Abstract
Repetitive stimulation often results in habituation of the elicited response. However, if the stimulus is sufficiently strong, habituation may be preceded by transient sensitization or even replaced by enduring sensitization. In 1970, Groves and Thompson formulated the dual-process theory of plasticity to explain these characteristic behavioral changes on the basis of competition between decremental plasticity (depression) and incremental plasticity (facilitation) occurring within the neural network. Data from both vertebrate and invertebrate systems are reviewed and indicate that the effects of depression and facilitation are not exclusively additive but, rather, that those processes interact in a complex manner. Serial ordering of induction of learning, in which a depressing locus precedes the modulatory system responsible for inducing facilitation, causes the facilitation to wane. The parallel and/or serial expression of depression and waning facilitation within the stimulus–response pathway culminates in the behavioral changes that characterize dual-process learning. A mathematical model is presented to formally express and extend understanding of the interactions between depression and facilitation.
Origins of the Dual-Process Theory of Plasticity
There have been principally two different approaches to the study of learning, the aggregate-field approach and the cellular-connection approach (see Kandel and Spencer 1968). The aggregate-field approach maintains that plasticity cannot be studied at the cellular level because learning is a property of large groups of neurons rather than of any single neuron. The cellular-connection approach holds the opposite view, that learning is encoded by changes in specific neurons and their synaptic connections to other neurons. According to the latter approach, there are three steps of investigation: (1) characterization of the neural circuit mediating a behavior, (2) localization of the sites of plasticity within that circuit, and (3) characterization of the mechanisms of plasticity at the cellular and synaptic levels (Hawkins et al. 1987). Over the past 30 years, application of these steps has achieved impressive advancements in our understanding of learning, with concurrent loss in popularity of the aggregate-field approach.
Nowhere has the cellular connection approach been more successful than in its application to the plasticity of withdrawal reflexes in invertebrates, especially in Aplysia (for reviews, see Jacklet and Lukowiak 1975; Carew and Sahley 1986; Byrne 1987; Hawkins et al. 1987, 1993). The neural circuits mediating such reflexes are comparatively simple and amenable to investigation. But even “simple” circuits are not so easily understood. For instance, the siphon-elicited siphon withdrawal reflex of Aplysia is mediated through both monosynaptic and polysynaptic pathways. Investigation of interneurons has led to increasing recognition of the polysynaptic pathway’s importance in mediating and modulating the siphon withdrawal reflex and coordinating this reflex with other behaviors (Byrne 1981; Hawkins et al. 1981a,b; Trudeau and Castellucci 1992; Cleary et al. 1995; Frost and Kandel 1995). This relatively low level of network complexity is sufficient to offer multiple loci at which plasticity can occur (e.g., Frost et al. 1988; Cohen et al. 1997). In the gill and siphon withdrawal (GSW) reflex, the synapses between sensory neurons (SNs) and motoneurons (MNs) exhibit homosynaptic depression and presynaptic facilitation (Castellucci et al. 1970; Castellucci and Kandel 1976) as well as post-tetanic potentiation (PTP) (Clark and Kandel 1984; Walters and Byrne 1984). The same homosynaptic learning processes are expressed at synapses between SN and excitatory interneurons (IN+s) (Hawkins et al. 1981a; Clark and Kandel 1984), though a heterosynaptic process like facilitation is not necessarily equally expressed at SN–MN and SN–IN+ synapses (Clark and Kandel 1984; Fitzgerald and Carew 1991; Trudeau and Castellucci 1993b). PTP and depression also occur, to some degree, at downstream IN+–MN synapses (for review, see Cleary et al. 1995). The interconnections between IN+ and inhibitory interneurons (IN−s) also change through a variety of mechanisms (Frost et al. 1988; Fischer and Carew 1993; Trudeau and Castellucci 1993a,b; Cleary et al. 1995). Even the neuromuscular junction and other peripheral sites are capable of plasticity (Jacklet and Rine 1977; Lukowiak and Colebrook 1988–1989; Cohen et al. 1997). In addition to synaptic plasticity, the excitability of SNs, IN+s, IN−s, and MNs can change consequent to past experience (e.g., Klein and Kandel 1978; Kanz et al. 1979; Frost et al. 1988; Trudeau and Castellucci 1993b).
So, while memory storage can be considered to occur at discrete loci (i.e., neurons and their synaptic connections), plastic loci occur throughout the network. Memory is distributed, not in the manner held by the aggregate-field hypothesis, but, rather, by one more consistent with parallel distributed processing (Rumelhart and McClelland 1986; Frost et al. 1988; Lockery and Sejnowski 1993). Multiple mechanisms can act at a single cellular locus to effect a variable level of change at that locus; at the network level, these variable changes at discrete loci combine in numerous permutations and allow a high degree of behavioral flexibility through learning (e.g., Lockery and Sejnowski 1993; White et al. 1993). Although much effort is spent investigating the cellular and molecular mechanisms of plasticity, the interactions of these mechanisms both at the cellular level and at the network level cannot be neglected if one’s ultimate goal is to explain learning at the behavioral level. These interactions include, for example, those between short, intermediate, and long-term memory (Kandel 1976; Christoffersen and Schilhab 1996; Mauelshagen et al. 1996; Sossin 1996). As well, simple nonassociative forms of learning might somehow combine and interact to produce more complex associative learning (Hawkins and Kandel 1984; Hawkins 1989; Buonomano et al. 1990). Even the efficacy of a particular mechanism for short-term nonassociative learning may be influenced by prior synaptic or cellular activity that may or may not have itself caused plasticity (Marcus et al. 1988; Fischer et al. 1997; for review of metaplasticity, see Abraham and Bear 1996); for example, spike broadening and vesicle mobilization both contribute to facilitation, but whereas the former mechanism is more important for effecting change at naive synapses, the balance shifts in favor of the latter for effecting change at depressed synapses (for reviews, see Klein 1995; Byrne and Kandel 1996). This example illustrates the interactions that can occur between decremental and incremental learning processes at the cellular level. Interactions between these processes can also occur at the network level (e.g., Groves and Thompson 1970; Fitzgerald et al. 1990; Hawkins et al. 1998).
The remainder of this paper will attempt to advance understanding of interactions of this last sort, namely, the interactions between short- to intermediate-term depression and facilitation at the network level. Plasticity in numerous systems representing both vertebrates and invertebrates will be reviewed. Based on the principles derived from this review and the results of a simple mathematical model, I will develop the central thesis: Depression occurs at loci early in the stimulus–response (S-R) pathway, upstream of the modulatory system necessary for the induction of facilitation, and consequently, depression not only competes directly with facilitation for the determination of behavioral change (by serial and/or parallel expression), but depression also precludes the ongoing development and maintenance of facilitation (by serial induction). The combination of these two interactions ultimately determines how the reflex will change and leads to the “bumpy” learning curves characteristic of dual-process learning.
DEFINING HABITUATION AND SENSITIZATION
Habituation can be defined as behavioral response decrement resulting from repeated stimulation (Harris 1943), the parametric characteristics of which were described by Thompson and Spencer (1966). Only those characteristics important for the current discussion are recounted here. First, response decrement is a negative exponential function of the number of stimulus presentations. Second, the rate and degree of decrement are directly proportional to stimulation frequency according to Thompson and Spencer (1966) though later publications (Hinde 1970; Thompson et al. 1973) have asserted that the number of stimuli is really the more significant variable. Third, the rate and degree of decrement are inversely proportional to the stimulus intensity, though this is a less significant factor than stimulus repetition (Groves and Thompson 1970; Thompson et al. 1973).
Following from the last point, strong stimuli may in fact cause sensitization instead of habituation. Sensitization is defined as behavioral response increment resulting from novel, strong, or noxious stimulation (Peeke and Petrinovich 1984). In contrast to habituation, stimulus intensity is a more important factor than stimulus repetition in determining the rate and degree of sensitization. From a teleological standpoint, these relationships seem logical: Habituation serves to decrease the response to a stimulus whose informational value has decreased as a result of its inconsequential repetition, whereas sensitization serves to rapidly increase the response to a stimulus whose informational value is judged as high on the basis of its initial novelty or strength, though stimulus repetition may ultimately prove the sensitization unnecessary and promote its reduction.
Likely because of interest in associative learning, sensitization has most often been studied by application of a strong stimulus to a different area on the body than the milder test stimulus. This sort of sensitizing stimulus is referred to as a remote or extra-stimulus. However, sensitization can be induced by the test stimulus itself (Davis and Wagner 1969; Groves et al. 1969a,b; Hinde 1970). To make the distinction, sensitization caused by stimulation remote to and/or qualitatively different (e.g., modality) from the test stimulus is called extrinsic sensitization (Groves and Thompson 1970; Davis and File 1984), whereas sensitization caused by stimulation to the same site and of the same modality as the test stimulus is called intrinsic sensitization, warm-up (Hinde 1970; Lockery and Kristan 1991), or iterative enhancement (Brown et al. 1996). In this paper I refer to intrinsic sensitization simply as sensitization and otherwise specify extrinsic.
As discussed by Peeke and Petrinovich (1984), definitions of habituation and sensitization may refer to either the processes causing change or the behavioral consequences of those processes, that is, mechanistic and operational definitions, respectively. The definitions presented above are operational. Mechanistic definitions are not used in this paper. Instead, cellular plasticity is referred to as depression or facilitation, which unless otherwise explained (e.g., because of inhibition), confers behavioral habituation or sensitization, respectively. This terminology is not meant to connote any mechanistic details.
Before proceeding, it is valuable to make a distinction between induction and expression of learning. For a homosynaptic process such as depression, both induction and expression occur in the S-R pathway. For a heterosynaptic process such as presynaptic facilitation, expression of the learning is in the S-R pathway, but this learning is induced by a modulatory system.
GROVES AND THOMPSON’S THEORY
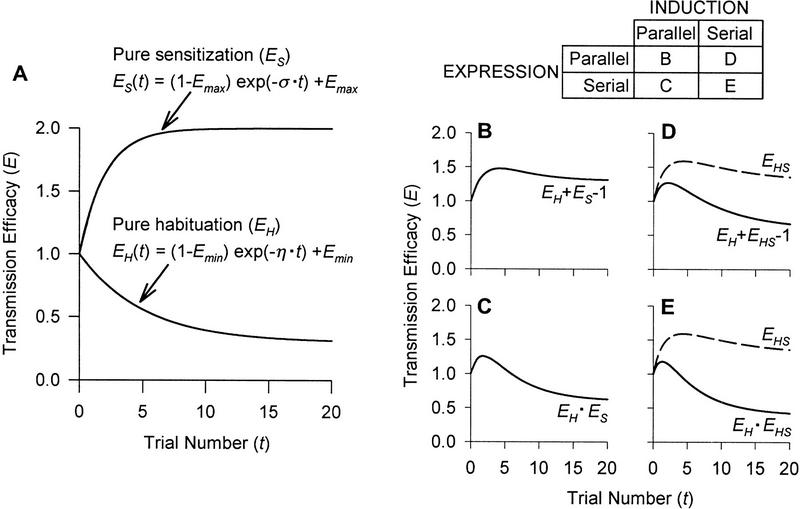
The dual-process theory of plasticity claims that two opposing processes, depression and facilitation, compete to determine the final behavioral outcome after a stimulus series (Fig. 1). The theory was formalized and given its name in 1970 by Groves and Thompson (see also Groves and Thompson 1973; Thompson et al. 1973), though very similar concepts were presented independently by Hinde (1970). The earliest conceptualization of this theory, however, dates back to Thompson and Spencer’s (1966) review of habituation in which they recognize that dishabituation is not the disruption of habituation but rather “a separate facilitatory process superimposed upon the habituated system.” Groves and Thompson (1970) also recognized the significance of intrinsic sensitization in that the same stimulus can simultaneously elicit depression and facilitation.
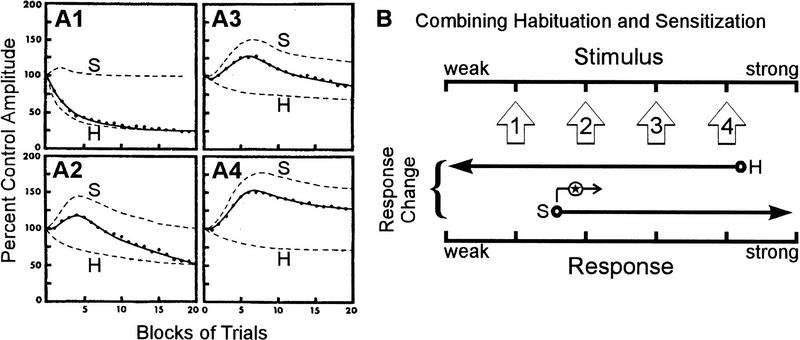
Figure 1.
The combination of habituation and sensitization to produce dual-process learning. (A1–A4) Decomposition of dual-process learning curve (solid line) into component habituation learning curve (broken line labeled H) and habituating sensitization learning curve (broken line labeled S) at four different stimulus intensities increasing between A1 and A4. Empirical data are shown as dots. (B) The contribution of opposing processes at different stimulus intensities. Stimulus intensities used in A are illustrated as open arrows pointing along the stimulus axis. Below each of these arrows are shown the learning processes recruited at that stimulus intensity. The arrow marked H indicates habituation (response decrement by depression); the arrow marked S indicates sensitization (response increment by facilitation). Stimulus 1 elicits strong habituation; stimulus 4 elicits only weak habituation but also elicits strong sensitization; stimuli 2 and 3 are intermediate. An important addition to this interaction is that the lower limit for eliciting sensitization increases (indicated by arrow marked with star) consequent to depression early in the neural circuit (see text), meaning that the balance between habituation and sensitization shifts in favor of habituation over time, explaining the bumpy shape of the curves seen in A. A is reprinted, with permission, from Groves and Thompson (1970). Copyright 1970 by the American Psychological Association.
The stimulus strength sets the balance between the opposing learning processes (Fig. 1B) and thereby determines the net magnitude and direction of plasticity at the network and behavioral levels (Fig. 1, cf. A1–A4). The bumpy shape of the curves in Figure 1, A1–A4 differs from canonical habituation learning curves and indicates that the balance between learning processes is dynamic, shifting as the stimulus is repeated. Extending beyond Groves and Thompson’s discussion, is there a physiological basis in the neural network for this changing balance? The data presented herein suggest that the kinetics of dual-process learning can be explained by the relative positioning of learning processes within the neural network.
Groves and Thompson’s ideas were based on investigations of the hindlimb flexion reflex of the acute spinal cat and the startle response of the intact rat, both of which exhibit dual-process learning (Groves and Thompson 1970). More complex behaviors such as the mobbing response of chaffinches (Hinde 1970) and aggression in three-spined sticklebacks (Peeke 1969, 1983; Peeke and Veno 1973) also exhibit plasticity consistent with the dual-process theory, as do certain human behaviors such as infant visual attention (Bashinski et al. 1985; Kaplan and Werner 1986). Dual-process learning is also common, though not ubiquitous, amongst invertebrate withdrawal responses. These responses include the withdrawal response in the earthworm (Roberts 1966), whole body withdrawal in the snail Lymnaea (Cook 1975), tentacle withdrawal in the snail Helix (Christoffersen et al. 1981; Zakharov and Balaban 1987; Balaban 1993; Prescott and Chase 1996; S.A. Prescott and R. Chase, in prep.), local bending in the leech (Lockery and Kristan 1991), escape response in the crab (Rakitin et al. 1991), swim response in Tritonia (Brown et al. 1996), and tail-induced siphon and tail withdrawal in Aplysia (Stopfer and Carew 1996). Data also suggest that dual-process learning may occur in the siphon-elicited GSW reflex (see below). The occurrence of dual-process learning is not explicitly recognized in all of these publications, but in each case, the reflex exhibits plasticity like that described by the learning curves in Figure 1, A1–A4, namely transient sensitization followed by habituation or, at least, a delayed onset of net habituation.
Review of Physiological Data
HINDLIMB FLEXION REFLEX IN THE CAT
The dual-process theory of plasticity stemmed largely from work on the spinal cat. In these experiments, electrical stimulation is applied to the skin or to a cutaneous nerve, and the reflex contraction of a flexor muscle is measured isometrically. Habituation of the flexion reflex occurs in the acute spinal cat with all nine parametric characteristics described by Thompson and Spencer (1966). The reflex also exhibits extrinsic sensitization (Spencer et al. 1966a; Thompson and Spencer 1966) and intrinsic sensitization (Groves et al. 1969a,b). Interestingly, both forms of sensitization habituate if the sensitizing stimulus is repeatedly applied (Spencer et al. 1966a; Thompson and Spencer 1966; Groves and Thompson 1970).
To date, the polysynaptic circuit mediating the reflex remains incompletely understood (see Moschovakis et al. 1992; Burke 1998), making it impossible to localize precisely the plastic loci. Data are consistent with depression at interneuronal sites (Spencer et al. 1966a–c; Thompson and Spencer 1966; Wickelgren 1967a,b; Groves and Thompson 1970; Durkovic 1983; for review, see Mendell 1984). The generalization (or transfer) of habituation between input pathways can be interpreted to indicate depression at synapses downstream of the primary afferent terminals; however, the generalization is incomplete and the receptive fields for decrement are narrow (Spencer et al. 1966a; Thompson and Spencer 1966), suggesting that depression occurs, in part, before pathway convergence from other stimulus input sites (see Wickelgren 1967b). Furthermore, depression occurs in both the spinal S-R pathway and the ascending pathways (Spencer et al. 1966b), consistent with depression occurring upstream of the point where the pathways diverge and/or depression occurring in both pathways after divergence. Note the widespread effects if the decremental process occurs before the central pathways diverge (which is not to be confused with input specificity and input pathway convergence).
While some have argued that habituation is caused by alterations in inhibitory transmission (e.g., Holmgren and Frenk 1961; Wickelgren 1967b; Wall 1970), data presented by Spencer et al. (1966a–c) argued that habituation is caused by reduced excitatory transmission independent of changes in inhibition (see also Horn 1967). More recent data have not definitively indicated which mechanism is responsible, and the mechanisms need not be mutually exclusive (for review see Mendell 1984). Stimulus parameters seem to determine which mechanism predominates. Based on their experiments, Groves and Thompson (1970) considered habituation to be caused by decremental changes intrinsic to the S-R pathway and probably mediated by a process such as homosynaptic depression. It is data from these experiments on which I will focus.
Homosynaptic mechanisms can produce sensitization with intense stimulation (e.g., PTP; Lloyd 1949), but Groves and Thompson (1970) maintained that for more moderate stimulation intensities, sensitization is predominantly induced by a separate modulatory system. In contrast to the comparative input-specificity of habituation (Hagbarth and Kugelberg 1958; Thorpe 1963; notwithstanding Thompson and Spencer 1966), a heterosynaptic mechanism allows the facilitatory effects to generalize between input pathways. Despite this, the effects of facilitation are not ubiquitous between the central pathways activated by a stimulus: Facilitation occurs in the spinal S-R pathway but not in the ascending pathways (Spencer et al. 1966b); this differs from depression, which tends to affect all central pathways (see above).
Given the approximate localization of the plastic processes and some understanding of the processes themselves, how do depression and facilitation interact at the network level? In their attempts to characterize the neural analogs of dual-process learning in the cat, Groves et al. (1969a; see also Groves and Thompson 1970; Egger 1978) recorded from interneurons in the spinal cord whose activity changed in markedly different ways as learning progressed (Fig. 2). There are nonplastic interneurons that exhibit a short-latency phasic response that does not change over the course of training. Type H interneurons, named after their tendency to habituate (depress), also show a short-latency phasic response that invariably decreases with training. Type S interneurons, named after their tendency to sensitize (facilitate) but that also depress, exhibit a phasic burst followed by a more prolonged response, suggesting that these cells may be farther downstream in the S-R pathway than the aforementioned interneurons. Consistent with this positioning, S neuron response plasticity closely parallels muscle response plasticity (Fig. 2); for instance, the response of S neurons does not increase under conditions in which the reflex fails to sensitize.
Figure 2.
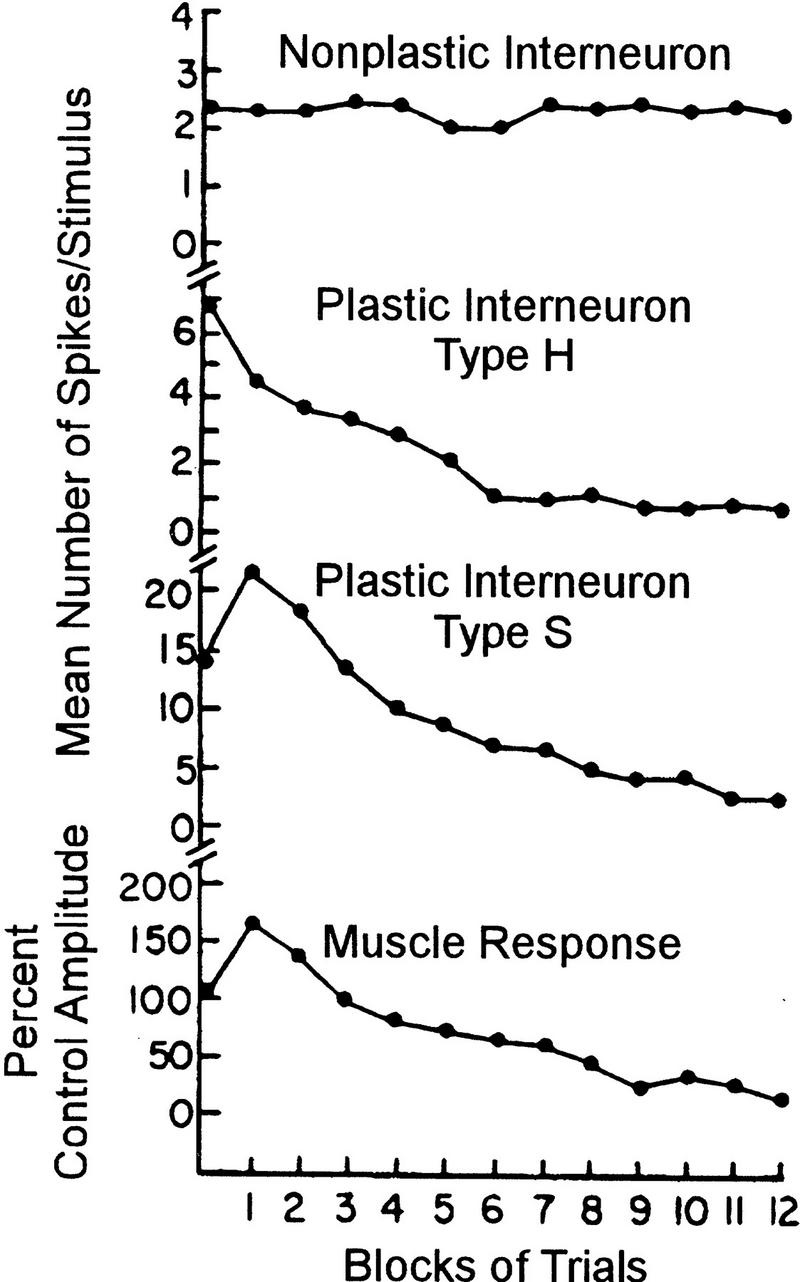
Plasticity of interneurons in the spinal cord of the acute spinal cat during habituation and sensitization of the flexion reflex. The activity of nonplastic interneurons does not change during the course of learning. Type H neurons exhibit only habituation, even when the behavioral response is concomitantly sensitizing. Type S neurons on the other hand exhibit plasticity that closely parallels changes in the muscle response, namely transient sensitization followed by habituation. Reprinted, with permission, from Groves and Thompson (1970). Copyright 1970 by the American Psychological Association.
ACOUSTIC STARTLE REFLEX IN THE RAT
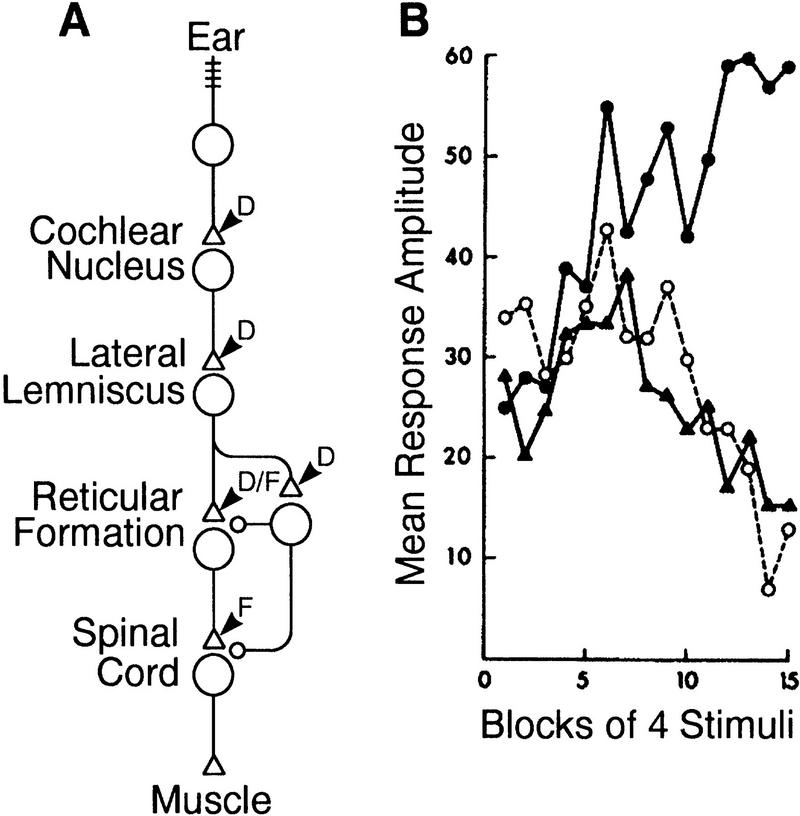
The second behavior considered briefly by Groves and Thompson (1970) was the acoustic startle reflex of the rat. This reflex has been shown to habituate, extrinsically sensitize (Prosser and Hunter 1936; Parker et al. 1974; Davis 1989b), and intrinsically sensitize (Hoffman and Fleshler 1963; Davis and Wagner 1969; Davis 1974). The gross circuitry underlying the acoustic startle reflex (Fig. 3A) has been worked out (Davis et al. 1982a; for reviews, see Davis 1989a; Yeomans and Frankland 1995) thereby allowing a better localization of plastic loci than for the spinal cat. As with repeated acoustic stimulation, repeated electrical stimulation of the posteroventral cochlear nucleus results in initial sensitization followed by habituation, whereas electrical stimulation downstream, at the reticular formation (in the nucleus reticularis pontis caudalis), results only in sensitization (Davis et al. 1982b) (Fig. 3B). These data indicate that depression occurs at or downstream of the cochlear nucleus but upstream of neurons in the reticular formation, some of which constitute a modulatory system. This is supported by the observation that neuronal activity in the reticular formation depresses (Lingenhöhl and Friauf 1994). A modulatory system contributing to intrinsic sensitization most likely descends in parallel with the S-R pathway to induce facilitation at the spinal level (Davis 1980; Davis et al. 1980; Astrachan and Davis 1981), though this does not rule out modulation in the reticular formation that itself can express facilitation (see below). Without being able to rule out facilitation in either the reticular formation or the spinal cord, Figure 3A shows the circuitry whereby incremental changes would be expressed at both sites.
Figure 3.
Acoustic startle reflex in the rat. (A) Neural circuitry mediating the acoustic startle reflex. The circuit diagram shows the S-R pathway (cells with triangular synapses) and modulatory system (cell with circular synapses). (B) Effects of stimulating at different positions along the S-R pathway: acoustic stimulation (▴), electrical stimulation of the cochlear nucleus (○), and electrical stimulation of the reticular formation (•). Response amplitude is measured as the velocity of cage displacement caused by the startle response. Notice that the first two forms of stimulation cause canonical dual-process learning, whereas the third form of stimulation elicits only sensitization. The interpretation of these results in terms of the location of expression of depression (D) and facilitation (F) within the S-R pathway (i.e., at triangular synapses) is shown in A. B is reprinted, with permission, from Davis et al. (1982b). Copyright 1982 by the American Association for the Advancement of Science.
Continued research on this preparation has yielded an increasingly detailed and complex story. The facilitation responsible for extrinsic sensitization has been localized to the reticular formation (Boulis and Davis 1989) where there is a high degree of input pathway convergence (Parker et al. 1974; Groves et al. 1976). The central nucleus of the amygdala is necessary for induction of extrinsic sensitization (Hitchcock et al. 1989) in contrast to intrinsic sensitization (Schanbacher et al. 1996). The difference in the location of the expression of facilitation mediating intrinsic and extrinsic sensitization may have consequences for the behavioral expression of learning (Pilz and Schnitzler 1996) and for learning kinetics, which is an important issue to be discussed again later.
What then does the acoustic startle reflex contribute to the current discussion of dual-process learning? Davis et al. (1982b) concluded that “sensitization may be related to the motor side of reflex arcs, whereas habituation may be related to the sensory side.” Similar conclusions have been reached with other vertebrate reflexes (Hagbarth and Kugelberg 1958; Sanes and Ison 1983) and are generally true of invertebrate reflexes (Menzel and Bicker 1987; see below). This view is consistent with the current paper’s working hypothesis: The modulatory system responsible for inducing facilitation is downstream of at least one depressing locus. This arrangement may account for the habituation of sensitization and, I will argue, is key to understanding dual-process learning kinetics.
SIPHON-ELICITED GSW REFLEX IN APLYSIA
As discussed at the start of this paper, the cellular-connection approach has been very successful in its application to simple systems and nowhere more so than in the GSW reflex of Aplysia. This review is not meant to be comprehensive, but rather, I will draw from the available data those principles of the neural circuitry and its plasticity (Fig. 4) that are relevant to dual-process learning.
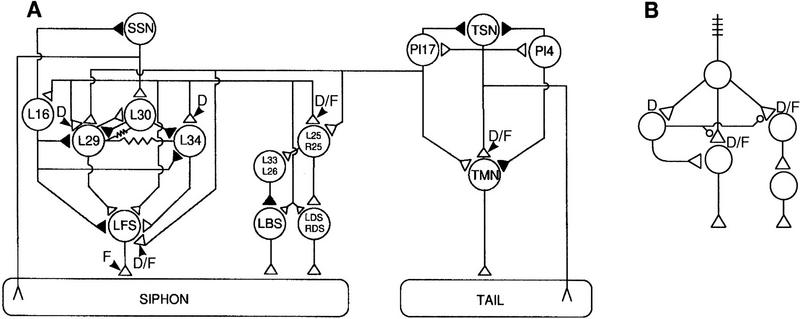
Figure 4.
Neuronal connectivity underlying dual-process learning. (A) The neural circuitry mediating siphon-elicited siphon withdrawal as well as tail-elicited tail and siphon withdrawal in Aplysia. (▵) Excitatory synapses; (▴) inhibitory synapses; modulatory synapses are not shown. Key locations of the expression of depression (D) and facilitation (F) are marked on the circuit diagram. Though mentioned only briefly in the text, the interneuron II network that is responsible for respiratory pumping is shown in the center of the figure as a set of four elements. This network can be recruited by sensory input to contribute to the withdrawal reflex; the plasticity of this recruitment contributes to behavioral plasticity (Eberly and Pinsker 1984; Frost et al. 1988). (SSN) Siphon sensory neurons; (TMN) tail motoneurons; (TSN) tail sensory neurons. (B) Simplified schematic of circuit mediating siphon withdrawal, including putative projections of modulatory neurons. Facilitatory synapses are shown as open circles. The monosynaptic pathway (shown down the center) and the interneuron II network (shown at right) exhibit both depression and facilitation. The polysynaptic pathway (shown at left) exhibits depression but does not feed back onto itself to cause facilitation of its own input. The basis for ubiquitous depression but differential facilitation, and therefore, differential net learning between the pathways, is evident in the network configuration illustrated here. Downstream expression of facilitation is not illustrated here although it cannot be ruled out. A is adapted, with permission, from Cleary et al. (1995).
Canonical dual-process learning in the GSW reflex of naive Aplysia has not been reported, but the system is nonetheless relevant to the ideas presented in this paper. SNs excite the L29 interneurons that are known to cause facilitation at SN–MN synapses (Hawkins et al. 1981a,b; Hawkins and Schacher 1989). Such connectivity conceivably allows for intrinsic sensitization, though the strength of this sensitization is not clear. Although the facilitation may be insufficiently strong to effect a net increase in transmission at the SN–MN synapse, this must be considered in light of the propensity of the SN–MN synapse to undergo depression, meaning that although the effects of facilitation may be less obvious for lack of incremental change, an occult incremental process could have significant influence on the net decremental change (Hawkins et al. 1981b). Compared with naive preparations, intrinsic sensitization is robust in preparations expressing long-term sensitization (Pinsker et al. 1973; for similar observation in rat, see Davis 1972).
Though often neglected, many invertebrate withdrawal reflexes are mediated in part by a peripheral S-R pathway (Peretz 1970; Peretz et al. 1976; Perlman 1979; Prescott and Chase 1996; Prescott et al. 1997). The central and peripheral pathways act in parallel, and each is sufficient to induce and express its own depression. Peripheral induction of facilitation can occur (Lukowiak and Jacklet 1972, 1975), but early data suggest that a central modulatory system contributes to peripheral expression of facilitation (Bullock and Horridge 1965; see also Prescott and Chase 1996). One can speculate that under conditions where centripetal input decreases (because of upstream depression), centrifugal modulation would progressively wane and dual-process learning would ensue.
The separation of mediating and modulatory roles between the peripheral S-R pathway and the central modulatory system, respectively, is beneficial to the investigation of dual-process learning (e.g., in the tentacle withdrawal reflex of Helix; Prescott and Chase 1996; S.A. Prescott and R. Chase, in prep.). However, in most cases, the central S–R pathway cannot be clearly delineated from the modulatory system. In Aplysia, a single cell type such as L29 serves both modulatory (Hawkins et al. 1981a,b; Hawkins and Schacher 1989) and mediating (Hawkins et al. 1981a; Fischer and Carew 1993) roles (for review, see Frost and Kandel 1995). Fortunately, the central S-R pathway can be subdivided into monosynaptic and polysynaptic pathways (Fig. 4A), as well as into the interneuron II network that will not be given detailed consideration here. Unlike the polysynaptic pathway, the monosynaptic pathway does not include modulatory interneurons and therefore serves a purely mediating role. Recording from a motoneuron, changes in the monosynaptic excitatory postsynaptic potential (EPSP) reflect plasticity in the monosynaptic pathway, whereas changes in the complex EPSP reflect the combined changes in both pathways. Comparatively recent data have shown that the polysynaptic pathway makes a significant contribution to the complex EPSP and hence to mediation of the behavior (Trudeau and Castellucci 1992; Fischer and Carew 1993). Prolonged activity in the polysynaptic pathway determines the duration of the reflex muscle contraction (Frost and Kandel 1995; Lieb and Frost 1997), whereas the short-latency phasic burst transmitted through the monosynaptic pathway is probably more influential in the determination of the rate or latency of reflex muscle contraction (Frost et al. 1988).
Decreased transmitter release from SN terminals resulting from homosynaptic depression (Castellucci and Kandel 1974) is widely held as the ultimate cause of habituation in this preparation. Because this decrease works through a homosynaptic mechanism, depression occurs at all SN output sites (Clark and Kandel 1984). This implies decreased input to peripheral MNs via collaterals (Bailey et al. 1979) and decreased input to central MNs and interneurons via projections to the CNS. Homosynaptic processes that increase SN output, for example, PTP, are similarly “cell wide.” In contrast, heterosynaptic facilitation is “branch specific,” meaning that output from different sites on the same SN can be facilitated to different degrees. Clark and Kandel (1984) showed that it is possible to facilitate transmission at either central or peripheral SN terminals independently based on where serotonin (5-HT) is exogenously applied. In the intact animal, differential delivery of 5-HT (or some other modulatory transmitter) probably depends on the patterns of projection of facilitatory neurons and the connections with their target SN (Clark and Kandel 1984). Differential distribution of receptors for the modulatory transmitter on the target cell might further contribute to the branch specificity (Trudeau and Castellucci 1993b; Sun et al. 1996).
The issue of branch-specific facilitation is important for dual-process learning because incremental changes mediating intrinsic sensitization have the possible effect of creating a positive feedback loop to the modulatory system, or, in other words, sensitizing sensitization. This might occur in the GSW reflex if input to certain components of the polysynaptic S-R pathway was facilitated, but this does not seem to be the case. Differential facilitation not only occurs between central and peripheral pathways (see above), but it also occurs between the central monosynaptic S-R pathway and the central polysynaptic S-R pathway/modulatory system. Trudeau and Castellucci (1993b) showed that exogenous application of 5-HT causes twice as much facilitation at SN–MN synapses than at SN–IN+ synapses. Despite 5-HT’s ability to enhance the EPSP recorded in hyperpolarized interneurons, 5-HT has a negligible (Trudeau and Castellucci 1992) or even inhibitory (Fitzgerald and Carew 1991) effect on the complex EPSP measured in MNs. This suggests that there is a compensatory balance after the SN–IN+ synapse to restrict incremental change (e.g., inhibition; see below). Interestingly, small cardioactive peptide B (SCPB) has the opposite selectivity, specifically, enhancing transmission through the polysynaptic pathway (Trudeau and Castellucci 1992).
Decremental changes, on the other hand, are the same for both central pathways given that SN depression is cell-wide (see above) and that interneuron output does not tend to depress so that no additional signal decrement occurs specifically in the polysynaptic pathway (Cleary et al. 1995; exceptions include L22 and L23). Under most stimulation conditions, PTP is probably not significantly induced when inhibitory pathways are intact (Trudeau and Castellucci 1993b; notwithstanding Frost et al. 1988). Given the differential facilitation, net plasticity differs between the two pathways (Fig. 4). Differential facilitation is also found in monosynaptically and polysynaptically mediated components of the human eye-blink reflex (Sanes and Ison 1983), the tentacle withdrawal reflex of Helix (S.A. Prescott and R. Chase, in prep.), and the tail-elicited tail withdrawal reflex of Aplysia (see below), though which component is affected depends on the specific system.
Another difference between the monosynaptic and polysynaptic pathways is the amount of inhibition impinging on each. For example, during the period of L16-mediated inhibition, the amplitude of the complex EPSP is reduced, whereas the amplitude of the monosynaptic EPSP is concurrently enhanced, suggesting that the polysynaptic pathway (specifically, the constituent interneurons) is subject to more inhibition (Wright et al. 1991). Consistent with this view are the inhibitory effects of L30 on L29 and L34. L30 is activated by a wide range of stimulus intensities via the SNs as well as via L29. The reciprocal connections between L29 and L30 mediate recurrent inhibition that is itself plastic, undergoing what Fischer and Carew (1993) call activity-dependent potentiation. Recurrent inhibition functions as a negative feedback mechanism to stabilize the intensity of the neural signal passing through the polysynapic pathway (Lieb and Frost 1997).
This differential inhibition may account for some of the differential facilitation described above. For example, SCPB exerts its facilitatory effect by the reduction of inhibitory input to IN+s, enhancing transmission specifically in the polysynaptic pathway (Trudeau and Castellucci 1993a,b). Fitzgerald and Carew (1991) suggested that different 5-HT receptors may mediate opposite facilitatory and inhibitory effects between different pathways. An alternative proposal is that increased output from SNs may enhance input to IN−s more than input to IN+s, the net effect being reduced activation of the IN+s (Fitzgerald and Carew 1991). In light of these more recent data, the earlier assumption that facilitation necessarily causes the same plasticity in monosynaptic and polysynaptic pathways is inaccurate.
Other questions still remain unanswered. Given that L29’s transmitter is neither 5-HT nor SCPB, it would be interesting to investigate the specific effects of L29-mediated modulation on network activity, including whether or not L29 modulates its own activity thereby forming a positive feedback loop. Hawkins et al. (1981b) reported that facilitation of the SN–MN synapse accounts for most of the L29’s modulatory effects, whereas Frost et al. (1988) and Trudeau and Castellucci (1993a) proposed that the removal of inhibition from the polysynaptic pathway contributes significantly to extrinsic sensitization. Might disinhibition be absent for intrinsic sensitization? This absence could help explain the comparative weakness of intrinsic sensitization and would be consistent with intrinsic sensitization’s tendency to habituate rather than to sensitize. Figure 4B shows the likely configuration in which there is differential facilitation and no positive feedback loop. Hawkins’s (1989) difficulty modelling sensitization’s tendency to habituate might be attributable to not considering the differential effects of facilitation. It would also be interesting to investigate the differences between sensitization and dishabituation (see Marcus et al. 1988) in the context of the ideas discussed above.
To summarize findings from the GSW reflex relevant to later discussion (see Fig. 4B), depression at the SN terminals causes decrement of the neural signal early in the neural circuit, resulting in decreased activity in the S-R pathway and reduced activation of the downstream modulatory system. To counteract this decrement, heterosynaptic facilitation acts at the SN terminals (and potentially elsewhere) to enhance the neural signal. Whereas the former process has cell-wide effects, the latter process has branch-specific effects, selectively enhancing transmission through certain pathways. This specificity may be determined presynaptically by the projection of facilitatory neurons or by the sensitivity of SN terminals to the modulatory transmitter, or the specificity may be determined postsynaptically by differential inhibition of monosynaptic and polysynaptic pathways. Combining specific incremental changes with ubiquitous decremental changes, it follows that the monosynaptic S-R pathway (and perhaps the peripheral S-R pathway and the interneuron II network) exhibits a net increase in transmission efficacy, whereas the polysynaptic S-R pathway/modulatory system exhibits a net decrease. This latter tendency causes sensitization to wane and also directly contributes to response habituation. So long as one contributing S-R pathway shows dual-process learning, pure decrement in the other S-R pathways serves only to dilute (to a degree consistent with the size of that pathway’s contribution) the behavioral manifestation of dual-process learning.
TAIL-ELICITED TAIL AND SIPHON WITHDRAWAL REFLEX IN APLYSIA
Stronger behavioral evidence exists for dual-process learning in the tail-elicited reflexes of Aplysia (Stopfer and Carew 1996) (Fig. 5A). Walters et al. (1983b) clearly showed that both intrinsic and extrinsic sensitization can occur in the tail withdrawal reflex. However, the neural circuitry mediating and modulating tail-elicited reflexes is not as well characterized as that for siphon-elicited reflexes. As shown in Figure 4, tail-induced tail withdrawal is mediated by parallel monosynaptic (Walters et al. 1983a) and polysynaptic pathways (Cleary and Byrne 1993; White et al. 1993), whereas tail-induced siphon withdrawal is mediated through a purely polysynaptic pathway (Cleary and Byrne 1993; Cleary et al. 1995). IN−s activated by tail stimulation have also been identified and shown to inhibit SNs, MNs, and IN−s (Buonomano et al. 1992; Small et al. 1992; Xu et al. 1994, 1995), but the data are insufficient to allow one to compare the inhibitory influences in monosynaptic and polysynaptic pathways.
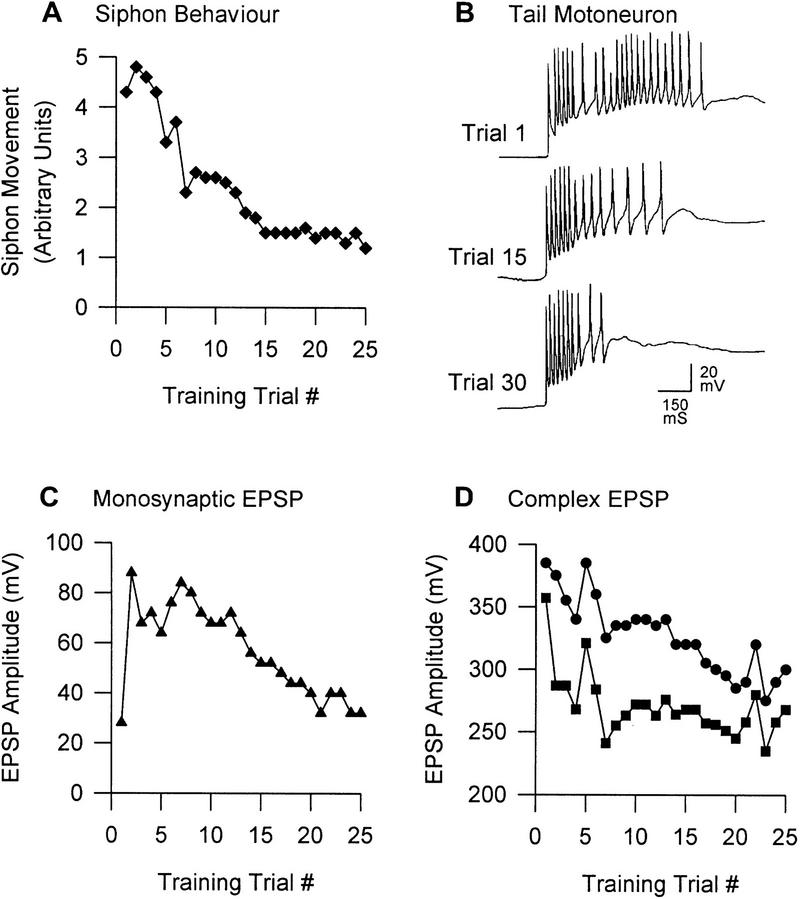
Figure 5.
Tail-elicited withdrawal reflexes in Aplysia. (A) Plasticity of the tail-elicited siphon withdrawal response. This behavior exhibits transient sensitization followed by habituation; these behavioral changes are parallelled by changes in the number of action potentials in siphon MNs (not shown here; see Stopfer and Carew 1996). Stopfer and Carew (1996) also show similar changes in tail-elicited tail MN activity and the tail withdrawal response, though the behavioral changes are quite erratic. (B) Tail MN activity. Short-latency spiking is mediated by monosynaptic pathways, whereas longer latency spiking is mediated by polysynaptic pathways (White et al. 1993). Note the dissociation of plasticity between these two pathways; between trials 1 and 30, the monosynaptic pathway exhibits facilitation, whereas the polysynaptic pathway exhibits depression. A strikingly similar dissociation of plasticity between the different phases of the neuronal response is seen in the S neurons of the spinal cat (Groves and Thompson 1970). (C) Monosynaptic EPSP in tail MN. When the tail MN is prevented from spiking, the monosynaptic EPSP shows sensitization consistent with the data in B but eventually habituates. (D) Complex EPSP in tail MN. The complex EPSP, consisting of monosynaptic and polysynaptic components (•), does not change analogously to the monosynaptic EPSP (C). If the monosynaptic component is subtracted (based on the assumption of linear additivity and four monosynaptic inputs; see White et al. 1993), one sees the polysynaptic component (█) that appears to exhibit fairly pure depression. Adapted, with permission, from Stopfer and Carew (1996).
Many of the phenomena described above for the siphon-elicited GSW reflex are similar for tail-elicited reflexes. In brief, Walters et al. (1983a) reported that repeated intracellular excitation of tail SNs results in progressive depression of their synaptic output. Cutaneous stimulation of the tail, on the other hand, does not cause an equivalent change, because this form of stimulation elicits a concomitant heterosynaptic facilitation to counteract the homosynaptic depression (Walters et al. 1983b; Stopfer and Carew 1996). Furthermore, the two types of S-R pathways are differentially affected by the facilitation mediating intrinsic sensitization. Cutaneous tail stimulation causes net incremental change in the monosynaptic pathway while concurrently causing net decremental change in the polysynaptic pathway (Stopfer and Carew 1996). The dissociation of plasticity between the pathways is evident in two ways. First, the early monosynaptically mediated burst of spikes in the tail MN is increased, whereas the later polysynaptically mediated firing is substantially reduced with repeated stimulation (Fig. 5B) (see also White et al. 1993). Second, the monosynaptic EPSP recorded in the tail MN increases (Fig. 5C), whereas the complex EPSP decreases (Fig. 5D).
Fitzgerald et al. (1990) stressed that the balance between inhibition and facilitation is largely responsible for determining the net magnitude and direction of reflex modulation. As in the GSW reflex, different transmitters have differential effects on cells underlying the tail withdrawal reflex (Xu et al. 1995). For instance, 5-HT reduces inhibition by reducing the activity of the inhibitory neuron Pl4, whereas SCPB has the opposite effect. Understanding the specific effects of such modulation on signal transmission through monosynaptic and polysynaptic pathways will surely benefit the understanding of behavioral plasticity.
SUMMARY OF PHYSIOLOGICAL DATA
On the basis of the above review of different systems and their plasticity, I will draw certain conclusions pertinent to dual-process learning. Habituation is mediated largely by a homosynaptic process (depression) acting at an upstream locus in the S-R pathway. The decremental effect is ubiquitously expressed among the pathways that diverge downstream of the depressed locus. Sensitization on the other hand is mediated largely by a heterosynaptic process (facilitation) that is induced by a modulatory system downstream of the first plastic locus. Facilitation may be expressed downstream of the depressing locus (i.e., in series) and/or upstream, at the same locus as depression (i.e., in parallel). Differential facilitation presynaptically or differential inhibition postsynaptically produces branch-specific plasticity, meaning that the incremental effect will not necessarily be the same for all pathways diverging at the plastic locus. Hence, net plasticity will vary between pathways. One consequence is that the modulatory system will tend not to cause increment of its own input but, rather, that its input will exhibit pure decrement causing sensitization to wane. It is plausible that decrement of the modulatory system’s output might contribute to habituation of sensitization, but this does not seem to occur. The implications of different configurations of plasticity’s induction and expression for dual-process learning will be considered in more detail below.
Model of Dual-Process Learning
In the first model of dual-process learning, Groves and Thompson (1970) worked backwards from dual-process learning curves (solid lines in Fig. 1A1–A4) to compute the hypothetical component single-process learning curves (broken lines in Fig. 1A1–A4). They assumed that decremental and incremental changes in the neural signal are simply added to determine the net plasticity. The present model asserts that interactions between depression and facilitation are more complex.
The single-process habituation learning curves (Fig. 1A1–A4, broken lines, labeled H) exhibit standard parametric features as described by Thompson and Spencer (1966). Each curve decreases exponentially to a minimum asymptote. As stimulus intensity is increased, the rate and degree of habituation decrease. Parametric features of the hypothetical “pure” decremental process are unaffected by sensitization. Certain interneurons, H cells, exhibit learning curves very similar to these hypothetical pure habituation learning curves (Fig. 2). Given the empirically derived dual-process learning curve and a good idea of the single-process habituation learning curve’s shape, the single-process sensitization learning curve (Fig. 1A1–A4, broken lines, labeled S) was calculated by subtraction (i.e., linear additivity is assumed). In simpler systems such as Helix or Aplysia, experimental techniques are available that can facilitate the decomposition of the dual-process learning curve (S.A. Prescott and R. Chase, in prep.).
Determining a single-process learning curve for sensitization as Groves and Thompson did does not explain the basis for that curve, although the curve’s shape does provide some insights. The rate and degree of sensitization are proportional to stimulus intensity (Fig. 1A). However, these learning curves do not simply increase exponentially to a maximum asymptote as might be expected if only pure sensitization were taking place (Fig. 3B; Hawkins 1989; S.A. Prescott and R. Chase, in prep.); instead, each curve rises and peaks early in the stimulus series but decreases, or habituates, as training progresses. Interestingly, the rate and degree of the habituation of sensitization parallels the rate and degree of the partner single-process habituation curve (Fig. 1, cf. A2, A3, and A4). Such a correlation suggests that a common decremental process might be responsible for both response habituation and the habituation of sensitization, though this evidence alone does not rule out multiple decremental processes acting in parallel (see below).
MATHEMATICAL FORMALISMS
Consider the expression of learning from an arithmetic point of view: By adjusting synaptic weights, depression reduces the signal intensity by a certain degree (division) and facilitation increases the signal intensity by a certain degree (multiplication) (Baxter and Byrne 1993). Changing synaptic weights equates to adjusting the efficacy of signal transmission through a locus. The extent of such changes is restricted by physiological constraints. The effects of learning can be described mathematically by differential equations.
Habituation:
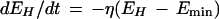 |
1 |
where η ⩾ 0; η∝1/stimulus intensity; 0 ⩽ Emin ⩽ 1; Emin ∝ stimulus instensity.
Sensitization:
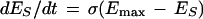 |
2 |
where σ ⩾ 0; σ∝stimulus intensity; Emax ⩾ 1; Emax∝stimulus intensity.
E refers to the transmission efficacy, which determines the factor by which neural signal intensity will change as the signal passes through the plastic locus. For a naive locus, E = 1 (i.e., causes no change to signal intensity), but this value can decrease or increase as a consequence of depression (habituation) or facilitation (sensitization), respectively. Subscripts identify the plasticity related to the change in E.
In equation 1, the constant of proportionality, η, sets the rate of habituation. The degree of habituation is described by the term Emin that sets the asymptote for allowable change in EH (degree of habituation ∝ 1/Emin). Therefore, the equation as written defines exponential decrease of EH at rate η to a minimum asymptote of Emin. Equation 2 defines exponential increase of ES at rate σ to a maximum asymptote of Emax. Equations 1 and 2 can be analytically integrated to give a new pair of equations allowing E to be plotted against discrete time steps (t) that correspond to stimulus trial number (Fig. 6A).
Figure 6.
Hypothetical learning curves to show the interactions of habituation and sensitization to produce dual-process learning. (A) Pure habituation and pure sensitization learning curves. The equations shown are based on the analytical integration of equations 1 and 2 in the text. Numerical values: Emin = 0.3; η = 0.2; Emax = 2; σ = 0.5; EH(0) = ES(0) = 1 (replaced by numerical value in equations). Notice that the value of σ is greater than η, which reflects the fact that sensitization occurs rapidly with the first few stimuli, whereas habituation develops more slowly, relying on stimulus repetition. The matrix shows the possible combinations of parallel and serial induction and expression of learning; the letter in each cell corresponds to the graphs below. (B) Parallel induction, parallel expression. (C) Parallel induction, serial expression. (D) Serial induction, parallel expression. (E) Serial induction, serial expression. The broken curves in D and E, labeled EHS, represent habituating sensitization (see text). The solid curves in B–E represent the net change in transmission efficacy based on combination of incremental and decremental changes according to the equation on each graph (see text). Changes in transmission efficacy are commensurate with changes in behavior.
The matrix in Figure 6 illustrates the possible configurations of induction and expression of depression and facilitation; the letter in each cell corresponds to a graph below showing the learning curve resulting from that particular configuration (Fig. 6B–E). Although each of these graphs exhibits a learning curve seemingly consistent with dual-process learning, one can narrow the field of possibilities by considering the physiological plausibility of each configuration.
INDUCTION OF LEARNING
As a homosynaptic process, depression is both induced and expressed in the S-R pathway. In contrast, for conditions conducive to dual-process learning, evidence indicates that facilitation is largely a heterosynaptic process, expressed in the S-R pathway but induced by a separate modulatory system (Ellaway and Trott 1975; Kandel 1976; Davis 1980; Astrachan and Davis 1981; Flicker et al. 1981).
As already described (see Fig. 1), sensitization tends to wane or habituate. There are two potential explanations for this, both of which imply serial induction: (1) decrement of input to the modulatory system and/or (2) decrement of output from the modulatory system. These conditions are not mutually exclusive, nor are the effects equivalent: Decreased input implies habituation that is input-specific, whereas decreased output implies habituation that is generalized assuming convergence of input pathways to a common modulatory system. In other words, habituation of intrinsic sensitization might generalize to cause habituation of extrinsic sensitization and dishabituation, or vice versa. Stimulation data from the acoustic startle reflex in rats and from the tentacle withdrawal reflex of Helix are consistent with decrement of input but not with decrement of output. Evidence from Aplysia tends to suggest the same conclusion: SN output exhibits robust depression, whereas the output of directly stimulated interneurons (some of which are facilitatory) does not depress. Hence, physiological data argue that serial induction of learning, specifically depression upstream of (i.e., decrement of input to) the modulatory system, is largely responsible for the habituation of sensitization’s induction. The tendency to habituate is also true for the induction of extrinsic sensitization and dishabituation (Lehner 1941; Thompson and Spencer 1966; Pinsker et al. 1970). In short, intrinsic and extrinsic sensitizing stimulation recruits the modulatory system via SNs or upstream interneurons whose output is prone to depression. Depending on how the circuit is organized, it is possible that depression at some loci causes habituation of the response, whereas depression at other loci causes habituation of sensitization. This may help explain differences in the rate of habituation and is easily accounted for in the model by incorporation of a scaling factor (see below).
Given the physiological data discussed above, I will focus on the learning curves resulting from serial induction (Fig. 6D,E). The habituation of sensitization can be expressed by modifying equation 2.
Habituating Sensitization:
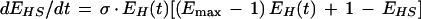 |
3 |
Both σ and Emax are directly proportional to stimulus intensity (see above). Neural signal intensity is supposed to be the neural analog of stimulus intensity, but signal intensity can change consequent to learning despite no change in stimulus intensity. Depression at upstream loci causes decreased input to facilitatory neurons and therefore a decreased rate of sensitization. This equates with a reduction of σ by EH, a factor reflecting the effects of habituation. A scaling factor or nonlinear transformation might also be incorporated to more accurately describe the rate at which sensitization habituates (see subsection Application of the Model). The degree of sensitization is also influenced by habituation and is accounted for in equation 3 by multiplying (Emax −1 ) by EH so as to reduce the maximum asymptote to a naive value of one. Because EH changes over time, analytical integration of equation 3 was not self-evident and, instead, integration was done numerically using the fourth order Runge–Kutta method with 0.1 time unit increments run on SigmaPlot 4.0 (SPSS, Inc.). The resulting habituating sensitization curves are shown on Figure 6, D and E (broken lines labeled EHS). The EHS curve must then be combined with the EH curve to determine the final changes to E, but the manner in which those curves are combined depends on the configuration of the expression of learning.
EXPRESSION OF LEARNING
In Aplysia, depression and facilitation are both expressed in the SN terminals, but this does not exclude plasticity elsewhere, and downstream expression of facilitation may contribute significantly to effecting behavioral change (Cohen et al. 1997). Data on the specific localization of plasticity are also inconclusive for vertebrate preparations. Without being able to eliminate either of the possibilities, both serial and parallel expression of plasticities will be considered to juxtapose the outcomes.
Because depression and facilitation are, respectively, dividing or multiplying the neural signal by some factor (as opposed to adding or subtracting a fixed value from the signal), the size of the neural signal influences the expression of learning in terms of absolute change in signal intensity. Configuration of the expression of learning therefore influences how one calculates the net change in transmission efficacy (Enet) for the neural circuit (see below) and gives rise to different learning kinetics (Fig. 6B–E). Changes in Enet are commensurate with changes in neural signal intensity and, ultimately, with changes in behavior.
Under conditions of parallel expression, the current model treats depression and facilitation as acting independently but simultaneously (i.e., at the same locus) on the neural signal with the resultant decremental and incremental effects adding to determine Enet. Specific subcellular changes effecting synaptic plasticity, including vesicle depletion and mobilization as well as calcium current down- and up-regulation (Gingrich and Byrne 1985; Byrne et al. 1989; Klein 1995; Byrne and Kandel 1996), may interact in complex ways, but given the redundancy of those changes, the ultimate decremental and incremental changes are assumed to be additive. Detailed consideration of specific interactions at the synaptic level is beyond the scope of this paper, but those interactions certainly warrant further investigation. Figure 6D shows the learning curve resulting from serial induction and parallel expression.
In the instance that depression’s expression precedes facilitation’s expression, depression divides the neural signal such that the subsequent multiplicative effect of facilitation (now acting on a signal smaller than that at the start of the circuit) will be reduced in efficacy. In contrast to parallel expression, facilitation under these conditions is purely “restorative” in that the process does not prevent the initial signal decrement but only tries to effect some recovery after the fact. If signal decrement is severe, subsequent increment might be insufficient to restore the signal to its original intensity. This is true even before taking into account that the induction of intrinsic sensitization would be reduced, meaning that not only is a smaller signal “multiplied” by a constant facilitation, but in fact, a progressively smaller signal is multiplied by a progressively smaller facilitation. These combined actions cause exaggeration of the falling phase of the learning curve resulting from serial induction and serial expression (Fig. 6E) compared with that in Figure 6D. The reverse order of expression, that is, facilitation followed by depression, is not suggested by physiological data.
Following from the above discussion, the configuration of expression has important implications for dishabituation. Given that dishabituation is not mediated by removal of habituation but rather by a separate facilitatory process (Thompson and Spencer 1966; Carew et al. 1971), the cellular changes mediating habituation and dishabituation need not be expressed at the same locus. However, the capacity of most reflexes to readily dishabituate may suggest that serial expression is less likely than parallel expression. Under the latter conditions, the multiplicative effect of facilitation, acting on the signal before that signal’s decrement, will be more efficacious by preventing signal decrement through the circuit rather than by trying to effect recovery from that decrement. But there is a caveat: Dishabituation, extrinsic sensitization, and intrinsic sensitization need not be mediated by the same mechanism and/or by changes at the same locus. Data from the rat (Schanbacher et al. 1996) and from Aplysia (Marcus et al. 1988) suggest differences between these forms of incremental plasticity.
APPLICATION OF THE MODEL
Thus far, the mathematical model presented here has been used to formalize the description of interactions between depression (habituation) and facilitation (sensitization). The current section has four purposes: (1) to show how the model can be applied to empirical data; (2) to identify what sorts of data are necessary for proper application of the model; (3) to offer modifications that may improve the model; and (4) to demonstrate the potential benefits of the model.
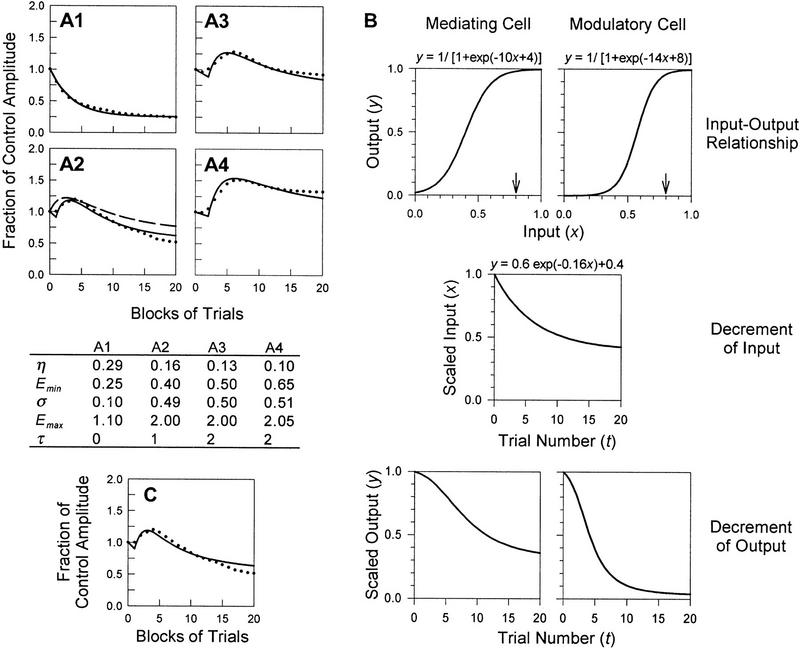
The data presented by Groves and Thompson (1970) demonstrate dual-process learning at four different stimulus intensities (see Fig. 7A). When trying to fit curves to these data using the current model, it becomes immediately obvious that too many parameters are free to change. Although the relationships between stimulus intensity and the parameters in equations 1–3 are known (see above), specific functions relating those parameters to stimulus intensity are not known, and moreover, the stimulus intensities corresponding to each of the four curves were not published. Hence, it is not possible to fit the curves by adjusting only stimulus intensity. To deal with this problem, I have assumed that Groves and Thompson’s calculation of pure habituation curves (Fig. 1A, broken lines, labeled H) is accurate despite a lack of empirical evidence for those specific curves (see below). The values of η and Emin were determined by fitting the pure habituation curves. Curve fitting was done visually by manually adjusting parameters in user-defined transforms performed on SigmaPlot 4.0. Having determined values for η and Emin, only σ and Emax were free to vary when fitting the dual-process learning curve. In all cases, parameters varied with stimulus intensity according to known relationships. Assuming that induction is serial, such that sensitization habituates, curves were fit to the data in Figure 7, A1–A4, for serial expression (solid lines) and for parallel expression (broken line; example shown only on A2); in the former case, parameter values are shown in the table below part A1–A4.
Figure 7.
Application of the model. (A1–A4) Curve fits of data from the spinal cat. A1–A4 correspond to A1–A4 in Fig. 1. Solid lines represent learning curves for conditions of serial induction and serial expression of learning; the broken line in A2 shows an example of a learning curve for serial induction and parallel expression (for discussion of curve fitting technique, see text). For the former case, parameter values are shown in the table. (B) Enhancement of habituation of sensitization compared with response habituation. The logistic function relates a neuron’s input to its output, resulting in a sigmoidal input–output curve (top graphs). Assuming a high threshold to recruit sensitization, the input–output curve for a modulatory cell is shifted right and is steep compared with the analogous curve for a mediating cell. Using the example of a stimulus of strength x = 0.8 (small arrows), equivalent decrement of input (middle graph) will cause more rapid decrement of modulatory cell output than mediating cell output (bottom graphs). The value of x is determined by the stimulus intensity; however, values for input and output are scaled to starting values of 1 for plotting against t, so as to be consistent with other learning curves. (C) Example of curve fit after nonlinear transformation. The data points are the same as those shown in A2. The solid line shows the improved fit (compare with broken curve in A2) after a transformation of the sort described in B, wherein the rate and degree of sensitization’s habituation is increased compared with response habituation.
The problems with the methodology described suggest how data collection could be improved to facilitate application of the model. First, if one could elicit depression and facilitation separately, one could characterize the kinetics of each process in isolation from the other. That is, learning by pure habituation could be curve fit by equation 1, whereas learning by pure sensitization could be curve fit by equation 2, therein determining η, Emin, σ, and Emax for use in equation 3 to predict dual-process learning (S.A. Prescott and R. Chase, in prep.). Even if only one process could be separately elicited, the process described in the preceding paragraph could be used without assuming any arbritrary dissection of the dual-process learning curve. Curve fitting should ideally be repeated over a range of stimulus intensities, thereby testing the model with different combinations of depression and facilitation. In so doing, η, Emin, σ, and Emax would be determined at a number of stimulus intensities, therein allowing those parameters to be written as functions of stimulus intensity and extending the predictive powers of this model. The results in the table in Figure 7 suggest that such functions might be highly nonlinear (see below), though as already mentioned, the stimulus intensities corresponding to A1–A4 are not known.
A number of potential ways for improving the model are also suggested by this prelimary attempt at applying it. A time delay (τ) in the expression of sensitization may help explain the inflection on the rising phase of the dual-process learning curves. Such a latency is not uncommon (e.g., Marcus et al. 1988). Different integer values were used to approximate τ for parts A1–A4 (see table in Fig. 7); the value was determined solely by the horizontal shift necessary for the rising phase of the curve to coincide with the data points. The onset of sensitization is approximated as a discrete event, though more accurately, the onset is gradual. Moreover, the stronger the sensitization, the more delayed is the onset of the full sensitizing effect, explaining the increase in τ between parts A1 and A4.
The solid curves (Fig. 7A1–A4) fit the data fairly well after inclusion of the time delay. On the other hand, the broken curve (Fig. 7A2) tends to diverge from the data points later in the stimulus series. This suggests that the rate and degree of habituation of sensitization are higher than for the corresponding habituation of the behavioral response. As previously alluded to, a scaling factor or nonlinear transformation can be incorporated into equation 3 to more accurately relate the rate and degree of response habituation to that of sensitization’s habituation. There is a plausible physiological basis for this if the input–output curves for mediating and modulatory neurons are not the same. Based on a modulatory cell’s higher threshold for activation, the sigmoidal input–output curve is steeper and displaced to the right compared with the curve for a mediating cell (i.e., a cell in the S-R pathway) (Fig. 7B). Consequently, although depression may cause equivalent reduction in input to the two cell types, the output is differentially affected such that the rate of sensitization’s habituation is increased. Applying this transformation (see figure legend for details) to the EH term used in equation 3 can improve curve fitting; Figure 7C shows an example of such a fit using the same data as in Figure 7A2.
As stated before, the rate of sensitization is thought to be strongly influenced by stimulus intensity. But as shown in the table in Figure 7, σ changes only slightly between part A2 and A4, suggesting that the incremental effects of sensitization become saturated. However, the rate of sensitization’s habituation can vary substantially and is likely prone to nonlinearities given the relatively steep sigmoidal shape expected for a modulatory cell’s input–output curve (Fig. 7B). Despite the restriction on σ, the capacity of sensitization to withstand habituation increases with stronger input. This can be thought of as “supermaximal” sensitization and is analogous to sub-zero habituation (Thompson and Spencer 1966).
Unlike previous models of dual-process learning (Groves and Thompson 1970; Bashinski et al. 1985), the current model does not simply assume habituation of sensitization, but rather, it attempts to explain how the positioning of learning processes within a neural network might cause sensitization to habituate and, furthermore, how expression of opposing changes might interact within the S-R pathway. Working from a plausible physiological mechanism, it is possible to mathematically describe the interaction between learning processes and predict dual-process learning kinetics. The formal mathematical description offered here, although still open to modifications, is necessary for eventually understanding the complex, nonlinear manner in which depression and facilitation may interact. The suggestion of supermaximal sensitization, for instance, would not be evident without having applied the current model.
Hopefully, the results presented here will direct future research to collect the sort of data necessary to test this model. For instance, if the effects of the modulatory system can be removed by lesions or drugs, the kinetics of pure habituation can be determined to facilitate curve fitting. The kinetics of pure sensitization can also be determined by adjusting stimulation parameters. If the modulatory system is identified, its activity can be monitored over the course of learning and related to the habituation of sensitization; it can also be directly stimulated to test whether its output depresses. These last two tests would indicate whether the depression responsible for causing sensitization to habituate occurs upstream or downstream of the modulatory system.
Conclusion
Dual-process learning has important behavioral consequences. The occurrence of intrinsic sensitization serves to rapidly increase responsiveness to a stimulus that is strong and/or novel. Some people might claim that habituation, on the other hand, results from weak stimulation, and they would therefore deduce that habituation and intrinsic sensitization are mutually exclusive. In keeping with Groves and Thompson (1970), I maintain that although habituation is affected by stimulus intensity, it primarily serves to decrease responsiveness to a stimulus, weak or strong, that experience has proven to be inconsequential, meaning that habituation is more heavily influenced by stimulus repetition than by stimulus intensity. Habituation and intrinsic sensitization are not mutually exclusive if they are thought of in this way, and the dual-process theory of plasticity attempts to explain how the processes interact when they occur together. Weak stimuli are shown to be inconsequential with comparatively little stimulus repetition, and thus, the evoked responses readily habituate. In contrast, strong stimuli initially elicit sensitization, and the response would remain sensitized were it not for repetition of the stimulus that serves to ultimately cause habituation, albeit more slowly because of the stimulus intensity.
The differential sensitivity of habituation and sensitization to different stimulation parameters, namely stimulus intensity and repetition, relates to the cellular mechanisms mediating those learning processes. When these processes occur together, they interact at the cellular level (for reviews, see Klein 1995; Byrne and Kandel 1996) and also at the network level as demonstrated in this paper. The evidence reviewed and incorporated into a simple mathematical model suggests that depression not only competes with facilitation to determine the final behavioral outcome, but that depression also works to reduce the induction of facilitation. The latter interaction results from the serial induction of plasticity, depression followed by facilitation. The former interaction results from serial and/or parallel expression of opposing changes within the same S-R pathway. The combination of interactions between depression and facilitation results in the kinetics of dual-process learning.
Acknowledgments
I thank R. Chase and L. Glass for their helpful advice. I also thank S. Ratté for her criticism of the manuscript. This research was supported by a postgraduate scholarship from the Natural Sciences and Engineering Research Council of Canada.
References
- Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Astrachan DI, Davis M. Spinal modulation of the acoustic startle response: The role of norepinephrine, serotonin and dopamine. Brain Res. 1981;206:223–228. doi: 10.1016/0006-8993(81)90121-9. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Castellucci VF, Koester J, Kandel ER. Cellular studies of peripheral neurons in siphon skin of Aplysia californica. J Neurophysiol. 1979;42:530–557. doi: 10.1152/jn.1979.42.2.530. [DOI] [PubMed] [Google Scholar]
- Balaban P. Behavioral neurobiology of learning in terrestrial snails. Prog Neurobiol. 1993;41:1–19. doi: 10.1016/0301-0082(93)90038-t. [DOI] [PubMed] [Google Scholar]
- Bashinski HS, Werner JS, Rudy JW. Determinants of infant visual fixation: Evidence for a two-process theory. J Exp Child Psychol. 1985;39:580–598. doi: 10.1016/0022-0965(85)90058-x. [DOI] [PubMed] [Google Scholar]
- Baxter DA, Byrne JH. Learning rules from neurobiology. In: Gardner D, editor. The neurobiology of neural networks. Cambridge, MA: MIT Press; 1993. pp. 71–105. [Google Scholar]
- Boulis NM, Davis M. Footshock-induced sensitization of electrically elicited startle reflexes. Behav Neurosci. 1989;103:504–508. doi: 10.1037//0735-7044.103.3.504. [DOI] [PubMed] [Google Scholar]
- Brown GD, Frost WN, Getting PA. Habituation and iterative enhancement of multiple components of the Tritonia swim response. Behav Neurosci. 1996;110:486–491. doi: 10.1037//0735-7044.110.3.478. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Horridge GA. Structure and function of the nervous system of invertebrates. San Francisco, CA: Freeman; 1965. pp. 1340–1353. [Google Scholar]
- Buonomano DV, Baxter DA, Byrne JH. Small networks of empirically derived adaptive elements simulate some higher-order features of classical conditioning. Neural Networks. 1990;3:507–523. [Google Scholar]
- Buonomano DV, Cleary LJ, Byrne JH. Inhibitory neuron produces heterosynaptic inhibition of the sensory-to-motor synapse in Aplysia. Brain Res. 1992;577:147–150. doi: 10.1016/0006-8993(92)90548-n. [DOI] [PubMed] [Google Scholar]
- Burke RE. Spinal cord: Ventral horn. In: Shepherd GM, editor. The synaptic organization of the brain. 4th ed. New York, NY: Oxford University Press; 1998. pp. 77–120. [Google Scholar]
- Byrne JH. Comparative aspects of neural circuits for inking behavior and gill withdrawal in Aplysia californica. J Neurophysiol. 1981;45:98–106. doi: 10.1152/jn.1981.45.1.98. [DOI] [PubMed] [Google Scholar]
- ————— Cellular analysis of associative learning. Physiol Rev. 1987;67:329–439. doi: 10.1152/physrev.1987.67.2.329. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: State and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JH, Gingrich KJ, Baxter DA. Computational capabilities of single neurons: Relationship to simple forms of associative and nonassociative learning in Aplysia. In: Hawkins RD, Bower GH, editors. Computational models of learning in simple neural systems. Toronto, Canada: Academic Press; 1989. pp. 31–63. [Google Scholar]
- Carew TJ, Sahley CL. Invertebrate learning and memory: From behavior to molecules. Annu Rev Neurosci. 1986;9:435–487. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Castellucci VF, Kandel ER. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci. 1971;2:79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Kandel ER. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci. 1974;71:5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976;194:1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Castellucci V, Pinsker H, Kupfermann I, Kandel ER. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Christoffersen GRJ, Schilhab TSS. Synaptic long-term depression alters recovery from, and frequency dependency of, short-term depression in Helix pomatia. Neuroscience. 1996;73:1009–1016. doi: 10.1016/0306-4522(96)00104-2. [DOI] [PubMed] [Google Scholar]
- Christoffersen GRJ, Frederiksen K, Johansen J, Kristensen BI, Simensen L. Behavioural modification of the optic tentacle of Helix pomatia; effect of puromycin, activity of S-100. Comp Biochem Physiol A. 1981;68:611–624. [Google Scholar]
- Clark GA, Kandel ER. Branch-specific heterosynaptic facilitation in Aplysia siphon sensory cells. Proc Natl Acad Sci. 1984;81:2577–2581. doi: 10.1073/pnas.81.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary LJ, Byrne JH. Identification and characterization of a multifunction neuron contributing to defensive arousal in Aplysia. J Neurophysiol. 1993;70:1767–1776. doi: 10.1152/jn.1993.70.5.1767. [DOI] [PubMed] [Google Scholar]
- Cleary LJ, Byrne JH, Frost WN. Role of interneurons in defensive withdrawal reflexes in Aplysia. Learn & Mem. 1995;2:133–151. doi: 10.1101/lm.2.3-4.133. [DOI] [PubMed] [Google Scholar]
- Cohen TE, Kaplan SW, Kandel ER, Hawkins RD. A simplified preparation for relating cellular events to behavior: Mechanisms contributing to habituation, dishabituation, and sensitization of the Aplysia gill-withdrawal reflex. J Neurosci. 1997;17:2886–2899. doi: 10.1523/JNEUROSCI.17-08-02886.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. The withdrawal response of a freshwater snail (Lymnaea stagnalis L.) J Exp Biol. 1975;62:783–796. [Google Scholar]
- Davis M. Differential retention of sensitization and habituation of the startle response in the rat. J Comp Physiol Psychol. 1972;78:260–267. doi: 10.1037/h0032811. [DOI] [PubMed] [Google Scholar]
- ————— Sensitization of the rat startle response by noise. J Comp Physiol Psychol. 1974;87:571–581. doi: 10.1037/h0036985. [DOI] [PubMed] [Google Scholar]
- ————— Neurochemical modulation of sensory-motor reactivity: Acoustic and tactile startle reflexes. Neurosci Biobehav Rev. 1980;4:241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- ————— Neural systems involved in fear-potentiated startle. Ann NY Acad Sci. 1989a;563:165–183. doi: 10.1111/j.1749-6632.1989.tb42197.x. [DOI] [PubMed] [Google Scholar]
- ————— Sensitization of the acoustic startle reflex by footshock. Behav Neurosci. 1989b;103:495–503. [PubMed] [Google Scholar]
- Davis M, Wagner AR. Habituation of the startle response under an incremental sequence of stimulus intensities. J Comp Physiol Psychol. 1969;67:486–492. doi: 10.1037/h0027308. [DOI] [PubMed] [Google Scholar]
- Davis M, File SE. Intrinsic and extrinsic mechanisms of habituation and sensitization: Implications for the design and analysis of experiments. In: Peeke HVS, Petrinovich L, editors. Habituation, sensitization, and behavior. Montreal, Canada: Academic Press; 1984. pp. 287–323. [Google Scholar]
- Davis M, Astrachan DI, Kass E. Excitatory and inhibitory effects of serotonin on sensorimotor reactivity measured with acoustic startle. Science. 1980;209:521–523. doi: 10.1126/science.7394520. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: Lesion and stimulation studies. J Neurosci. 1982a;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Parisi T, Gendelman DS, Tischler M, Kehne JH. Habituation and sensitization of startle reflexes elicited electrically from the brainstem. Science. 1982b;218:688–690. doi: 10.1126/science.7134967. [DOI] [PubMed] [Google Scholar]
- Durkovic RG. Classical conditioning of the flexion reflex in spinal cat: Features of the reflex circuitry. Neurosci Lett. 1983;39:155–160. doi: 10.1016/0304-3940(83)90069-1. [DOI] [PubMed] [Google Scholar]
- Eberly LB, Pinsker HM. Neuroethological studies of reflex plasticity in intact Aplysia. Behav Neurosci. 1984;98:609–630. doi: 10.1037//0735-7044.98.4.609. [DOI] [PubMed] [Google Scholar]
- Egger MD. Sensitization and habituation of dorsal horn cells in cats. J Physiol. 1978;279:153–166. doi: 10.1113/jphysiol.1978.sp012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Trott JR. The mode of action of 5-hydroxytryptophan in facilitating a stretch reflex in the spinal cat. Exp Brain Res. 1975;22:145–162. doi: 10.1007/BF00237685. [DOI] [PubMed] [Google Scholar]
- Fischer TM, Carew TJ. Activity-dependent potentiation of recurrent inhibition: A mechanism for dynamic gain control in the siphon withdrawal reflex of Aplysia. J Neurosci. 1993;13:1302–1314. doi: 10.1523/JNEUROSCI.13-03-01302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TM, Blazis DEJ, Priver NA, Carew TJ. Metaplasticity at identified inhibitory synapses in Aplysia. Nature. 1997;389:860–865. doi: 10.1038/39892. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K, Carew TJ. Serotonin mimics tail shock in producing transient inhibition in the siphon withdrawal reflex of Aplysia. J Neurosci. 1991;11:2510–2518. doi: 10.1523/JNEUROSCI.11-08-02510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K, Wright WG, Marcus EA, Carew TJ. Multiple forms of non-associative plasticity in Aplysia: A behavioural, cellular and pharmacological analysis. Phil Trans R Soc Lond B Biol Sci. 1990;329:171–178. doi: 10.1098/rstb.1990.0162. [DOI] [PubMed] [Google Scholar]
- Flicker C, McCarley RW, Hobson JA. Aminergic neurons: State control and plasticity in three model systems. Cell Mol Neurobiol. 1981;1:123–166. doi: 10.1007/BF00710716. [DOI] [PubMed] [Google Scholar]
- Frost WN, Kandel ER. Structure of the network mediating siphon-elicited siphon withdrawal in Aplysia. J Neurophysiol. 1995;73:2413–2427. doi: 10.1152/jn.1995.73.6.2413. [DOI] [PubMed] [Google Scholar]
- Frost WN, Clark GA, Kandel ER. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol. 1988;19:297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Byrne JH. Simulation of synaptic depression, posttetanic potentiation, and presynaptic facilitation of synaptic potentials from sensory neurons mediating gill-withdrawal reflex in Aplysia. J Neurophysiol. 1985;53:652–669. doi: 10.1152/jn.1985.53.3.652. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- ————— . A dual-process theory of habituation: Neural mechanisms. In: Peeke HVS, Herz MJ, editors. Habituation. Vol. II. Physiological substrates. New York, NY: Academic Press; 1973. pp. 175–205. [Google Scholar]
- Groves PM, DeMarco R, Thompson RF. Habituation and sensitization of spinal interneuron activity in acute spinal cat. Brain Res. 1969a;14:521–525. doi: 10.1016/0006-8993(69)90129-2. [DOI] [PubMed] [Google Scholar]
- Groves PM, Lee D, Thompson RF. Effects of stimulus frequency and intensity on habituation and sensitization in acute spinal cat. Physiol Behav. 1969b;4:383–388. [Google Scholar]
- Groves PM, Wilson CJ, Miller SW. Habituation of the acoustic startle response: A neural systems analysis of habituation in the intact animal. In: Riesen AH, Thompson RF, editors. Advances in psychobiology vol. 3. Toronto, Canada: John Wiley and Sons; 1976. pp. 327–379. [PubMed] [Google Scholar]
- Hagbarth KE, Kugelberg E. Plasticity of the human abdominal skin reflex. Brain. 1958;81:305–318. doi: 10.1093/brain/81.3.305. [DOI] [PubMed] [Google Scholar]
- Harris JD. Habituatory response decrement in the intact organism. Psychol Bull. 1943;40:385–422. [Google Scholar]
- Hawkins RD. A biologically based computational model for several simple forms of learning. In: Hawkins RD, Bower GH, editors. Computational models of learning in simple neural systems. Toronto, Canada: Academic Press; 1989. pp. 65–108. [Google Scholar]
- Hawkins RD, Kandel ER. Is there a cell-biological alphabet for simple forms of learning? Psychol Rev. 1984;91:375–391. [PubMed] [Google Scholar]
- Hawkins RD, Schacher S. Identified facilitator neurons L29 and L28 are excited by cutaneous stimuli used in dishabituation, sensitization, and classical conditioning of Aplysia. J Neurosci. 1989;9:4236–4245. doi: 10.1523/JNEUROSCI.09-12-04236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Castellucci VF, Kandel ER. Interneurons involved in mediation and modulation of gill-withdrawal reflex in Aplysia. I. Identification and characterization. J Neurophysiol. 1981a;45:304–314. doi: 10.1152/jn.1981.45.2.304. [DOI] [PubMed] [Google Scholar]
- ————— Interneurons involved in mediation and modulation of gill-withdrawal reflex in Aplysia. II. Identified neurons produce heterosynaptic facilitation contributing to behavioral sensitization. J Neurophysiol. 1981b;45:315–326. doi: 10.1152/jn.1981.45.2.315. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Clark GA, Kandel ER. Cell biological studies of learning in simple vertebrate and invertebrate systems. In: Mountcastle VB, Plum F, editors. Handbook of physiology, sec. 1: The nervous system V. Baltimore, MD: American Physiological Society; 1987. pp. 25–83. [Google Scholar]
- Hawkins RD, Kandel ER, Siegelbaum SA. Learning to modulate transmitter release: Themes and variations in synaptic plasticity. Annu Rev Neurosci. 1993;16:625–665. doi: 10.1146/annurev.ne.16.030193.003205. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Cohen TE, Greene W, Kandel ER. Relationships between dishabituation, sensitization, and inhibition of the gill- and siphon-withdrawal reflex in Aplysia californica—effects of response measure, test time, and training stimulus. Behav Neurosci. 1998;112:24–38. doi: 10.1037//0735-7044.112.1.24. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Behavioural habituation. In: Horn G, Hinde RA, editors. Short-term changes in neural activity and behaviour. Cambridge, UK: Cambridge University Press; 1970. pp. 3–40. [Google Scholar]
- Hitchcock JM, Sananes CB, Davis M. Sensitization of the startle reflex by footshock: Blockade by lesions of the central nucleus of the amygdala or its efferent pathway to the brainstem. Behav Neurosci. 1989;103:509–518. doi: 10.1037//0735-7044.103.3.509. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Fleshler M. Startle reaction: Modification by background acoustical stimulation. Science. 1963;141:928–930. doi: 10.1126/science.141.3584.928. [DOI] [PubMed] [Google Scholar]
- Holmgren B, Frenk S. Inhibitory phenomena and “habituation” at the neuronal level. Nature. 1961;192:1294–1295. doi: 10.1038/1921294a0. [DOI] [PubMed] [Google Scholar]
- Horn G. Neuronal mechanisms of habituation. Nature. 1967;215:707–711. doi: 10.1038/215707a0. [DOI] [PubMed] [Google Scholar]
- Jacklet JW, Lukowiak K. Neural processes in habituation and sensitization in model systems. Prog Neurobiol. 1975;4:1–56. [Google Scholar]
- Jacklet JW, Rine J. Facilitation at neuromuscular junctions: Contribution to habituation and dishabituation of the Aplysia gill withdrawal reflex. Proc Natl Acad Sci. 1977;74:1267–1271. doi: 10.1073/pnas.74.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. Cellular basis of behavior. San Francisco, CA: W.H. Freeman; 1976. [Google Scholar]
- Kandel ER, Spencer WA. Cellular neurophysiological approaches in the study of learning. Physiol Rev. 1968;48:65–134. doi: 10.1152/physrev.1968.48.1.65. [DOI] [PubMed] [Google Scholar]
- Kanz JE, Eberly LB, Cobbs JS, Pinsker HM. Neuronal correlates of siphon withdrawal in freely behaving Aplysia. J Neurophysiol. 1979;42:1538–1556. doi: 10.1152/jn.1979.42.6.1538. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Werner JS. Habituation, response to novelty, and dishabituation in human infants: Tests of a dual-process theory of visual attention. J Exp Child Psychol. 1986;42:199–217. doi: 10.1016/0022-0965(86)90023-8. [DOI] [PubMed] [Google Scholar]
- Klein M. Modulation of ion currents and regulation of transmitter release in short-term synaptic plasticity: The rise and fall of the action potential. Invert Neurosci. 1995;1:15–24. doi: 10.1007/BF02331828. [DOI] [PubMed] [Google Scholar]
- Klein M, Kandel ER. Presynaptic modulation of voltage-dependent Ca2+ current: Mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci. 1978;75:3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner GFJ. A study of the extinction of unconditioned reflexes. J Exp Psychol. 1941;29:435–456. [Google Scholar]
- Lieb JR, Jr, Frost WN. Realistic simulation of the Aplysia siphon-withdrawal reflex circuit: Roles of circuit elements in producing motor output. J Neurophysiol. 1997;77:1249–1268. doi: 10.1152/jn.1997.77.3.1249. [DOI] [PubMed] [Google Scholar]
- Lingenhöhl K, Friauf E. Giant neurons in the rat reticular formation: A sensorimotor interface in the elementary acoustic startle circuit? J Neurosci. 1994;14:1176–1194. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DPC. Post-tetanic potentiation of response in monosynaptic reflex pathways of the spinal cord. J Gen Physiol. 1949;33:147–170. doi: 10.1085/jgp.33.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockery SR, Kristan WB., Jr Two forms of sensitization of the local bending reflex of the medicinal leech. J Comp Physiol A. 1991;168:165–177. doi: 10.1007/BF00218409. [DOI] [PubMed] [Google Scholar]
- Lockery SR, Sejnowski TJ. A lower bound on the detectability of nonassociative learning in the local bending reflex of the medicinal leech. Behav Neural Biol. 1993;59:208–224. doi: 10.1016/0163-1047(93)90974-m. [DOI] [PubMed] [Google Scholar]
- Lukowiak K, Jacklet JW. Habituation and dishabituation: Interactions between peripheral and central nervous systems in Aplysia. Science. 1972;178:1306–1308. doi: 10.1126/science.178.4067.1306. [DOI] [PubMed] [Google Scholar]
- ————— Habituation and dishabituation mediated by the peripheral and central neural circuits of the siphon of Aplysia. J Neurobiol. 1975;6:183–200. doi: 10.1002/neu.480060206. [DOI] [PubMed] [Google Scholar]
- Lukowiak K, Colebrook E. Neuronal mechanisms of learning in an in vitro Aplysia preparation: Sites other than the sensory-motor neuron synapse are involved. J Physiol (Paris) 1988–1989;83:198–206. [PubMed] [Google Scholar]
- Marcus EA, Nolen TG, Rankin CH, Carew TJ. Behavioral dissociation of dishabituation, sensitization, and inhibition in Aplysia. Science. 1988;241:210–213. doi: 10.1126/science.3388032. [DOI] [PubMed] [Google Scholar]
- Mauelshagen J, Parker GR, Carew TJ. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LE. Modifiability of spinal synapses. Physiol Rev. 1984;64:260–324. doi: 10.1152/physrev.1984.64.1.260. [DOI] [PubMed] [Google Scholar]
- Menzel R, Bicker G. Plasticity in neuronal circuits and assemblies of invertebrates. In: Changeux J-P, Konishi M, editors. The neural and molecular bases of learning. Toronto, Canada: John Wiley and Sons; 1987. pp. 433–472. [Google Scholar]
- Moschovakis AK, Solodkin M, Burke RE. Anatomical and physiological study of interneurons in an oligosynaptic cutaneous reflex pathway in the cat hindlimb. Brain Res. 1992;586:311–318. doi: 10.1016/0006-8993(92)91641-q. [DOI] [PubMed] [Google Scholar]
- Parker MV, Miller SW, Groves PM. Neuronal habituation and sensitization in the reticular formation of the rat. Physiol Psychol. 1974;2:464–470. doi: 10.1016/0031-9384(72)90079-0. [DOI] [PubMed] [Google Scholar]
- Peeke HVS. Habituation of the conspecific aggression in the three-spined stickleback (Gasterosteus aculeatus L.) Behaviour. 1969;35:137–156. [Google Scholar]
- ————— Habituation, sensitization, and redirection of aggression and feeding behavior in the three-spined stickleback (Gasterosteus aculeatus L.) J Comp Psychol. 1983;97:43–51. [PubMed] [Google Scholar]
- Peeke HVS, Petrinovich L. Approaches, constructs, and terminology for the study of response change in the intact organism. In: Peeke HVS, Petrinovich L, editors. Habituation, sensitization, and behavior. Montreal, Canada: Academic Press; 1984. pp. 1–14. [Google Scholar]
- Peeke HVS, Veno A. Stimulus specificity of habituated aggression in three-spined sticklebacks (Gasterosteus aculeatus L.) Behav Biol. 1973;8:427–431. doi: 10.1016/s0091-6773(73)80083-5. [DOI] [PubMed] [Google Scholar]
- Peretz B. Habituation and dishabituation in the absence of a central nervous system. Science. 1970;169:379–381. doi: 10.1126/science.169.3943.379. [DOI] [PubMed] [Google Scholar]
- Peretz B, Jacklet JW, Lukowiak K. Habituation of reflexes in Aplysia: Contribution of the peripheral and central nervous systems. Science. 1976;191:396–399. doi: 10.1126/science.1246622. [DOI] [PubMed] [Google Scholar]
- Perlman AJ. Central and peripheral control of siphon-withdrawal reflex in Aplysia californica. J Neurophysiol. 1979;42:510–529. doi: 10.1152/jn.1979.42.2.510. [DOI] [PubMed] [Google Scholar]
- Pilz PKD, Schnitzler H-U. Habituation and sensitization of the acoustic startle response in rats: Amplitude, threshold, and latency measures. Neurobiol Learn Mem. 1996;66:67–79. doi: 10.1006/nlme.1996.0044. [DOI] [PubMed] [Google Scholar]
- Pinsker H, Kupfermann I, Castellucci V, Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Pinsker HM, Hening WA, Carew TJ, Kandel ER. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science. 1973;182:1039–1042. doi: 10.1126/science.182.4116.1039. [DOI] [PubMed] [Google Scholar]
- Prescott SA, Chase R. Two types of plasticity in the tentacle withdrawal reflex of Helix aspersa are dissociated by tissue location and response measure. J Comp Physiol A. 1996;179:407–414. [Google Scholar]
- Prescott SA, Gill N, Chase R. Neural circuit mediating tentacle withdrawal in Helix aspersa, with specific reference to the competence of the motoneuron C3. J Neurophysiol. 1997;78:2951–2965. doi: 10.1152/jn.1997.78.6.2951. [DOI] [PubMed] [Google Scholar]
- Prosser CL, Hunter WS. The extinction of startle responses and spinal reflexes in the white rat. Am J Physiol. 1936;117:609–618. [Google Scholar]
- Rakitin A, Tomsic D, Maldonado H. Habituation and sensitization to an electrical shock in the crab Chasmagnathus. Effect of background illumination. Physiol Behav. 1991;50:477–487. doi: 10.1016/0031-9384(91)90533-t. [DOI] [PubMed] [Google Scholar]
- Roberts MBV. Facilitation in the rapid response of the earthworm, Lumbricus terrestris L. J Exp Biol. 1966;45:141–150. doi: 10.1242/jeb.45.1.141. [DOI] [PubMed] [Google Scholar]
- Rumelhart DE, McClelland D, editors. Parallel distributed processing. Cambridge, MA: MIT Press; 1986. [Google Scholar]
- Sanes JN, Ison JR. Habituation and sensitization of components of the human eyeblink reflex. Behav Neurosci. 1983;97:833–836. doi: 10.1037//0735-7044.97.5.833. [DOI] [PubMed] [Google Scholar]
- Schanbacher A, Koch M, Pilz PKD, Schnitzler H-U. Lesions of the amygdala do not affect the enhancement of the acoustic startle response by background noise. Physiol Behav. 1996;60:1341–1346. doi: 10.1016/s0031-9384(96)00242-9. [DOI] [PubMed] [Google Scholar]
- Small SA, Cohen TE, Kandel ER, Hawkins RD. Identified FMRFamide-immunoreactive neuron LPl16 in the left pleural ganglion of Aplysia produces presynaptic inhibition of siphon sensory neurons. J Neurosci. 1992;12:1616–1627. doi: 10.1523/JNEUROSCI.12-05-01616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin WS. Mechanisms for the generation of synapse specificity in long-term memory: The implications of a requirement for transcription. Trends Neurosci. 1996;19:215–218. doi: 10.1016/0166-2236(96)20016-5. [DOI] [PubMed] [Google Scholar]
- Spencer WA, Thompson RF, Neilson DR. Response decrement of the flexion reflex in the acute spinal cat and transient restoration by strong stimuli. J Neurophysiol. 1966a;29:221–239. doi: 10.1152/jn.1966.29.2.221. [DOI] [PubMed] [Google Scholar]
- ————— Alterations in responsiveness of ascending and reflex pathways activated by iterated cutaneous afferent volleys. J Neurophysiol. 1966b;29:240–252. doi: 10.1152/jn.1966.29.2.240. [DOI] [PubMed] [Google Scholar]
- ————— Decrement of ventral root electrotonus and intracellularly recorded PSPs produced by iterated cutaneous afferent volleys. J Neurophysiol. 1966c;29:253–274. doi: 10.1152/jn.1966.29.2.253. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Carew TJ. Heterosynaptic facilitation of tail sensory neuron synaptic transmission during habituation in tail-induced tail and siphon withdrawal reflexes of Aplysia. J Neurosci. 1996;16:4933–4948. doi: 10.1523/JNEUROSCI.16-16-04933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZY, Kauderer B, Schacher S. Differential distribution of functional receptors for neuromodulators evoking short-term heterosynaptic plasticity at Aplysia sensory neurons. J Neurosci. 1996;16:7540–7549. doi: 10.1523/JNEUROSCI.16-23-07540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Groves PM, Teyler TJ, Roemer RA. A dual-process theory of habituation: Theory and behavior. In: Peeke HVS, Herz MJ, editors. Habituation. Vol. I. Behavioral studies. New York, NY: Academic Press; 1973. pp. 239–271. [Google Scholar]
- Thorpe WH. Learning and instinct in animals. Cambridge, MA: Harvard University Press; 1963. [Google Scholar]
- Trudeau L-E, Castellucci VF. Contribution of polysynaptic pathways in the mediation and plasticity of Aplysia gill and siphon withdrawal reflex: Evidence for differential modulation. J Neurosci. 1992;12:3838–3848. doi: 10.1523/JNEUROSCI.12-10-03838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Functional uncoupling of inhibitory interneurons plays an important role in short-term sensitization of Aplysia gill and siphon withdrawal reflex. J Neurosci. 1993a;13:2126–2135. doi: 10.1523/JNEUROSCI.13-05-02126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Sensitization of the gill and siphon withdrawal reflex of Aplysia: Multiple sites of change in the neuronal network. J Neurophysiol. 1993b;70:1210–1220. doi: 10.1152/jn.1993.70.3.1210. [DOI] [PubMed] [Google Scholar]
- Wall PD. Habituation and post-tetanic potentiation in the spinal cord. In: Horn G, Hinde RA, editors. Short-term changes in neural activity and behaviour. Cambridge, UK: Cambridge University Press; 1970. pp. 181–210. [Google Scholar]
- Walters ET, Bryne JH. Post-tetanic potentiation in Aplysia sensory neurons. Brain Res. 1984;293:377–380. doi: 10.1016/0006-8993(84)91247-2. [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol. 1983a;50:1522–1542. doi: 10.1152/jn.1983.50.6.1522. [DOI] [PubMed] [Google Scholar]
- ————— Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimulation. J Neurophysiol. 1983b;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- White JA, Ziv I, Cleary LJ, Baxter DA, Byrne JH. The role of interneurons in controlling the tail-withdrawal reflex in Aplysia: A network model. J Neurophysiol. 1993;70:1777–1786. doi: 10.1152/jn.1993.70.5.1777. [DOI] [PubMed] [Google Scholar]
- Wickelgren BG. Habituation of spinal motoneurons. J Neurophysiol. 1967a;30:1404–1423. doi: 10.1152/jn.1967.30.6.1404. [DOI] [PubMed] [Google Scholar]
- ————— Habituation of spinal interneurons. J Neurophysiol. 1967b;30:1424–1438. doi: 10.1152/jn.1967.30.6.1424. [DOI] [PubMed] [Google Scholar]
- Wright WG, Marcus EA, Carew TJ. A cellular analysis of inhibition in the siphon withdrawal reflex of Aplysia. J Neurosci. 1991;11:2498–2509. doi: 10.1523/JNEUROSCI.11-08-02498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Cleary LJ, Byrne JH. Identification and characterization of pleural neurons that inhibit tail sensory neurons and motor neurons in Aplysia: Correlation with FMRFamide immunoreactivity. J Neurosci. 1994;14:3565–3577. doi: 10.1523/JNEUROSCI.14-06-03565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Pieroni JP, Cleary LJ, Byrne JH. Modulation of an inhibitory interneuron in the neural circuitry for the tail withdrawal reflex of Aplysia. J Neurophysiol. 1995;73:1313–1318. doi: 10.1152/jn.1995.73.3.1313. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: Neurons and connections. Brain Res—Brain Res Rev. 1995;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Zakharov IS, Balaban PM. Neural mechanisms of age-dependent changes in avoidance behaviour of the snail Helix lucorum. Neuroscience. 1987;23:721–729. doi: 10.1016/0306-4522(87)90089-3. [DOI] [PubMed] [Google Scholar]