Abstract
The most commonly measured mouse behavior in fear conditioning tests is freezing. A technical limitation, particularly for genetic studies, is the method of direct observation used for quantifying this response, with the potential for bias or inconsistencies. We report the use of a computerized method based on latency between photobeam interruption measures as a reliable scoring criterion in mice. The different computer measures obtained during contextual fear conditioning tests showed high correlations with hand-scored freezing; r values ranged from 0.87 to 0.94. Previously reported strain differences between C57BL/6J and DBA/2J in context-dependent fear conditioning were also detected by the computer-based system. In addition, the use of computer-scored freezing of 199 (BALB/cJ × C57BL/6J)F2 mice enabled us to detect a suggestive gender-dependent chromosomal locus for contextual fear conditioning on distal chromosome 8 by QTL analysis. Automation of freeze scoring would significantly increase efficiency and reliability of this learning and memory test.
Emotional responses such as fear are rapidly acquired through classical conditioning. Fear responses are elicited by previously neutral conditioned stimuli, such as a distinctive chamber (context) or auditory cue after the conditioned stimulus has been paired with an aversive unconditioned stimulus, such as footshock. In mice, freezing is a common and easily measured response used as an index of fear conditioning (Blanchard and Blanchard 1988; Graef 1994). Fanselow (1990) and Paylor et al. (1994) define freezing as the absence of any movement except for respiratory-related movements. Freezing behavior is measured by direct observation, scoring an animal as either freezing or active per interval of time, usually every 5–10 sec (Fanselow 1990; Paylor et al. 1994) or measuring freezing duration with a stopwatch (Phillips and Le Doux 1992).
One of the central goals of cognitive science is to elucidate the molecular mechanisms of learning and memory. Genetic analysis, when combined with the powerful tools of molecular biology, promises to address this need. Fear conditioning is a good candidate for genetic analysis because it appears subject to complex genetic regulation. Behavioral analyses of targeted mutants have demonstrated roles for specific genes such as αCaMKII, PKCγ, SynII, mGluR1, or CREB in contextual fear conditioning (Abeliovich et al. 1993; Aiba et al. 1994; Bourtchuladze et al. 1994; Chen et al. 1994; Silva et al. 1996). Inbred strains of mice differ in both contextual and cued fear conditioning, and analyses of recombinant inbred strains as well as segregating generations (intercrosses or backcrosses) have indicated both significant heritability and polygenic control for these traits (Paylor et al. 1994; Owen et al. 1997a,b; Wehner et al. 1997). Recently, quantitative trait loci (QTL) analyses have identified multiple candidate gene loci for contextual fear conditioning (Caldarone et al. 1997; Owen et al. 1997a; Wehner et al. 1997). The loci identified could be limited by the number of parental strains involved, and more loci affecting this behavior could be identified as more strain combinations are examined.
A variety of approaches may be applied to the examination of the genetic regulation of complex behaviors such as fear conditioning and the identification of candidate genes involved in learning and memory processes (Takahashi et al. 1994). Genetic methods such as QTL analyses or mutagenesis screens all necessitate quantifying the behavior of large numbers of animals, which could extend over a period of months. Observer-based measures are slow and labor intensive. In addition, they are open to subjective bias and the assessment may vary both over time and between observers. The automation of learning and memory tests such as fear conditioning could speed and enhance the reliability, consistency, and practicality of such tests.
Here we report the results of three experiments. The first two experiments were intended to test the validity of a computer system based on the measure of latency between photobeam interruption in the detection and quantification of freezing behavior; the third experiment used computer-based freezing measures for QTL analysis of contextual fear conditioning.
Materials and Methods
SUBJECTS
All animals of the inbred strains and F1 progeny were purchased from the Jackson Laboratory (Bar Harbor, ME). CD1 mice were originally obtained from Harlan, and were bred in the Center for Experimental Animal Resources (CEAR) at Northwestern University. CB6F2 progeny also were bred at Northwestern University. Mice were grouped-housed (six per cage) in the animal facility, where the light/dark (LD) cycle was LD 12:12 (lights on at 5 a.m.) and the temperature was maintained constant at 23 ± 2°C. Food (Harlan Teklad) and water were available ad libitum. In experiments I and III, mice were handled daily by the experimenter 3 days before training and on the day of training the cages were moved to the experimental room. In experiment II, 3 days prior to training mice were grouped-housed 3 per cage, moved to the experimental room, and placed in ventilated, light-tight wooden cabinets where the conditions were the same as in the animal facility. For experiment I, 12 B6 female mice 2–3 months old were randomly divided into two groups: controls (n = 6, no shock given during training) and experimentals (n = 6, three shocks presented during training). For experiment II, 12 B6, 12 D2, 12 B6D2F1/J, and 10 CD1 mice, all 2–3 months old males were used. In experiments II and III, all animals were in the experimental group (i.e., no unshocked controls were run). For experiment III, B6, C, and CB6F1/J mice, all 2 months old were used. Six males and six females from each strain were tested. For the QTL analysis 199 CB6F2 mice of both sexes from 2–5 months old, were used.
APPARATUS
The Freeze Monitor (San Diego Instruments) consisted of a transparent acrylic conditioning chamber (33 cm high × 25 cm wide × 21 cm deep). A grid floor made of stainless steel rods separated by 0.5 cm was connected to a shock generator (Coulberg). The test chamber was cleaned with 70% ethanol between subjects. A frame (33 × 33 cm) with 16 infrared photobeams (2.5 cm between beams) in the horizontal plane surrounded the chamber. The freeze monitor software (San Diego Instruments) controlled the shock generator and recorded data from the photobeams. In experiment II, the conditioning chamber and surrounding frame were located inside a sound-attenuated enclosure (interior dimensions were 50 cm high × 65 cm wide × 47 cm deep). The inside of the enclosure was covered with gray acoustical foam. A 15W light bulb was centered on the ceiling. Two night lights (Limelite, Austin Instruments, TX) were placed on the sides of the sound enclosure. A small fan was located on the top of the right wall. A white noise generator (Sleep Machine, Radio Shack) was used to deliver the auditory cue (85 dB). The speaker was placed on the floor to the left side of the test chamber. Distinct geometric shapes of white paper were also located on the inside walls of the sound chamber.
TESTING PROCEDURE
All training and testing occurred during the light phase, between 10 a.m. and 5 p.m. In experiments I and III the training session consisted of placing the animal in the conditioning chamber for 6 min. After 3 min in the enclosure three shocks (1 sec, 0.6 mA) were given at 1-min intervals. The mice in experiment I that were assigned to the no-shock condition were simply placed in the test chamber for 6 min. All mice were tested for acquisition of the conditioned fear response 24 hr after the training session. The test session consisted in placing each mouse in the enclosure for 6 min (experiment I) or 8 min (experiment III) and scored for freezing using the sampling procedure described below. No shock was delivered during testing.
In experiment II, the mice were first placed in the test chamber and 2 min later a 30-sec auditory cue (white noise) was presented. Immediately after the auditory cue terminated a 2-sec, 0.6 mA foot shock was delivered. Mice were removed from the test chamber 30 sec later and returned to their home cage. Twenty-four hr later, subjects were placed into the same training chamber for 5 min and their freezing behavior was scored as described below. One to 3 hr later, subjects were tested for their freezing to the auditory cue. For the auditory cue test, the training chamber and sound enclosure were altered by placing a green plastic cover over the grid floor, illuminating the sound chamber with two green lights (0.5–1.0 lux at the level of the rods), covering with white paper the entire inside of the sound chamber, and placing a container with vanilla extract (5 ml) inside the sound chamber. The test chamber was also cleaned with 1% acetic acid between subjects. During the auditory cue test, mice were scored for 6 min; the white noise generator was turned on for the final 3 min of the session. Before the auditory testing a different person transported the animal in a transfer cage with paper towel in the place of bedding while the lights of the experimental room were off.
RESPONSE MEASURES
The basic measure of the freeze monitor is photobeam interruptions. Total activity was the total number of beam breaks per session. The software translates beam interruption in latency between photobeam interruptions. For this the whole session is divided in 5-sec bins and the latency to break the first three new photobeams in each 5-sec interval was recorded. That is, the latency from the beginning of each 5-sec interval to the first new beam interruption within that interval was (termed latency 1); the latency between the beginning of each 5-sec interval to the second new beam interruption within that interval was called latency 2; and the latency between the beginning of each 5-sec interval to the third new beam interruption within that interval was called latency 3. If a new beam interruption never occurred during the 5-sec interval, a score of 5 sec was recorded. For each animal, we computed the total amount of time to break the first, second, and third photobeams for the entire session.
In addition, we established four different criteria that were used to approximate the type of freezing scores obtained using the hand-scored procedures. First, we counted the number of 5-sec intervals in which the animals took >1 sec to cross the first new beam (termed 1sec5sec). Second, we counted the number of 5-sec intervals in which the animal took more than 2 sec to cross the first new beam (2sec5sec). The same criteria were also applied to every other 5-sec interval, the number of 10-sec intervals in which the animal took more than 1 sec to cross the first new beam of the interval and the number of 10-sec intervals in which the animal took more than 2 sec to cross the first new beam of the interval were counted (1sec10sec and 2sec10sec, respectively). For each of these measures, we computed the percentage of intervals during which the mouse was freezing.
Simultaneously with the computer scoring, the number of freezes was scored through direct observation by a time-sampling procedure. This was done by two different methods in order to compare the computer scoring with different methods of observer scoring. In experiments I and III, every 5-sec observation of the animal would start and continued for the whole 5 sec unless freezing was observed. If freezing occurred, observation was halted until the beginning of the next 5-sec interval. In experiment II, every 10 sec the animal was observed for 1 sec and judged as either freezing or active, this judgment being made at the instant that the sample was taken (Paylor et al. 1994; Owen et al. 1997b; Wehner et al. 1997). Freezing was defined as the absence of visible movement, except for the minor movements required by respiration. All other behavior was considered active. One observer scored experiment I. In experiment II each session was scored by at least two observers; some sessions were scored by three observers.
GENOTYPING OF F2 MICE
High-molecular-weight genomic DNA was extracted from tissue samples (either tail biopsies or liver) collected from each of the 199 CB6F2 progeny using a standard proteinase K digestion and phenol–chloroform extraction procedure (Ausubel et al. 1995). Genotyping of all F2 DNAs used simple sequence length polymorphisms (SSLPs; Copeland et al. 1993; Dietrich et al. 1994, 1996), obtained as MapPairs from Research Genetics. SSLP genotyping methods were slightly modified from those of Dietrich et al. (1992), and are described here. PCR reactions were carried out in 10-μl volumes, using ∼25–40 ng template DNA in 5 μl of H2O, 0.25 units of Amplitaq DNA (Perkin Elmer), 200 nm each dNTP, 0.85× GeneAmpPCR buffer II (1× buffer: 50 mm KCl, 10 mm Tris-HCl at pH 8.3), 1 μg/μl bovine serum albumin (BSA), and 1.275 mm MgCl. One hundred ten nanomolar of each primer was used, with all of the forward primer aliquots for each reaction labeled with [γ-32P]ATP (sp. act. 6000 Ci/mmole, DuPont/NEN), using T4 polynucleotide kinase. PCR reactions were carried out on either a 96- or 192-well PTC-100 thermal cycler (MJ Research). The thermocycling profile was 94°C for 4 min, 27–35 cycles of (94°C for 15 sec, 55°C for 2 min, 72°C for 2 min), followed by a 7 min extension step at 72°C. PCR products were separated on 7% denaturing acrylamide sequencing gels and visualized by autoradiography. The genotype, either homozygous B6 or C, or heterozygous (B6/C), was scored for 121 loci throughout the mouse genome.
QTL ANALYSIS
A total of 199 CB6F2 mice were genotyped for 121 SSLPs spaced at ∼20- to 30-cM intervals throughout the genome. Five different computer-measured freeze scores were used as phenotypic traits for the quantitative trait loci analysis. These were 1sec5sec, 2sec5sec, 1sec10sec, 2sec10sec, and latency 3. Genotypic and phenotypic scores were entered into an MS-Excel worksheet. The order of SSLP markers was based on the MIT/Whitehead Institute map (Dietrich et al. 1996), and for some chromosomal regions in which additional markers were analyzed, the most likely order and genetic distances between markers was calculated using the program MAPMAKER (Lander et al. 1987). Genome-wide scans for linkage of freeze scores were done using the program MapManager QT (Manly 1993) on a Macintosh computer, as well as the program MAPMAKER/QTL 1.1 (Lander et al. 1987; Paterson et al. 1988; Lander and Botstein 1989) on a Sun computer. lod [logarithm of the (odds of linkage/odds of no linkage)] scores for candidate regions were calculated for all phenotypic scores using MAPMAKER/QTL. Data were analyzed first using a “free” genetic model (that assumes no phenotypic effect of C alleles), and subsequently using additive, dominant, and recessive models, in which dominant or recessive refers to the B6 allele in each case.
STATISTICAL ANALYSIS
For all statistical analyses, values were calculated as percentages (e.g., bouts of 1sec10sec measures/total possible 1sec10sec bouts). In experiment I t-tests were performed on hand scores, 1sec5sec, and latency 3 measures of the testing sessions to compare control and experimental groups. Correlation analysis quantified the strength of association between hand-scored freezes and the different computer measures and between the hand-scored freezes of the different observers. In experiment II one-way analyses of variance (ANOVA) with strain as the grouping variable were performed on observer freeze scores and 1sec10sec computer measures for both the context and auditory-cue testing sessions. For analysis, a net baseline-corrected (net) 1sec10sec was derived by subtracting the freezing level in the first 2 min of the training session [before the unconditioned stimulus (US) was presented] from the 1sec10sec freezing value recorded during the context test. This measure was also analyzed through a one-way ANOVA. CD1s were excluded from the ANOVA because they are not an isogenic group. In experiment III, variances were not equal between strains. Significant differences in variances were assessed by F-test. Comparisons between groups (either strains or genders) were done by Kruskall–Wallis tests. All statistical analyses were performed using the program NCSS 6.0 (NCSS, Kaysville, UT) on a Windows 95 PC.
Results
EXPERIMENT I
To assess the Freeze Monitor’s ability to detect differences in conditioned freezing behavior, two groups (shock and no shock) of B6 mice were simultaneously scored by an experimenter and by the Freeze Monitor system.
All computer measures were significantly (P < 0.0003) correlated with hand scoring. Correlation coefficients ranged from 0.87 (latency 1) to 0.94 (latency 3) (Table 1).
Table 1.
Correlation values (r) between observer-scored freezing and each computer-based measure
| Measure
|
Experiment I
|
Experiment II
|
Experiment III
|
|||
|---|---|---|---|---|---|---|
| context
|
context
|
net context
|
cued
|
context
|
net context
|
|
| Latency 1 | 0.87 | 0.78 | 0.79 | 0.73 | 0.85 | 0.81 |
| Latency 2 | 0.92 | 0.76 | 0.75 | 0.74 | 0.87 | 0.80 |
| Latency 3 | 0.94 | 0.75 | 0.76 | 0.74 | 0.84 | 0.75 |
| Activity | −0.93 | −0.69 | −0.73 | −0.69 | −0.84 | −0.76 |
| 1sec5sec | 0.91 | 0.76 | 0.77 | 0.69 | 0.84 | 0.72 |
| 2sec5sec | 0.89 | 0.79 | 0.80 | 0.72 | 0.85 | 0.79 |
| 1sec10sec | 0.92 | 0.78 | 0.79 | 0.62 | 0.81 | 0.65 |
| 2sec10sec | 0.88 | 0.78 | 0.80 | 0.66 | 0.84 | 0.79 |
Figure 1 presents the freezing data during the training and test sessions. The percentage freezing obtained from direct observation (Fig. 1A) is shown with two selected computer measures, the 1sec5sec (Fig. 1B) and latency 3 scores (Fig. 1C). Even though all computer measures showed high correlations with hand scores, latency 3 was chosen to graph because it had the highest r value (0.94). The 1sec5sec measure was chosen because it is dichotomous and is based on a 5-sec interval similar to the hand-scoring procedure used in this experiment. Low levels of freezing were recorded during the preshock part of the training session followed by an increase in freezing during shock presentation. These patterns of data were obtained for all computer measures. Both hand-scored and computer-scored freezing showed that there were significantly higher levels of freezing in experimental animals than in controls (Ps < 0.01).
Figure 1.
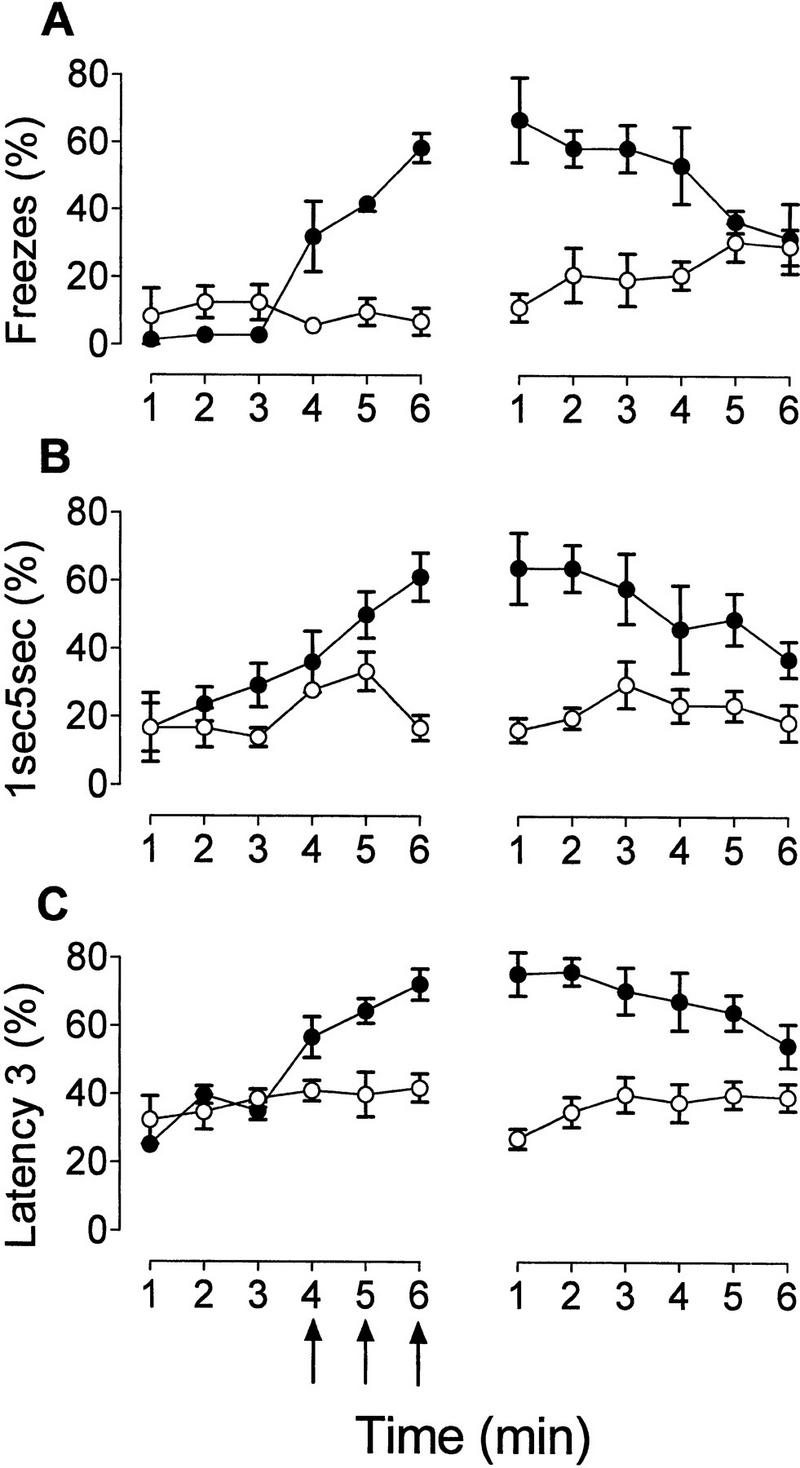
Mean ± s.e.m. number per minute of percent hand-scored freezes (A), percent 1sec5sec computer-scored freezing (B), percent latency 3 computer-scored freezing (C) for training (left) and testing (right) sessions of experiment I. The control group was not exposed to shocks during training (○), the experimental group was submitted to three shocks during training (•). Arrows indicate 1-sec, 0.6-mA shocks given to the experimental group.
EXPERIMENT II
Because different strains of mice have different behavior patterns and levels of activity, we considered the possibility that the same scoring criterion may not be ideal for every strain. To address this issue, B6, D2, B6D2F1/J, and CD1 mice were tested in context and cued fear-conditioning paradigms. These strains were selected because it is known that B6 mice display more contextual, but not auditory-cued, conditioned fear than D2 mice (Paylor et al. 1994) and because CD1 mice have been observed to have low levels of freezing using similar procedures (R. Paylor, unpubl.).
Correlations between hand-scored freezing and all the computer measures were highly significant (Ps < 0.0001) with r values ranging from 0.69 (activity) to 0.79 (2sec5sec) (Table 1). Figure 2 depicts the correlation plots between observer-scored freezing and 1sec10sec (Fig. 2A). Even though all computer measures had significant correlations with hand-scoring, 1sec10sec was chosen because of its high r value (0.78) and because it is a dichotomous measure based on 10-sec sampling intervals as was the hand-scoring method used in this experiment. The correlation graphs of all the computer measures were very similar. As expected, the freezing values of D2 mice (crosses) were on the lower part of the hand-scored and computer-scored freezing scales, whereas those of B6 (solid circles) tended to be at the higher part of the scales. CD1s (squares) had low values and F1s (×s) tended to have intermediate values. It is important to note that the levels of freezing were higher in all the computer measures, particularly in animals with low observational freezing scores. This was especially apparent for D2 mice and yielded non-zero y-intercepts (Fig. 2A).
Figure 2.
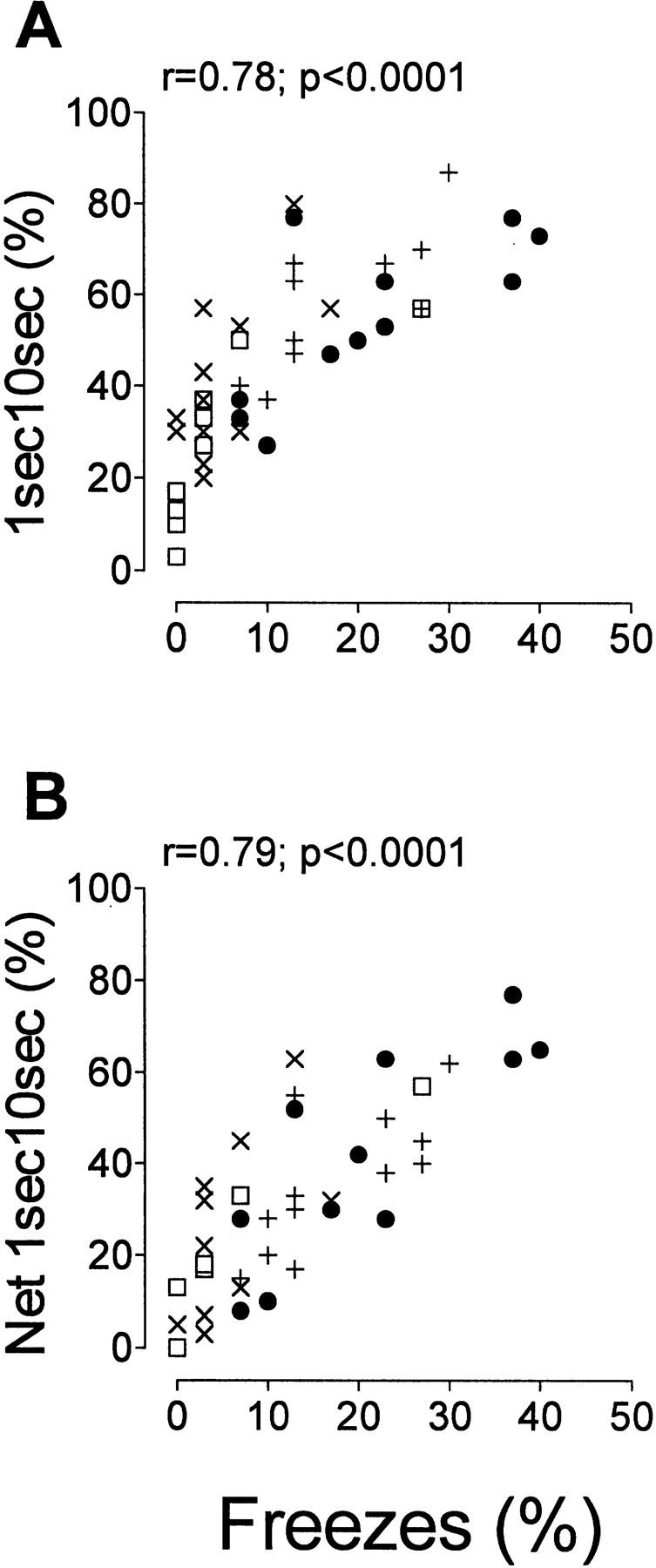
Linear correlation plots between percentage of hand-scored freezes and percent 1sec10sec (A) and percent net 1sec10sec (B), per testing sessions of experiment II.
We considered possible sources of this apparent false-positive rate in computer-scored freezing, as well as methods to correct for it. The freeze monitor system is not able to detect small head movements that would be scored as “active” by an observer, thus this system tends to have higher levels of freezing when testing mice that show low levels of freezing as measured by an observer. Thus an animal that is relatively inactive or moves slowly may be erroneously scored as freezing. This indicates that under certain circumstances when low levels of freezing are recorded using the hand-scored protocol, the computer-derived scores will record more freezing behavior. We have attempted to compensate for this overscoring by generating a net 1sec10sec measure. We considered the level of freezing in the first 2 minutes of the training session (before the US was presented) as a measure of the baseline false-positive freeze rate for the 1sec10sec computer-derived measure. By subtracting this value from the 1sec10sec freezing value recorded during the context test, we were able to correct for different baseline scores. This manipulation sets the baseline level of freezing to zero, which may not be true for all animals. However, we did find that the mean level of hand-scored freezing during the pre-US part of the training session was not significantly different from zero for any of the strains tested. Correlation coefficients were higher for the baseline-corrected (net) values (Table 1; Fig. 2B).
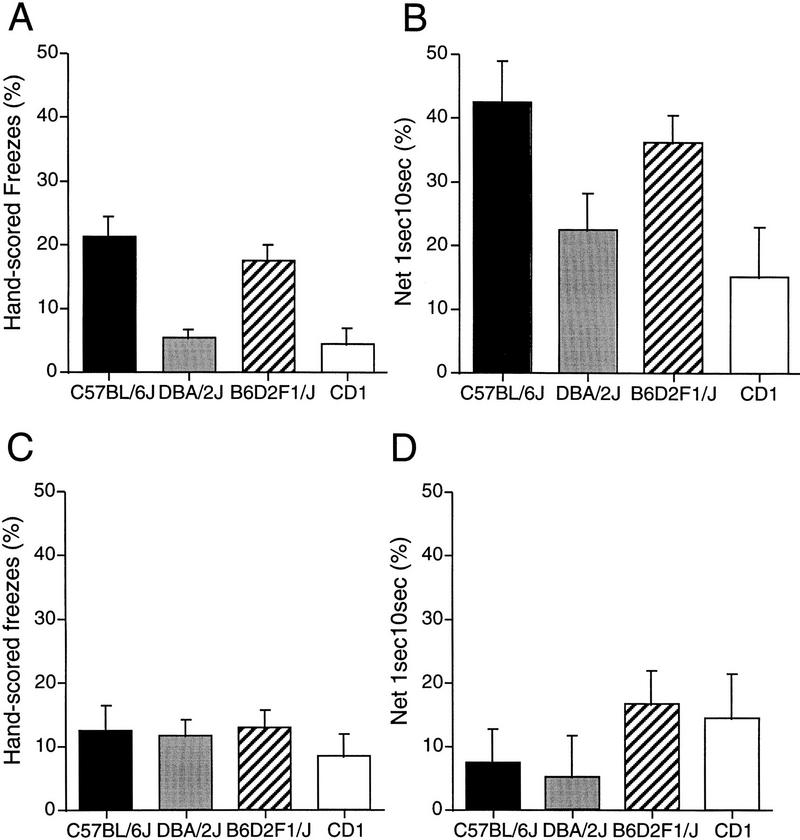
The 1sec10sec baseline-corrected computer measure was chosen for statistical analysis between strains. The ANOVA of hand-scored freezes during testing revealed a significant main effect of strain, F(2,33) = 10.04, P < 0.001. Post hoc analysis with the Newman–Keuls’ test indicated that B6 animals were significantly (P < 0.05) different from D2 (Fig. 3A). Similarly, when analyzing the baseline-corrected 1sec10sec values during testing, the ANOVA revealed a significant main effect of strain, F(2,33) = 3.35, P < 0.05. The post hoc analysis indicated that B6 animals were significantly (P < 0.05) different from D2 (Fig. 3B). No significant effect of strain was detected with uncorrected 1sec10sec values.
Figure 3.
Mean ± s.e.m. of percent hand-scored freezes during context testing (A), percent 1sec10sec baseline-corrected measures during context testing (B), percent hand-scored freezes during auditory-cued testing (C), percent 1sec10sec measures during auditory-cued testing (D), for C57BL/6J, DBA/2J, B6D2F1/J, and CD1 strains of experiment II.
Correlations between observer scores and computer measures were also analyzed for the auditory-cued fear conditioning testing during conditioned stimulus (CS) presentation. Correlation values were highly significant (P < 0.0001) with r values ranging from 0.62 (1sec10sec) to 0.74 (latency 2 and latency 3) (Table 1). Figure 3C and D shows the freezing levels for each strain during auditory-cued testing as measured by hand scores (Fig. 3C) and net 1sec10sec computer scores (Fig. 3D). Consistent with Paylor et al. (1994), no difference between D2 and B6 during auditory-cued fear conditioning was detected either with hand-scored or any of the computer-scored freezing measures (Ps > 0.05).
EXPERIMENT III
We undertook a QTL analysis to identify candidate chromosomal regions containing loci that influence contextual fear conditioning as an example for the use of the Freeze Monitor, in a protocol similar to experiment I. F2 intercross progeny between BALB/cJ and C57BL/6J were used as the segregating generation (n = 199) for the QTL analysis.
To determine the most appropriate of the Freeze Monitor measures to use for the QTL analysis, B6, C, and CB6F1 mice were scored simultaneously by observer and the computer. All computer measures obtained in the context testing sessions of parentals and F1 intercross showed significant (P < 0.0001) correlations with hand-scored measures; r values ranging from 0.81 (1sec10sec) to 0.87 (latency 2) (Table 1). The correction for baseline activity (net values) actually resulted in decreased correlation coefficients (Table 1), consequently the net values were considered inappropriate for these strains, and were not used.
Both observer-scored and computer-scored freezing showed unequal variances among the isogenic generations. In general B6 animals had significantly lower variance than the other groups. When analyzing hand-scored measures the F-tests for equal variance showed that only B6 and F1 had unequal variances [F(10,11) = 5.05, P < 0.01]. Latency 3 measures had unequal variances between B6 and C [F(10,11) = 6.75, P < 0.005], B6 and F1 [F(10,11) = 5.70, P < 0.005] and between F1 and F2 [F(11,222) = 1.06, P < 0.05]. Measures of 1sec10 sec showed unequal variances between B6 and C [F(10,11) = 9.96, P < 0.001] and between B6 and F1 [F(10,11) = 8.98, P < 0.001]. All groups showed normal distributions as revealed by Kolmogorov–Smirnov normality tests. Transformation of the data to facilitate use of a parametric statistical test was not possible because some groups do show equal variance. The nonparametric Kruskall–Wallis analysis of observer-scored freezes during testing of parental and F1 mice revealed a significant main effect of strain [F(2,32) = 12.53, P < 0.001]. Post hoc Z tests for pairwise comparisons revealed no difference between parental strains, whereas F1 animals showed significantly more hand-scored freezing than B6 and C. When analyzing 1sec10sec values during testing of parentals, F1s, and F2s, a significant main effect of strain was observed [F(3,232) = 3.91, P < 0.001]. F1 animals showed significantly different values than B6, C, and F2, whereas no difference between parental strains was detected. Similarly, latency 3 analysis during testing also showed a significant main effect of strain [F(3,232) = 3.21, P < 0.05]. F1 animals were significantly different from B6, C, and F2 and, again, no difference between parental strains was evident.
The frequency distributions for 1sec10sec and latency 3 respectively, of B6, C, CB6F1 and CB6F2 mice are shown in Figure 4. These two computer measures were graphed because they yielded the highest lod scores. The significantly higher level of freezing behavior of the intercross F1 detected by hand scoring was also detected by the latency 3 and 1sec10sec computer measures (Fig. 4), consistent with overdominance for this trait. The mean values of the CB6F2 are intermediate between the CB6F1 and the parental values, also consistent with dominance towards high freezing. A continuous distribution is observed in CB6F2 mice for both measures, suggesting a polygenic mode of inheritance. The variance of the CB6F2 population is not significantly different than the CB6F1. However, the variances of the two parental groups also are unequal; B6 mice have lower variance than C and CB6F1 mice, so comparison of variance of F1 to F2 may not reflect genetic variance.
Figure 4.
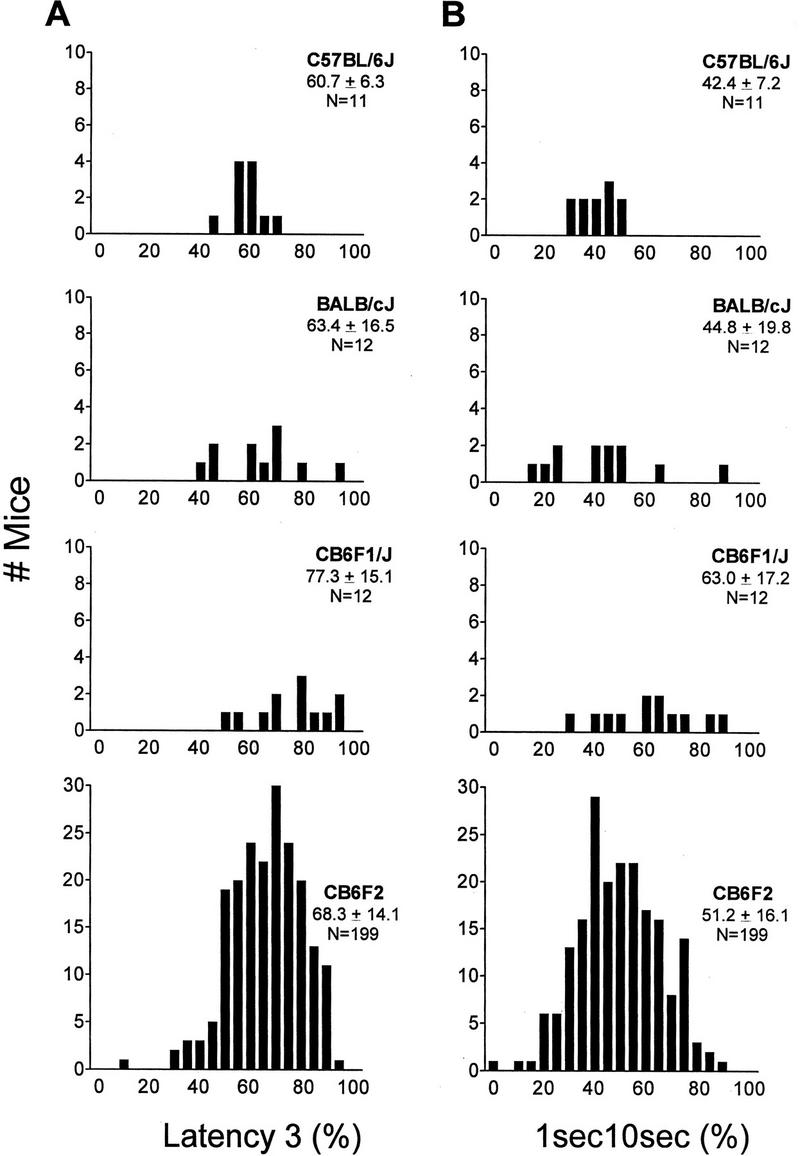
Frequency distribution graphs for percent 1sec10sec computer measure (A) and percent latency 3 computer measure (B) of C57BL/6J and BALB/cJ, CB6F1/J, and CB6F2 mice of experiment III.
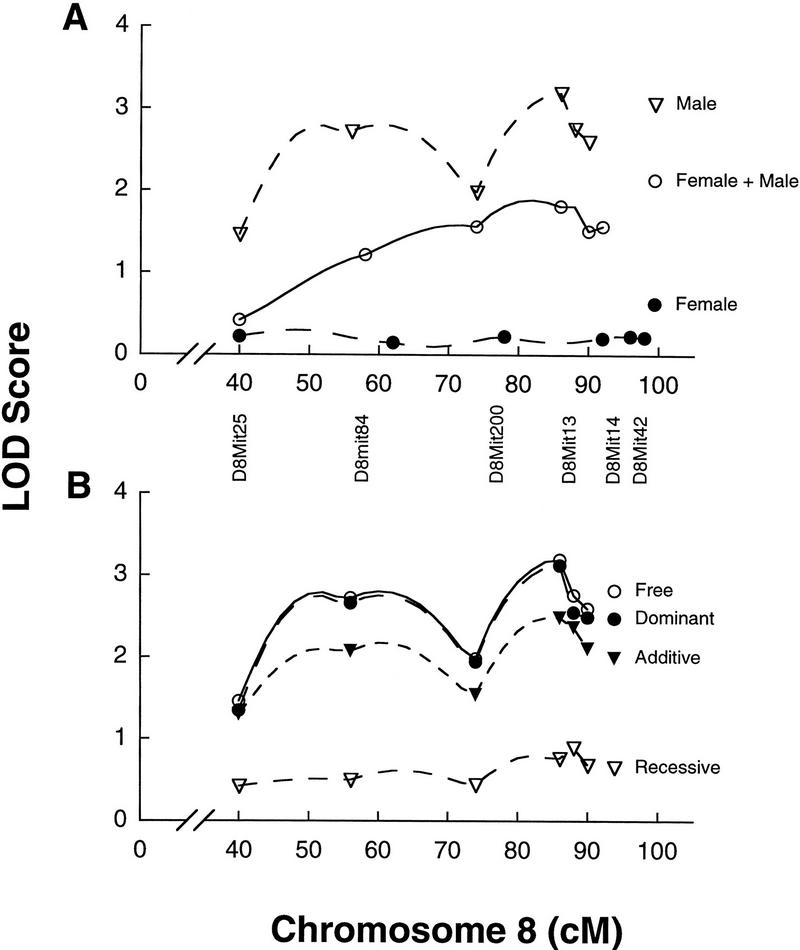
The results of the linkage analysis are shown in Table 2, in which the candidate chromosomal regions with lod scores over 2.0 were selected. A suggestive QTL (lod scores between 2.8 and 4.1) was detected in chromosome 9 when using the 2sec10sec measure. Segregation at D9Mit2 accounts for 8.2% of the phenotypic variance in this computer measure. Similar lod score values for this location were found for the 1sec5sec and the 1sec10sec measures. Another suggestive QTL in distal chromosome 8, linked to D8Mit13 was located when analyzing males alone. The same result arose when using all the computer-generated freeze measures. Both 1sec5sec and 1sec10sec had lod scores >2.8 with free genetic models. Segregation at D8Mit13 accounts for 12% of the phenotypic variance in 1sec5sec, and 13.4% of the phenotypic variance in 1sec10sec. The position of this QTL is shown as described by MAPMAKER/QTL (Figure 5). A suggestive lod score for latency 3 measure linked to D8Mit25 was also found in males, however no other computer measure at this chromosomal region supported this. The analysis of only females revealed lod scores >2.0 for all computer measures on chromosome 16 linked to D16Mit27.
Table 2.
Summary of genome-wide QTL analysis reporting lod scores (upper number) for linkage of candidate chromosomal regions for loci affecting fear conditioning and percentage of phenotypic variance explained (lower number)
| Genetic interval
|
Trait
|
||||
|---|---|---|---|---|---|
| 1sec5sec
|
2sec5sec
|
1sec10sec
|
2sec10sec
|
Latency 3
|
|
| A. All F2 progeny | |||||
| D3Mit11–D3Mit17 | 2.00 | 2.32 | – | – | 2.09 |
| 8.6% | 10% | 9.3% | |||
| D9Mit2–D9Mit4 | 2.41 | – | 2.62 | 2.81 | – |
| 6.6% | 7.6% | 8.2% | |||
| D8Mit13–D8Mit14 | – | 2.07 | – | – | 2.29 |
| 4.7% | 5.2% | ||||
| D16Mit27–D16Mit106 | – | – | – | – | – |
| B. Female F2 progeny | |||||
| D3Mit11–D3Mit17 | 2.22 | – | 2.29 | 2.16 | 2.29 |
| 13.9% | 14% | 12% | 14.1% | ||
| D3Mit131–D3Mit6 | – | 2.01 | – | – | – |
| 12.7% | |||||
| D9Mit2–D9Mit4 | – | – | – | – | – |
| D8Mit13–D8Mit14 | – | – | – | – | – |
| D16Mit27–D16Mit106 | 2.21 | 2.66 | 2.08 | 2.3 | 2.25 |
| 13.4% | 18% | 11.8% | 14.5% | 12.8% | |
| C. Male F2 progeny | |||||
| D3Mit11–D3Mit17 | – | – | – | – | – |
| D9Mit2–D9Mit4 | – | – | – | – | – |
| D8Mit13–D8Mit14 | 2.84 | 2.63 | 3.2 | 2.25 | 2.55 |
| 12% | 11.2% | 13.4% | 9.7% | 10.4% | |
| D8Mit25–D8Mit200 | – | – | – | – | 2.92 |
| 13.8% | |||||
| D16Mit27–D16Mit106 | – | – | – | – | – |
(−) lod scores < 2.0.
Figure 5.
lod plot on chromosome 8 of contextual learning using 1sec10sec computer measures for males, females + males, and females alone (A), using a free genetic model, or only males (B) using free, dominant, additive, and recessive genetic models. lod scores are plotted on the vertical axis. Genetic distances along the chromosome (in centimorgans) are plotted on the horizontal axis with markers used for the assessments.
Discussion
Taken together, these data constitute convincing evidence that the Freeze Monitor is a good tool to study conditioned fear as measured by behavioral inhibition that is reflected by increased latencies to break new beams. This behavioral inhibition, clearly detected by the equipment is highly correlated with the freezing measure that is typically scored by experimenters. We conclude that the Freeze Monitor is capable of obtaining reliable measures of the freezing response during fear-conditioning tests.
In experiment I, the levels and patterns of freezing were similar when comparing the hand-scored data to those obtained from the computer. High correlation values were obtained between hand scores and all the different computer measures. These results suggest that using various computer-derived measures from the Freeze Monitor system, it is possible to obtain reliable conditioned-freezing scores in B6 mice.
Different strains of mice have been shown to exhibit significantly different locomotor and exploratory activity (Lhotellier et al. 1993) and reaction to novelty (Rodgers and Cole 1993). There are also strain differences in learning performance (e.g., Upchurch and Wehner 1988; Yamada et al. 1992; Paylor et al. 1993; Roullet et al. 1993; Mori and Makino 1994; Paylor et al. 1994; Owen et al. 1997b). As with any behavioral measure, it would be essential to validate the testing or scoring procedure when adapting it for use in a new strain. The purpose of experiment II was to extend the use of the Freeze Monitor to some other strains. D2 mice and the F1 intercross of this strain with B6 were tested. Again, high correlation values were obtained between hand scores and computer scores. In addition, the previously reported difference between B6 and D2 mice during context testing (Paylor et al. 1994) was also detected with computer scores. However, it is important to point out that we obtained lower r values in experiment II than in experiment I. Currently, we do not fully understand the nature of these differences but it is likely caused by a combination of factors including different strains, different training procedures, and differences in the hand-scored protocols. Findings from experiment II also indicate that the Freeze Monitor is apparently less sensitive when mice display low levels of freezing. This is an issue that requires more testing to confirm.
In the first part of experiment III, C, B6 and CB6F1/J were tested for context fear conditioning. CB6F1/J animals showed a significantly higher level of freezing compared to B6 and C, indicative of overdominance. The intermediate mean values of CB6F2 mice also suggest this type of trait. Overdominance is predicted for phenotypes that have strong direct survival or reproductive effects and is invoked as a basis for so-called hybrid vigor (Falconer 1981). Hence, overdominance is not surprising in the case of learning processes. Indeed, there is evidence of overdominance in the hybrids 129B6F1 and FVB129F1 that show better scores than either of the parental strains during context-fear-conditioning tests and during Morris probe trails (Owen et al. 1997b). The behavioral analysis of the F2 intercross showed that several computer measures were sufficient to detect suggestive QTLs for context fear conditioning on chromosomes 8 and 9. Other studies using hand scoring during context testing have indicated that there are several genetic regions that have strong influences on performance in this paradigm. QTLs on chromosomes 1 and 16 were detected in a study of the BxD recombinant inbred strains (Owen et al. 1997a). Wehner et al. (1997) tested an intercross between B6 and D2 animals and found that QTLs for context-fear conditioning were associated with specific regions in chromosomes 1, 2, 3, 10, and 16. Similarly, Caldarone et al. (1997) identified strong QTLs in the distal and proximal ends of chromosome 1 in a backcross population generated from B6 and C3H/HeJ mice. Using F2 intercross mice from C and B6 progenitors, our results indicate that there are some additional QTLs to be considered. The suggestive QTL on chromosome 8 was only present in males. Even though no gender difference was observed in the mean freezing levels, context learning is a complex test in which the same ability to perform may reflect different strategies and/or different sensory inputs. C3H animals that become blind as adults show an increase in freezing in response to a context paired previously with a shock (Owen et al. 1997b) despite being visually impaired, suggesting that other nonvisual cues are being used to identify the context. In the present experiment, males and females could be relying on different sensory pathways; consequently the genes involved could be different. The suggestive QTL on chromosome 9 was detected when considering males and females together. None of the suggestive values obtained matched the results of the already published data, however a different strain combination was used so it can be expected that different loci or sets of genes responsible for this trait would be detected.
It is important to note that the genetic models used here (free, dominant, additive, and recessive) do not predict exactly the allelic effects at an overdominant locus. None of these models were designed for this kind of effect, consequently the actual linkage values may be higher than the values reported here. As might be expected for a trait in which overdominance is present, the lod score estimates are highest for the free genetic model, followed by the dominant genetic model. Additive and recessive genetic models predict lower lod scores for all freeze measures.
Testing more animals is necessary to confirm these results and further refinement of the interval on chromosomes 8 and 9 will be required to determine the behavioral specificity of the QTL found.
In general, some computer-scored measures more accurately reflect hand-scored freezes than others. The selection of which should be used or the use of baseline-corrected values will depend on the strain used and the method of hand scoring normally used. Other manipulations can be done to try to increase the sensitivity of the Freeze Monitor like adjusting the frequency of measurements, height, position, or number of the photobeams to optimize the measure for other strains’ behavior patterns. The possibilities are many and the data shown here are a first step towards improving the method.
These observations support the Freeze Monitor as a way to automate the measure of freezing response. As with any automation method, the benefits are obvious: increase in efficiency, elimination of the subjective component that characterizes direct observation and the possibility of testing more animals and in more diverse conditions such as darkness. The recent increase in the use of genetic techniques for mapping genes related to learning and memory could significantly benefit with the automation of learning tests.
Acknowledgments
We are indebted to Erik Naylor for assistance with Freeze Monitor data analysis, and to Anne-Marie Chang and Peter Zemenides for assistance with breeding or genotyping mice, respectively. The National Council of Scientific Development and Technology (CNPq) of the Brazilian Government provided a fellowship to Verónica S. Valentinuzzi. This research was supported by the National Science Foundation (NSF) Science and Technology Center for Biological Timing (J.S.T.), Bristol-Myers Squibb Unrestricted Grant in Neurosciences (J.S.T.) and National Institutes of Health grants P01 AG11412 (F.W.T. and J.S.T.), R01 AG10870 (F.W.T.), and R01 AG09297 (F.W.T.). J.S.T. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKCγ mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seideman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York, NY: John Wiley and Sons; 1995. [Google Scholar]
- Blanchard RD, Blanchard RJ. Ethoexperimental approaches to the biology of emotions. Annu Rev Psychol. 1988;39:43–68. doi: 10.1146/annurev.ps.39.020188.000355. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli R, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Caldarone B, Saavedra C, Tartaglia K, Wehner JM, Dudek BC, Flaherty L. Quantitative trait loci analysis affecting contextual conditioning in mice. Nat Genet. 1997;17:335–337. doi: 10.1038/ng1197-335. [DOI] [PubMed] [Google Scholar]
- Copeland NG, Jenkins NA, Gilbert DJ, Eppig JT, Maltais LJ, Miller JC, Dietrich WF, Weaver A, Lincoln SE, Steen RG. A genetic linkage map of the mouse: Current applications and future prospects. Science. 1993;262:57–66. doi: 10.1126/science.8211130. [DOI] [PubMed] [Google Scholar]
- Dietrich W, Katz H, Lincoln SE, Shin HS, Friedman J, Dracopoli NC, Lander ES. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992;131:423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WF, Miller JC, Steen RG, Merchant M, Damron D, Nahf R, Gross A, Joyce DC, Wessel M, Dredge RD. A genetic map of the mouse with 4006 simple sequence polymorphisms. Nat Genet. 1994;7:220–245. doi: 10.1038/ng0694supp-220. [DOI] [PubMed] [Google Scholar]
- Dietrich WF, Miller J, Steen R, Merchant MA, Damron-Boles D, Husain Z, Dredge R, Daly MJ, Ingalls KA, O’Connor TJ. A comprehensive genetic map of the mouse genome. Nature. 1996;380:149–152. doi: 10.1038/380149a0. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Introduction to quantitative genetics. 2nd ed. New York, NY: Longman; 1981. [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- Graef FG. Neuroanatomy and neurotransmitter regulation of defensive behaviors and related emotions in mammals. Braz J Med Biol Res. 1994;27:811–829. [PubMed] [Google Scholar]
- Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newberg L. MAPMAKER: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Le Doux JE. Emotion, memory and brain. Sci Am. 1994;270:50–57. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- Lhotellier L, Perez-Diaz F, Cohen-Salmon C. Locomotor and exploratory activity in three inbred strains of mice from young adulthood to senescence. Exp Aging Res. 1993;19:177–187. doi: 10.1080/03610739308253930. [DOI] [PubMed] [Google Scholar]
- Manly KF. A Macintosh program for storage and analysis of experimental genetic mapping data. Mamm Genome. 1993;4:303–313. doi: 10.1007/BF00357089. [DOI] [PubMed] [Google Scholar]
- Mori T, Makino J. Response type to shock and avoidance learning in inbred strains of mice. Jpn J Psychol. 1994;65:295–302. doi: 10.4992/jjpsy.65.295. [DOI] [PubMed] [Google Scholar]
- Owen EH, Christensen SC, Paylor R, Wehner JM. Identification of quantitative trait loci involved in contextual and auditory-cued fear conditioning using BXD recombinant inbred strains. Behav Neurosci. 1997a;111:292–300. doi: 10.1037//0735-7044.111.2.292. [DOI] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: Implications of genetics background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997b;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Paterson AH, Lander ES, Hewitt JD, Peterson S, Lincoln SE, Tanksley SD. Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature. 1988;335:721–726. doi: 10.1038/335721a0. [DOI] [PubMed] [Google Scholar]
- Paylor R, Baskall L, Wehner J. Behavioral dissociations between C57BL/6 and DBA/2 mice on learning and memory tasks: A hippocampal-dysfunction hypothesis. Psychobiology. 1993;21:11–26. [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear conditioning but not in auditory fear conditioning. Behav Neurosci. 1994;108:1–8. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC. Influence of social isolation, gender, strain, and prior novelty on plus-maze. Physiol Behav. 1993;54:729–736. doi: 10.1016/0031-9384(93)90084-s. [DOI] [PubMed] [Google Scholar]
- Roullet P, Lassalle JM, Jegat R. A study of behavioral and sensorial bases of radial maze learning in mice. Behav Neural Biol. 1993;59:173–179. doi: 10.1016/0163-1047(93)90926-9. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Rosahl TW, Chapman OF, Marowitz Z, Friedman E, Frankland PW, Cestari V, Cioffi D, Südhof TC, Bourtchuladze R. Impaired learning in mice with abnormal short-lived plasticity. Curr Biol. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Pinto LH, Vitaterna MH. Forward and reverse genetic approaches to behavior in the mouse. Science. 1994;264:1724–1733. doi: 10.1126/science.8209253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch M, Wehner JM. Differences between inbred strains of mice in Morris water maze performance. Behav Genet. 1988;18:55–68. doi: 10.1007/BF01067075. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Radcliffe RA, Rosmann ST, Christensen SC, Rasmussen DL, Fulker DW, Wiles M. Quantitative trait locus analysis of contextual fear conditioning in mice. Nat Genet. 1997;17:331–334. doi: 10.1038/ng1197-331. [DOI] [PubMed] [Google Scholar]
- Yamada K, Satoh M, Tokoi J, Tsubi M, Nagasaka T. Strain difference of mice in learning of swimming behavior and effect of hemicholinium and vasopressin. Observation by a simple water maze. J Pharm Soc Jpn. 1992;112:824–831. doi: 10.1248/yakushi1947.112.11_824. [DOI] [PubMed] [Google Scholar]