Biochemical Corp., Lakewood, NJ, USA), which had previously been activated with 0.4 mg ml−1 cysteine (Sigma-Aldrich Co., St Louis, MO, USA), followed by two washes of HBSS. This was followed by a 10 min incubation in a HBSS solution containing 1 mg ml−1 collagenase type II (Worthington) and 4 mg ml−1 dispase II (Boehringer-Mannheim, Gaithersburg, MD, USA). Reaction was stopped with tissue culture medium (F12, GIBCO and 10% fetal calf serum). Ganglia were then triturated 10 times through a flame-polished Pasteur pipette until the tissue was fully dispersed into a single-cell suspension. The suspension was subjected to low speed centrifugation for 5 min at 1000 rpm (136 g) to remove myelin debris. The resulting neuron pellet was resuspended in tissue culture medium and layered onto coverslips pre-coated with PureCol (Inamed, Gauting, Germany). Cells were kept in an incubator at 37°C (5% CO2) for 12–24 h.
Whole cell patch clamp recordings
Whole-cell patch clamp recordings were performed 12–24 h after neuron isolation. Coverslips containing nodose ganglion neurons were placed in a recording chamber and mounted on an inverted microscope (Axiovert 10, Zeiss, Montreal Canada). Labelled neurons were identified by their bright blue fluorescence. Glass microelectrodes were pulled with a PC-10 puller (Narishige, Japan). The pipette resistance was between 2 and 4 MΩ. Signals were amplified by using a Multiclamp 700B voltage clamp amplifier and digitized with a Digidata 1440A A/D converter (Molecular Devices, Sunnyvale, CA, USA). After obtaining whole cell access, membrane access resistance was stable reaching less than 10 MΩ. Using the amplifier circuitry, series resistance was compensated up to 75–80%. In current clamp mode, electrophysiological properties were assessed. To assess the rheobase, 500 ms current pulses were applied to the cells from −10 to 290 pA with an increment of 10 pA. Action potentials were evoked with 15 ms depolarizing current pulses. Recordings were analysed by the Clampfit 10.0 software (Molecular Devices) and Origin (OriginLab Corp., Northampton, MA, USA).
Calcium imaging
Nodose ganglion neurons were incubated at room temperature with Fura-2 AM for 30 min (4 μm final concentration in culture media). After the incubation, the cells were superfused with Hepes buffer for 30 min. The experiments were performed at room temperature using calcium imaging system and software ImageMaster (PTI, NJ, USA) and an inverted epifluorescence microscope (Olympus 1X71, Olympus Canada, Markham, Ontario) with a fluorescence lens. The images were captured with a CCD Camera (Cascade 512B, Roper Scientific, Tuscon, AZ, USA). The data were expressed as the 340/380 nm ratio. A positive response was defined as an increase in fluorescence ratio of twice baseline. All data were analysed in Origin. All data are expressed as means ± SEM. Significance was determined by an independent Student's t test (P < 0.05).
Solutions for patch clamp and calcium imaging experiments
For patch clamp experiments, the extracellular solution used contained (mM): NaCl, 135; KCl, 5; MgCl2, 1; CaCl2, 2; Hepes, 10; glucose, 10; pH adjusted to 7.4 using NaOH. The intracellular solution contained (mM): KCl, 140; Hepes, 5; MgCl2, 5; EGTA, 0.05; pH adjusted to 7.2 using KOH. Identical extracellular solutions were used to superfuse the cells in Ca2+ imaging experiments. All drugs were prepared as stock solution, then diluted to their final concentrations in bath solution. Drugs were dissolved in distilled water or DMSO. Sulfated cholecystokinin octapeptide (CCK-8S), was purchased from Tocris Bioscience (Bristol, UK), 5-HT from Sigma-Aldrich, and Fura-2 AM from Molecular Probes (Eugene, OR, USA).
Results
Induction of diet induced obesity
Mice were randomly divided into two groups, a control group fed a commercially available low-fat diet (10% calories from fat, low fat fed, LFF) and an obese group fed a nutrient matched high-fat diet (60% of calories from fat, high fat fed, HFF). Mice fed the HFF diet began to gain significantly more weight after 6 weeks on the diet compared to mice fed the LFF diet (P < 0.001, 2-way ANOVA, Bonferroni post hoc test), this increased weight gain continued for the duration of the study and at the time of experimentation (12–14 weeks of age) HFF mice were significantly heavier at 32.2 ± 1.0 g (n = 25) than LFF mice whose mean body weight was 25.9 ± 0.3 g (n = 25, P < 0.0001, Student's t test, Fig. 1A–C).
Figure 1. Effect of HFF diet on body weight, fat mass and blood glucose.
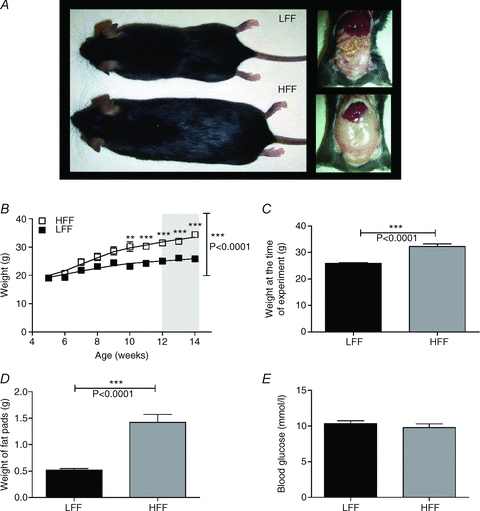
A, mice fed a high fat diet were significantly larger and had more visceral fat compared to mice fed a low fat diet. B, body weight of HFF and LFF animals over a 10 week period of feeding; HFF animals gained significantly more weight after 6 weeks of feeding compared to LFF animals (P < 0.0001, 2-way ANOVA and Bonferroni post hoc test, n = 25 each). All experiments were conducted on mice at 12–14 weeks of age. C, the mean body weight of HFF animals was significantly greater than the mean body weight of LFF animals at the time of experimentation. D, HFF animals had larger epididymal fat pads than LFF (n = 8 each). E, blood glucose levels (fed) were not significantly different in the HFF group compared to the LFF group (n = 11 LFF, 10 HFF).
In order to quantify the relative amounts of visceral fat, the epididymal fat pads were removed and weighed. LFF mice had a mean fat pad weight of 0.5 ± 0.03 g (n = 8); HFF mice had significantly heavier fat pads at 1.4 ± 0.1 g, (n = 8, P < 0.006, Student's t test) demonstrating that mice fed a high fat diet had increased amounts of visceral fat (Fig. 1D). To ensure that the data in this study were not confounded by the onset of diabetes and hyperglycaemia, resting blood glucose levels were examined in the fed state. Mean blood glucose level drawn at 09.00 h after ad lib feeding overnight for LFF mice was 10.3 ± 0.4 mmol l−1 (n = 11); this was unaltered in the HFF population (n = 10 Fig. 1E).
Diminished intestinal afferent responses to satiety mediators in HFF mice
In order to evaluate the function of afferent nerves innervating the intestine, whole nerve multiunit extracellular recordings were conducted. There afferent nerves typically exhibit a low level spontaneous discharge during such recordings. Spontaneous afferent discharge rate arising from isolated segments of jejunum was ∼70% lower in preparations from HFF animals compared to LFF animals (P < 0.0001, Student's t test, n = 10 and 10, respectively, Fig. 2A).
Figure 2. Attenuated intestinal afferent responses to chemical stimulation in HFF mice.
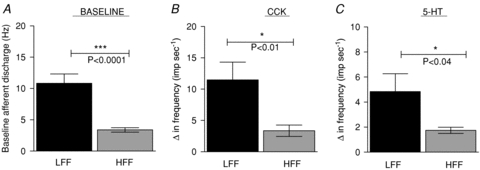
A, baseline afferent discharge arising from the isolated jejunum was significantly attenuated in HFF mice compared to LFF mice (P < 0.001 Student's t test). B, the mesenteric afferent response to CCK application (100 nm) was significantly lower from HFF mice compared to LFF mice. C, the mesenteric afferent response to 5-HT application (10 μm) was significantly lower from HFF mice compared to LFF mice (Student's t test, n = 10 for each group).
The response of these jejunal afferents to the satiety mediators CCK (100 nm) and 5-HT (10 μm) was tested. In preparations from LFF animals, application of CCK increased afferent discharge by 11.5 ± 2.8 Hz (n = 10). However in preparations from HFF animals, application of CCK caused significantly smaller responses (3.4 ± 0.9 Hz, P < 0.01, Student's t test, n = 10, Fig. 2B). A similar phenomenon was observed following application of 5-HT whereby jejunal afferent responses to application of 5-HT were significantly lower in preparations from HFF animals (n = 10) compared to LFF animals (n = 10, P < 0.04, Student's t test).
Jejunal afferent mechanosensitivity was attenuated in HFF mice
In addition to investigating altered baseline activity and chemosensitivity in chronic diet induced obesity, the impact of chronic high fat feeding on mechanosensitivity of jejunal afferents was examined. Ramp distension of isolated segments of jejunum induced a typical biphasic increase in afferent discharge which was concomitant with increased intraluminal pressure. In preparations from HFF animals the afferent response to distension was significantly attenuated (P < 0.0001, 2-way ANOVA, Bonferroni post hoc test, Fig. 3). This attenuation was most apparent between 10 and 30 mmHg where afferent response to distension was ∼40% less in HFF jejunal preparations (n = 8) compared to LFF preparations (n = 6), suggesting a reduction in the sensitivity of low-threshold mechanoreceptors. To determine whether changes in muscle compliance were responsible for this altered mechanosensitivity, the pressure–volume relationship was measured. In preparations from both HFF (n = 8) and LFF (n = 6) animals the pressure–volume relationship was identical (Fig. 3C).
Figure 3. Diminished intestinal afferent mechanosensitivity in HFF mice.
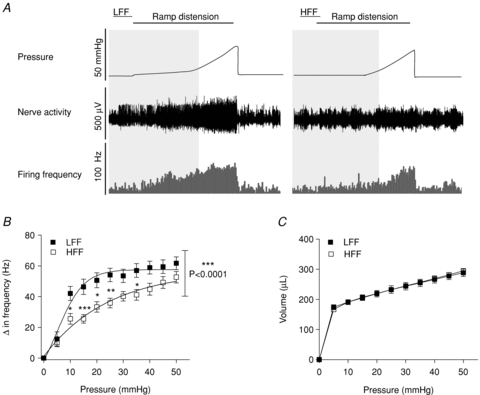
A, representative traces showing the distension induced biphasic multiunit afferent response to jejunal distension in preparations from LFF and HFF mice. B, pressure–response relationship. Multiunit afferent response to increased intraluminal pressure was significantly attenuated (P < 0.0001, 2-way ANOVA and Bonferroni post hoc test) in HFF mice (n = 8) compared to LFF mice (n = 6). C, pressure–volume relationship. Muscle compliance was unaltered in the HFF mice compared to the LFF mice.
Using the single unit discrimination function of Spike2, it is possible to discriminate single units from a multiunit recording based on action potential morphology (Fig. 4). This then allows examination of the stimulus–response relationship of each of the individual fibres. Using these techniques, a total of 33 single mechanosensitive afferent units were discriminated from multiunit recordings from LFF jejunal segments (n = 6 animals) and 42 single mechanosensitive units were identified in preparations from HFF animals (n = 8). The units were classified as either low-threshold afferents (respond to distension at <15 mmHg) or high-threshold afferents (respond to distension at >15 mmHg). The proportions of low-threshold and high-threshold units identified were not significantly different between the two groups of mice (Fig. 5C), nor did the number of units discriminated per multiunit preparation. The low-threshold afferent response to jejunal distension was significantly inhibited in HFF preparations compared to LFF preparations by ∼50% (P < 0.0001, 2-way ANOVA and Bonferroni post hoc test). In contrast, the afferent response to distension for high-threshold units was similar between the two groups (Fig. 5A and B).
Figure 4. Single unit analysis of jejunal afferent responses to distension.
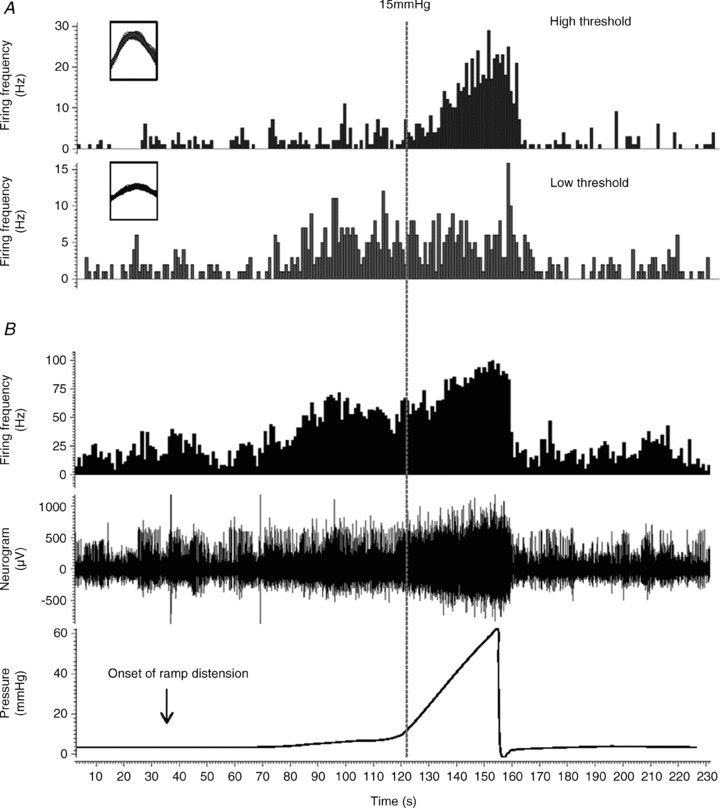
A, single unit analysis was used to identify distinct single units contributing to the whole nerve discharge (shown in B, middle trace) (see Methods). Overlaid action potentials are shown in the insets, which illustrates the distinct amplitude and duration of 2 distinct single units. Each inset represents a period of 2 ms. Note that each unit has a distinct threshold and response profile which is used to classify individual units into LT and HT afferents. B, representative traces of increased intraluminal pressure (lower trace), concurrent increase in afferent discharge (middle) and whole nerve spike rate histogram (upper).
Figure 5. Selective impairment of low threshold mechanosensitivity induced by HFF diet.
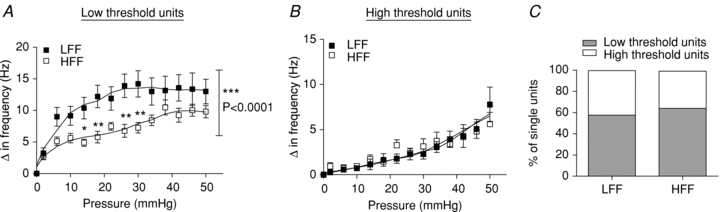
Single units were discriminated from the whole nerve recording using computer software and classified into low and high threshold units. A, pressure–response relationship of low threshold units. Low threshold afferent activity was significantly attenuated in preparations from HFF mice (n = 27 units) compared to preparations from LFF mice (n = 19 units, P < 0.0001, 2-way ANOVA and Bonferroni post hoc test). B, pressure–response relationship of high threshold units. High threshold afferent activity was not altered in preparations from HFF mice (n = 15 units) compared to preparations from LFF mice (n = 14 units). C, the proportion of low threshold and high threshold afferents was similar between the two groups (P > 0.05, Fisher's exact test).
Membrane excitability was decreased in nodose ganglion neurons from HFF mice
In vitro whole cell current clamp recordings were conducted to examine the active and passive membrane properties of randomly selected dissociated nodose ganglion neurons from LFF and HFF mice. Current-clamp studies using sequential stepwise current pulses revealed an elevated rheobase (minimum current necessary to elicit a single action potential) in nodose neurons from HFF mice (125.4 ± 11.8 pA; n = 13) compared to LFF mice (67.3 ± 12.3 pA; n = 15; Student's t test, independent, P < 0.05, Fig. 6C) and a significant reduction in the number of action potentials elicited at twice rheobase (1 ± 0; n = 8 vs. 3.7 ± 0.9; n = 13 in LFF; Student's t test, independent, P < 0.05, Fig. 6E). Additionally input resistance (Rinput) was significantly lower in neurons from HFF mice (185.6 ± 51.5 MΩ; n = 13) compared to neurons from LFF mice (448.2 ± 103.1 MΩ; Student's t test, independent, P < 0.05, Fig. 6D), suggesting a global decrease in the excitability of HFF nodose ganglion neurons. The membrane properties and action potential parameters were also compared. The resting membrane potential was not significantly different between the two populations, nor were characteristics of action potentials such as the duration, threshold, maximum rise slope, overshoot amplitude and maximum decay slope. Action potentials were followed by a fast after-hyperpolarization (fAHP <10 ms); no significant difference in the amplitude of this AHP was observed in the HFF nodose neurons compared to controls.
Figure 6. Impact of diet induced obesity on nodose ganglion neuron excitability.
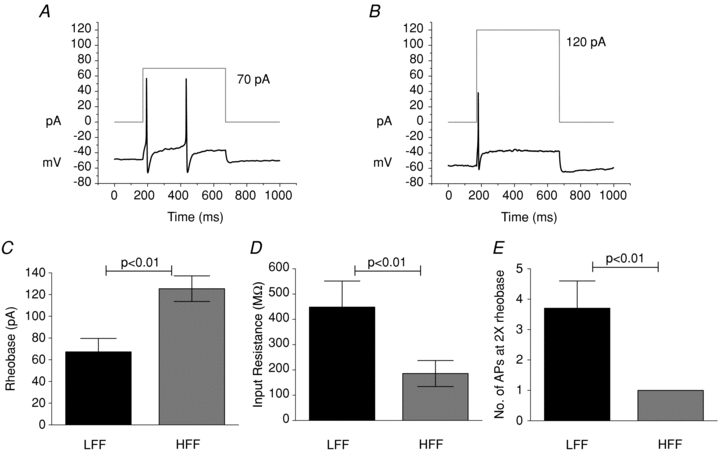
The rheobase was determined as the lowest current leading to action potential. The representative action potential firing elicited by a 500 ms current pulse (grey square wave) in LFF (A) and HFF (B) nodose ganglion neuron. More current is required to elicit an action potential in HFF neurons. C, rheobase was significantly increased while there was a significant reduction in input resistance (D) (Student's t test, n = 15 LFF, 13 HFF). At twice rheobase, significantly more action potentials were elicited in neurons from LFF comapred to HFF animals. E, Student's t test, n = 13 LFF, 8 HFF).
To determine the effect of diet induced obesity on the vagal afferent population specifically innervating the small intestine, we also performed experiments on nodose neurons labelled by fast blue injection into the wall of the small intestine. When fluorescent neurons were studied (n = 10 LFF, 12 HFF), similar results were seen, compared to unlabelled neurons (Fig. 7). In labelled neurons from HFF mice, rheobase was significantly greater than in neurons from LFF mice (96.7 ± 9.7 pA vs. 47.0 ± 16.5 pA, P < 0.05, unpaired t test). The number of action potentials at twice rheobase was also fewer in HFF neurons (1.0 ± 0 vs. 4.3 ± 1.1, P < 0.05) and the input resistance was less (154.8 ± 30.9 MΩ, HFF vs. 430.3 ± 73.4 MΩ, LFF). Other parameters such as resting membrane potential, duration, threshold, maximum rise slope, overshoot amplitude, afterhyperpolarization and maximum decay slope were unaffected.
Figure 7. Impact of diet induced obesity on excitability of intestine-projecting nodose neurons.
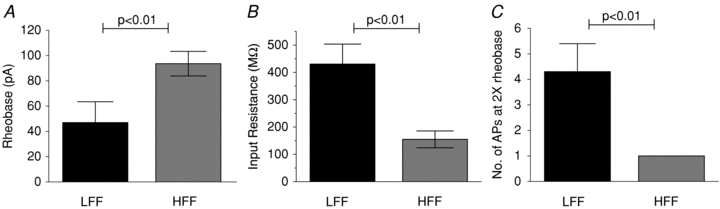
High fat feeding-induced obesity resulted in decreased excitability of fast blue labelled, intestine projecting vagal afferents. This was manifested by an elevation of rheobase (A), fall in input resistance and diminished numbers of action potentials at twice rheobase, similar to the effect seen in unlabelled neurons (unpaired Student's t test, n = 15 LFF, 13 HFF).
Fewer nodose neurons respond to CCK and 5-HT in high fat fed mice
Ratiofluorometric calcium imaging was used to screen nodose neurons for responsiveness to both CCK and 5HT in nodose neurons from LFF and HFF mice. Of nodose neurons from LFF animals, 25.4% responded to CCK with an elevation in intracellular calcium (100 nmn = 67) and 20.9% of LFF neurons responded to 5-HT (1 μm, n = 67). The proportion of neurons from HFF mice responsive to both CCK and 5-HT was markedly lower at 13.2% and 9.5% respectively (Fig. 8, P < 0.05, Fisher's exact test). In responsive cells the magnitude of the increase did not differ between HFF and LFF groups.
Figure 8. Fewer nodose neurons respond to satiety mediators in HFF mice.
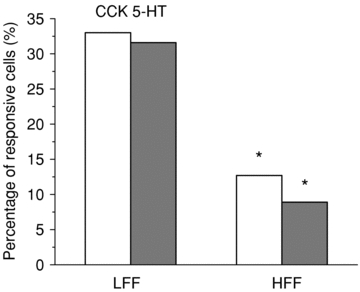
Ratiofluorometric calcium imaging was used to screen nodose neurons for responsiveness to both CCK and 5-HT in nodose neurons from LFF (left) and HFF (right) mice. CCK (white bars) and 5-HT (grey bars) induced an all or none Ca2+ increase. Thirty-three per cent of nodose neurons from LFF animals responded to CCK with an elevation in intracellular calcium (100 nmn = 100) and 31.6% of LFF neurons responded to 5-HT (1 μm, n = 136). The proportion of neurons from HFF mice responsive to both CCK and 5-HT was markedly lower at 12.7% and 8.9%, respectively (*P < 0.0005, Fisher's exact test).
Discussion
An important unanswered question crucial to the understanding of the pathophysiology of overeating and obesity is, how is it possible, in the face of multiple overlapping controls limiting food intake, to eat in excess of nutrient requirements and develop obesity? This study has provided insight into this question by demonstrating that when obesity is induced with a high fat diet, there is a resultant reduction in intestinal afferent responses to satiety signals, and that this may in part be due to decreased electrical excitability of the vagal afferents themselves. As satiety signals arising from the gut are thought to be critically important in regulating meal size, this may be a key event in either allowing overeating to occur, or in perpetuating the obese state once initiated.
The high fat fed C57Bl6/J mouse is an appropriate model for diet induced obesity as it combines a genetic predisposition with an environmental factor (high fat feeding) to result in obesity (Surwit et al. 1995; Collins et al. 2004), which ultimately can lead to type 2 diabetes (Surwit et al. 1988), replicating many cases of human obesity. This and other animal models also develop hyperphagia when fed a high fat diet (Lin et al. 2000a; Petro et al. 2004; Gallou-Kabani et al. 2007), suggesting impaired intrameal satiety signalling. We confirmed, in our hands, excess weight gain in animals fed a high fat diet for 12–14 weeks, and that this increase in body weight was accompanied by increased visceral fat mass. Importantly at the time of killing, there was no difference in fed blood glucose levels in the low or high fat fed mice, as hyperglycaemia and diabetes may have confounded the results due to development of diabetic visceral sensory neuropathy (Beyak et al. 2009).
The finding of decreased intestinal afferent responsiveness provides an explanation for a number of previous observations. For example, it has previously been demonstrated that in rat models of obesity that (i) satiety responses induced by CCK are attenuated (Covasa & Ritter, 1998), (ii) that satiety responses to intraluminal lipid are attenuated (Covasa & Ritter, 1999), and (iii) activation of neurons of the nucleus of the solitary tract (the site of termination of vagal afferents) in response to these stimuli is also diminished in obesity (Covasa et al. 2000a,b; Donovan et al. 2009). As the effects of 5-HT and CCK are almost solely due to activation of vagal afferent fibres, we hypothesized that that there may be a decreased responsiveness of these fibres to these mediators. Furthermore both of these mediators have been shown to be involved in satiating effects of both lipid and non-lipid nutrients (Hayes & Covasa, 2005; Savastano et al. 2005). Our study is the first to date to directly examine the response of afferent fibres innervating the intestine to CCK and 5-HT in obesity, demonstrating direct evidence for altered responses at the level of the afferent terminal in the intestine. While it is acknowledged that the present experiments recorded from a mix of both vagal and spinal afferents, there is abundant evidence in the literature that the effects of these compounds is likely to be limited to the vagal subpopulation (Richards et al. 1996; Hillsley & Grundy, 1998; Zhu et al. 2001), especially responses relevant to control of food intake (Schwartz et al. 1993; Moran et al. 1997). The potential mechanisms of the change in responsiveness are likely to be multiple. For example it is possible that due to increased stimulation, there is increased local release of 5-HT and CCK in the microenvironment where these afferent terminals lie, and this leads to receptor downregulation or alterations in ion channels that mediate the downstream effects. Some evidence for receptor downregulation is provided by Nefti et al.(2009), where after a short term high fat diet, CCK and 5HT3 receptor expression was decreased in vagal afferents; however these investigators did not examine the effects of more chronic high fat feeding. In addition, work in our study suggests the possibility that more global changes, i.e. in excitability of the neuronal membrane itself, are also at play.
In addition to altered chemosensitive responses in intestinal afferents, we also demonstrated significant alterations in mechanosensitivity. Jejunal afferents in both the rat and mouse have been shown previously to respond to distension in different ways. There is an initial activation of low threshold units, and these are primarily believed to be vagal as this component of the response is eliminated by vagotomy, and as the distension pressure increases, there is a graded activation of higher threshold, likely to be spinal, endings (Booth et al. 2008). The effect of chronic high fat feeding was to selectively attenuate responses at low distension pressures, suggesting an effect on these low threshold mechanosensitive endings. Previous work in the mouse jejunum has identified both low and high threshold units, based on responses to different distension pressures (Rong et al. 2004). Using similar single unit analysis techniques to discriminate individual mechanosensitive units based on their response profiles, we were able to demonstrate that the function of low threshold units was impaired, while high threshold units had their function preserved. As the high threshold units are believed to be spinal this suggests a selective effect on vagal mechanosensitive fibres innervating the jejunum. This altered mechanosensory function has a possible correlate in obese humans who can tolerate greater volumes of ingestion than can lean subjects, suggesting that similar impairment of mechanosensory function may exist in the GI tract of obese humans (Camilleri, 2009), though we acknowledge that volume of intake is likely to depend more on gastric than intestinal mechanisms. The exact pathways involved are open to speculation, and our demonstration of altered excitability of vagal afferents is likely to play an important role. However with recent developments in the ion channels underlying visceral mechanosensitivity, it is also logical to investigate alterations in channels such as TRPV1, ASIC, TRPV4 and TRPA1, which have been shown to play a role in mechanosensory function in gastrointestinal afferents (Brierley, 2010).
Since basal afferent discharge as well as chemical and mechanosensitivity was decreased in afferents from HFF mice, we examined whether there may be a reduction in excitability at the level of the cell membrane. A reduction in membrane excitability may be a unifying mechanism to explain decreased responses to diverse stimuli. As assessment of membrane excitability at the level of the sensory terminal is impossible, we used whole cell recordings from nodose ganglion neurons (vagal afferent cell bodies) as a model. Recordings were performed on both randomly selected as well as neurons labelled by fast blue injection into the intestinal wall. In nodose ganglion neurons from high fat fed mice there was a significant increase in rheobase (current threshold), which would effectively require a greater stimulus to elicit action potential firing from these afferents. Since at the level of the nerve terminal it is the generator potential elicited by either the chemical (e.g. satiety mediator) or mechanical stimulus (gut distension) that must exceed the current threshold to result in action potential firing, this has significant implications for the function of these afferents (Carr, 2005). Simply put, a greater degree of stimulus, whether that is greater concentrations of satiety hormone or greater gut distension, is required for signals to get from the gut to the brain. Furthermore we show that at a suprathreshold stimulus (twice rheobase), fewer action potentials are elicited. Thus for a given stimulus, it is more difficult to elicit afferent transmission, and when elicited, the strength of the signal (in terms of action potential numbers) is diminished. These alterations are accompanied by a decreased input resistance of the membrane – that is the cell membrane accommodates more to a given electrical stimulus. The significant alteration in input resistance points to a key mechanism of decreased responsiveness of vagal afferents in this obese model as it indicates altered membrane conductances at rest. As the resting membrane resistance is determined for the most part by potassium conductances, such as the two pore domain channels, these are a potential target, but other channels may play a role such as voltage-gated potassium channels or impairment of rapidly repriming sodium currents (Stewart et al. 2003; Beyak et al. 2004). Definitive identification of the specific channels responsible, however, requires further detailed study. Importantly, similar changes were found in both small intestine-projecting as well as randomly selected nodose ganglion neurons, indicating that changes in excitability affect the whole population of vagal afferents, not just those projecting to the GI tract. This may point to altered circulating mediators as an underlying cause for these changes.
In addition we demonstrated, using calcium imaging, that the number of nodose neurons responding to CCK and 5-HT is also diminished by chronic high fat feeding. This could result from two possibilities. The first is that due to changes in excitability that we have seen, the proportion of cells reaching threshold for activation by CCK and 5-HT is significantly less, because much of the calcium influx is via voltage gated calcium channels (Lankisch et al. 2002) that are activated when the neuron is depolarized by activation of CCK and 5HT3 receptors. Alternatively, there may be additional changes in receptor expression. For example Nefti et al.(2009) have recently shown that short term high fat feeding reduced mRNA for both CCK1 and 5HT3 receptors. Other studies have demonstrated alterations in feeding related mediator receptor expression in high fat fed rodents (Paulino et al. 2009). Our study lays the groundwork for further investigation into the effects of long term high fat feeding on satiety mediator receptor expression and function, but this idea awaits the results of definitive studies measuring levels of receptor expression using PCR or Western blot techniques.
The underlying mechanism for these changes clearly requires further study. The fact that we have observed changes in the global excitability of randomly selected nodose neurons suggests that circulating factors may be at play. Circulating lipid levels would be expected to be higher, which has been reported to be associated with peripheral nerve dysfunction. In addition, these mice would be expected to have elevated levels of circulating adipose derived mediators such as leptin and also would be hyperinsulinaemic, in this prediabetic model. The effect of chronic elevation of circulating levels of adipose derived mediators on afferent nerve function is unknown, as is the effect of hyperinsulinaemia. Overall our results cannot distinguish between the relative roles of the altered diet alone, vs. the alteration in circulating factors in obesity that may also play a role, and this will be a critical question for further investigation. In addition, using a similar model of high fat induced obesity Obrosova et al.(2007) have demonstrated somatic sensory neuropathy, very similar in nature to diabetic neuropathy, involving some of the same pathways (e.g. the aldose reductase pathway). However the finding of euglycaemia in our study strongly suggests that diabetes mellitus and hyperglycaemia is not responsible for decreased function of these afferent fibres. However when diabetes mellitus ensues, the impairment of afferent function is likely to be magnified. Furthermore given recent evidence that afferent neural pathways may play important roles in regulating glucose metabolism (Vahl et al. 2007; Wang et al. 2008; Cheung et al. 2009), out results may have relevance to the early development of glucose intolerance in obesity. Indeed a recent paper by Cheung et al.(2009) suggests that CCK causes a neurally mediated reduction in glucose production, and that this action is impaired in high fat fed animals. Our observations may provide the underlying mechanisms for this effect.
In summary, the present paper has demonstrated that chronic consumption of a high fat, obesogenic diet results in a significant reduction in response of intestinal afferents to the satiety mediators CCK and 5HT, and to mechanical stimulation of the intestine by distension. A likely mechanism responsible for this alteration is a reduction in membrane excitability, likely to be due to altered membrane conductances at rest. This impairment of meal related vagal afferent function is likely to be involved in decreased satiety signalling from the gut to the brain and may be an important mechanism by which overeating and obesity occur, and are maintained. Given recently demonstrated roles for intestinal vagal afferents in regulating glucose homeostasis, these results may also have significant implications in the development of obesity-associated type 2 diabetes mellitus. Strategies to enhance vagal afferent excitability may therefore prove fruitful in treating and preventing obesity, and improving insulin sensitivity. Furthermore the knowledge that chronic consumption of unhealthy diets can alter the function of important controls on eating may help to inform public policy and individuals regarding dietary choices.
Acknowledgments
The authors wish to specially thank Iva Kosatka for her expert technical assistance. The assistance of Dr Stephen Vanner, Dr Alan Lomax, Ala'a Al Helaili and Drew Webster in reading the manuscript and providing helpful advice is appreciated. This work was funded in part by an AGA/FDHN Research Scholar Award and CAG/CIHR/Nycomed New Investigator Award to M.J.B. D.D. was supported by a CAG/CIHR/Axcan Postdoctoral Research Fellowship. W.C.V. was the recipient of a CAG/CIHR summer Studentship award.
Glossary
Abbreviations
- CCK
cholecystokinin
- GLP-1
glucagon like peptide-1
- HFF
high fat fed
- 5-HT
5-hydroxytryptamine
- LFF
low fat fed
- PYY
peptide YY
Author contributions
All authors were involved in collection, analysis and interpretation of data, drafting and revision of the manuscript and statistical analysis. M.J.B. supervised the study and obtained grant support. None of the authors have any conflicts of interest to declare.
Author's present address
D. M. Daly: Florey Building, Department of Biomedical Sciences, University of Sheffield, Western Bank, Sheffield, UK.
References
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Avenell A, Sattar N, Lean M. ABC of obesity. Management: Part I – behaviour change, diet, and activity. BMJ. 2006;333:740–743. doi: 10.1136/bmj.333.7571.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20(Suppl 1):64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyak MJ, Bulmer DC, Sellers D, Grundy D. Impairment of rectal afferent mechanosensitivity in experimental diabetes in the rat. Neurogastroenterol Motil. 2009;21:678–681. doi: 10.1111/j.1365-2982.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–G855. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- Booth CE, Shaw J, Hicks GA, Kirkup AJ, Winchester W, Grundy D. Influence of the pattern of jejunal distension on mesenteric afferent sensitivity in the anaesthetized rat. Neurogastroenterol Motil. 2008;20:149–158. doi: 10.1111/j.1365-2982.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- Brierley SM. Molecular basis of mechanosensitivity. Auton Neurosci. 2010;153:58–68. doi: 10.1016/j.autneu.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, Hellstrom PM. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil. 2009;21:978–e78. doi: 10.1111/j.1365-2982.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics and role in influencing neurochemical phenotype. Am J Physiol Gastrointest Liver Physiol. 2010;299:G63–G69. doi: 10.1152/ajpgi.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Community Health Survey. 2005. xxxx. Statistics Canada.
- Camilleri M. Peripheral mechanisms in the control of appetite and related experimental therapies in obesity. Regul Pept. 2009;156:24–27. doi: 10.1016/j.regpep.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MJ. Chemical transduction in vagal afferent endings. In: Unden B, Weinreich D, editors. Advances in Vagal Afferent Neurobiology. Boca Raton: CRC Press; 2005. pp. 167–189. [Google Scholar]
- Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000a;86:83–88. doi: 10.1016/s0167-0115(99)00084-1. [DOI] [PubMed] [Google Scholar]
- Covasa M, Grahn J, Ritter RC. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton Neurosci. 2000b;84:8–18. doi: 10.1016/S1566-0702(00)00176-4. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19:1407–1415. doi: 10.1016/s0196-9781(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Reduced sensitivity to the satiation effect of intestinal oleate in rats adapted to high-fat diet. Am J Physiol Regul Integr Comp Physiol. 1999;277:R279–R285. doi: 10.1152/ajpregu.1999.277.1.R279. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lartigue G, Dimaline R, Varro A, Raybould H, De la Serre CB, Dockray GJ. Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology. 2010a;138:1479–1490. doi: 10.1053/j.gastro.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology. 2010b;151:3589–3599. doi: 10.1210/en.2010-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ. The versatility of the vagus. Physiol Behav. 2009;97:531–536. doi: 10.1016/j.physbeh.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 2011;201:313–321. doi: 10.1111/j.1748-1716.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Paulino G, Raybould HE. Activation of hindbrain neurons in response to gastrointestinal lipid is attenuated by high fat, high energy diets in mice prone to diet-induced obesity. Brain Res. 2009;1248:136–140. doi: 10.1016/j.brainres.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Baronowsky EA, Byerly MS, Jones S, Powley TL. Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J Neurosci. 2001;21:8602–8615. doi: 10.1523/JNEUROSCI.21-21-08602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Byerly MS, Baronowsky EA, Chi MM, Powley TL. Selective loss of vagal intramuscular mechanoreceptors in mice mutant for steel factor, the c-Kit receptor ligand. Anat Embryol (Berl) 2002;205:325–342. doi: 10.1007/s00429-002-0261-x. [DOI] [PubMed] [Google Scholar]
- Gallou-Kabani C, Vige A, Gross MS, Boileau C, Rabes JP, Fruchart-Najib J, Jais JP, Junien C. Resistance to high-fat diet in the female progeny of obese mice fed a control diet during the periconceptual, gestation, and lactation periods. Am J Physiol Endocrinol Metab. 2007;292:E1095–E1100. doi: 10.1152/ajpendo.00390.2006. [DOI] [PubMed] [Google Scholar]
- Grundy D, Hillsley K, Kirkup AJ, Richards W. Mesenteric afferent sensitivity to cholecystokinin and 5-hydroxytryptamine. Dtsch Tierarztl Wochenschr. 1998;105:466–468. [PubMed] [Google Scholar]
- Hayes MR, Covasa M. CCK and 5-HT act synergistically to suppress food intake through simultaneous activation of CCK-1 and 5-HT3 receptors. Peptides. 2005;26:2322–2330. doi: 10.1016/j.peptides.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Grundy D. Serotonin and cholecystokinin activate different populations of rat mesenteric vagal afferents. Neurosci Lett. 1998;255:63–66. doi: 10.1016/s0304-3940(98)00690-9. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol. 1998;506:551–561. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- Lankisch TO, Tsunoda Y, Lu Y, Owyang C. Characterization of CCKA receptor affinity states and Ca2+ signal transduction in vagal nodose ganglia. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1002–G1008. doi: 10.1152/ajpgi.00313.2001. [DOI] [PubMed] [Google Scholar]
- Lean M, Finer N. ABC of obesityManagement: part II – drugs. BMJ. 2006;333:794–797. doi: 10.1136/bmj.333.7572.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res. 2000a;875:89–95. doi: 10.1016/s0006-8993(00)02580-4. [DOI] [PubMed] [Google Scholar]
- Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000b;24:639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 1997;272:R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- Nefti W, Chaumontet C, Fromentin G, Tome D, Darcel N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1681–R1686. doi: 10.1152/ajpregu.90733.2008. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56:2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, Raybould HE. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296:E898–E903. doi: 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004;53:454–457. doi: 10.1016/j.metabol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Richards W, Hillsley K, Eastwood C, Grundy D. Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. J Physiol. 1996;497:473–481. doi: 10.1113/jphysiol.1996.sp021781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savastano DM, Carelle M, Covasa M. Serotonin-type 3 receptors mediate intestinal Polycose- and glucose-induced suppression of intake. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1499–R1508. doi: 10.1152/ajpregu.00745.2004. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Berkow G, McHugh PR, Moran TH. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol Regul Integr Comp Physiol. 1993;264:R630–R637. doi: 10.1152/ajpregu.1993.264.3.R630. [DOI] [PubMed] [Google Scholar]
- Shields M, Tjepkema M. Trends in Adult Obesity. Health Reports. 2006;17:53–59. [PubMed] [Google Scholar]
- Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol. 2003;552:797–807. doi: 10.1113/jphysiol.2003.046409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


