Abstract
Metabotropic glutamate receptors (mGluRs) have been implicated in long-term potentiation and in learning and memory formation. In this study, we tested the effects of group I mGluR inhibition on synaptic plasticity and learning of rats at different levels of organization (1) in the hippocampal slice preparation; (2) in freely moving animals implanted with chronic hippocampal electrodes; and (3) in different spatial learning paradigms. To allow a direct comparison of the effects obtained the same doses were used in all paradigms. Bath-application of the selective group I mGluR antagonist (S)4-carboxyphenylglycine (4-CPG) impaired a decremental long-term potentiation (LTP) induced by a weak tetanization paradigm, but failed to affect a robust LTP generated by strong tetanization. In contrast, 4-CPG impaired a robust LTP in freely moving animals if applied 30 min before tetanization. The same dose of 4-CPG only impeded spatial learning mildly in the eight-arm radial maze and had no effect on a simple configuration of the Y-maze spatial alternation task. In the more difficult configuration of this task, however, 4-CPG caused complete amnesia. The lack of state-dependent 4-CPG actions and the absence of any 4-CPG effects in the open-field test classify the obtained retention deficit as a selective impairment of memory storage. Our results indicate a specific role of group I mGluRs in certain types of synaptic plasticity and of spatial learning.
The discovery of metabotropic glutamate receptors (mGluRs) ∼10 years ago, allowed more thorough insights in the functional cross talk between ionotropic and metabotropic glutamate actions. Since then, much effort has been centered on the cloning and characterization of the different subtypes and splice variants of mGluRs and the elucidation of their physiological function in multifarious regions of the brain. To date, eight subtypes of mGluRs have been cloned and divided into three groups according to their sequence homology, pharmacological characterization, and coupling to second messenger pathways. Activation of group I mGluRs (mGluR1, 5) gives rise to the hydrolysis of phosphatidylinositol 4,5-bisphosphate into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol, which are required for intracellular Ca2+ release and activation of protein kinase C (PKC), respectively (Nakanishi 1994). In contrast, mGluRs of group II (mGluR2, 3) and group III (mGluR4, 6, 7, 8) are negatively coupled to adenylyl cyclase (Conn and Pin 1997).
Although many studies support a role of mGluRs in synaptic plasticity and memory formation (for review, see Nakanishi 1994; Riedel et al. 1996; Conn and Pin 1997), the involvement of different mGluR groups and subtypes in particular physiological circuits and functions such as hippocampal synaptic plasticity and learning is still a matter of controversial debate. For instance, some authors described an inhibition of hippocampal long-term potentiation (LTP) by the class I/II specific antagonist S-α-methyl-4-carboxyphenylglycine (MCPG) (Bashir et al. 1993; Bortolotto et al. 1994; Brown et al. 1994; Richter-Levin et al. 1994; Little et al. 1995; Riedel et al. 1995a), whereas in other studies the MCPG actions could not be confirmed (Chinestra et al. 1993; Izumi and Zorumski 1994; Manzoni et al. 1994; Selig et al. 1995; Thomas and O’Dell 1995; Martin and Morris 1997). A clue to resolve the controversy was presented by Bortolotto et al. (1994) who postulated that activation of mGluRs before LTP sets an input-specific molecular switch that then negates the necessity of further mGluR-activation during LTP-induction, assigned subsequently as “molecular switch” hypothesis. Other groups, however, failed to find experimental evidence for the existence of this molecular switch (Selig et al. 1995; Thomas and O’Dell 1995; Martin and Morris 1997). Studies using mGluR 1 knockout mice confounded the issue further. Although Aiba et al. (1994) found a significantly lower magnitude of LTP in the CA1 area of the hippocampus, Conquet et al. (1994) did not observe a change of CA1 and dentate LTP in these mutants.
With regard to the function of mGluRs in learning, in most studies, either MCPG or the mGluR group I/II agonist 1-aminocyclopentane-1,3-dicarboxylic acid (ACPD) were used. Whereas MCPG was reported to have detrimental effects on learning in the water maze (WAM) (Richter-Levin et al. 1994; Bordi et al. 1996) and in passive avoidance learning (Bianchin et al. 1994; Hölscher 1994; Rickard and Ng 1995), the results with ACPD are wide-ranging, encompassing reports of an improvement of olfactory memory (Kaba et al. 1994) and retention in passive avoidance (Hölscher 1994), missing effects on passive avoidance (Bianchin et al. 1994), as well as an impairment of learning in the WAM (Pettit et al. 1994) and in the radial maze (RAM) (Hölscher et al. 1997). Therefore, these studies together implicated mGluRs in certain types of learning, depending on experimental procedure, substances, doses, and times of injection. The lack of subtype-specific agonists and antagonists, however, hampered a detailed investigation of the distinct roles of mGluR subtypes in synaptic plasticity and learning.
To characterize the role of group I mGluRs in synaptic plasticity and learning in a contiguous, comparable way at different degrees of cellular organization we employed the specific group I mGluR antagonist (S)4-carboxyphenylglycine (4-CPG) (Davies et al. 1995; Sekiyama et al. 1996) to examine the functional consequences of group I mGluR inhibition, at three physiological levels (1) in the hippocampal slice preparation; (2) in freely moving animals implanted with chronic hippocampal electrodes, and (3) in two spatial learning paradigms. Our findings lead us to conclude, that the functional impact of group I mGluRs in hippocampal synaptic plasticity is contingent on distinct circumstances, such as the type and the strength of the stimulus applied and the particular properties of the spatial learning paradigm employed. Therefore, group I mGluRs may be involved in the fine tuning of hippocampal synaptic plasticity and learning.
Materials and Methods
ANIMALS
For all experiments, male Wistar rats of the outbred strain MOL: WIST (SHOE) 7–8 weeks old were used. The animals were housed under standard laboratory conditions with light on between 6 a.m. and 6 p.m. and with free access to food and water.
ELECTROPHYSIOLOGICAL RECORDINGS IN VITRO
SLICE PREPARATION
Seven- to eight-week-old animals were decapitated, the brain dissected in cold oxygenated physiological solution (ACSF: 124 mm NaCl, 4.9 mm KCl, 1.3 mm MgSO4, 2.5 mm CaCl2, 1.2 mm KH2PO4, 25.6 mm NaHCO3, and 10 mm d-glucose, saturated with 95% O2, 5% CO2 at pH 7.4) and the hippocampus cut into 400-μm-thick slices using a tissue chopper. Thereafter, the slices were immediately transferred to a submerged-type slice chamber and permanently perfused with 32°C ACSF.
RECORDING
After an incubation of at least 1 hr duration, a lacquer-coated stainless-steel stimulating electrode and a glass recording electrode (filled with ACSF, 1-4 MΩ) were placed into the stratum radiatum of the CA1-region to record excitatory postsynaptic field potentials (fEPSPs). The initial slope of the fEPSP served as a measure of this potential. After constructing input/output curves, the stimulation strength was adjusted to 35% of the maximum and kept constant at this level throughout the experiment. During baseline recording, four single stimuli (0.1 msec pulse width; 10 sec interval) were averaged every 5 min. Once a stable baseline had been established, LTP was induced by one of the following tetanization paradigm.
Strong tetanization—Ten bursts of four stimuli at 100 Hz, separated by 200 msec (0.2 msec pulse width) = theta-burst stimulation (TBS); induced a robust potentiation of fEPSP of at least 4 hr duration in control experiments. Immediately after tetanus recordings were taken at the min 1, 4, 7, and 10, and then every 10 min for at least 240 min.
Weak tetanization—Single train of 400 msec duration at 100 Hz and 0.2-msec pulse width. These weak tetanization protocols triggered a potentiation that returned to baseline levels within 3 hr. Subsequent to tetanization, recordings were collected at the 1st and 5th min and then every 5 min over a period of at least 120 min.
DRUGS
(S)4-carboxyphenylglycine (4-CPG) (Tocris, Northpoint, UK) was dissolved in ACSF and applied via the perfusion line 10 min before and up to 5 min after tetanization. All solutions were adjusted to a pH of 7.2.
ELECTROPHYSIOLOGICAL RECORDINGS IN VIVO
SURGICAL PREPARATION
Seven- to 8-week-old animals were prepared under Nembutal anesthesia (40 mg/kg, i.p.) as described previously (Manahan-Vaughan 1997). Briefly, a monopolar recording electrode [coordinates anteroposterior (AP) −2.8, lateral (L) 1.8 from bregma] and a bipolar stimulation electrode (coordinates AP −3.1, L 3.1), both made from lacquer-coated stainless steel wire, were implanted stereotaxically into the stratum radiatum of the CA1 region and into the Schaffer collaterals, respectively, in the right hemisphere. The electrodes were adjusted such that the initial slope of the fEPSP was maximal. For drug application, a cannula was chronically implanted in the right lateral ventricle [AP −0.8, L 1.6 from bregma; coordinates according to Paxinos and Watson (1998)]. All animals were allowed at least 8–10 days to recover from surgery, during which period they had free access to food and water.
RECORDING
Throughout each experiment, the animals could move freely in purpose-designed experimental boxes (40 × 40 × 40 cm). The electrodes were connected by a flexible cable to a differential amplifier (Inhvers+, Science Products, Germany). The recorded responses were filtered by band-pass filters at 0.1 Hz and 5 kHz, transformed via an A/D interface (CED 1401, Cambridge Electronic Design, UK) and stored online on PC. By means of input/output curves, the maximum fEPSP (at 0.1 msec pulse width) was determined and a stimulus intensity that evoked 40% of this maximum was used as standard for all recordings except LTP induction by high-frequency stimulation. Robust LTP was generated by 10 bursts of 10 pulses, at 100 Hz (interburst interval 10 sec, 0.1 msec duration each stimulus). The high-frequency tetanus was delivered at a stimulus intensity that evoked 20% of the maximum and resulted in a potentiation that remained stable for at least 24 hr. For each time point during the experiment, five responses evoked every 10 sec, were averaged. During baseline, recordings were collected every 10 min. After tetanization, recordings were taken at t = 5, 10, and 15 min and then every 15 min up to 4 hr.
DRUGS
For drug or vehicle application, the injection cannula was inserted before the baseline measurements were taken and left in place for the duration of the experiment to circumvent artefacts attributable to the handling of the cannula. All drugs were first dissolved in 5 μl of NaOH (1 mm), further diluted with 0.9% saline, adjusted to a neutral pH with 1 mm HCl and finally made up to a volume of 100 μl with 0.9% saline. 4-CPG (29 μg) was injected in a 5-μl volume over a 5-min period via a Hamilton syringe 30 min before tetanization. According to previous investigations (Manahan-Vaughan et al. 1998), which employed a radioactive-labeled NMDA antagonist, this type and schedule of drug application results in a primarily hippocampal localization of the drug around the time of tetanization.
BEHAVIORAL EXPERIMENTS
For drug application, animals were chronically implanted with a microcannula into the right lateral ventricle under nembutal anesthesia as described above and allowed to recover for at least 1 week.
OPEN-FIELD TEST
The open-field arena consisted of a 1 × 1-m quadratic, gray plastic box, divided into 16 equally sized squares and confined by walls 40-cm high. The arena was indirectly lit with four 60 W bulbs. The open-field test was conducted on 2 successive days between 9 a.m. and 1 p.m. At the beginning of the 10-min session, the rat was placed in the center of the open field. Ambulation was recorded and analyzed by means of a computer-aided video analysis system. The following parameters were determined—number of crossings, path length, number of rearings, number of grooming bouts, and number of fecal boli. All behavioral measures were counted per min and calculated for the 10-min observation period. Drugs were injected intracerebroventricularly (ICV) at a total volume of 5 μl and a flow rate of 1 μl/min in 5 min, 30 min before the open-field session on the first day. Control rats received 5 μl of 0.9% saline.
EIGHT-ARM RAM
The apparatus was constructed of gray vinylchloride plates and had eight equally spaced arms (14 × 40 cm) projecting outward from a central octagonal area (37 cm across). The side walls of the arms were made of transparent Plexiglas. A semicircular food cup, 3 cm in diameter and 1-cm deep, was located at the outer end of the arms. At the inner end of the arms, and directly above the food cup, infrared photocell sensors were positioned that were connected to a computer-controlled analysis system allowing an automatic evaluation of the learning session. The whole apparatus was elevated 90 cm from the floor in a soundproof chamber, lit with two 40 W bulbs. Surrounding the maze were several distal cues (e.g., bottles, posters, and lamps) that remained in a constant location from trial to trial.
Three days before starting the experiment, rats were subjected to a food deprivation regimen that reduced their body weight to ∼85% of their initial weight. On the first day, animals received two habituation trials at an interval of 5 min. During these trials, all eight arms were baited with a single standard micro food pellet (45 mg). Habituation session lasted until all pellets were found. On the second day, only three arms were baited and animals were trained two times a day for 8 days with the second trial starting 5 min after completion of the first one. The baited and nonbaited arms remained constant throughout training. The experiment started by placing a rat on the central platform. Entry into an unbaited arm was scored a reference memory (RM) error, arm reentries were scored as working memory (WM) errors. Data were averaged across blocks of two sessions. The maze was cleaned after each test to prevent animals from following scent trails.
Y-MAZE SPATIAL ALTERNATION TASK
The Y-maze spatial alternation task represents a variant of the common hippocampus-dependent Y-maze procedure (Matthies 1978; Grecksch and Matthies 1980; Wetzel et al. 1980) in which the spatial component was strengthened by forcing the animal to acquire an alternation between two arms of the maze in complete darkness. For the procedure, a computer-controlled Y-maze consisting of three equal arms (30 × 15 × 15 cm) with a stainless-steel grid floor was used as described earlier (Riedel et al. 1994b). After a 5-min habituation period in the Y-maze, the rats had to learn a foot shock-motivated right–left spatial alternation. At the beginning of the 40-trial training session, a foot shock (0.7–1.3 mA, depending on individual sensitivity) was given in the start arm and the animal had to escape into the right alley (correct run, no foot shock in this arm), whereas entry into the left alley (error) was punished by further foot shock. In the next trial, the former goal arm served as start arm and the animal had to run into the left alley to avoid punishment. In the third trial, the animal had to run into the right alley, and so on. Therefore, no handling between the trials was necessary. The intertrial interval was 1 min. After the twentieth trial, the rat was removed from the goal arm and was replaced by hand into the formerly wrong alley, now serving as start arm for the next series of trials. This design was used to avoid the animals learning the spatial alternation reaction simply by avoiding one particular arm of the Y-maze (Riedel et al. 1994b). Twenty-four hours after the training session, retention of the Y-maze spatial alternation task (SAT) was tested using the same behavioral procedure as during training.
These experiments were conducted with six groups of rats that received all injections ICV at a volume of 5 μl and a flow rate of 1 μl/min, 30 min before the training session. Group 1 (n = 21) was treated with 29 μg of the group I mGluR antagonist (S)4-carboxyphenylglycine (4-CPG) (Tocris), group 2 (n = 20) served as control and was injected with 5 μl 0.9% of NaCl; group 3 received the same amount of 4-CPG, but before both the training and the retention session, to test for state-dependent effects of 4-CPG (4-CPG/sd group; n = 20). Group 4 was the control group for state-dependent effects receiving 5 μl of 0.9% NaCl before training and retention session (n = 13). Group 5 (n = 15) and group 6 (n = 19) were treated as groups 1 and 2, but the test was modified by omitting the transfer of the animals to a new arm after 20 runs.
The following parameters were evaluated—number of errors, percent savings (number of training errors − number of retention errors/number of training errors), number of intertrial crossings, and mean foot-shock intensity.
DATA ANALYSIS
For statistical analysis, the Kruskal-Wallis H-test and the Mann-Whitney U-test were used to assess between-group differences whereas the Wilcoxon matched pairs signed rank test served to evaluate within-group differences. Statistical differences against zero were tested with the Wilcoxon median signed rank test.
Results
LTP IN VITRO
In the first set of experiments, we investigated whether inhibition of group I mGluRs by 4-CPG has functional consequences on a robust potentiation generated by strong tetanic stimulation. As depicted in Figure 1B, none of the three 4-CPG concentrations applied (50, 100, 150 μm) resulted in discernible changes of a robust LTP, that is, neither the induction of potentiation (4-CPG 50 μm: 219.1 ± 14.8, n = 4; 4-CPG 100 μm: 249.3 ± 24.1, n = 4; 4-CPG 150 μm: 202.1 ± 15.1, n = 4; control: 218.4 ± 13.3, n = 8) nor its maintenance were affected (at 240 min: 4-CPG 50 μm: 171.5 ± 11.7; 4-CPG 100 μm: 163.3 ± 21.4; 4-CPG 150 μm: 158.6 ± 7.6; control: 156.5 ± 13.7). Therefore, we examined the effects of 4-CPG on an LTP induced by weak tetanic stimulation that can be suggested to be more susceptible to a diminution of the [Ca2+]i elevation during tetanization. Such a reduction of the tetanic [Ca2+]i rise was reported recently after the inhibition of group I mGluRs resulting in the blockade of Ca2+ release from IP3-sensitive intracellular Ca2+ stores (Wilsch et al. 1998). Whereas application of 4-CPG (50 μm) 10 min before a weak tetanization of 400 msec at 100 Hz did not affect the initial potentiation (4-CPG group: 204.6 ± 9.9, n = 9; control: 210.5 ± 7.4, n = 9), it resulted in a significant impairment of LTP maintenance, that is, the recordings of the 4-CPG group returned to baseline already after 30 min (Fig. 1C). The controls, in contrast, still showed a significant potentiation after 120 min (116.2 ± 3.1; 4-CPG 96.1 ± 5.3). The differences between the two groups were statistically significant beginning 35 min post-tetanus (P< 0.05).
Figure 1.
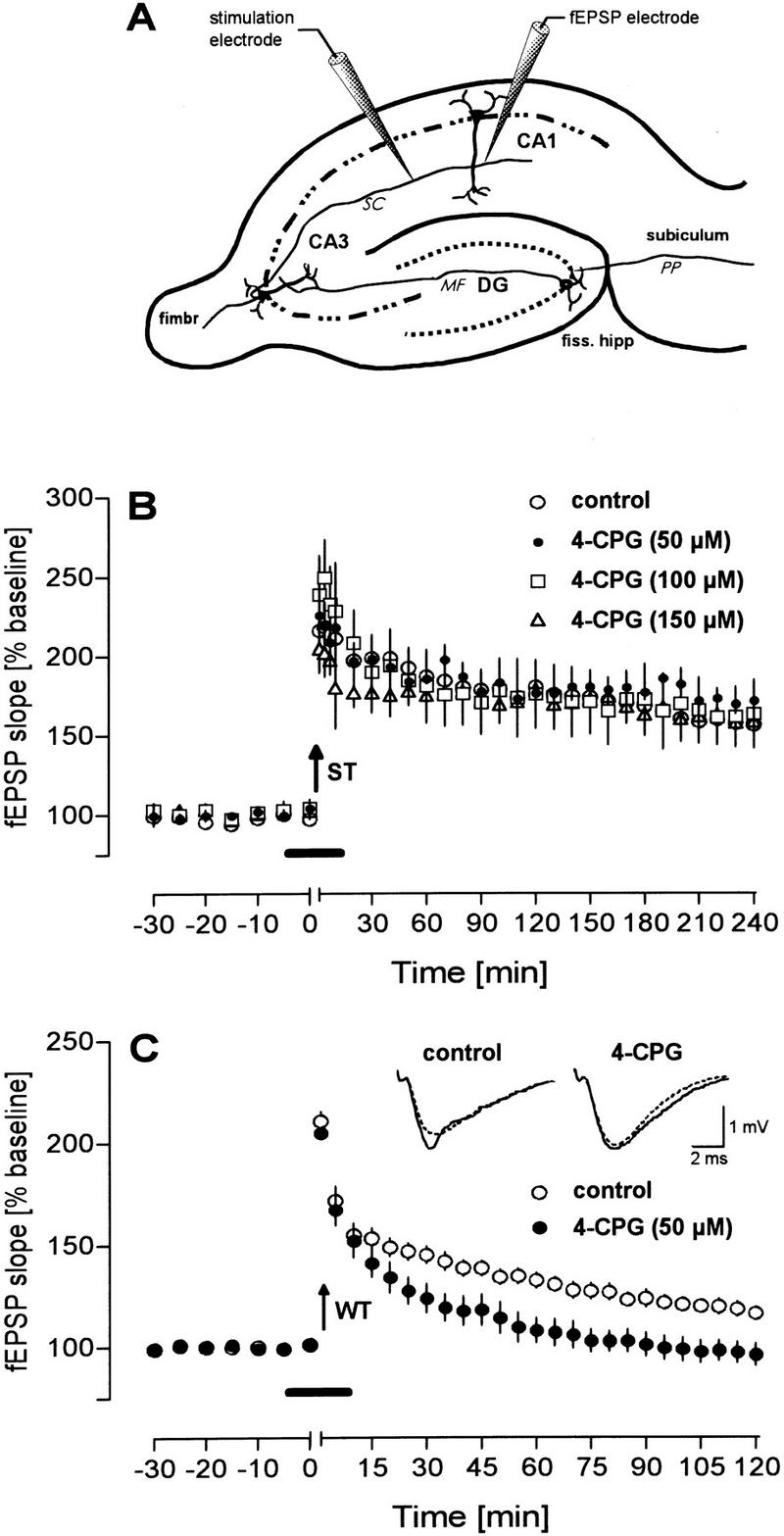
Under in vitro conditions, the mGluR group I antagonist 4-CPG impairs LTP induced by weak tetanization (WT) but not by strong tetanization (ST). (A) Scheme of the placement of recording electrodes in the CA1 region. (B) 4-CPG applied in increasing concentrations does not impair a potentiation induced by a strong tetanization paradigm (ST; 10 bursts of four stimuli at 100 Hz, separated by 200 msec) but was effective (C) if a weak tetanization protocol (WT; 100 Hz, 400-msec duration) was used to generate LTP. Analog traces depict typical responses taken immediately before tetanization (broken line) and 60 min thereafter (solid line). Arrows indicate the time of tetanization and horizontal bars indicate the bath application of 4-CPG.
LTP IN VIVO
The deterioration of a weak CA1 LTP in vitro after mGluR group I inhibition prompted us to examine the effects of 4-CPG in the intact hippocampus of freely moving animals. Surprisingly, ICV injection of 29 μg of 4-CPG (30 mm) a dose that is in about the same range as the concentration used in vitro, impaired a robust LTP in vivo (Fig. 2B). As found with the weak tetanization in the in-vitro experiments, application of 4-CPG resulted in decremental potentiation, which declined to baseline values 105 min after tetanization (P < 0.01). In contrast, control rats displayed a robust potentiation for at least 240 min (at 240 min: fEPSP 140.0 ± 7.0, n = 5).
Figure 2.
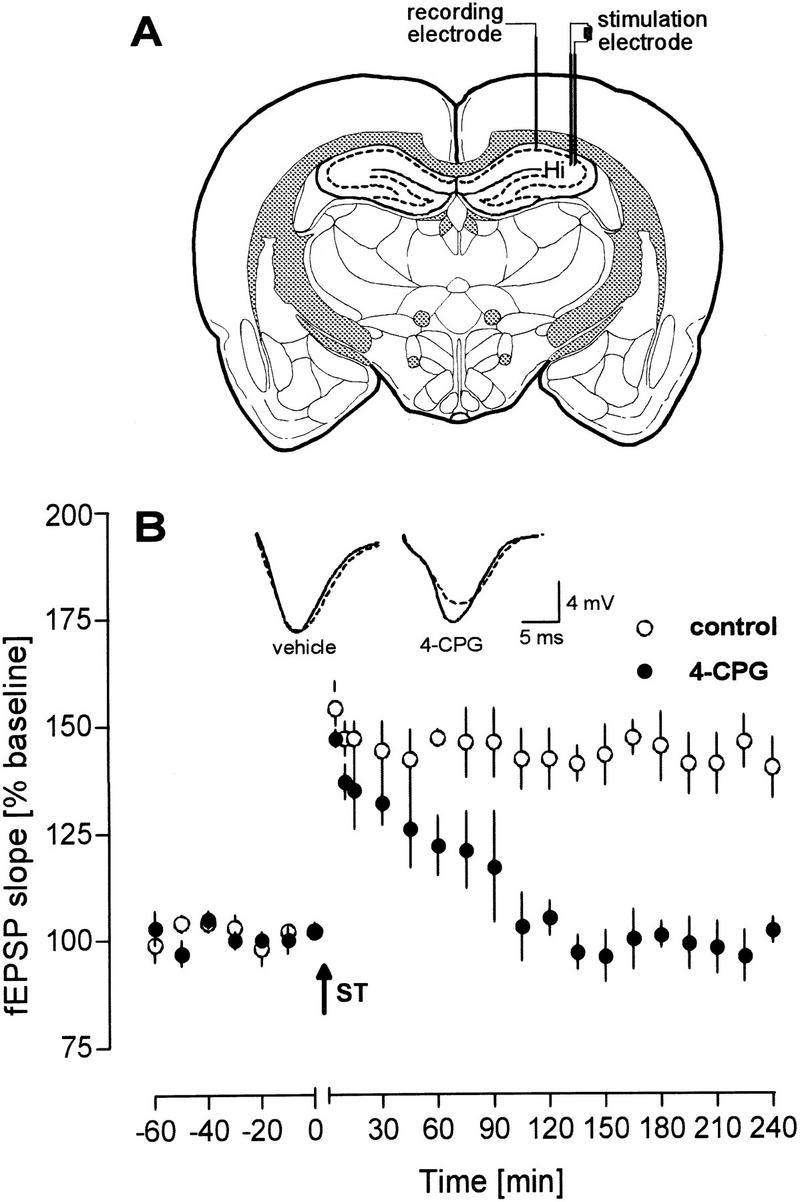
4-CPG Applied ICV to freely moving animals impaired effectively an LTP induced by a strong tetanization paradigm (ST; 10 bursts of 10 pulses, 100 Hz, interburst interval 10 sec, 0.1-msec duration each stimulus). (A) Schematic diagram of electrode placement for the recording from the CA1 area of the right hemisphere. (B) Time course of the potentiation of the fEPSP slope. Analog traces represent typical recordings from a 4-CPG-treated animal (right) and a control animal (left), taken 5 min (solid line) and 240 min (broken line) after tetanization. The arrow indicates the time of tetanization.
OPEN-FIELD TEST
Because of the detrimental effects of 4-CPG on hippocampal LTP in vitro and in vivo, it was tempting to speculate whether application of 4-CPG might affect spatial learning. To exclude that the results of the learning studies are confounded with general effects of 4-CPG on behavior, we first tested the exploratory behavior in an open field 30 min after application of the same 4-CPG concentration as used in the LTP studies in vivo. Because the repetition of the open-field test allows the evaluation of short- and long-term habituation, the procedure was conducted twice with an intertest interval of 1 day.
The analysis of all parameters obtained in the open-field test did not reveal any behavioral abnormalities of the 4-CPG-treated animals. This is evidenced by the nearly identical total number of crossings of the 4-CPG group and the control group on day 1 (Fig. 3A). and supported further by lack of any difference in the total number of rearings and grooming bouts (Fig. 3C,E) as well as in the path length and the number of fecal boli (data not shown). Furthermore, 4-CPG application on the first day did not affect exploratory behavior 24 hr later, as clearly indicated by missing inter-group differences in the number of crossings, rearings, and grooming bouts (right bars in Fig. 3A,C,E). A more detailed analysis of the behavioral measures over time (Fig. 3B,D,F) confirmed these conclusions with the exception that a statistically significant between-group difference was detected in the rearing behavior during the seventh minute of day 1 (4-CPG: 0.11 ± 0.11, n = 9; control: 0.78 ± 0.28, n = 9, P < 0.05). In addition, the between-day comparison displayed a clear trend of a reduction in the number of crossings and grooming bouts from day 1 to day 2, which was supported by a significant decline of the number of crossings of 4-CPG-treated animals (51.4 ± 8.0 vs. 36.2 ± 6.8, P < 0.05; controls: 48.9 ± 7.0 vs. 38.0 ± 9.2, NS) and by a significant reduction in the number of grooming bouts of controls (35.7 ± 3.8 vs. 23.1 ± 3.9, P < 0.05; 4-CPG group: 32.8 ± 5.9 vs. 24.3 ± 5.4 NS).
Figure 3.
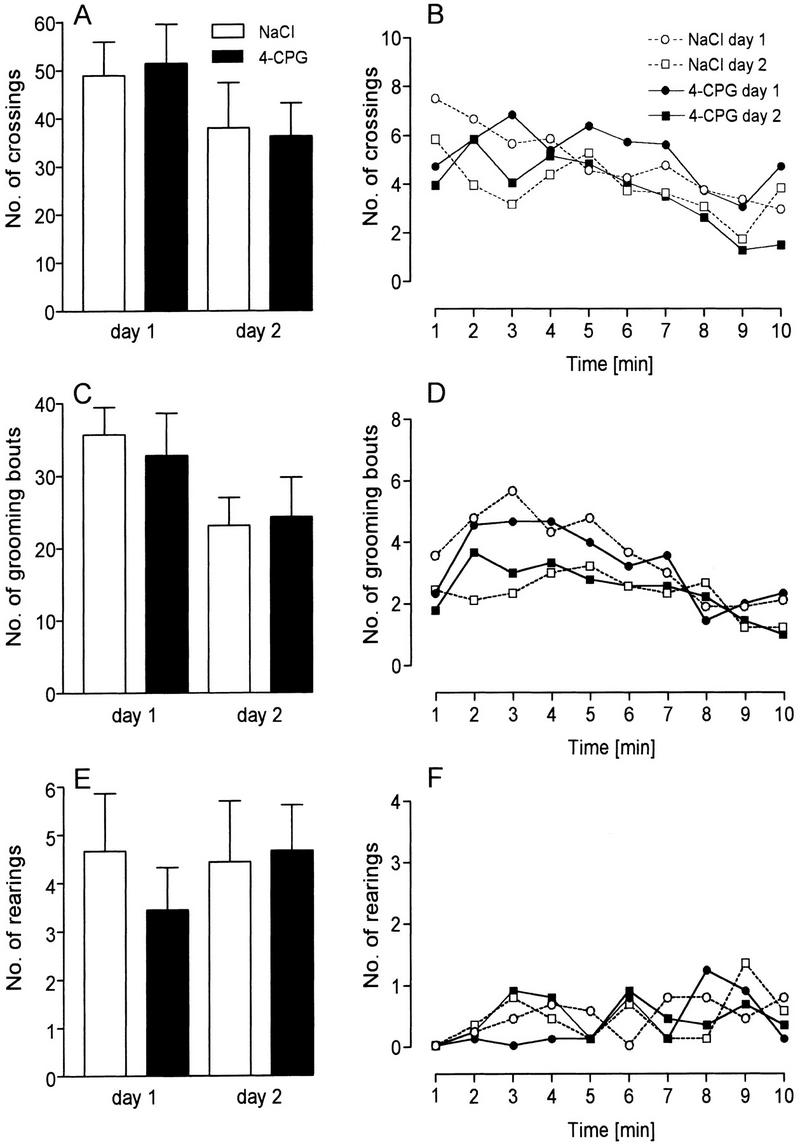
The application of 4-CPG had no influence on behavior in the open field. Bars depict the mean number of crossings (A), grooming bouts (C), and rearings (E), as calculated for each day of testing. In the graphs (B, D, and F, respectively) at the 1-min time courses of the same parameters are given. The only between-group difference that was statistically significant was in the rearing behavior of the 4-CPG group and controls at the seventh minute of day 1 (P < 0.05).
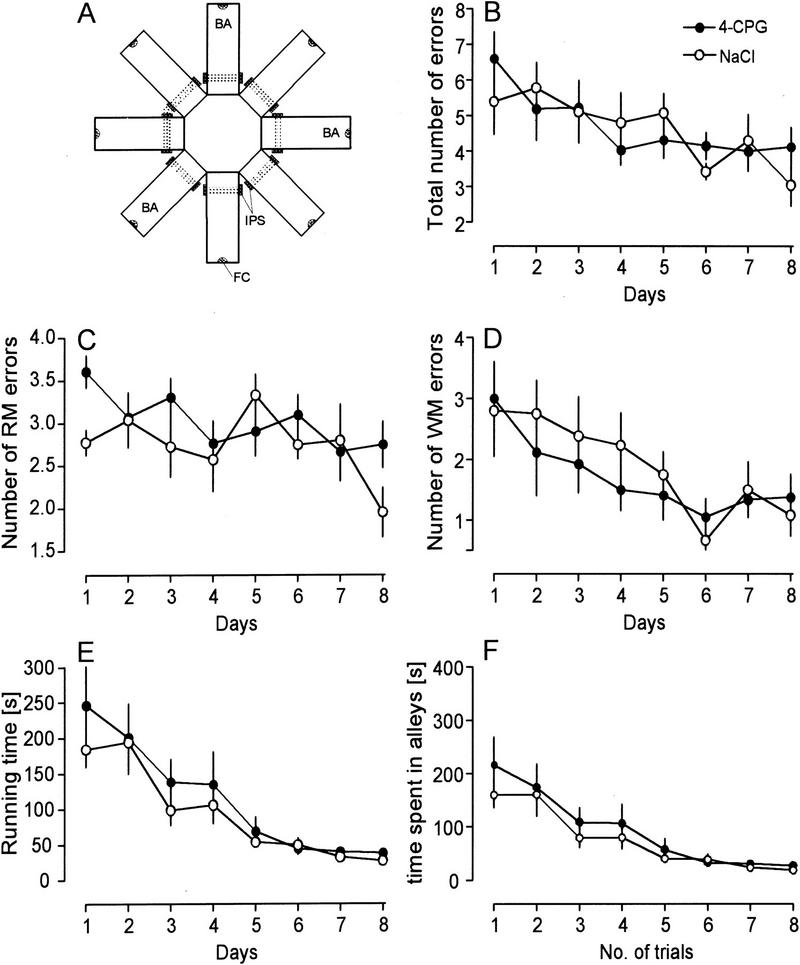
RAM
The overall learning performance in the eight-arm RAM was not affected significantly by 4-CPG application as indicated by a similar reduction in the total number of errors, the running time, and the time spent in alleys in both groups (Fig. 4B,E,F). Therefore, 4-CPG-treated animals decreased their total errors from 6.6 ± 0.7 on day 1 to 4.1 ± 0.5 on day 8 (n = 12), controls from 5.4 ± 0.9 to 3.0 ± 0.6 (n = 12). Similarly, the 4-CPG group reduced the running time from 246.3 ± 54.9 on the first day to 38.2 ± 4.4 on day 8; controls from 184.4 ± 24.9 to 27.7 ± 2.0, the respective alley times were reduced from 216.5 ± 51.9 to 27.5 ± 3.9 and from 159.8 ± 24.1 to 19.2 ± 1.3. If, however, the performance in WM and RM was compared between groups, the number of RM errors on day 1 and day 8 were higher in 4-CPG animals than in controls (day 1, 3.6 ± 0.2 vs. 2.8 ± 0.1, P < 0.01; day 8, 2.8 ± 0.3 vs. 1.9 ± 0.3, P < 0.05).
Figure 4.
4-CPG only mildly affected spatial learning in the eight-arm RAM. The means of two sessions per day are given. The only difference that was detected concerns the number of reference memory (RM) errors, which was higher on day 1 and day 8 in 4-CPG animals than in controls (C) (P < 0.05). In the schematic of the eight-arm RAM shown in A the dotted lines indicate the triple-beam infrared detector. (BA) Baited arm; (FC) food cup; (IPS) infrared photosensors; (WM) working memory.
Y-MAZE SPATIAL ALTERATION TASK
To verify the effects of an inhibition of group I mGluRs in another spatial learning paradigm, the efficacy of 4-CPG was examined in two configurations of the Y-maze spatial alteration task (SAT). In the first configuration, which was described previously (Riedel et al. 1994b), the animals are transferred after half of the trials (20 runs) to another alley that enhances the difficulty of the task (dSAT). In the second configuration, this transfer was omitted resulting in a task that could be acquired more easily (eSAT). In dSAT 4-CPG given ICV 30 min before training did not affect acquisition on day 1 (percent errors: 4-CPG 34.8 ± 1.6, n = 21; NaCl 36.7 ± 2.2, n = 20) (Fig. 5A) but significantly impaired retention on the following day (percent errors: 4-CPG 33.7 ± 2.6, NaCl 27.9 ± 1.9) resulting in percent savings of the 4-CPG group that were not significantly different from zero (4-CPG 3.1 ± 7.6, NaCl 24.1 ± 6.6) (Fig. 5B; P < 0.05). To exclude that the observed amnesia following 4-CPG application was a state-dependent effect, in subsequent experiments the drug was given twice—before the training and the retention session. As depicted in Figure 5, C and D, the results of these experiments resembled the previous ones. Whereas no differences were discernible on day 1 (percent errors: 4-CPG 33.5 ± 2.4, n = 20; NaCl 33.3 ± 2.0, n = 13), on day 2 the retention of the 4-CPG group was deteriorated (percent errors: 4-CPG 32.1 ± 2.3, NaCl 28.3 ± 1.7) as clearly evidenced by the lack of significant savings of the 4-CPG group (4-CPG 4.1 ± 7.6%, NaCl 15.0 ± 3.4%; P < 0.05).
Figure 5.
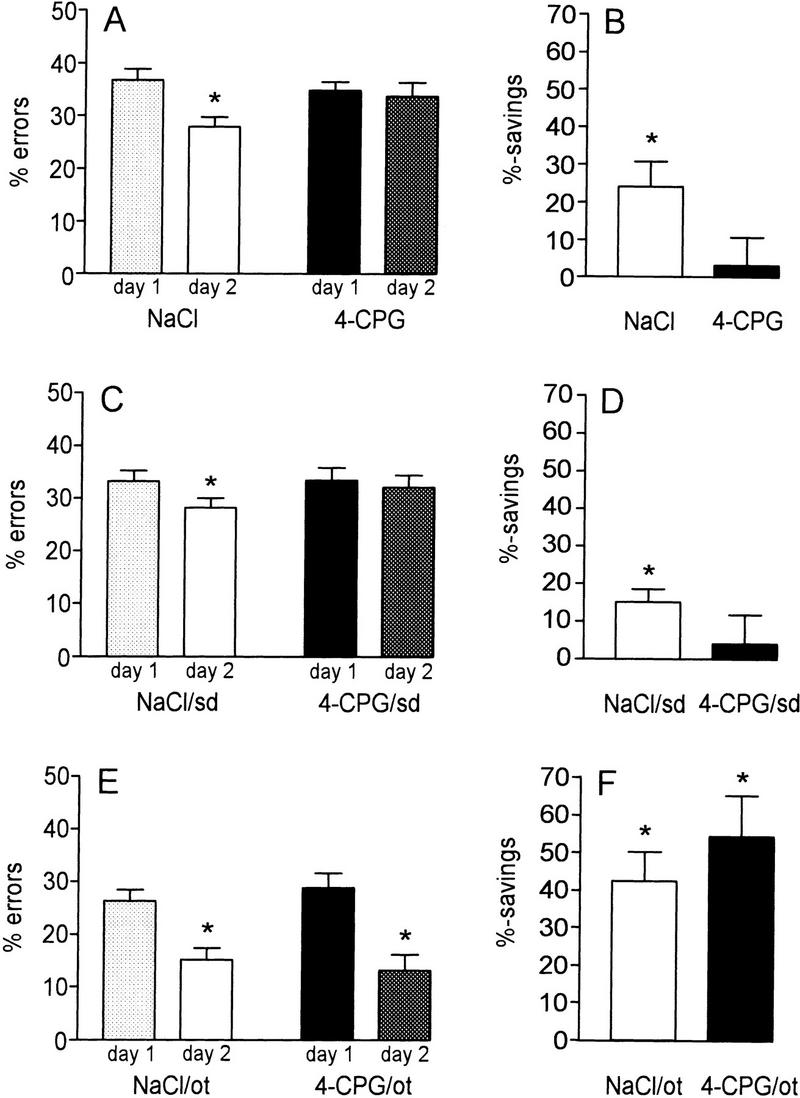
The effect of 4-CPG on learning of the SAT was contingent on the difficulty of the particular task configuration. (A–D) Effects of 4-CPG in the more difficult configuration of SAT (dSAT), including a transfer of the experimental animals to another alley after half on the session (20 runs). (A,B) Under these conditions, the ICV application of 4-CPG before the training session prevented the decline of errors on day 2 resulting in percent savings that were not significantly different from zero. Controls, in contrast, displayed a significant retention on the second day. (C,D) The impairment of SAT learning was not state-dependent (sd), as the effects of 4-CPG given before both the training and the retention session (4-CPG/sd) resembled the findings obtained after application of the drug before training depicted above. (E,F) Omission of the transfer (ot) after half of the session (20 runs) resulted in a configuration of SAT that could be acquired more easily (eSAT) as indicated by the higher percent savings as compared with dSAT. This configuration abolished the effect of 4-CPG.
In the next set of experiments, 4-CPG was applied in eSAT, the more easy configuration of SAT. As expected, the number of errors committed was lower and the percent savings were higher than in dSAT. During acquisition both groups attained an error level beneath 30% (4-CPG 28.8 ± 2.8%, n = 15; NaCl 26.3 ± 2.1%, n = 19; Fig. 5E). Surprisingly, not only the control but also the 4-CPG group displayed a further decline of errors in the retention test (4-CPG 13.2 ± 3.0%, P < 0.05; NaCl 15.1 ± 2.3%; P < 0.05) resulting in significant percent savings of both groups (4-CPG 54.3 ± 10.8, P < 0.05; NaCl 42.5 ± 7.8; P < 0.05) (Fig. 5F). As a consequence of the simple task configuration, not only the eSAT control group but also the 4-CPG-treated animals attained a level of percent savings that was higher than the values attained by the control group in the dSAT configuration (P < 0.01). Therefore, in the eSAT, 4-CPG failed to impair spatial learning.
Discussion
THE EFFECT OF GROUP I mGluR INHIBITION ON LTP IN VITRO DEPENDS ON THE STRENGTH OF TETANIZATION
The results of the in-vitro studies indicate that the role of group I mGluRs in LTP depends on the strength of the tetanization paradigm employed. Whereas increasingly higher concentrations of 4-CPG were ineffective in blocking LTP generated by a strong tetanization, the drug reliably blocked a potentiation induced by a weak tetanization paradigm. Our finding that inhibition of group I mGluRs may impair LTP is consistent with the results of other laboratories showing that activation of group I receptors may facilitate the induction of LTP or even induce a potentiation on their own (McGuinness et al. 1991; Otani and Ben-Ari 1991; Ben-Ari et al. 1992; Bortolotto and Collingridge 1992, 1995; Behnisch and Reymann 1993; Manahan-Vaughan and Reymann 1995, 1996; O’Leary and O’Connor 1997). Studies using antagonists of group I mGluRs, however, have produced conflicting results. Whereas some groups have reported an impairment of LTP after application of L-2-amino-3-phosphonopropionate (L-AP3) (Behnisch and Reymann 1993), or MCPG (Bashir et al. 1993; Bortolotto et al. 1994; Brown et al. 1994; Richter-Levin et al. 1994; Little et al. 1995) others have failed to reproduce these effects (Chinestra et al. 1993; Izumi and Zorumski 1994; Manzoni et al. 1994; Selig et al. 1995; Thomas and O’Dell 1995). Even studies employing mGluR 1 and mGluR 5 knockout mice could not resolve the controversy. Aiba et al. (1994) found in mGluR 1 knockouts a significantly lower magnitude of LTP in the CA1 area of the hippocampus, whereas Conquet et al. (1994) did not observe a change of CA1 and dentate LTP in these mutants. In contrast, mutant mice deficient in mGluR5 were impaired in CA1 and dentate LTP, but showed a normal potentiation in the CA3 region (Lu et al. 1997).
The dependence of the 4-CPG action on the strength of tetanization, found in our study, might be related to the action of group I mGluRs on the intracellular calcium metabolism. As initial confocal Ca2+-measurements support, agonists of group I mGluRs, such as 3,5-dihydroxyphenylglycine (DHPG), cause an increase of the intracellular Ca2+ level, most likely by release of Ca2+ from intracellular stores (data not shown). On the other hand, tetanizations at various strengths may result in a differential recruitment of two other important sources of intracellular Ca2+–NMDA receptors and voltage-gated calcium channels. As a result, the attained intracellular Ca2+ level, which was suggested to determine the properties and the type of synaptic plasticity (Artola and Singer 1993; Cummings et al. 1996; Tsumoto and Yasuda 1996; Hänsel et al. 1997), might vary as a function of tetanization strength. Therefore, it is conceivable that Ca2+ might be limited under some conditions whereas it is available in excess under others. Consequently, the block of Ca2+ release by group I mGluRs after 4-CPG application would be expected to result in an impairment of potentiation if intracellular Ca2+ is meager (e.g., during weak tetanization) but to have no effect if sufficient Ca2+ is provided by other sources (e.g., during strong tetanization).
4-CPG IMPAIRS ROBUST LTP IN THE INTACT HIPPOCAMPUS IN VIVO
In this study, LTP in the CA1 area of the intact hippocampus, that is, in the freely moving animal, was consistently impaired by ICV application of 4-CPG given 30 min before tetanization. Interestingly, the same dose of 4-CPG also caused an impairment of robust dentate gyrus LTP (data not shown). These findings corroborate previous reports on detrimental effects of MCPG on LTP in vivo. Riedel et al. (1995a) described a complete block of dentate gyrus LTP under almost the same experimental conditions as used here, if MCPG was given before but not after tetanization. Richter-Levin et al. (1994) pursued the actions of MCPG given ICV under urethane anesthesia in the dentate gyrus and found a reduction in fEPSP potentiation, but not in population-spike potentiation. If MCPG, however, was directly infused into the recording region via a push–pull cannula, MCPG also impaired population-spike potentiation. Quite similar to the contradictory findings in vitro, some researchers found neither an effect of MCPG (Bordi and Ugolini 1995; Martin and Morris 1997) nor of 4-CPG (Bordi and Ugolini 1995) on LTP in vivo. The reasons for the conflicting results are difficult to identify. All studies that failed to obtain an effect of the antagonist on LTP in vivo were performed under urethane anesthesia. Although urethane anesthesia can be suggested to result in multifarious metabolic changes as compared with the freely moving state, this may impede but apparently does not prevent the antagonists’ effects as indicated by the findings of Richter-Levin et al. (1994). Another factor that has to be considered is an increase in the induction threshold of LTP that was reported to occur under urethane anesthesia (Riedel et al. 1994a). The higher tetanization strength necessary to induce LTP under anesthesia (as compared with an LTP generated in freely moving animals) would be in accordance with our in vitro findings that LTP induced by strong tetanic stimulation is not susceptible to the action of MCPG (Wilsch et al. 1998) and 4-CPG (see above). Last but not least, the strain (and age) of animals may account for the contradictory results. Comparative studies suggest that the magnitude of LTP induced by identical protocols varies across strains (D. Manahan-Vaughan, unpubl.).
In this study, inhibition of group I mGluRs impaired LTP in vitro, which was generated by a weak but not by a strong tetanization protocol, whereas under in vivo conditions the expression of robust LTP was prevented as well. This difference is likely to be caused by the preserved intrahippocampal connectivity in the intact hippocampus resulting in a higher inhibition as compared with the slice preparation. This higher inhibition leads to fairly different dendritic conditions. For instance, active back propagation of somatic spikes into dendrites (Stuart et al. 1997) occurs less frequently in vivo and is more regionally confined (Buzsaki et al. 1996). Because the frequency of sodium spikes governs the dendritic Ca2+ dynamics (Svoboda et al. 1997), a certain tetanization paradigm is likely to trigger a smaller increase of the intracellular Ca2+ concentration in vivo than in vitro. This holds true in particular for distal dendritic regions. Therefore, in contrast to the in vitro conditions, in the intact hippocampus Ca2+ may be a critical resource even during strong tetanization protocols. Under these conditions a blockade of Ca2+ release from intracellular stores by inhibiton of group I mGluRs may prevent the induction of a robust LTP.
APPLICATION OF 4-CPG DOES NOT CAUSE GROSS BEHAVIORAL CHANGES
The evaluation of the different behavioral measures obtained in the open-field test provides clear evidence that pretest application of 4-CPG in the same dose as used in the LTP experiments does not induce any behavioral alteration. 4-CPG-Treated animals displayed ambulation, rearing, grooming, and defecation that did not differ from that of controls. Furthermore, the two groups showed a comparable decline of crossings during the 10-min session on both days and between day 1 and day 2 as well as a similar reduction of grooming bouts from day 1 to day 2, altogether indicative of a normal habituation to novelty. Therefore, our results clearly exclude group I mGluRs from being critically involved in open-field behavior, thereby extending previous findings that reported only minor effects of the broad-spectrum mGluR–antagonist MCPG and the group 1 agonist trans-azetidine-2,4-dicarboxylic acid (tADA) on behavior in the open field (Wetzel et al. 1995). Importantly, the present results of the open field indicate that under the conditions employed, 4-CPG is devoid of behavioral side effects. The preclusion of such behavioral side effects is of particular relevance because mutant mice lacking the group I receptor mGluR 1 (Aiba et al. 1994; Conquet et al. 1994) or mGluR 5 (Lu et al. 1997) were reported to suffer from impaired motor coordination and decreased sensory sensitivity.
GROUP I mGluR INHIBITION ONLY MILDLY AFFECTS SPATIAL LEARNING IN THE EIGHT-ARM RAM
Learning in the RAM has been shown repeatedly to be hippocampus-dependent (Olton et al. 1978; Olton and Papas 1979; Jarrard 1986; Kawabe et al. 1998). In this study, we found only mild effects of 4-CPG on spatial learning in the RAM, that is, both groups acquired the task equally well as indicated by the decaying error scores and the clear-cut reduction of the running time and the time spent in alleys (to ∼15% and 12%, respectively). One of the merits of the RAM consists of allowing the distinction of both WM and RM components. Trial-specific WM allows the animal to recall arms entered previously on the current trial and therefore avoid reentries. RM, on the other hand, refers to stored representations and rules that are useful for all trials. In our study the number of total errors and WM errors, committed under the same dose of 4-CPG as used in LTP in vivo and in the open field test, did not differ significantly between groups. Nevertheless, an analysis of the RM errors revealed significantly higher RM errors of 4-CPG-treated animals on the first and last day of training. The worse RM of the 4-CPG group on day 1 could reflect an effect of 4-CPG on acquisition of the three-baited-arms RAM configuration. On day 1, the task configuration (three baited arms) met by the animals does not conform to the stored reference memory of the preceding habituation trials (eight baited arms, data not shown). Under these somewhat conflicting conditions, activation of group I mGluRs might be required to attain the same performance as controls. The absence of differences on the days thereafter is indicative of a subsidiary function of group I mGluRs during this phase of spatial learning. Given that also under these conditions small effects are still present, one would expect that the effect needs some time (of accumulation) to become discernible. The appearance of an overt 4-CPG effect on RM errors on day 8 appears to support this postulate. As another possibility, the repeated exposure to 4-CPG may have diminished the effect of 4-CPG. This appears to be unlikely, however, because to our knowledge, there are no reports so far about a desensitization of group I receptors following repeated antagonists application.
THE EFFECT OF 4-CPG IN THE SAT DEPENDS ON THE DIFFICULTY OF THE PARADIGM
In our hands, application of the mGluR group I antagonist does not impair spatial learning in an easy type of the SAT (eSAT) but was effective in disrupting SAT learning in the more difficult standard configuration (dSAT), in which the transfer of the experimental subject to another alley after half of the session results in an immediate increase of the committed errors, which is indicative of a conflicting situation. Because the effects of 4-CPG in the dSAT configuration were not state-dependent, they may be assigned as a specific impairment of retention. A decreased SAT performance was reported after the application of the broad-spectrum mGluR antagonist MCPG (Riedel et al. 1994b). In the latter study in which the dSAT configuration was used, application of MCPG 30 min before training resulted in amnesia that was not state-dependent. The similarity of these MCPG effects and the 4-CPG actions obtained here indicates that MCPG, an inhibitor of group I mGluRs and with a somewhat lower efficacy of group II mGluRs (Davies et al. 1995; Sekiyama et al. 1996), impeded spatial learning by antagonizing group I mGluRs.
THE ROLE OF GROUP I mGluRs IN SPATIAL LEARNING
Although there are several reports about an involvement of mGluRs in learning and memory (for reviews, see Riedel et al. 1996; Conn and Pin 1997), studies approaching the role of mGluRs in spatial learning are rare and these studies mostly used mGluR agonists and antagonists that act on more than one group of mGluR receptors (e.g., ACPD and MCPG). For example, Bordi et al. (1996) and Richter-Levin et al. (1994) reported that WAM learning was lessened by MCPG and Hölscher et al. (1997) found only a mild impairment of learning in the WAM and the RAM after ICV injection of ACPD. In RAM learning, ACPD increased the number of WM errors but not the number of RM errors. Another mGluR agonist, L(+)-2-amino-4-phosphonobutyric acid (L-AP4), which acts on mGluRs 4, 6, 7, and 8 was found to exert detrimental effects on learning in WAM and RAM although not affecting open-field behavior (Hölscher et al. 1996). In the same study, (S)-2-amino-2-methyl-4-phosphonobutanoic acid (MAP4), an antagonist at presynaptic L-AP4-sensitive receptors, impaired spatial learning in the same two paradigms. The application of L-AP4 and MAP4 alone increased both the number of WM and RM errors. Joint application of the two drugs prevented the impairment of spatial learning. Whereas studies elaborating the role of group I mGluRs in spatial learning are completely lacking, a few investigations approached their function in other types of learning. Recently, Nielsen et al. (1997) reported that intraperitoneal injection of the group I mGluR antagonist 1-aminoindan-1,5-dicarboxylic acid (AIDA) blocked hippocampus-dependent contextual fear, but not hippocampus-independent cue conditioning in rats. This is in contrast to Bordi et al. (1996), who obtained no alteration of context-dependent fear conditioning after application of the broad-spectrum antagonist MCPG.
The distinct 4-CPG effect on spatial learning in this study and the related findings with MCPG and knockout mice indicate a function of group I mGluRs in certain types of hippocampus-dependent tasks. Mutant mice that were deficient of the mGluR 1 gene were impaired moderately in the hippocampus-dependent nonspatial, context-dependent fear conditioning (Aiba et al. 1994) but displayed a severe deterioration in the spatial version of the Morris WAM (Conquet et al. 1994). In contrast, they performed like wild-type controls in the hippocampus-independent cue conditioning. Mutant mice, deficient for mGluR 5 showed a deterioration in two hippocampus-dependent tasks, the Morris WAM and contextual fear conditioning (Lu et al. 1997). In earlier investigations, we have found, that the same dose of MCPG as impaired dSAT learning (Riedel et al. 1994b) had no effect on Y-maze brightness discrimination, which is a non-spatial, but hippocampus-dependent paradigm (Riedel et al. 1995b).
The differential effects of 4-CPG in the various types of hippocampus-dependent, spatial learning highlight the problem that different spatial learning paradigms do not measure the same things. Therefore, depending on the structural and procedural characteristics of the employed experimental paradigm, the experimental subjects can demonstrate very different learning performance, which indicates the heterogeneity of the sensorial and behavioral processes underlying the various spatial learning tasks.
A critical evaluation of SAT and RAM reveals that the two methods differ considerably with regard to the complexity of the task as well as the sensory and cognitive mechanisms that can be suggested to be implicated. RAM learning represents a very complex spatial learning task thought to use predominantly allocentric extramaze visuospatial cues (Hodges 1996) for spatial navigation, although other factors such as intramaze cues and associative mechanisms may contribute to the formation of a spatial map (Brown et al. 1993). In the eight-arm RAM configuration used in this study, the animals learn the firm location of a food reward in a spatial map. In contrast, SAT learning, a type of egocentric spatial learning, depends on other types of sensory information. It does not allow the use of any extramaze visuospatial cues because all walls of the maze and the cover-lid are made of opaque plastic and the learning trial is conducted without any additional illumination. Therefore, the rats have to rely on olfaction, vibrissae movements, and the scattered light penetrating through the slits of the lid to get spatial information. Another difference to RAM concerns the subject of learning, that is, what is learned. Whereas RAM learning requires the formation and storage of a fixed spatial map, during SAT learning the animals have to adopt a particular spatial strategy. Furthermore, whereas SAT represents a fast (2 days), aversively motivated task with a rapidly decaying error rate, the weaker motivational drive of the appetitive motivation in RAM learning results in long experiments (several days to weeks) with slowly decaying error scores. Therefore, the different means used by rats in SAT and RAM learning, can be suggested to represent complementary subsets of spatial learning mechanisms.
Bearing these aspects in mind, the disparity of the 4-CPG effects in SAT and RAM is likely to be caused by two factors: (1) the type of learning paradigm employed, which determines the subsets of sensory and cognitive mechanism involved in the acquisition of the respective spatial map and navigation strategy; and (2) the level of difficulty of a given paradigm, as determined by the particular configuration of this paradigm. The second factor is reminiscent of the differential involvement of group I mGluRs in hippocampal LTP in vitro. Whereas in the LTP studies the strength of tetanization determined whether or not group I mGluRs have a functional role, in the learning studies, the difficulty of the task appeared to be one critical variable. Therefore, activation of group I mGluRs seems to be of functional importance (1) if crucial cellular resources are limited, such as Ca2+ in LTP induction; and (2) under conditions of a conflicting learning situation or a high task difficulty.
Acknowledgments
The excellent technical assistance of S. Hartmann, U. Lerke, U. Stolz and S. Vieweg is gratefully acknowledged. This work was supported by Biomed BMH4-CT96-0228.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Beretta N, Irving AJ, Sea AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Behnisch T, Reymann KG. Co-activation of metabotropic glutamate and N-methyl-D-aspartate receptors is involved in mechanisms of long-term potentiation maintenance in rat hippocampal CA1 neurons. Neuroscience. 1993;54:37–47. doi: 10.1016/0306-4522(93)90381-o. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Aniksztejn L, Bregestowski P. Protein kinase-C modulation of NMDA currents—an important link for LTP induction. Trends Neurosci. 1992;15:333–339. doi: 10.1016/0166-2236(92)90049-e. [DOI] [PubMed] [Google Scholar]
- Bianchin M, Da Silva RC, Schmitz PK, Medina JH, Izquierdo I. Memory of inhibitory avoidance in the rat is regulated by glutamate metabotropic receptors in the hippocampus. Behav Pharmacol. 1994;5:356–359. doi: 10.1097/00008877-199406000-00014. [DOI] [PubMed] [Google Scholar]
- Bordi F, Ugolini A. Antagonists of the metabotropic glutamate receptor do not prevent induction of long-term potentiation in the dentate gyrus of rats. Eur J Pharmacol. 1995;273:291–294. doi: 10.1016/0014-2999(94)00756-w. [DOI] [PubMed] [Google Scholar]
- Bordi F, Marcon C, Chiamulera C, Reggiani A. Effects of the metabotropic glutamate receptor antagonist MCPG on spatial and context-specific learning. Neuropharmacology. 1996;35:1557–1565. doi: 10.1016/s0028-3908(96)00101-3. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collingridge GL. Activation of glutamate metabotropic receptors induces long-term potentiation. Eur J Pharmacol. 1992;214:297–298. doi: 10.1016/0014-2999(92)90135-q. [DOI] [PubMed] [Google Scholar]
- ————— On the mechanism of long-term potentiation induced by (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD) in rat hippocampal slices. Neuropharmacology. 1995;34:1003–1014. doi: 10.1016/0028-3908(95)00054-a. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- Brown MF, Rish PA, von Culin JE, Edberg JA. Spatial guidance of choice behavior in the radial-maze. J Exp Psychol. 1993;19:195–214. [PubMed] [Google Scholar]
- Brown RE, Rabe H, Reymann KG. (RS)-α-methyl-4-carboxyphenylglycine (MCPG) does not block theta burst-induced long-term potentiation in area CA1 of rat hippocampal slices. Neurosci Lett. 1994;170:17–21. doi: 10.1016/0304-3940(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Penttonen M, Nadasdy Z, Bragin A. Pattern and inhibition-dependent invasion of pyramidal cell dendrites by fast spikes in the hippocampus in vivo. Proc Natl Acad Sci. 1996;93:9921–9925. doi: 10.1073/pnas.93.18.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinestra P, Aniksztejn L, Diabira D, Ben-Ari Y. (RS)-alpha-methyl-4-carboxyphenylglycine neither prevents induction of LTP nor antagonizes metabotropic glutamate receptors in CA1 hippocampal neurons. J Neurophysiol. 1993;70:2684–2689. doi: 10.1152/jn.1993.70.6.2684. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin J-P. Pharmacology and function of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16:825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- Davies CH, Clarke VRJ, Jane DE, Collingridge GL. Pharmacology of postsynaptic metabotropic glutamate receptors in rat hippocampal CA1 pyramidal neurons. Brit J Pharmacol. 1995;116:1859–1869. doi: 10.1111/j.1476-5381.1995.tb16674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecksch G, Matthies HJ. Two sensitive periods for the amnestic effect of anisomycin. Pharmacol Biochem Behav. 1980;12:663–665. doi: 10.1016/0091-3057(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Hänsel C, Artola A, Singer W. Relation between dendritic Ca2+ levels and the polarity of synaptic long-term modifications in rat visual cortex neurons. Eur J Neurosci. 1997;9:2309–2322. doi: 10.1111/j.1460-9568.1997.tb01648.x. [DOI] [PubMed] [Google Scholar]
- Hodges H. Maze procedures: The radial-arm and water maze compared. Cognitive Brain Res. 1996;3:167–181. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Hölscher C. Inhibitors of metabotropic glutamate receptors produce amnestic effects in chicks. Neuroreport. 1994;5:1037–1040. doi: 10.1097/00001756-199405000-00005. [DOI] [PubMed] [Google Scholar]
- Hölscher C, McGlinchey L, Rowan MJ. L-AP4 (L-(+)-2-amino-4-phosphonobutyric acid) induced impairment of spatial learning in the rat is antagonized by MAP4 ((S)-2-amino-2-methyl-4-phosphonobutanoic acid) Behav Brain Res. 1996;81:69–79. doi: 10.1016/s0166-4328(96)00045-9. [DOI] [PubMed] [Google Scholar]
- Hölscher C, McGlinchey L, Anwyl R, Rowan MJ. HFS-induced long-term potentiation and LFS-induced depotentiation in area CA1 of the hippocampus are not good models for learning. Psychopharmacology. 1997;130:174–182. doi: 10.1007/s002130050226. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Developmental changes in the effects of metabotropic glutamate receptor antagonists on CA1 long-term potentiation in rat hippocampal slices. Neurosci Lett. 1994;176:89–92. doi: 10.1016/0304-3940(94)90878-8. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Selective hippocampal lesions and behavior. Implications for current research and theorizing. In: Isaacson RL, Pribram KH, editors. The hippocampus. Vol. 4. New York, NY: Plenum Press; 1986. pp. 93–126. [Google Scholar]
- Kaba H, Hayashi Y, Higuchi T, Nakanishi S. Induction of an olfactory memory by the activation of a metabotropic glutamate receptor. Science. 1994;265:262–264. doi: 10.1126/science.8023145. [DOI] [PubMed] [Google Scholar]
- Kawabe K, Ichitani Y, Iwasaki T. Effects of intrahippocampal AP5 treatment on radial-arm maze performance in rats. Brain Res. 1998;781:300–306. doi: 10.1016/s0006-8993(97)01256-0. [DOI] [PubMed] [Google Scholar]
- Little Z, Grover LM, Teyler TJ. Metabotropic glutamate receptor antagonist, (R,S)-alpha-methyl-4carboxyphenyglycine, blocks two distinct forms of long-term potentiation in area CA1 of rat hippocampus. Neurosci Lett. 1995;201:73–76. doi: 10.1016/0304-3940(95)12141-p. [DOI] [PubMed] [Google Scholar]
- Lu Y-M, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Reymann KG. 1S,3R-ACPD dose-dependently induces a slow-onset potentiation in the rat hippocampal CA1 region in vivo. Neuropharmacology. 1995;34:1103–1105. doi: 10.1016/0028-3908(95)00108-i. [DOI] [PubMed] [Google Scholar]
- ————— Metabotropic glutamate receptor subtype agonists facilitate LTP within a distinct time window in the dentate gyrus in vivo. Neuroscience. 1996;74:723–731. doi: 10.1016/0306-4522(96)00162-5. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell K-H, Reymann KG. Subtype-specific involvement of metabotropic glutamate receptors in two forms of long-term potentiation in the dentate gyrus of freely moving rats. Neurosciences. 1998;86:709–721. doi: 10.1016/s0306-4522(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Weisskopf MG, Nicoll RA. MCPG antagonizes metabotropic glutamate receptors but not long-term potentiation in the hippocampus. Eur J Neurosci. 1994;6:1050–1054. doi: 10.1111/j.1460-9568.1994.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Morris RGM. (R,S)-α-methylphenylglycine (MCPG) fails to block long-term potentiation under urethane anaesthesia in vivo. Neuropharmacology. 1997;36:1339–1354. doi: 10.1016/s0028-3908(97)00129-9. [DOI] [PubMed] [Google Scholar]
- Matthies HJ. Learning and Memory. In: Dumont C, editor. Advances in pharmacology and therapeutics. 5, neuropsycho-pharmacology. Oxford, UK: Pergamon Press; 1978. pp. 117–135. [Google Scholar]
- McGuinness N, Anwyl R, Rowan M. TransACPD enhances long-term potentiation in the hippocampus. Eur J Pharmacol. 1991;19:231–232. doi: 10.1016/0014-2999(91)90529-y. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: Synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Nielsen KS, Macphail EM, Riedel G. Class I mGlu receptor antagonist 1-aminoindan-1,5-dicarboxylic acid blocks contextual but not cue conditioning in rats. Eur J Pharmacol. 1997;326:105–108. doi: 10.1016/s0014-2999(97)85402-7. [DOI] [PubMed] [Google Scholar]
- O’Leary DM, O’Connor JJ. Potentiation of synaptic transmission in the rat dentate gyrus in vitro by (S)-3,5-dihydroxyphenylglycine ((S)-DHPG) Neurosci Lett. 1997;229:29–32. doi: 10.1016/s0304-3940(97)00404-7. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker FH, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Otani D, Ben-Ari Y. Metabotropic receptor mediated long-term potentiation in rat hippocampal slices. Eur J Pharmacol. 1991;205:325–326. doi: 10.1016/0014-2999(91)90920-l. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Pettit HO, Lutz D, Gutierrez C, Eveleth D. I.c.v. infusions of ACPD(1S,3R) attenuate learning in a Morris water maze paradigm. Neurosci Lett. 1994;178:43–46. doi: 10.1016/0304-3940(94)90285-2. [DOI] [PubMed] [Google Scholar]
- Rickard NS, Ng KT. Blockade of metabotropic glutamate receptors prevents long-term memory consolidation. Brain Res Bull. 1995;36:355–359. doi: 10.1016/0361-9230(94)00222-m. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Errington ML, Maegawa H, Bliss TV. Activation of metabotropic glutamate receptors is necessary for long-term potentiation in the dentate gyrus and for spatial learning. Neuropharmacology. 1994;33:853–857. doi: 10.1016/0028-3908(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Riedel G, Seidenbecher T, Reymann KG. LTP in hippocampal CA1 of urethane-narcotized rats requires stronger tetanization parameters. Physiol Behav. 1994a;55:1141–1146. doi: 10.1016/0031-9384(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Riedel G, Wetzel W, Reymann KG. Computer-assisted shock-reinforced Y-maze training: A method for studying spatial alternation behaviour. Neuroreport. 1994b;5:2061–2064. doi: 10.1097/00001756-199410270-00018. [DOI] [PubMed] [Google Scholar]
- Riedel G, Casabona G, Reymann KG. Inhibition of long-term potentiation in the dentate gyrus of freely moving rats by the metabotropic glutamate receptor antagonist MCPG. J Neurosci. 1995a;15:87–98. doi: 10.1523/JNEUROSCI.15-01-00087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G, Wetzel W, Reymann KG. Metabotropic glutamate receptors in spatial an nonspatial learning in rats studied by means of agonist and antagonist application. Learn & Memory. 1995b;2:243–265. doi: 10.1101/lm.2.5.243. [DOI] [PubMed] [Google Scholar]
- Riedel G, Wetzel W, Reymann KG. Comparing the role of metabotropic glutamate receptors in long-term potentiation and in learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:761–789. doi: 10.1016/0278-5846(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Sekiyama N, Hayashi Y, Nakanishi S, Jane DE, Tse H-W, Birse EF, Watkins JC. Structure-activity relationships of new agonists and antagonists of different metabotropic glutamate receptor subtypes. Br J Pharmacol. 1996;117:1493–1503. doi: 10.1111/j.1476-5381.1996.tb15312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig DK, Lee HK, Bear MF, Malenka RC. Re-examination of the effects of MCPG on hippocampal LTP, LTD and depotentiation. J Neurophysiol. 1995;74:1075–1082. doi: 10.1152/jn.1995.74.3.1075. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Häusser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Denk W, Kleinfeld D, Tank DW. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature. 1997;385:161–165. doi: 10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, O’Dell TJ. The molecular switch hypothesis fails to explain the inconsistent effects of the metabotropic glutamate receptor antagonist MCPG on long-term potentiation. Brain Res. 1995;695:45–52. doi: 10.1016/0006-8993(95)00757-h. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Yasuda H. A switching role of postsynaptic calcium in the induction of long-term potentiation or long-term depression in visual cortex. Semin Neurosci. 1996;8:311–319. doi: 10.1016/0168-0102(95)01001-7. [DOI] [PubMed] [Google Scholar]
- Wetzel W, Getsova VM, Jork R, Matthies H. Effect of serotonin on Y-maze retention and hippocampal protein synthesis in rats. Pharmacol Biochem Behav. 1980;12:319–322. doi: 10.1016/0091-3057(80)90378-0. [DOI] [PubMed] [Google Scholar]
- Wetzel W, Wagner T, Reymann KG, Riedel G. Open field behaviour in rats: Role of metabotropic glutamate receptors. Neuroreport. 1995;6:2389–2393. doi: 10.1097/00001756-199511270-00027. [DOI] [PubMed] [Google Scholar]
- Wilsch V, Behnisch T, Jäger T, Reymann KG, Balschun D. When are class I metabotropic glutamate receptors necessary for long-term potentiation? J Neurosci. 1998;18:6071–6080. doi: 10.1523/JNEUROSCI.18-16-06071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]