Abstract
We examined whether swimming and inking, two defensive responses in Aplysia fasciata, are facilitated by a classical conditioning procedure that has been shown to facilitate a third defensive response, respiratory pumping. Training consisted of pairing a head shock (UCS) with a modified seawater (85%, 120%, or pH 7.0 seawater—CSs). Animals were tested by re-exposing them to the same altered seawater 1 hr after the training. For all three altered seawaters, only respiratory pumping is specifically increased by conditioning. Swimming is sensitized by shock, and inking is unaffected by training, indicating that the conditioning procedure is likely to affect a neural site that differentially controls respiratory pumping. Additional observations also indicate that the three defensive responses are differentially regulated. First, different noxious stimuli preferentially elicit different defensive responses. Second, the three defensive responses are differentially affected by shock. Inking is elicited only immediately following shock, whereas swimming and respiratory pumping are facilitated for a period of time following the shock. Third, swimming and respiratory pumping are differentially affected by noxious stimuli that are delivered in open versus closed environments. These data confirm that neural pathways exist that allow Aplysia to modulate separately each of the three defensive behaviors that were examined.
Neural circuits controlling withdrawal reflexes and other defensive behaviors in Aplysia are used as model systems for examining the cellular mechanisms underlying learning and memory (for review, see Carew and Sahley 1986; Byrne 1987; Byrne and Kandel 1996). Aplysia defensive behaviors are affected by a variety of associative and nonassociative learning paradigms. One training paradigm, termed “learned fear,” facilitates a number of defensive behaviors but also inhibits feeding, indicating that learning probably occurs by modulating a central motivational state (Walters et al. 1981). Other training paradigms affect only a particular defensive response, sometimes only a response to a very localized noxious stimulus (Carew et al. 1971, 1983; Scholz and Byrne 1987; Walters 1987), indicating that they probably occur by modifying only lower level circuitry that controls a specific aspect of behavior. Examining whether a particular learning task affects a number of defensive behaviors in synchrony or affects only a particular aspect of a single defensive response can provide insight into the neural sites at which the learning occurs and the possible cellular mechanisms underlying the learning.
The present study examines whether a classical conditioning procedure that was shown previously to affect one defensive behavior in Aplysia fasciata, respiratory pumping, also affects other defensive behaviors. If the training procedure affects respiratory pumping and other defensive responses in tandem, it is reasonable to hypothesize that the primary neural site affected by learning is a higher order motivational center that regulates many defensive behaviors. In contrast, if the training procedure only modifies respiratory pumping, the learning is likely to be localized to lower level neural circuitry that is concerned with regulating only a subset of defensive behaviors.
Previous studies in our laboratory showed that increases and decreases in seawater concentration (Levy et al. 1993), as well as an increase in seawater pCO2 that is measured by a decrease in pH (Levy et al. 1989), elicit increases in the rate of respiratory pumping in A. fasciata. A number of observations supported the conclusion that respiratory pumping in response to these stimuli does not have a respiratory or a volume regulatory function but, rather, is a defensive response (Levy et al. 1993). First, the temporal patterning of respiratory pumping in response to these altered seawaters is similar to that in response to a noxious stimulus, head shock (Levy et al. 1993). Second, surgical procedures that block the increased respiratory pumping in response to changes in seawater concentration increase the ability of an animal to maintain its volume rather than decreasing it (Levy and Susswein 1993; Levy et al. 1993). Third, respiratory pumping in response to an increased pCO2 is associated with a decrease in oxygen consumption rather than with an increase (Levy et al. 1989). Fourth, increases and decreases in seawater concentration also elicit escape locomotion and inking, bona fide defensive responses (Levy et al. 1993). Fifth, a classical conditioning procedure in which a noxious stimulus (head shock) is paired with a subthreshold exposure to an altered seawater causes a pairing-specific increase in respiratory pumping in response to these stimuli an hour following the pairing (Levy and Susswein 1990; Levy et al. 1994b).
In this paper we examine whether a training procedure that leads to pairing-specific increases in respiratory pumping when animals are stimulated with an altered seawater conditioned stimulus (CS) also causes increases in other defensive responses, such as swimming and inking. Previous studies have suggested that somewhat different neural pathways, which use different neurotransmitters, may be activated in response to a variety of altered seawaters that can modulate respiratory pumping (Levy and Susswein 1993). For this reason, it was of interest to determine the effects of training on a number of different altered seawaters. We report that similar pairing-specific effects are seen when the CS is an increase in seawater concentration, a decrease in concentration, or a decrease in pH. For all three CSs, pairing-specific effects are restricted to respiratory pumping, suggesting that the primary events underlying the learning are restricted to the neural circuitry controlling this behavior.
Materials and Methods
Most of the data reported in this paper were collected along with data that have been reported previously on the effects of altered seawaters on respiratory pumping (Levy et al. 1989, 1993) and on learned changes in these effects (Levy and Susswein 1990; Levy et al. 1994b). We now report additional data collected along with those previously reported on two additional defensive behaviors, swimming and inking. In addition, much of the earlier data on respiratory pumping were reanalyzed to provide a direct comparison with the data on swimming and inking. A small number of additional experiments were also performed, to supplement the data that were already available from the previous experiments.
Data were combined from experiments using identical treatments, which were performed at different times. Cursory examination of the data showed no differences in the results obtained. For most treatments, combining data provided us with very large ns, thereby adding to the reliability of the findings.
ANIMALS
A. fasciata weighing 80–160 grams were collected along the Mediterranean coast of Israel. Animals were stored in 940-liter tanks of aerated, filtered Mediterranean seawater at 19°C with lighting at Light/dark periods of 12:12 and were fed two to three times weekly with Ulva lactuca that was gathered along with animals and was stored frozen. Experiments were performed from 1 week to 1 month after the animals were collected.
PREPARATION OF DIFFERENT SOLUTIONS
Concentrated seawaters were prepared by boiling natural Mediterranean seawater to make a 200% stock solution and then diluting the stock solution with 100% Mediterranean seawater, to end up with solutions varying from 110% to 140% seawater. Solutions of 75%–90% seawater were prepared by diluting Mediterranean seawater with deionized water. The concentration of seawater achieved was measured with a refractometer (Tamco Industries).
Low-pH seawaters were prepared by bubbling CO2 into the water. The pH achieved was monitored by a feedback loop that controlled the rate of bubbling. The apparatus used to control pH has been described previously (Levy et al. 1989).
MEASUREMENT OF BEHAVIORS AND STATISTICAL TESTS
On the day of an experiment, animals were transferred to a 400-liter tank of aerated, filtered Mediterranean seawater at 19°C. At the start of each experiment, the animals were transferred to 8-liter experimental aquaria in which their behaviors were observed.
Respiratory pumping, swimming, and inking movements were observed visually. The intensity of a movement was not monitored. A contraction of the parapodia, mantle, and siphon not elicited by an external stimulus was classified as a respiratory pump. A secretion of a purple substance was classified as inking. Parapodial flapping with the foot not attached to the substrate was classified as swimming.
For respiratory pumping, the number of pumps observed for a 10-min test was determined. Thus, for this behavior, parametric statistical tests could be used (either t-tests or one-way analyses of variance, followed by multiple-comparison tests). For swimming and inking, in experiments in which the animals were trained and in experiments measuring threshold, the presence or absence of swimming or inking at any time during the 10-min test was noted, but the length of time devoted to the behavior or the number of bouts of a behavior were not noted. Thus, each 10-min test provided only a single yes–no observation of swimming or inking, and parametric tests could not be used. χ2 tests were used to determine whether swimming or inking was changed by an experimental procedure. In some cases, groups were combined to provide larger ns when testing swimming and inking, whereas the groups were tested separately when using parametric tests on respiratory pumping. For observations in open versus closed environments, the length of time spent swimming was measured, and therefore, t-tests were used.
TRAINING PROCEDURE
The training procedures are illustrated in Figure 1. The control rate of respiratory pumping before training was measured during a 10-min immersion in an 8-liter aquarium of normal Mediterranean seawater (100%, pH 7.8 seawater). The animals were then transferred to a second chamber, where they received one of four training procedures, which are described below. The animals remained in the training chamber for 5 min, and during this period, all incidence of inking and swimming were noted, but the number of respiratory pumps was not recorded, because in many cases animals inked, and the number of pumps could not be counted reliably. At the end of this period, the animals were transferred to a new chamber of 100% seawater. They remained in this chamber for 1 hr. Respiratory pumping, swimming, and inking were noted during the first and last 10 min of this hour. At the end of the hour, animals were transferred to another test chamber, where they were tested by exposure to one of three altered seawaters (85%, 120%, or pH 7.0). Each animal was tested with only one of these stimuli and was not subsequently reused. For animals that were exposed to an altered seawater during the training (paired, unpaired, or CS alone—see below), the altered seawater during the test was the same as that during the training. Respiratory pumping, swimming, and inking were measured during a 10-min exposure to this stimulus.
Figure 1.
Training procedures. Before all training procedures, the rate of respiratory pumping, as well as the presence of swimming and inking, were measured during 10 min in 100% (pH 7.8) seawater (Before Training). Animals were then transferred to a second chamber, where they received one of four treatments: UCS and CS paired, UCS and CS unpaired, UCS alone, or CS alone. Exposure during a treatment to the CS, an altered seawater (85%, 120%, or pH 7.0 seawater), is depicted by a shaded bar, whereas the presence of normal seawater is depicted by an open bar. The UCS, shock, is depicted by an arrowhead. The animals remained in the training chamber for 5 min. Inking and swimming (but not respiratory pumping) in response to the training procedures were noted. The animals were then transferred for 1 hr to a new chamber of 100% seawater. Respiratory pumping, swimming, and inking were measured during the first and last 10 min of this hour. At the end of the hour, animals were transferred to a test chamber with an altered seawater concentration. Respiratory pumping, swimming, and inking were measured during 10 min in this solution. Responses during this period were compared with those in naive animals, which had not been trained.
Handling the animals was minimized when animals were transferred from one chamber to another. The animals were transferred by placing a plastic mesh cage underneath them, lifting the cage out of the chamber, and then placing it into the second chamber, where animals were released. Control experiments (n = 10) in which animals were transferred between containers of 100% seawater showed that the transfer alone did not elicit defensive behaviors.
The four training procedures were as follows (1) Paired: Aplysia were placed in one of three altered seawater solutions (85% seawater, n = 16; 120% seawater, n = 17; and pH 7.0 seawater, n = 15) for 5 min. The altered seawaters represent the CS. At 2.5 min after being placed in this seawater, the animals received a series of shocks to the head. Shock was the unconditioned stimulus (UCS). (2) CS, UCS, unpaired: Animals were transferred to one of the altered seawater CSs for 5 min (for 85% seawater, n = 16; for 120% seawater, n = 17; and for pH 7.0 seawater, n = 13). They were then transferred to 100% seawater and there received the UCS (shock). (3) UCS (shock) alone: Animals were transferred to normal (100%) Mediterranean seawater and there received only the UCS (shock) 2.5 min after being transferred (for animals subsequently tested in 85% seawater, n = 12; for animals tested in 120% seawater, n = 14; and for animals tested in pH 7.0 seawater, n = 23). (4) CS (altered seawater) alone: Animals were transferred to one of the altered seawater CSs but were not stimulated with the UCS, shock (for 85% seawater, n = 8; for 120% seawater, n = 8; and for pH 7.0 seawater, n = 8).
Shocks were delivered by touching to the head (between the rhinophores) a bipolar platinum electrode. When the head withdrew, the experimenter followed it, so that the electrode maintained contact with the head. Shocks were delivered as a 30-sec train of 50-mA AC pulses at 0.33 Hz (1.5-sec pulse width).
BLIND PROCEDURES
In approximately half of the experiment in which animals were trained, both training and testing were done using blind experimental procedures (see Levy and Susswein 1990; Levy et al. 1994b). For blind training, shocks were delivered without the experimenter knowing the nature of the ambient water. For blind testing, the nature of the preceding training was not revealed to the experimenter until after the termination of the experiment. With respect to data on respiratory pumping, we have reported previously that there are no significant differences between data gathered from experiments using blind and nonblind procedures (Levy and Susswein 1990; Levy et al. 1994b). For swimming and inking, casual analysis of the data also showed no obvious differences between data from blind and nonblind procedures. For these reasons, in the analyses presented below, data from both blind and nonblind procedures were combined.
MEASUREMENTS OF THRESHOLD
Respiratory pumping, swimming, and inking were measured during a 10-min exposure to various increases (110%, n = 23; 120%, n = 40; 125%, n = 18; 130%, n = 19; 140%, n = 21) and decreases (90%, n = 19; 85%, n = 20; 80%, n = 20; 75%, n = 15) in seawater concentration, as well as decreases in pH (7.5, n = 15; 7.0, n = 32; 6.5, n = 15; 6.0, n = 26; and 5.5, n = 12). The values were compared with those measured in 111 control animals that were observed for 10 min in 100% (pH 7.8) seawater.
Results
Previous experiments have shown that shock and exposure to altered seawaters (hypertonic, hypotonic, and low pH seawaters) lead to increases in three defensive responses: (1) respiratory pumping, (2) escape swimming, (3) inking (Levy et al. 1993). Previous data (Levy and Susswein 1990; Levy et al. 1994b) also examined the effects of a number of training procedures on one of these defensive responses, respiratory pumping. The previous studies did not examine whether the training procedures also affect swimming or inking. We now present data on the effects of various training procedures on swimming and inking and compare these data to those on respiratory pumping.
PAIRING-SPECIFIC FACILITATION IS RESTRICTED TO RESPIRATORY PUMPING
We re-examined the data from the previous experiments to determine whether any of four training procedures (paired, unpaired, UCS alone, CS alone; Fig. 1) elicits changes in respiratory pumping, swimming, or inking when animals are tested for 10 min in an altered seawater CS 1 hr after the training. The four training procedures were applied using three separate stimuli as a CS: 85% seawater, 120% seawater, and pH 7.0 seawater. For each of these stimuli, respiratory pumping, swimming, and inking were found to be differentially affected by the four training procedures.
EFFECT ON INKING
Inking differed from respiratory pumping and swimming in that inking was never observed in any of the animals tested, in any of the altered seawaters, following any of the four training procedures. These results suggest that none of the training procedures is effective in causing animals to ink in response to an altered seawater. However, an alternate possibility is that the animals did not ink in response to the altered seawaters because they were unable to release ink in response to any stimulus. Because many of the animals had inked in response to shock delivered during the training procedure 1 hr previously (see below), it is possible that the ink gland was depleted or that there is a refractory period of >1 hr between bouts of inking. Furthermore, it is possible that animals that did not ink in response to shock during the training are unable to ink in response to all noxious stimuli. To test these possibilities, 16 animals were shocked in either 120% (n = 10) or pH 7.0 (n = 6) seawater and were then shocked a second time a 1 hr later in the same altered seawater, to determine whether they were able to ink. Thirteen of the 16 animals inked in response to the first shock. Nine of the 13 inking animals subsequently responded to the second shock as well, whereas 1 of the 3 animals that did not ink in response to the first shock inked in response to the second shock. To determine whether the two animals that did not respond to either shock were able to ink, these animals were exposed to an additional shock of 200 mA, which is 4 times larger than the shocks used previously. Both animals inked in response to the more powerful shock. These data indicate that animals are generally able to ink 1 hr after being shocked, independent of whether or not the shock induces inking. Thus, the lack of inking in response to altered seawater most likely reflects a failure of the altered seawater stimulus to induce inking, rather than an inability of the animals to ink.
PAIRING SPECIFICITY
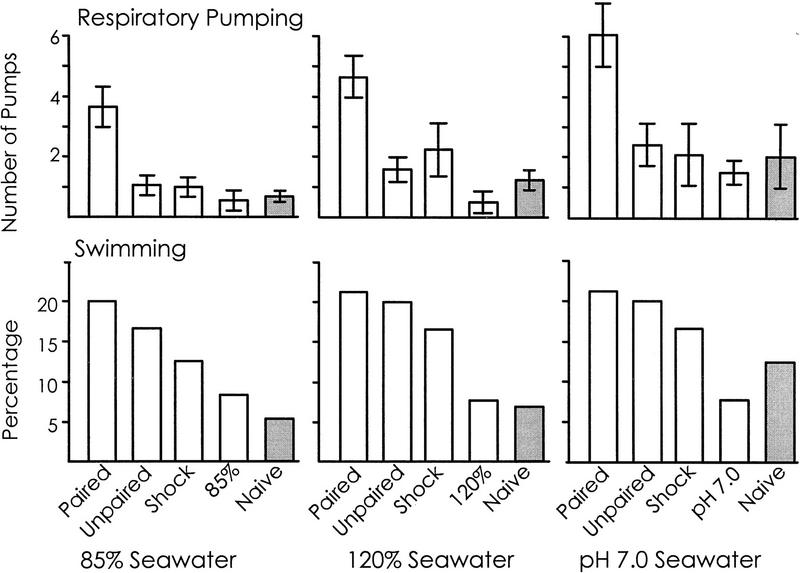
For respiratory pumping and swimming, we reexamined the previously published data (Levy and Susswein 1990; Levy et al. 1994b) to compare the response in altered seawaters following paired versus unpaired shock, to determine whether these behaviors are increased as a specific result of pairing (Fig. 2). Pairing shock with either 85%, 120%, or pH 7.0 seawater was found to cause increases in the respiratory pump rate when animals are re-exposed to the same altered seawater a 1 hr later, with respect to the values seen when the two stimuli are presented unpaired [for 85% seawater, P = 0.013, t(30) = 2.63; for 120% seawater, P = 0.011, t(32) = 2.70; for pH 7.0 seawater, P = 0.012, t(26) = 2.71; two-tailed t-tests]. In contrast, there were no significant differences between swimming in animals that had experienced paired and unpaired shock [for 85% seawater, P = 0.82, χ2(1) = 0.05; for 120% seawater, P = 0.92, χ2(1) = 0.01; for pH 7.0 seawater, P = 0.85, χ2(1) = 0.04; χ2 tests].
Figure 2.
Respiratory pumping and swimming in altered seawaters after conditioning. The effects of four treatments are shown on respiratory pumping and swimming in response to exposure to 85%, 120%, or pH 7.0 seawaters. Treatments occurred an hour before the tests shown in the figure. (Paired) Shock delivered in an altered seawater; (Unpaired) exposure to an altered seawater followed by shock; (Shock) exposure to a shock delivered in 100% seawater; (85%, 120%, or pH 7.0 seawater) exposure to one of these altered seawaters. Respiratory pumping and swimming in naive animals exposed to the three altered seawaters is also shown (shaded bars). For respiratory pumping, s.e.s are shown. The data show pairing-specific increases in respiratory pumping in response to all three altered seawaters, as well as sensitization of swimming in response to 85% and 120% seawaters.
SENSITIZATION
The possible sensitization of either respiratory pumping or of swimming by the preceding shock was examined by comparing the responses after shock (unpaired or alone) with those seen in naive animals, during a 10-min exposure to the altered seawater solutions (Fig. 2). There were no significant differences in respiratory pumping between naive animals and animals that had been shocked previously, for any of the three altered seawaters (P > 0.30, t < 1.01). In contrast, there were significant increases in swimming in 85% [P = 0.040, χ2 (1) = 4.21] and in 120% seawaters [P = 0.007, χ2(1) = 7.24] after shock, either alone or unpaired (data were combined from these two groups). There was no significant increase in swimming in pH 7.0 seawater [P = 0.15, χ2(1) = 2.06].
The experiment above indicated that shock causes a sensitization of swimming, but not of respiratory pumping, when animals are exposed 1 hr later to 85% or 120% seawater. A second experiment was performed to replicate this finding. In this experiment, respiratory pumping and swimming were examined when animals were immersed for 10 min in either 85% (n = 13) or 120% (n = 12) seawater. Twenty-four hours later, these animals were shocked in 100% seawater, as described above. One hour after the shock, the response to 10 min of immersion in either 85% or 120% seawater was tested again. Data were compared with those collected 24 hr earlier, before animals had been shocked. Shock was not found to cause an increase in the rate of respiratory pumping in either 85% [P = 0.60, t(12) = 0.54] or 120% seawater [P = 0.180, t(11) = 1.43; two-tailed paired t-tests]. In contrast, after the shock, 3 of 13 animals swam in response to 85% seawater, and 3 of 12 animals swam in response to 120% seawater, whereas no swimming was seen in response to either stimulus before the shock. The difference in swimming before and after shock was significant [P = 0.005, χ2(1) = 7.89; values for 85% and 120% seawaters were combined]. These data confirm the finding that shock sensitizes swimming but not respiratory pumping.
UNCONDITIONED RESPONSES TO ALTERED SEAWATERS
The previous experiment showed that pairing an altered seawater with shock causes increases in respiratory pumping and swimming. The increases in respiratory pumping are dependent on the pairing of shock with an altered seawater, whereas the increases in swimming are dependent on shock alone. Increases in inking do not occur. The differences in the effects on the three defensive responses could be explained in two ways. (1) The different training procedures differentially affect the various neural circuits that separately control each of the three defensive responses. The circuit controlling respiratory pumping is affected as a result of pairing, and the circuit affecting swimming is sensitized by shock, whereas the inking circuitry is unaffected by the training procedures. (2) The various training procedures affect a single site that responds to altered seawaters and that controls the activation of all three defensive behaviors. However, the three behaviors are affected at different thresholds. Low levels of noxious stimulation are sufficient to elicit only swimming. Higher levels of stimulation are needed to also cause an increase in respiratory pumping, whereas still stronger noxious stimuli are needed to induce inking. Shock alone elicits a mild sensitization of the site that controls all three behavior. This is sufficient to cause sensitization of swimming in response to an altered seawater 1 hr later but not to elicit an increase in respiratory pumping or inking. Shock paired with an altered seawater elicits a more powerful, pairing-specific facilitation of this site. This leads to an increase in respiratory pumping in response to altered seawaters, but the facilitation is still insufficient to elicit inking in response to altered seawaters.
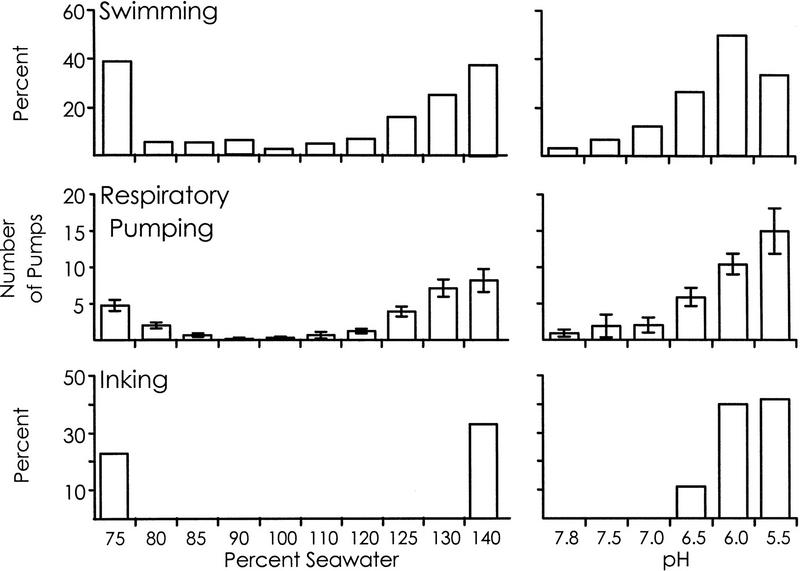
If the latter hypothesis is correct, one would predict that in naive, untrained animals the threshold for eliciting swimming in response to any noxious stimulus will be lower than is the threshold for facilitating respiratory pumping or inking. In addition, the threshold for facilitating respiratory pumping will be lower than is that for eliciting inking. We tested this prediction by measuring the thresholds needed to elicit an increase in respiratory pumping and to elicit swimming or inking in response to a number of different noxious stimuli (Fig. 3). Previous studies (Levy et al. 1993) have shown that changes in seawater concentration, as well as a decrease in pH, affect all three defensive behaviors, presumably because these are noxious stimuli. For these tests, animals were immersed for 10 min in either 100% (pH 7.8 seawater) or in seawaters of increased (110%, 120%, 125%, 130%, 140%) or decreased (90%, 85%, 80%, 75%) concentration or in low-pH seawaters (pH: 7.5, 7.0, 6.5, 6.0, 5.5). We noted whether animals responded at any time during the 10-min exposure by swimming or inking, as well as counting the number of respiratory pumps. To examine the thresholds for swimming and inking, a series of χ2 tests examined whether the percent occurrence of the behavior in an altered seawater is different from that in 100% (pH 7.8) seawater. For respiratory pumping, a one-way analysis of variance was performed for each of the three stimuli (increase and decrease in seawater concentration and decrease in pH). A Duncan’s post hoc test (α = 0.05) was then used to determine which stimuli elicited responses that were significantly different from that in 100% (pH 7.8) seawater.
Figure 3.
Defensive behaviors elicited by the graded application of three stimuli. The thresholds required to elicit swimming, respiratory pumping, and inking were measured by stimulating animals with different levels of seawater of increased and decreased concentration and with different levels of seawater with a decreased pH.
Our data did not support the hypothesis that the three defensive responses are differentially regulated by differences in threshold, because the threshold for swimming was not systematically lower than that for respiratory pumping and inking, and the threshold for respiratory pumping was not always lower than that for inking.
For a decrease in seawater concentration, the threshold for eliciting changes in any of the three defensive behaviors was the same. All three behaviors were significantly increased only in 75% seawater [for swimming, P < 0.001, χ2(1) = 79.34; for inking, P < 0.001, χ2(1) = 10.15].
For an increase in seawater concentration, the threshold for eliciting swimming and an increase in respiratory pumping was the same, 125% seawater [in 125% seawater, for swimming, P = 0.0002, χ2(1) = 13.35; in 120% seawater, P = 0.06, χ2(1) = 3.5], whereas the threshold for eliciting inking was higher, 140% seawater [P < 0.001, χ2(1) = 15.04].
For a decrease in pH, the threshold for eliciting swimming was pH 7.0 (P < 0.001, χ2(1) = 11.68), whereas an increase in respiratory pumping and inking were both elicited at pH 6.5 [for inking, P < 0.001, χ2(1) = 11.17].
UNCONDITIONAL RESPONSES
The data on thresholds for eliciting defensive responses support the suggestion that respiratory pumping, swimming, and inking can be separately controlled. This finding is consistent with the suggestion that a classical conditioning procedure differentially affects the three defensive responses. Further support that the three defensive responses are differentially regulated was gathered by examining the dynamics of the three defensive behaviors in response to shock delivered as an UCS (either alone, paired, or unpaired with an altered seawater) during the training. Previous data have shown that shock elicits increased respiratory pumping, and the pump rate remains elevated for some time after the shock. The pump rate returns to control values 40–50 min after the shock (Levy and Susswein 1990; Levy et al. 1993). We examined whether similar dynamics also were seen for inking and swimming following the shock.
INKING
Inking differed from respiratory pumping and swimming in that animals inked only immediately after being shocked. No subsequent inking was seen during the first or last 10 min of the subsequent hour, when animals were immersed in 100% seawater. There were significant differences in the likelihood to ink in response to shock that was delivered alone, paired, or unpaired with an altered seawater [P < 0.001, χ2(2) = 12.4]. Shock delivered alone or unpaired elicited inking in 44% of all animals, whereas shock delivered in an altered seawater elicited inking in 70% of the animals. Data were similar for shock delivered in the three altered seawaters (85%, 120%, and pH 7.0 seawater).
RESPIRATORY PUMPING
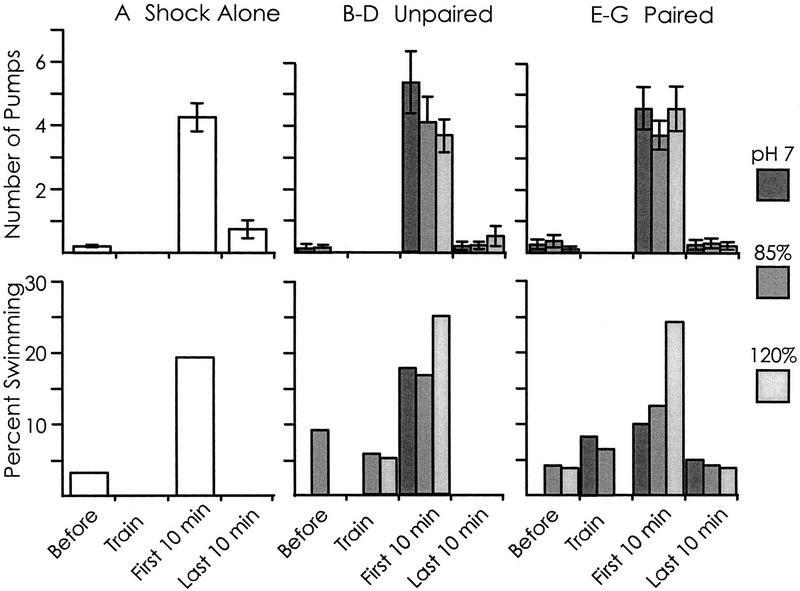
Most of the animals responded to shock with respiratory pumping, although the rate could not be reliably measured, because the animal was often obscured by ink. For all seven treatments (shock alone, paired with pH 7.0, with 85% or with 120% seawater, and unpaired with the three altered seawaters), the respiratory pump rate remained significantly elevated over control levels during the first 10 min in 100% seawater (Fig. 4). Respiratory pumping returned to control levels during the last 10 min in 100% seawater.
Figure 4.
Defensive behaviors elicited by shock during and subsequent to the training. Animals were examined for 10 min before receiving one of seven treatments. (A) Animals were shocked in 100% seawater. (B–D) Animals were pre-exposed for 5 min to one three altered seawaters (B, dark-shaded bar; pH 7.0; C, medium-shaded bar, 85%; D, light-shaded bar, 120%), then transferred to 100% seawater where they were shocked. (E–G) Animals were shocked in one of the three altered seawaters (E, dark-shaded bar, pH 7.0; F, medium-shaded bar, 85%; G, light-shaded bar, 120%). After this treatment, animals were transferred to a new chamber of 100% seawater. Data are shown separately for respiratory pumping (means and s.e.s of the number of pumps in each 10-min period) and swimming (percent of the animals tested that responded with this behavior) during the 10 min before the training, during the training in which they were shocked, and during the first and last 10 min of the hour in 100% seawater after the training. For each of the seven experimental treatments shown, respiratory pumping during the first and last 10 min in 100% seawater was compared with respiratory pumping before the training procedure. All seven comparisons were significant for the first 10 min in altered seawater [for shock alone, t(32) = 8.88; for pH 7.0 unpaired, t(24) = 5.14; for pH 7.0 paired, t(28) = 6.09; for 85% unpaired, t(14) = 3.29; for 85% paired, t(14) = 6.53; for 120% unpaired, t(32) = 6.55; for 120% paired, t(32) = 6.04; for all seven tests, P < 0.001; two-tailed t-tests], whereas none of the seven comparisons was significant during the last 10 min in altered seawater (P ⩾ 0.10, t ≤ 1.7). For each of the seven treatments, a χ2 test was used to determine whether swimming was increased over baseline values during either the first or the last 10 min in 100% seawater. All seven treatments led to a significant increase in swimming during the first 10 min (for shock alone, χ2 = 28.22; for pH 7.0 unpaired, χ2 = 14.44; for pH 70 paired, χ2 = 4.05; for 85% unpaired, χ2 = 8.90; for 85% paired, χ2 = 8.76; for 120% unpaired, χ2 = 37.81; for 120% paired, χ2 = 58.22; for all seven tests, P < 0.05; χ2 tests with 1 df) but no significant increase during the last 10 min (P ⩾ 0.31, χ2 ≤ 1.02).
SWIMMING
Swimming differed from respiratory pumping in that only ∼5% of the animals responded by swimming immediately after being shocked. Significant increases in swimming over control values were also seen in all seven groups during the first 10 min after animals were returned to 100% seawater (Fig. 4). Swimming declined to baseline values in all seven groups by the last 10 min in 100% seawater.
These data confirm that respiratory pumping, swimming, and inking are differentially affected by shock. Large increases in respiratory pumping and inking are elicited by the shock. Respiratory pumping and swimming are maintained at significantly elevated rates for some time after animals are returned to 100% seawater, whereas inking is seen only immediately following the shock.
DIFFERENTIAL INITIATION OF DEFENSIVE RESPONSES
The data above are consistent with the hypothesis that respiratory pumping, swimming, and inking can be separately controlled. If this is so, one might predict that different environmental conditions could change the likelihood to respond with a particular defensive response. We examined this possibility.
We reasoned that swimming is an appropriate defensive response in an open environment, because swimming will effectively move the animals away from a noxious stimulus. In contrast, respiratory pumping may be a more appropriate response in an enclosed space, in which animals are unable to locomote over any substantial distance but may be able to pump away a noxious solution. Accordingly, in this experiment we allowed animals a period of time to explore the space about them that is potentially available for escape locomotion and then presented them with a noxious stimulus. We tested whether there is an increase in swimming in an open space and an increase in respiratory pumping when animals are in a closed space.
In this experiment, the effects of four noxious stimuli were examined: (1) shock delivered to the head, (2) pH 6.0 seawater, (3) 140% seawater, and (4) application to the aquarium of ink that was secreted by another animal. These stimuli were delivered in two environments. In one, the animals were kept individually in small (12 × 12 cm) plastic mesh cages that were immersed in larger tanks (20-liter in volume). In the other, an animal was free to locomote in the large 20-liter tanks. In both conditions, each animal was placed in the 20-liter tank 24 hr before a noxious stimulus was applied. One hour before being stimulated, an animal was either placed in the small cage or was left free in the large tank. Respiratory pumping and swimming were measured for 10 min after the noxious stimuli were applied.
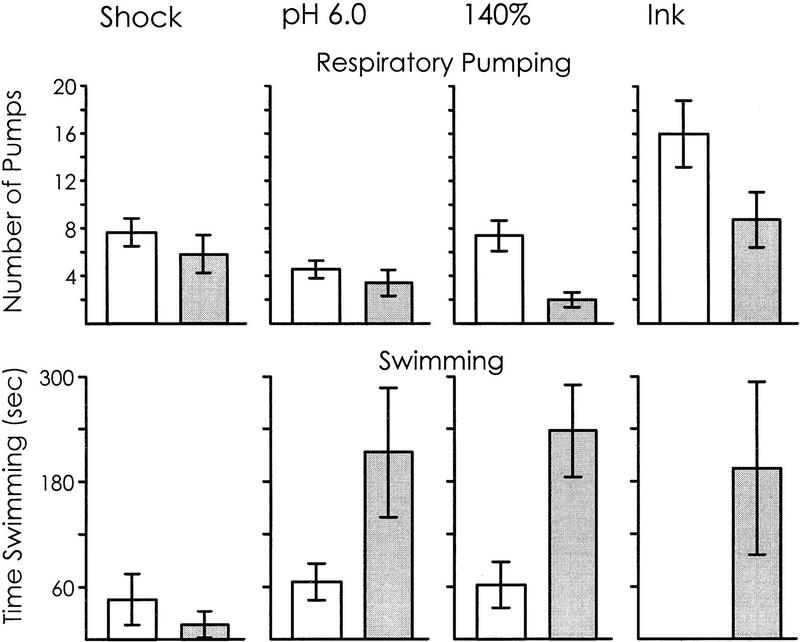
The results were consistent with the hypothesis that swimming is facilitated when space is available for escape and respiratory pumping is facilitated when no space is available (Fig. 5). There were significant increases in respiratory pumping in the closed space in response to two noxious stimuli (140% seawater and ink), with no significant differences in response to the additional two stimuli (shock and pH 6.0 seawater). There were also significant increases in swimming in the open space in response to three stimuli (pH 6.0 and 140% seawaters and ink), with no difference seen in response to shock.
Figure 5.
Respiratory pumping and swimming are differentially affected in open (shaded bars) and closed (open bars) spaces. Animals were stimulated with one of four noxious stimuli: shock, pH 6.0 seawater, 140% seawater, or ink collected from another animal. The number of respiratory pumps in response to the stimulus, as well as the time spent swimming, were then measured over a 10-min period. These measurements were made in a large, open space, as well as in a small cage that was immersed within the open space. There was no significant difference in respiratory pumping between animals in open and closed spaces when the animals were shocked [P = 0.19, t(10) = 0.92] or were exposed to pH 6.0 seawater [P = 0.2, t(12) = 0.86]. However, there was significantly more respiratory pumping in the closed space in animals that were stimulated with 140% seawater [P = 0.001, t(14) = 3.77] or with ink [P = 0.04, t(10) = 1.97]. For swimming, there was no significant difference between open and closed spaces in animals that had been shocked [P = 0.40, t(10) = 0.87], but swimming was significantly increased in open spaces in response to pH 6.0 seawater [P = 0.04, t(12) = 1.92], 140% seawater [P = 0.001, t(14) = 3.77] and ink [P = 0.03, t(10) = 2.24; all comparisons are one-tailed t-tests]. These data show that environmental conditions, such as whether animals are in closed or open areas, can differentially affect the likelihood of animals to respond to a stimulus by swimming or by respiratory pumping.
These data support those above that indicated that Aplysia can selectively regulate the different defensive responses.
Discussion
Respiratory pumping was initially identified as an intermittent, coordinated pattern of synaptic activity that could be recorded from many neurons in the isolated abdominal ganglion of Aplysia. The pattern was attributed to the firing of an unidentified neuron (or groups of neurons) in the Aplysia abdominal ganglion that was termed Interneuron II (Kandel et al. 1967). Later studies showed that this neural pattern is correlated with a distinct behavior characterized by contraction of the gill and siphon, inhibition of the heart, and closing of the parapodia (Kupfermann and Kandel 1969; Peretz 1969; Koester et al. 1974; Kupfermann et al. 1974; Jahan-Parwar and Fredman 1978; Peretz 1969; Perlman 1979; Sawada et al. 1981). Because the pattern seemed to be designed to flush the cavity surrounding the gill with fresh seawater, while simultaneously pumping fresh hemolymph through the gill, the behavior was assumed to have a respiratory function and was therefore named respiratory pumping (Kandel 1976). Subsequent studies have shown that the rate at which the behavior occurs is modulated by a number of different types of environmental stimuli (Koester et al. 1979; Croll 1985; Levy et al. 1989, 1993, 1994a, 1997a,b; Kanz and Quast 1990, 1992), and the behavior may have a number of different functions. Noxious stimuli that elicit other defensive behaviors such as the gill withdrawal reflex or inking also facilitate respiratory pumping (Walters and Erickson 1986; Levy et al. 1993), indicating that one of its functions is defensive. Neurophysiological studies have succeeded in identifying the L25 and R25 cells (Byrne 1983; Koester 1989), which act as both command cells and central pattern generators for respiratory pumping. These neurons directly activate many motor neurons that innervate the gill, siphon, parapodia, and heart. Additional neurons whose firing can modify the L25 and R25 neurons have also been identified (Schaefer and Brownell 1986; Alevizos et al. 1989, 1991; Cleary and Byrne 1993).
In this paper we have examined whether training Aplysia with a classical conditioning procedure affects three defensive responses. In this procedure, animals were exposed to a CS (an altered seawater) that did not elicit defensive responses. This stimulus was paired with a UCS (shock) that did elicit such responses. The conditioning procedure caused a pairing-specific increase in only one defensive response, respiratory pumping. A second response, swimming, was sensitized by the UCS, either alone, or paired or unpaired with the CS. The third response, inking, was unaffected by the training procedure. These data indicate that the pairing-specific conditioning is likely to arise as a result of changes that occur at a neural site that differentially affects respiratory pumping, rather than at a site that controls all three defensive responses in tandem. Differential regulation of the three defensive behaviors was also expressed in three additional ways: (1) There were differences in the relative thresholds of the defensive responses to various altered seawaters; (2) the UCS, shock, elicited the three defensive responses with different temporal patterns, and (3) there were differences in the response to noxious stimuli that were presented in open versus closed environments. These data confirm that neural pathways exist that allow Aplysia to modulate and separately control each of the three defensive behaviors.
DIFFERENTIAL EFFECTS OF CONDITIONING
Pairing head shock with any of three altered seawaters causes an increase in respiratory pumping when animals are re-exposed to the same altered seawater 1 hr after training (Fig. 2; Levy and Susswein 1990; Levy et al. 1994b). This facilitation can be attributed to classical conditioning, in which an altered seawater represents a CS, and shock is the UCS. A change in respiratory pumping, the conditioned response (CR), is also elicited as an unconditional response (UCR) to shock (Fig. 4), as well as to higher intensities of the altered seawaters (Fig. 3), but not to the specific altered seawaters used as CSs in the training procedure (Levy et al. 1989, 1993). The increase in respiratory pumping in response to the CSs is not seen following training with either the CS or the UCS alone or following an unpaired presentation of the two stimuli (Fig. 2).
Swimming and inking are not affected in the same manner as is respiratory pumping. The conditioning procedure leads to an increase in swimming in response to all three CSs examined, similar to that seen for respiratory pumping. However, this increase is also seen after a shock that is presented either alone or unpaired with the altered seawaters, thereby indicating that the increase in swimming is caused by sensitization as a result of shock (Fig. 2). In contrast to the effects on respiratory pumping and swimming, neither the conditioning procedure nor shock alone causes an increase in inking in response to the three stimuli tested. We cannot exclude the possibility that some of the training procedures might have affected inking in response to stimuli that were not tested. The thresholds for inking could have been lowered from those in naive animals but were not lowered to such an extent that the stimuli tested would elicit inking. This possibility is unlikely for the response to a decrease in pH, because the threshold stimulus for inking in naive animals is close to the stimulus used as a CS in our study. However, for increases and decreases in seawater concentration, thresholds could have been changed to values between those tested and those in naive animals.
A number of additional associative learning procedures that affect defensive responses have been demonstrated in Aplysia. In one classical conditioning paradigm, illumination of the siphon was used as a CS, which was paired with touch of the gill. After pairing, light elicited the CR, gill withdrawal (Lukowiak and Sahley 1981). In another paradigm, touch to the siphon was paired with tail shock (Carew et al. 1981). After pairing, tactile stimulation of the siphon produced larger withdrawal reflexes than previously. Subsequent studies showed that electrical shocks delivered to different parts of the body elicit somewhat different siphon withdrawal responses (Walters and Erickson 1986). Pairing siphon touch with either tail or mantle stimulation caused a change in the form of the siphon movement, so that it resembled that elicited by the UCS (Hawkins et al. 1989; Walters 1989). Thus, pairing affects only a specific defensive siphon movement, rather than all such movements. However, these studies did not examine the possible effects of the training paradigm on behaviors other than siphon withdrawal. In contrast, other studies (Walters et al. 1979, 1981) paired shock with the presence in the water of a shrimp extract. After multiple pairings, animals reacted as though fearful when re-exposed to the shrimp. Fear was demonstrated by pairing-specific increases in crawling, head withdrawal, siphon withdrawal, and inking, as well as by inhibition of feeding, in the presence of shrimp extract. Our training procedure is relatively similar to this procedure. In both, a chemically altered seawater (either flavored with shrimp or via changes in salinity or pH) is paired with shock. Nonetheless, we found no increases in inking on exposure to altered seawater, and the increase in locomotion (swimming) that we observed was not contingent on pairing. It is possible that shrimp extract stimulating the head modulates different defensive responses than do altered seawaters, which are sensed by the osphradium (Croll 1985; Levy and Susswein 1993). A second possibility is that learned fear arises from the multiple pairings, whereas we examined the effect of only a single pairing. An additional possibility is that learned fear does not affect the three defensive responses that we examined.
A number of nonassociative learning tasks that affect defensive responses in Aplysia also produce effects that are restricted to a small subpopulation of stimuli. For example, habituation of the gill withdrawal reflex that is elicited by repeated stimulation of the siphon does not generalize to stimulation of the mantle, and vice versa (Carew et al. 1971). In addition, both short-term and long-term habituations of siphon withdrawal that is elicited by stimulating one side of the tail do not generalize to stimuli delivered across the midline of the animal (Stopfer et al. 1996). Shocks delivered to one side of the body also elicit long-term sensitization that is restricted to stimuli delivered ipsilaterally, whereas contralateral stimuli are not sensitized (Scholz and Byrne 1987; Walters 1987). In these studies intracellular recordings from reduced preparations confirmed that some of the neural changes underlying the plasticity are localized to sites that control narrow, local responses, rather than occurring at sites that control many aspects of a defensive behavior.
DIFFERENTIAL RESPONSES OF DEFENSIVE BEHAVIORS
Two hypotheses were proposed to account for the differential effects of conditioning on the three defensive behaviors. (1) Aplysia can control each defensive behavior separately. Plasticity occurs in circuit elements that separately control each behavior. (2) Aplysia control the three defensive behaviors as a unit, but differences in the threshold for eliciting each behavior give rise to differences in the expression of the three behaviors. Both shock alone and the classical conditioning procedure affect a neural site that controls all three behaviors. Differences in the effect of shock and of conditioning on the three behaviors arise from differences in the relative efficacy of shock and of classical conditioning in facilitating this common site.
Our data strongly support the first hypothesis, because differential control of the three behaviors was observed in a number of experiments.
DIFFERENCES IN THRESHOLD TO ALTERED SEAWATERS
If the three defensive responses are controlled at a common site but at different thresholds, one would expect that the relative threshold for activating each defensive response remains constant. For all stimuli that initiate defensive responses, the threshold for one response should consistently be the lowest, the threshold for a second response should be intermediate, and the threshold for the third response should be the highest. However, when the thresholds for the three defensive responses were examined when animals were challenged with three different noxious stimuli, the relative thresholds did not remain constant (Fig. 3). In response to a decrease in pH, the threshold for swimming was lower than that for respiratory pumping and inking, which were the same. In contrast, in response to an increased seawater concentration, the threshold for respiratory pumping and swimming were the same and were lower than that for inking. In response to a decrease in seawater concentration, all three defensive responses had the same threshold.
DIFFERENTIAL RESPONSE TO SHOCK
The training procedure used head shock as a UCS, allowing us to determine whether this noxious stimulus differentially affects the three defensive behaviors that were examined. Head shock affected all three of the defensive behaviors, but there were major differences in the patterning of each behavior. Immediately after head shock, inking was elicited in >50% of all animals, whereas swimming was elicited in only ∼5% of the animals (Fig. 4). Head shock also elicited a large increase in respiratory pumping, but the number of pumps was not quantified. Previous data (Walters and Erickson 1986) showed that respiratory pumping accompanies ink release, and at least some of the respiratory pumping in response to shock is likely to have this function. The relative lack of swimming in response to shock probably reflects our use of head shock rather than tail shock. Noxious stimuli elicit turning that leads to locomotion away from the irritating stimulus (Walters and Erickson 1986). Because Aplysia are only able to swim forward, swimming would move animals toward the noxious stimulus.
Subsequent to the shock, both swimming and respiratory pumping were seen when animals were transferred to 100% seawater, whereas inking was not seen at this time. The increase in respiratory pumping and swimming declined over the hour in 100% seawater, and by the end of the hour, respiratory pumping and swimming were at control levels. Thus, inking differs from respiratory pumping and swimming in that it is elicited briefly immediately following a noxious stimulus, but not subsequently.
DIFFERENCES IN RESPONSE IN OPEN VS. CLOSED ENVIRONMENTS
Defensive responses were also differentially affected by the size of the container in which noxious stimuli were delivered (Fig. 5). Stimuli were more likely to elicit swimming in a larger area, where space is available to escape from the stimulus, whereas respiratory pumping was more likely in a smaller space. Aplysia could potentially use a number of cues to determine the size of the space available. It was not our goal to identify these stimuli, merely to show that animals can differentially control defensive responses. However, an intriguing possibility is that animals locomoted and explored the experimental space during the hour preceding the application of a noxious stimulus. This activity may provide the animals with information regarding the dimensions of their local environment, and animals may use this information in deciding which defensive response to elicit when subsequently challenged with a noxious stimulus. This is the first indication that Aplysia may have an internal representation of their local environment and that this representation can affect the animal’s behavior. Additional studies (M. Botzer and A.J. Susswein, unpubl.) have shown that the total time budgeted to swimming is reduced in a small container, with respect to that seen in a larger container. However, we have not ruled out the possibility that stimuli not directly related to the size of the space and the animal’s previous exploration of it might also contribute the ability of an animal to distinguish between an open and closed environment. For example, small differences in the local turbulence or in local concentrations of dissolved substances might be somewhat different in the open versus closed environments. One stimulus that is unlikely to signal to the animal its presence in a smaller environment is its encountering the wall of the container, because previous studies have shown that A. fasciata will continue swimming straight into the wall of an aquarium for many hours (Ziv et al. 1991).
It is important to note that the size of the container did not affect the defensive response chosen to all of the noxious stimuli. The response to shock was unaffected by the size of the container, whereas the responses to 140% seawater and to ink were strongly affected. Our use of ink to elicit defensive responses is of particular interest, as this indicates that Aplysia are able to sense the presence of ink in the environment and use this stimulus as a signal for initiating defensive responses. A previous report (E.T. Walters, P.A. Illich, and C. Hickie, unpubl.) mentions that ink can trigger locomotion in a quiescent Aplysia, as well as causing enhanced escape behavior and head withdrawal. In contrast, ink has been shown to inhibit siphon withdrawal in response to tail stimulation (Stopfer et al. 1993). This has been interpreted as a facilitation of inking in response to tail stimulation (Nolen et al. 1995). The ink is directed toward the tail via siphon movements that are incompatible with withdrawal movements (Illich et al. 1994).
SWIMMING AND RESPIRATORY PUMPING AS MUTUALLY EXCLUSIVE BEHAVIORS
Respiratory pumping and swimming both affect a common motor organ, the parapodia. However, the two behaviors cause different movements. Respiratory pumping produces a contraction, whereas swimming is caused by cyclical parapodial flapping (von der Porten et al. 1980). Therefore, it is likely that these behaviors may be mutually exclusive. A previous study found that respiratory pumping was never observed simultaneously with swimming (Levy et al. 1994a), suggesting that the neural circuits organizing respiratory pumping and swimming may inhibit one another. If this is so, the individual control and modulation of these behaviors by different stimuli and by learning could be a trivial consequence of their mutual inhibition. However, a number of observations have shown that stimuli can simultaneously facilitate both respiratory pumping and swimming, in spite of their never occurring at precisely the same time, indicating that the separate effects of training on these behaviors cannot be explained by their mutual inhibition. First, both respiratory pumping and swimming are elevated after shock, during the first 10 min after animals are restored to 100% seawater (Fig. 4). Second, both behaviors are facilitated in response to changes in the seawater concentration or to a decrease in pH (Fig. 3). Third, after paired training both behaviors are facilitated (Fig. 2), although the increase in respiratory pumping is pairing specific, whereas the increase in swimming is not. These data show that facilitation of mutually inhibitory behaviors can and does occur, provided that the behaviors are not expressed precisely simultaneously.
RELATIONSHIP BETWEEN CRs AND UCRs
Traditional descriptions of classical conditioning emphasized that pairing of the CS and UCS leads to a new CR that is elicited by the CS. This new response, termed the β response, is generally similar to the UCR elicited by the UCS. In some cases, pairing between the CS and UCS causes an amplification of the pre-existing α response that is elicited by the CS before conditioning (Schreurs 1989). Both α and β conditioning have been shown to affect Aplysia defensive behaviors (Carew et al. 1981, 1983; Hawkins et al. 1989; Walters 1989). A number of neural models have been proposed to account for both α and β conditioning of Aplysia defensive responses (Hawkins et al. 1989; Walters 1989).
Conditioning that arises from pairing an altered seawater with head shock is not easily classified as either α or β conditioning. The learning differs from α conditioning in that the concentrations of altered seawaters used do not elicit increased respiratory pumping above that seen in normal seawater, although higher concentrations do. However, higher concentrations of altered seawaters also elicit swimming and inking, whereas the conditioning procedure only facilitates respiratory pumping. The learning also differs from β conditioning, because shock elicits all three defensive responses. Thus, the CR in our learning paradigm differs from those elicited before conditioning by either the CS or UCS. Classical conditioning in which the CR differs substantially from the UCR (or is opposite in sign to the UCR) has also been described in other systems (Turkkan 1989).
POSSIBLE SITES OF PLASTICITY
The data above, coupled with those presented previously (Levy et al. 1994b), indicate that classical conditioning of respiratory pumping occurs at neural sites that receive convergent input from some, but not all, altered seawaters and that affect respiratory pumping but not swimming or inking. These data rule out a number of potential neural sites at which the conditioning could occur. Previous studies have shown that altered seawaters are sensed by the osphradium (Croll 1985; Levy and Susswein 1993). The primary mechanism causing plasticity is unlikely to be a neuron-wide change in afferents in the osphradium that sense altered seawaters or a reduced pH, because such changes would lead to a pairing-specific facilitation of all defensive responses affected by the afferents. For this reason, the conditioning is also is unlikely to be localized in previously identified higher order neurons that facilitate multiple defensive responses (Mackey et al. 1989; Cleary and Byrne 1993). The conditioning mechanism is also unlikely to be localized within the L25 and R25 command and central pattern generator neurons for respiratory pumping (Byrne 1983; Koester 1989), because a previous study showed that conditioning generalizes between 85% and 120% seawater but not between these two stimuli and pH 7.0 seawater (Levy et al. 1994b). If the conditioning were localized in the L25 and R25 neurons, one would expect generalization to all stimuli having access to these cells. It has also been shown that conditioning in response to changes in salinity, but not to a reduced pH, is maintained when the pleural–abdominal connectives are severed (Levy and Susswein 1993). Conditioning in which the CS is a change in seawater concentration is likely to be localized in neurons within the abdominal ganglion that excite the L25 and R25 neurons commanding respiratory pumping but not interneurons organizing swimming (McPherson and Blankenship 1991) or the L14 ink neurons (Carew and Kandel 1977), and that receive convergent input from receptors sensing increases and decreases in salinity but not a decrease in pH. Conditioning in which the CS is a decreased pH is apparently localized in other neurons that may be located in the head ganglia and that receive input from sensors responding to a decrease in pH but not to changes in salinity. The neurons responsible for conditioning must also receive modulatory inputs from head nociceptors that sense the UCS. To this find such neurons, we have recently developed a reduced preparation in which altered seawater stimuli are directly applied to the osphradium, and respiratory pumping is monitored via intracellular recordings from a number of neurons, as well as via gill contraction. In such a preparation, head shock can be simulated by electrical stimulation of the pleural–abdominal connective or by stimulating cerebral or pedal ganglion nerves that innervate the head.
Acknowledgments
We thank Sylvia Markovich for help in performing some of the experiments and Aron Weller for comments on an earlier draft of the manuscript. This work was supported by grant 561/93 awarded by the Israel Science Foundation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Alevizos A, Weiss KR, Koester J. SCP-containing R20 neurons modulate respiratory pumping in Aplysia. J Neurosci. 1989;9:3058–3071. doi: 10.1523/JNEUROSCI.09-09-03058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Synaptic actions of identified peptidergic neuron R15 in Aplysia. I. Activation of respiratory pumping. J Neurosci. 1991;11:1263–1274. doi: 10.1523/JNEUROSCI.11-05-01263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JH. Identification and initial characterization of a cluster of command and pattern-generating neurons underlying respiratory pumping in Aplysia californica. J Neurophysiol. 1983;49:491–508. doi: 10.1152/jn.1983.49.2.491. [DOI] [PubMed] [Google Scholar]
- ————— Cellular analysis of associative learning. Physiol Rev. 1987;67:329–439. doi: 10.1152/physrev.1987.67.2.329. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: State and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TJ, Kandel ER. Inking in Aplysia californica: I. Neural circuit of an all-or-none behavioral response. J Neurophysiol. 1977;40:692–707. doi: 10.1152/jn.1977.40.3.692. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Sahley CL. Invertebrate learning and memory: From behavior to molecules. Annu Rev Neurosci. 1986;9:435–487. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
- Carew T, Castellucci VF, Kandel ER. An analysis of dishabituation and sensitization of the gill withdrawal refelc in Aplysia. Int J Neurosci. 1971;2:79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Walters ET, Kandel ER. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981;1:1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TJ, Hawkins RD, Kandel ER. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983;219:397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- Cleary LJ, Byrne JH. Identification and characterization of a multifunction neuron contributing to defensive arousal in Aplysia. J Neurophysiol. 1993;70:1767–1776. doi: 10.1152/jn.1993.70.5.1767. [DOI] [PubMed] [Google Scholar]
- Croll RP. Sensory control of respiratory pumping in Aplysia californica. J Exp Biol. 1985;117:15–27. [Google Scholar]
- Hawkins RD, Lalevic N, Clark GA, Kandel ER. Classical conditioning of the Aplysia siphon-withdrawal reflex exhibits response specificity. Proc Natl Acad Sci. 1989;86:7620–7624. doi: 10.1073/pnas.86.19.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illich PA, Joynes RL, Walters ET. Response-specific inhibition during general facilitation of defensive responses in Aplysia. Behav Neurosci. 1994;108:614–623. doi: 10.1037//0735-7044.108.3.614. [DOI] [PubMed] [Google Scholar]
- Jahan-Parwar B, Fredman SM. Control of pedal and parapodial movements in Aplysia. I. Proprioceptive and tactile reflexes. J Neurophysiol. 1978;41:600–608. doi: 10.1152/jn.1978.41.3.600. [DOI] [PubMed] [Google Scholar]
- Kandel ER. Cellular basis of behavior: An introduction to behavioral neurobiology. San Francisco, CA: W.H. Freeman; 1976. [Google Scholar]
- Kandel ER, Frazier WT, Waziri R, Coggeshall RE. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967;30:1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Kanz JE, Quast WD. Respiratory pumping seizures: A newly discovered spontaneous stereotyped behavior pattern in the opisthobranch mollusc Aplysia. J Comp Physiol A. 1990;166:619–627. [Google Scholar]
- ————— Respiratory pumping behavior in the marine snail Aplysia californica as a function of ambient hypoxia. Physiol Zool. 1992;65:35–54. [Google Scholar]
- Koester J. Chemically and electrically coupled interneurons mediate respiratory pumping in Aplysia. J Neurophysiol. 1989;62:1113–1126. doi: 10.1152/jn.1989.62.5.1113. [DOI] [PubMed] [Google Scholar]
- Koester J, Mayeri E, Liebeswar G, Kandel ER. Neural control of circulation in Aplysia. II. Interneurons. J Neurophysiol. 1974;37:476–496. doi: 10.1152/jn.1974.37.3.476. [DOI] [PubMed] [Google Scholar]
- Koester J, Dieringer N, Mandelbaum DE. Cellular neuronal control of molluscan heart. Am Zool. 1979;19:103–116. [Google Scholar]
- Kupfermann I, Kandel ER. Neuronal controls of a behavioral response mediated by the abdominal ganglion of Aplysia. Science. 1969;164:847–850. doi: 10.1126/science.164.3881.847. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Carew TJ, Kandel ER. Local, reflex and central commands controlling gill and siphon movements in Aplysia. J Neurophysiol. 1974;37:996–1019. doi: 10.1152/jn.1974.37.5.996. [DOI] [PubMed] [Google Scholar]
- Levy M, Susswein AJ. Learned changes in rate of respiratory pumping in Aplysia fasciata. Behav Neural Biol. 1990;54:218–233. doi: 10.1016/0163-1047(90)90606-7. [DOI] [PubMed] [Google Scholar]
- ————— Separate neural pathways respond to different noxious stimuli affecting respiratory pump frequency in Aplysia fasciata. Brain Res. 1993;616:218–229. doi: 10.1016/0006-8993(93)90212-6. [DOI] [PubMed] [Google Scholar]
- Levy M, Achituv Y, Susswein AJ. Relationship between respiratory pumping and oxygen consumption in Aplysia depilans and Aplysia fasciata. J Exp Biol. 1989;141:389–405. [Google Scholar]
- Levy M, Susswein MO, Susswein AJ. Respiratory pumping in Aplysia fasciata as part of an integrated defensive response to increase and decrease in seawater concentration. J Comp Physiol A. 1993;172:749–758. [Google Scholar]
- Levy M, Markovich S, Susswein AJ. Modulation of respiratory pump rate in freely behaving pairs of Aplysia fasciata. Behav Neural Biol. 1994a;61:93–98. doi: 10.1016/s0163-1047(05)80048-2. [DOI] [PubMed] [Google Scholar]
- Levy M, Weller A, Susswein AJ. Learned changes in the rate of respiratory pumping in Aplysia fasciata in response to increases and decreases in seawater concentration. Behav Neurosci. 1994b;108:161–170. doi: 10.1037//0735-7044.108.1.161. [DOI] [PubMed] [Google Scholar]
- Levy M, Blumberg S, Susswein AJ. The rhinophores sense pheromones regulating multiple behaviors in Aplysia fasciata. Neurosci Lett. 1997a;225:113–116. doi: 10.1016/s0304-3940(97)00200-0. [DOI] [PubMed] [Google Scholar]
- Levy M, Levy I, Susswein AJ. Respiratory pumping in Aplysia fasciata in natural and artificial tide pools. J Comp Physiol A. 1997b;180:81–90. [Google Scholar]
- Lukowiak K, Sahley C. The in vitro classical conditioning of the gill withdrawal reflex of Aplysia californica. Science. 1981;212:1516–1518. doi: 10.1126/science.212.4502.1516. [DOI] [PubMed] [Google Scholar]
- Mackey SL, Kandel ER, Hawkins RD. Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglion of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci. 1989;9:4227–4235. doi: 10.1523/JNEUROSCI.09-12-04227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson DR, Blankenship JE. Neural control of swimming in Aplysia brasiliana. III. Serotonergic modulatory neurons. J Neurophysiol. 1991;66:1366–1379. doi: 10.1152/jn.1991.66.4.1366. [DOI] [PubMed] [Google Scholar]
- Nolen TG, Johnson PM, Kickligher CE, Capo T. Ink secretion by the marine snail Aplysia californica enhances its ability to escape from a natural predator. J Comp Physiol A. 1995;176:239–254. [Google Scholar]
- Peretz B. Central neuron initiation of periodic gill movements. Science. 1969;166:1067–1072. doi: 10.1126/science.166.3909.1167. [DOI] [PubMed] [Google Scholar]
- Perlman A. Central and peripheral control of siphon-withdrawal reflex in Aplysia. J Neurophysiol. 1979;42:510–529. doi: 10.1152/jn.1979.42.2.510. [DOI] [PubMed] [Google Scholar]
- Sawada M, Blankenship JE, McAdoo DJ. Neural control of a molluscan blood vessel, the anterior aorta of Aplysia. J Neurophysiol. 1981;46:967–986. doi: 10.1152/jn.1981.46.5.967. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Brownell PH. Modulation of a respiratory motor program by peptide-secreting neurons in Aplysia. J Neurobiol. 1986;17:121–126. doi: 10.1002/neu.480170207. [DOI] [PubMed] [Google Scholar]
- Scholz KP, Byrne JH. Long-term sensitization in Aplysia: Biophysical correlates in tail sensory neurons. Science. 1987;235:685–687. doi: 10.1126/science.2433766. [DOI] [PubMed] [Google Scholar]
- Schreurs BG. Classical conditioning of model systems: A behavioral review. Psychobiology. 1989;17:145–155. [Google Scholar]
- Stopfer M, Chen X, Carew TJ. Evoked ink release in Aplysia produces inhibition of the siphon withdrawal reflex in neighboring conspecifics. Behav Neural Biol. 1993;60:196–202. doi: 10.1016/0163-1047(93)90352-i. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Chen X, Tai Y-T, Huang GS, Carew TJ. Site specificity of short-term and long-term habituation in the tail-elicited siphon withdrawal reflex of Aplysia. J Neurosci. 1996;16:4923–4932. doi: 10.1523/JNEUROSCI.16-16-04923.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkkan JS. Classical conditioning: The new hegemony. Behav Brain Sci. 1989;12:121–179. [Google Scholar]
- von der Porten K, Redmann G, Rothman B, Pinsker H. Neuroethological studies of freely swimming Aplysia brasiliana. J Exp Biol. 1980;84:245–257. doi: 10.1242/jeb.84.1.245. [DOI] [PubMed] [Google Scholar]
- Walters ET. Site-specific sensitization of defensive reflexes in Aplysia: A simple model of long-term hyperalgesia. J Neurosci. 1987;7:400–407. doi: 10.1523/JNEUROSCI.07-02-00400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Transformation of siphon responses during conditioning of Aplysia suggests a model of primitive-response association. Proc Natl Acad Sci. 1989;86:7616–7619. doi: 10.1073/pnas.86.19.7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Erickson MT. Directional control and the functional organization of defensive responses in Aplysia. J Comp Physiol A. 1986;159:339–352. doi: 10.1007/BF00603980. [DOI] [PubMed] [Google Scholar]
- Walters ET, Carew TJ, Kandel ER. Classical conditioning in Aplysia californica. Proc Natl Acad Sci. 1979;76:6675–6679. doi: 10.1073/pnas.76.12.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Associative learning in Aplysia: Evidence for conditioned fear in an invertebrate. Science. 1981;211:504–506. doi: 10.1126/science.7192881. [DOI] [PubMed] [Google Scholar]
- Ziv I, Lustig C, Ben-Zion M, Susswein AJ. Daily variation of multiple behaviors in Aplysia fasciata: Integration of feeding, reproduction and locomotion. Behav Neural Biol. 1991;55:86–107. doi: 10.1016/0163-1047(91)80129-3. [DOI] [PubMed] [Google Scholar]