Abstract
Globally inhibiting CaM kinase activity in Drosophila, using a variety of genetic techniques, disrupts associative memory yet leaves visual and chemosensory perception intact. These studies implicate CaM kinase in the plastic processes underlying learning and memory but do not identify the neural circuitry that specifies the behavior. In this study, we use the GAL4/UAS binary expression system to define areas of the brain that require CaM kinase for modulation of courtship conditioning. The CaM kinase-dependent neurons that determine the response to the mated female during conditioning and those involved in formation and expression of memory were found to be located in distinct areas of the brain. This supports the idea that courtship conditioning results in two independent behavioral modifications: a decrement in courtship during the conditioning period and an associative memory of conditioning. This study has allowed us for the first time to genetically determine the circuit of information flow for a memory process in Drosophila. The map we have generated dissects the behavior into multiple components and will provide tools that allow both molecular and electrophysiological access to this circuit.
Introduction
In the courtship-conditioning assay male flies are programmed to reduce courtship toward a previously mated female (Siegel and Hall 1979). Several studies have demonstrated that a learned association takes place for the test males in this assay. The association is based on temporal pairing of the male’s courtship response to the attractive pheromones produced by all females with the aversive pheromonal cues the mated trainer female produces as the test male courts (Tompkins et al. 1983; Ackerman and Siegel 1986). Although chemosensory cues are required for this association to take place, other sensory stimuli can affect the quality of the association (Joiner and Griffith 1997). Sensing of the aversive stimulus has been localized to the basiconica-like sensilla in the maxillary palps (Stocker and Gendre 1989). The formation of memory for this association can be tested by placing the test male with a virgin female that emits only the courtship-stimulating pheromone. A test male with intact memory will display little courtship toward this female. Test males retain normal activity levels and will court young male flies that have attractive pheromone(s) different from those of fertilized and virgin females (Tompkins et al. 1983; Gailey et al. 1991). Memory of the association lasts for a few hours after the conditioning period (Siegel and Hall 1979). The experience-dependent courtship response has been established as a reliable assay for evaluating the effects of learning and memory mutations (Tompkins 1984). Mosaic studies suggest that this association and male courtship behavior is controlled by neurons in the dorsal posterior part of the brain (Hall 1979; Siegel et al. 1984).
Historically, the circuitry of behaviors mediated by central neurons has been less well defined in adult Drosophila than in larger insects in which electrophysiology has defined connectivity. Anatomical circuits contributing to learning in bees are understood at the cellular level (Hammer 1997). The relative lack of electrophysiological access to the intact nervous system has made mapping of behavioral circuits involved in learning and memory in Drosophila very difficult. With the advent of genetic tools for cell-specific expression of transgenes (Brand and Dormand 1995), these technical barriers can be overcome. The fly brain is organized into major structures as defined by groups of neuropil regions that are encapsulated by a thin glial lamella (Miller 1950; Hanesch et al. 1989), fibrous pathways, and other poorly delimited glomeruli (Power 1943). These structures express distinct sets of genes and therefore can also be defined by molecular means (Prokop and Technau 1993; Strauss and Heisenberg 1993; Tejedor et al. 1995). Subcompartments of the major structures have been further defined by enhancer-trap expression patterns (Yang et al. 1995; Han et al. 1996; Crittenden et al. 1998) and antibody labeling (Nighorn et al. 1991; Buchner et al. 1993).
Studies contributing to our understanding of the anatomical basis of behavior in flies have usually involved just one neuropil region (Han et al. 1992; Bouhouche et al. 1993; Skoulakis et al. 1993; de Belle and Heisenberg 1994; Connolly et al. 1996; Davis and Han 1996). Using a constitutively active Gαs, Connolly et al. (1996) found that the mushroom bodies but not the fan-shaped body or the ellipsoid body of the central complex are required for Gαs-dependent learning. Chemically ablating the mushroom bodies abolishes olfactory-mediated associative learning (de Belle and Heisenberg 1994). A similar ablation study shows that the mushroom bodies are required for the conditioned response to immature males (Neckameyer 1998). Data from structural mutants have also contributed to the study of the function of neuronal structures. Mutations disrupting the mushroom bodies or the ellipsoid body, fan-shaped body, or the protocerebral bridge of the central complex have implicated these individual structures in learning (Heisenberg 1980; Heisenberg et al. 1985; Bouhouche et al. 1993; Bouhouche and Choulli 1994). None of these studies attempt to define a complete circuit for a behavior.
Studies on the biochemical basis of behavior in flies have implicated a large number of individual molecules and signal transduction pathways (Dubnau and Tully 1998). Most of these findings involve mutations or transgenes that affect the entire nervous system. One such molecule, calcium/calmodulin-dependent protein kinase II (CaM kinase), has been shown to be required for learning and memory of courtship conditioning both through the use of dominant transgenes and mutations (Griffith et al. 1993; Joiner and Griffith 1997). This molecule has also been shown to be important in plasticity in a variety of other organisms including mammals (Kelly 1991). The circuitry responsible for courtship conditioning and the cells in the circuit that require CaM kinase were unknown.
The present study was undertaken without any assumptions about the number of different areas or the actual neurons that may be required for learning and memory in courtship conditioning. To define the full circuit, we used the GAL4/UAS system to inhibit CaM kinase in a number of areas of the fly brain and examined the effects of this manipulation on learning and memory. CaM kinase is inhibited by expressing the ala-inhibitor peptide (Griffith et al. 1993, 1994) under control of the yeast transcription factor GAL4 (Fischer et al. 1988; Brand and Perrimon 1993). Combining these behavioral and anatomical data with the anatomical data of others relating to the connectivity within the adult central nervous system, we are able to propose a circuit for the information flow of the CaM kinase pathway in this associative learning task.
Materials and Methods
DROSOPHILA STRAINS
Fly cultures were kept at 25°C with a 12-hr light/dark cycle on autoclaved cornmeal, yeast, sucrose, and agar food. The genetic background used for the behavior experiments was either from Canton-S or the line w1118(isoCJ1) a w, Canton-S isogenic stock (Yin et al. 1994). In this text, w refers to this allele. Unless otherwise stated, genotypes are described in Lindsley and Zimm (1992). The GAL4/UAS system is described by Brand and Perrimon (1993), the UAS line used in this study has the upstream activator sequences linked to the ala-inhibitor peptide. The ala-inhibitor peptide is a synthetic peptide based on the sequence of the rat αCaM kinase II autoregulatory domain (Griffith et al. 1993). F1 males that were tested in the learning assay resulted from crossing virgin females, homozygous for the P[GAL4] insert, to males homozygous for the UAS–ala insert. F1 males that were sectioned and stained resulted from crossing virgin females, homozygous for the P[GAL4] insertion, to males homozygous for the P[UAS–lacZ] insert, which express cytoplasmic β-galactosidase or the w+; P[wMc = UAS–GFP S65T]T2 (Bloomington stock no. 1521) insert, which expresses cytoplasmic green fluorescent protein (GFP). New P[GAL4] insert lines were generated by crossing FM7a females with a P[GAL4] insert on the balancer chromosome to w; MKRS, Δ2-3/TM2, Δ2-3 males. FM7a,P[GAL4];MKRS, Δ2-3 or FM7a,P[GAL4];TM2, Δ2-3 virgin females were crossed individually to w males. w+, non-FM7a, nonmottled-eyed progeny, which represented new stable mobilizations of the P[GAL4] transposon, were singly crossed to w flies. The Y chromosome is not indicated for hemizygous males.
SECTIONING AND STAINING
P[GAL4];UAS–lacZ flies were put into a fly collar (Jäger and Fischbach 1987) such that their heads are aligned and facing the same way. Heads were frozen in Tissue Tek and 12-mm frontal cryostat sections were loaded on Superfrost Plus slides. After the tissue is dried onto the slides, it is fixed [1.5% (wt/vol) glutaraldehyde, 2.0% (wt/vol) formaldehyde, 38.0% (wt/vol) sucrose, 1.0% (wt/vol) CaCl2, 1.0% (wt/vol) gum arabic, 0.05 m cacodylic acid, adjusted to pH 7.3] for 10 min at room temperature, then washed twice in 1× PBS (0.02 m sodium phosphate, 0.15 m NaCl) for 10 min at room temperature. The slides are incubated 1 hr or overnight in the X-Gal staining solutions [8% X-Gal in DMSO diluted 1/30 in Fe/NaP buffer (pH 7.2): 1.8 ml of 0.2 m Na2HPO4, 0.7 ml of 0.2 m NaH2PO4, 1.5 ml of 5 m NaCl, 50 ml of 1 m MgCl2 3.05 ml of 50 mm K3[Fe(CN)6], 3.05 ml of 50 mm K4[Fe(CN)6], distilled water to 50 ml] at 37°C. After staining, the slides are rinsed twice for 10 min in distilled water and mounted (Crystal Mount, Biomeda Corporation and Permount, Fisher Scientific). Photographs were taken using Kodachrome T160 slide film using phase-contrast or Nomarski optics.
Confocal images were done either on a Bio-Rad Confocal with a BHS filter and collected with COMOS software or on a Leica Lasertechnik with an argon laser at 50% using Leica TSC-NT V1.5 software. Analyses were performed on a Macintosh computer using the public domain National Institutes of Health (NIH) image program (developed at the NIH and available on the internet at http://rsb.info.nih.gov/nih-image/). Adult brains were dissected out in 1× PBS and suspended in 1× PBS on a microscope slide under a coverslip supported by two coverslips on either side of the brain, then examined immediately.
BEHAVIOR ASSAYS
COURTSHIP-CONDITIONING ASSAY
Singly housed, 5-day-old test males were placed with 4-day-old females, mated the previous day, in single-pair-mating chambers (8 mm diam. × 3 mm high) for 1 hr. The first and last 10-min periods of this conditioning period were videotaped. Two to five minutes after conditioning, the males were paired in a clean mating chamber with anesthetized virgin females that had been collected that day. The pairs were videotaped for the 10-min test period. For each of the 10-min periods, a courtship index (CI) was measured for each male tested. The fraction of time a male spent courting (orienting towards the female and wing flapping are two observable behaviors) in a 10-min interval, constitutes the CI. The response to the mated female is calculated by dividing the final CI (CIfin) by the CI of the initial 10-min period (CIin). Because males used for the test period are not the same as those in the sham test, memory is measured by dividing the test CI (CIt) with the average (mean) sham test CI (mCIs). As a control, sham tests are done in which the males are kept alone in the mating chamber for the first hour, then paired with anesthetized virgin females for the 10-min test period. Females used in this assay were collected from a shits/Y/C(1)DX, y w f stock. When kept at 29°C, only C(1)DX, y w f females emerge from this stock. Experiments were performed at 25°C and 75% humidity. Unless otherwise indicated, all behavior experiments were done under red light (two lamps with 25-watt red photographic light bulbs were placed 20 cm from the mating wheel). For each genotype n is indicated and n ≥ 20 for all CIshams. UAS–ala/+ flies were shown to behave normally in the courtship conditioning assay (Joiner and Griffith 1997).
DISCRIMINATION TEST
Data from the courtship-conditioning assay is used for this test. Here we compare CIin with CIsham to determine whether GAL4/+; UAS–ala/+ males can distinguish between mated and virgin females, based on the pheromones each set of females produces (Siegel and Hall 1979; Tompkins et al. 1983; Gailey et al. 1986).
LOCOMOTOR ACTIVITY ASSAY
Spontaneous locomotor activity was measured by counting the number of times a fly crosses a line drawn across an 8-mm-diam. circular chamber in a 4-min period.
STATISTICS
Data for the courtship-conditioning assay were analyzed with Wilcoxon’s signed-rank test using Statview software version 4.5 for the Macintosh. P values and n for each experiment are presented in Table 1. Data are presented as means with levels of significance indicated by P values: (*) <0.05; (**) <0.005; and (***) <0.0005.
Table 1.
P values for Wilcoxon’s nonparametric rank sums tests
|
P[GAL4] line
|
Statistical data for the courtship conditioning assay
|
|
|---|---|---|
| mated female effect (n for GAL4/+, n for GAL4/+; UAS–ala/+)
|
memory of mated female* (n for shams of GAL4/+, GAL4/+; UAS–ala/+)
|
|
| Mushroom body P[GAL4] lines | ||
| 29BD | 0.3942 (21, 20) | 0.0137 (20, 22) |
| 201Y | 0.0299 (22, 33) | 0.0208 (26, 29) |
| 30Y | 0.5862 (23, 28) | 0.0013 (32, 21) |
| c309 | 0.9721 (20, 23) | 0.0100 (20, 24) |
| c739 | 0.4328 (24, 21) | 0.1119 (30, 24) |
| Central complex P[GAL4] lines | ||
| c232 | 0.3139 (24, 24) | 0.9826 (25, 21) |
| MJ126a | 0.7971 (27, 26) | <0.0001 (20, 22) |
| OK348 | 0.6378 (20, 23) | 0.0479 (25, 24) |
| J183 | 0.7174 (21, 20) | 0.0019 (26, 34) |
| Lateral protocerebrum P[GAL4] lines | ||
| MJ146 | 0.0200 (20, 25) | 0.1165 (25, 23) |
| MJ286c | 0.3824 (20, 20) | 0.0008 (22, 21) |
| MJ63 | 0.9594 (22, 21) | 0.0002 (24, 20) |
| Antennal lobe P[GAL4] lines | ||
| MJ94 | 0.0129 (31, 22) | 0.1240 (21, 20) |
| GH86 | 0.7782 (24, 30) | 0.9353 (30, 31) |
| Mushroom body and antennal lobe P[GAL4] lines | ||
| MJ250 | 0.6949 (20, 26) | 0.0003 (23, 20) |
| MJ270 | 0.0057 (20, 23**) | 0.0019 (23, 22) |
| MJ162a | 0.0052 (21, 23) | 0.0836 (23, 21) |
| 40B | 0.3458 (24, 20) | 0.3037 (20, 24) |
| Statistical data for the discrimination test | |
|---|---|
| discrimination (n for mated female, n for virgin female) | |
| 201Y | 0.0013 (33, 29) |
| MJ146 | 0.0045 (25, 23) |
| MJ94 | 0.2111 (22, 20) |
| MJ270 | 0.2989 (23, 22) |
| MJ162a | 0.0015 (23, 21) |
(*) n values for the memory test are the same as for the test of the mated female effect.
(**) One of the test males was lost during the transfer; therefore, n = 22 for the memory test.
Results
COURTSHIP CONDITIONING IN DROSOPHILA
The behavioral paradigm of courtship conditioning (Siegel and Hall 1979; for review, see Hall 1994) has been shown to have characteristics of associative learning (Tompkins et al. 1983). Mature male flies have an innate and vigorous response to virgin female flies. They perform a stereotyped courtship ritual, which culminates in copulation. When a male encounters a female who has mated already, he initially courts vigorously but soon becomes uninterested. This depression of courtship activity lasts several hours. During this time, even if presented with a virgin female, the male fly is unlikely to court.
The assay consists of two parts. First, we expose a test male to a mated female trainer for 1 hr. During the first and last 10 min of the hour, a CI (fraction of the 10-min spent in courtship activity) is measured. A normal male will show a decrement in courtship activity when CIin and CIfin are compared. This decrement is a measure of the change in behavior toward the mated female that occurs during the conditioning period and is expressed as a ratio of CIfin/CIin. For wild-type flies, this ratio is normally ≤0.5. If the line is defective in the conditioning response, the value will be >0.5 (Joiner and Griffith 1997). In all experiments, conditioning scores for P[GAL4]/+;UAS–ala/+ flies, which express ala in particular regions of the fly brain, were compared with conditioning scores for P[GAL4]/+ flies that do not express the ala-inhibitory peptide. When tested as hemizygotes or heterozygotes, each of the independent P[GAL4] lines we used in this study has a ratio of CIfin/CIin < 0.5.
During the second part of the assay, the test male is paired with a virgin female for 10 min. A courtship index is obtained for this period (CIt) and ratioed to the mean of that obtained from males who have been sham conditioned in a chamber with no mated female (mCIs). A normal male who has been conditioned will not court the virgin, whereas a sham-conditioned male will court vigorously. Normal males will show a CIt/mCs ≤ 0.5. If the line is defective for memory the value will be >0.5. In all experiments, P[GAL4]/+;UAS–ala results were compared with results for the same P[GAL4]/+ line that does not express the ala-inhibitory peptide. When tested as hemizygotes or heterozygotes, each of the independent P[GAL4] lines we used has a ratio of CIt/mCs < 0.5 with one exception. GH86 has a ratio for the memory assay >0.5, but this difference is not statistically significant when the data are compared to Canton-S (P > 0.6).
CaM KINASE ACTIVITY IS REQUIRED IN THE MUSHROOM BODIES FOR MEMORY BUT NOT INITIAL RESPONSE TO THE MATED FEMALE
When ala peptide was expressed in the mushroom bodies, driven by each of four P[GAL4] insertions (29BD, 30Y, c309, and c739), no disruption in the response to the mated female during conditioning was seen (P > 0.1; Fig. 1A; Table 1). In line 201Y, there was a significant disruption of this response (P < 0.5; Fig. 1A; Table 1). Memory was disrupted in four of the P[GAL4] lines when they express UAS–ala (201Y, 29BD, 30Y, c309; P ≤ 0.05; Fig. 1B; Table 1). Memory is not disrupted significantly in line c739 (P > 0.1; Fig. 1B). The patterns of expression for each of these five P[GAL4] inserts as transheterozygotes with UAS–lacZ show predominant expression in the mushroom bodies (Fig. 2). Confocal imaging using UAS–green fluorescent protein (UAS–GFP) was done on three of the lines (29BD, 201Y, and 30Y) and four of the lines (201Y, 30Y, c309, and c739) are also described in further detail elsewhere (Connolly et al. 1996; Yang et al. 1995; see http://flybrain.org). Confocal reconstruction shows that 29BD expresses in most of the mushroom body cells, specifically in the core of the α- and β-lobes and throughout the γ-lobes. Neurons connecting the β-lobes, cells in the dorsal–lateral subesophageal ganglion, and a group of cells in the medial–lateral protocerebrum show GFP expression (Fig. 2A,B). Mushroom body expression is much the same in 30Y as in 29BD, including the connections between the β-lobes, except that 30Y expresses more heavily throughout each of the lobes and in extrinsic cells surrounding the mushroom bodies (Fig. 2C). Line 201Y shows specific expression in the core of the α- and β-lobes and throughout the γ-lobes of the mushroom bodies and, uniquely, several cells of the lateral protocerebrum and subesophageal ganglion (Fig. 2D; Yang et al. 1995). c309 shows expression throughout the mushroom bodies; confocal reconstruction describes weak expression in the core of the α- and β-lobes (Yang et al. 1995; Connolly et al. 1996) and light staining throughout the central nervous system (Fig. 2E). c739 limits expression to the α- and β-lobes and faint staining in the antennal glomeruli and lateral protocerebrum (Fig. 2F). These lines suggest that the entire mushroom body may contribute to normal memory formation, although the γ-lobe may be most important. When ala is expressed extensively throughout the mushroom bodies or primarily in the γ-lobe, we see more severe memory defects than when ala is expressed primarily in the α- and β-lobes (Fig. 1; c739).
Figure 1.
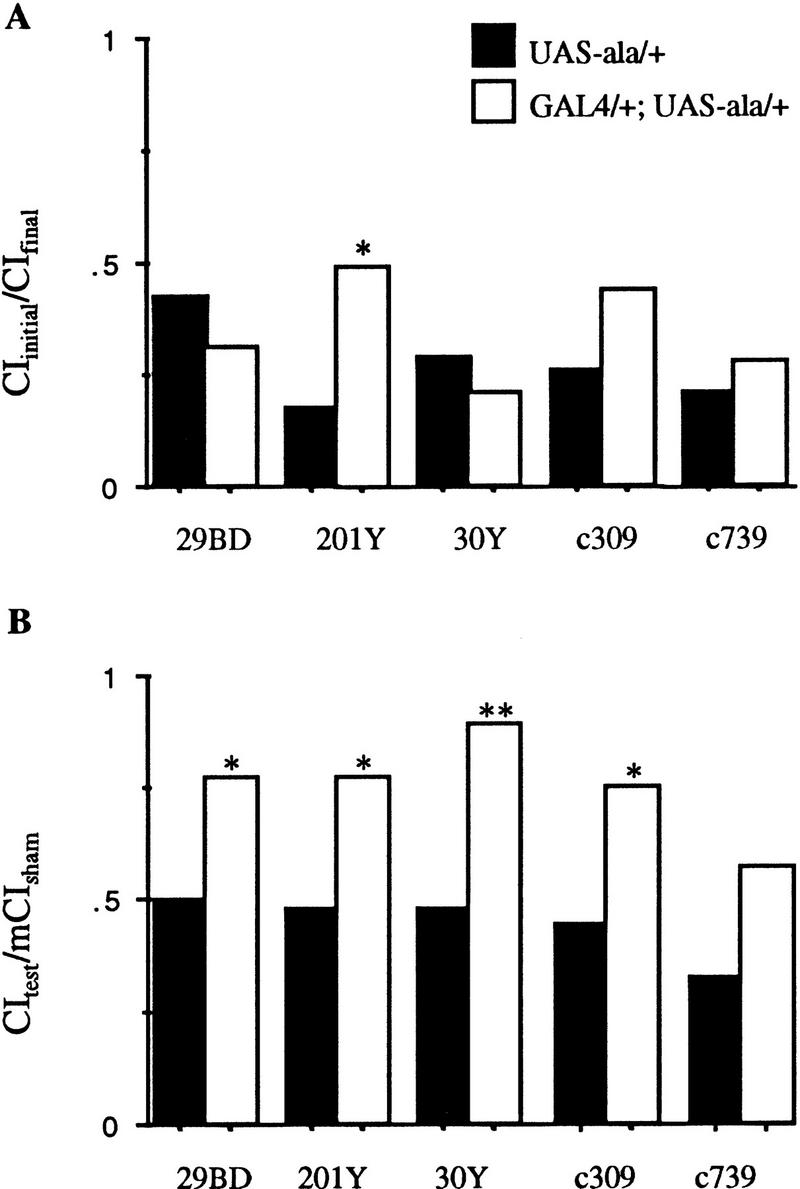
Response to the mated female during conditioning, and memory for flies with CaM kinase activity reduced primarily in the mushroom bodies: Males for the GAL4 lines indicated were trained by exposure to a female for 1 hr then placed with an anesthetized virgin female. For sham training, flies of the same genotype were manipulated identically, except that no female was present in the chamber during training. All manipulations were performed at 25°C, 75% humidity in dim red light. (A) Conditioning response was measured by calculating a courtship index (CI) for the initial (CIi) and the CI final (CIf) 10 min of the conditioning period. Data are expressed as the ratio of means, CIf/CII ± s.e.m. Values >0.5 indicate defects in response to the female during the conditioning period, with a failure to decrease courtship during the training period. (B) Memory was tested by measuring the CI for the initial 10 min of exposure to an anesthetized virgin female after training with a mated female (CIt) or after sham training (CIs). Data are expressed as the ratio of CIt/mean CIs. Solid bars represent data for P[GAL4] hemizygotes or heterozygotes; open bars represent data for P[GAL4] transheterozygous with UAS–ala. Statistical significance was assessed by Wilcoxon’s signed-rank test. Data from GAL4/+;UAS–ala/+ males were compared to data from control males of the genotype GAL4/+ for each line. (*) Significantly different from the genotype control with level of significance as indicated: (*) P < 0.05, (**) P < 0.005, and (***) P < 0.0005. P values and n for each group are shown in Table 1.
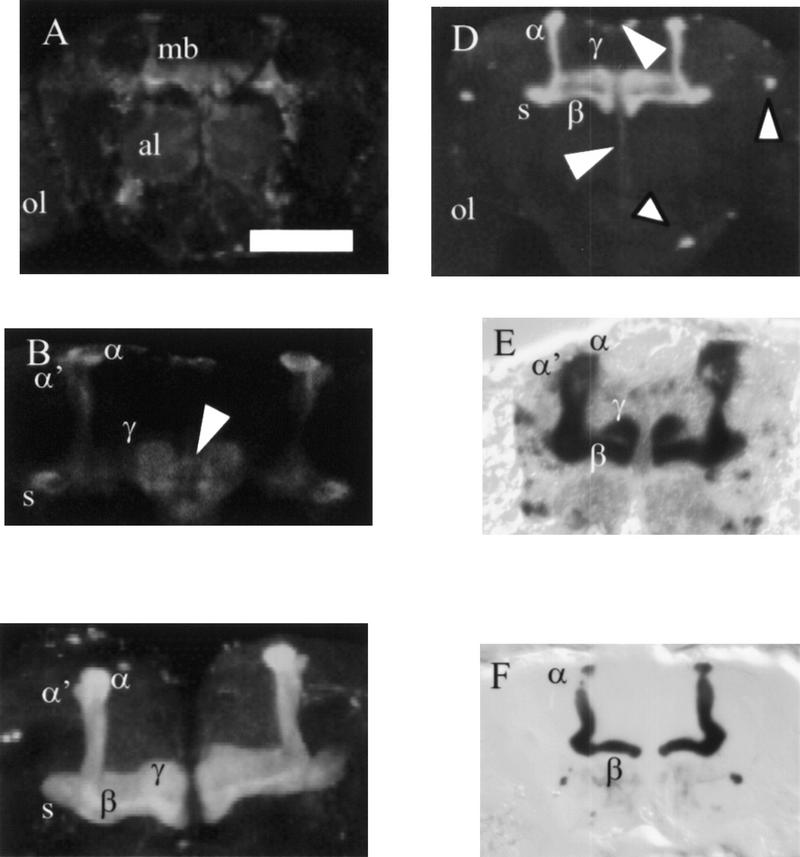
Figure 2.
Adult brain expression patterns of mushroom body P[GAL4] lines. (A–D) Confocal reconstruction of P[GAL4] transheterozygous with UAS–GFP. (E,F) β-Gal staining of P[GAL4] transheterozygous with UAS–lacZ. Dorsal is up in all images. (A) 20 × 3.06-μm sections of 29BD show expression in all the mushroom body (mb) lobes. Scattered cells show GAL4 expression in the optic lobes (ol) and lateral protocerebrum (lp) and light general staining in the antennal lobes (al). (B) 3 × 3.06-μm sections of mushroom bodies of 29BD show staining is restricted to the core of the lobes indicated. Arrow indicates the spherical connecting neurons between the γ-lobes. (C) 10 × 2.34-μm sections of 30Y indicate a high level of expression throughout all the mushroom body lobes with individual neurons surrounding the mushroom bodies. Connecting neurons between the γ-lobes express GAL4 in this line are not shown here. (D) 9 ×3.60-μm sections of 201Y show expression in the α-, β-, and γ-lobes and spur (s). (Top large arrowhead) Cell bodies of pars intercerebralis (pi); (bottom large arrowhead) axons of the pars intercerebralis forming the median bundle. Small arrowheads point to large neural cell bodies. (E) 12-μm frontal cryostat section of c309. β-Gal staining throughout mushroom body lobes and spur (not shown) with general light staining throughout the central CNS. (F) c739 frontal cryostat section (12 μm). β-Gal staining restricted to the α- and β-lobes of the mushroom bodies with some antennal lobe staining. Scale bar, 50 μm for A and D; 20 μm for B and C; 30 μm for E and F.
SELECTED AREAS OF THE CENTRAL COMPLEX REQUIRE CaM KINASE FOR MEMORY, BUT NOT CONDITIONING
When CaM kinase activity is disrupted in selected areas of the central complex by each of four P[GAL4] insertions (c232, MJ126a, OK348, and J183), the conditioning phase of the courtship-conditioning assay is not affected (P > 0.3 for all four lines when each is compared to the results for animals with the P[GAL4] insert alone; Fig. 3A; Table 1). Memory, however, is disrupted in three of these lines when ala is expressed (MJ126a, OK348, and J183; P < 0.05; Fig. 3B; Table 1). Expression of ala under control of c232 does not affect memory (P > 0.9; Fig. 3B; Table 1). Predominant expression in each of these lines occurs in either the ellipsoid body or the fan-shaped body (Fig. 4). c232 expression is isolated to the ellipsoid body (Fig. 4B). MJ126a expresses in the ellipsoid body, pars intercerebralis, and a few lateral cells (Fig. 4A). When rotated, both the pars intercerebralis and ellipsoid body appear bistratified (data not shown). Multiple strata are not seen in the confocal reconstruction of line c232, which may indicate that it represents an unstratified element of the ellipsoid body that is not required for learning or memory (Connolly 1996; see http://flybrain.org; Fig. 3B). Another possibility is that the number of neurons expressing ala in this line is insufficient to cause a memory deficit. OK348 strongly expresses in the fan-shaped body and a few scattered cells in the lateral protocerebrum (Fig. 4C). J183 expresses in a subset of the fan-shaped body, possibly in the superior arch (Hanesch et al. 1989; Fig. 4D–G). P[GAL4] lines that express in the central complex tend to be limited to the subparts of this structure, with little staining elsewhere in the brain, compared to mushroom body-expressing lines that show less specificity for the mushroom bodies. Unlike the mushroom bodies, not all of the central complex appears to be used for memory; only subparts of the ellipsoid body and fan-shaped body are required.
Figure 3.
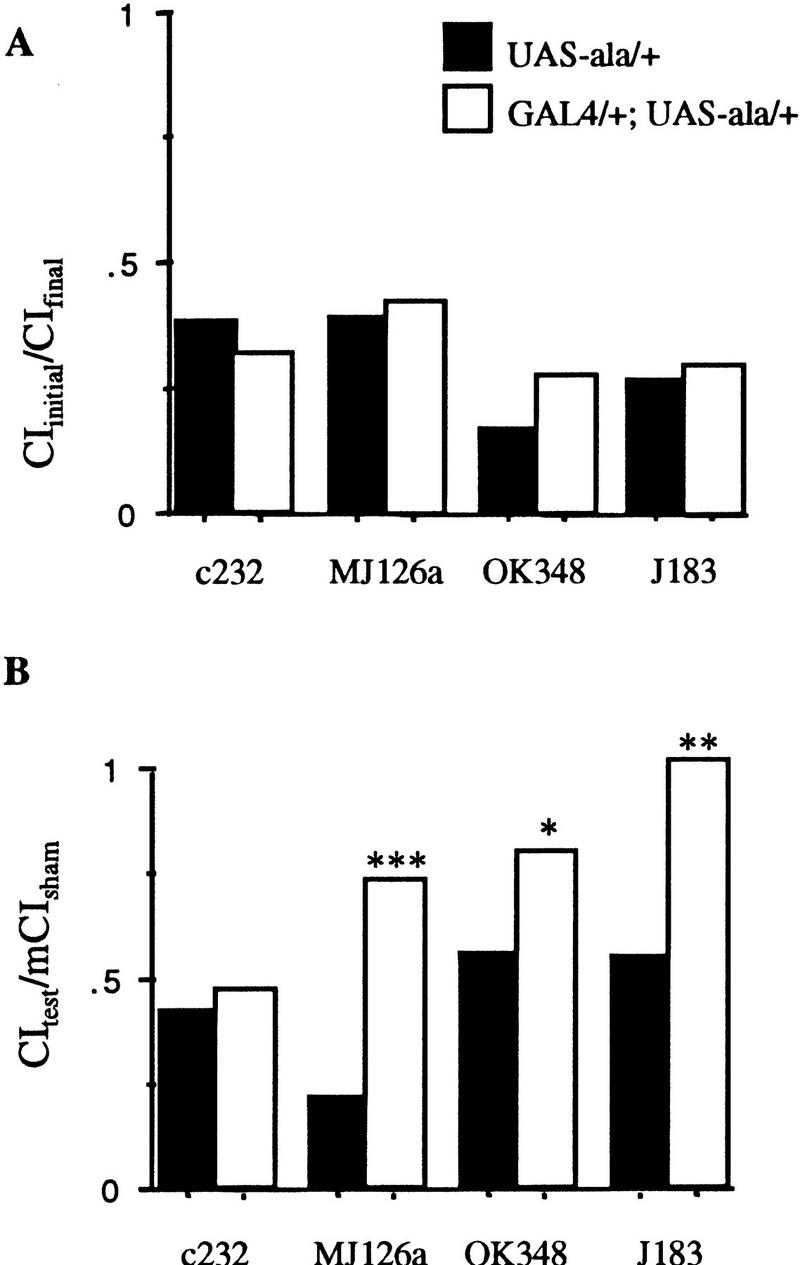
Response to the mated female during conditioning, and memory for flies with CaM kinase activity primarily reduced in the central complex. Solid bars represent data for P[GAL4] hemizygotes or heterozygotes; open bars represent data for P[GAL4] transheterozygous with UAS–ala. (See Fig. 1 legend for details.)
Figure 4.
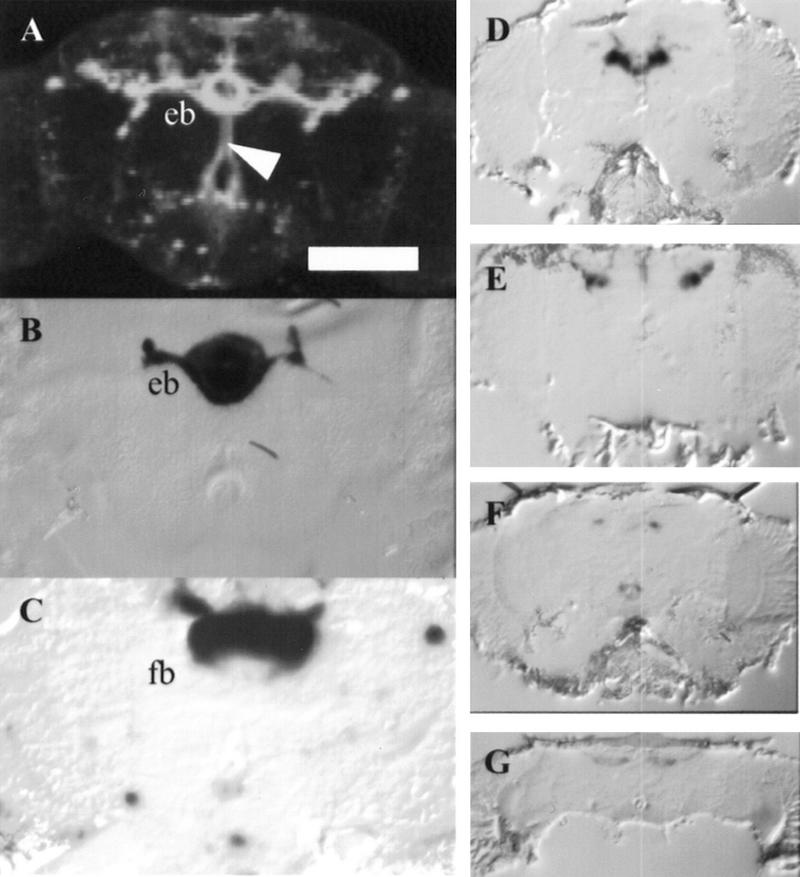
Adult brain expression patterns of central complex P[GAL4] lines. (A) Confocal reconstruction of P[GAL4] transheterozygous with UAS–GFP. (B–G) β-Gal staining of P[GAL4] lines transheterozygous with UAS–lacZ. Dorsal is up for all images. (A) 25 × 3.06-μm sections of MJ126a show expression in ring neurons of the ellipsoid body (eb), medial bundle (arrow), and some scattered cells in the optic lobe. (B) c232 frontal cryostat section (12 μm) shows predominant β-Gal staining in the ellipsoid body. (C) OK348 frontal cryostat section (12 μm) shows predominant β-Gal staining in the fan-shaped body (fb). (D–G) Four anterior to posterior, 12 μm frontal cryostat sections of J183 showing predominant β-Gal staining in the central brain. Scale bar, 50 μm for A; 30 μm for B and C, 100 μm for D–G.
EITHER CONDITIONING OR MEMORY IS DISRUPTED WHEN CaM KINASE ACTIVITY IN THE LATERAL PROTOCEREBRUM IS DECREASED
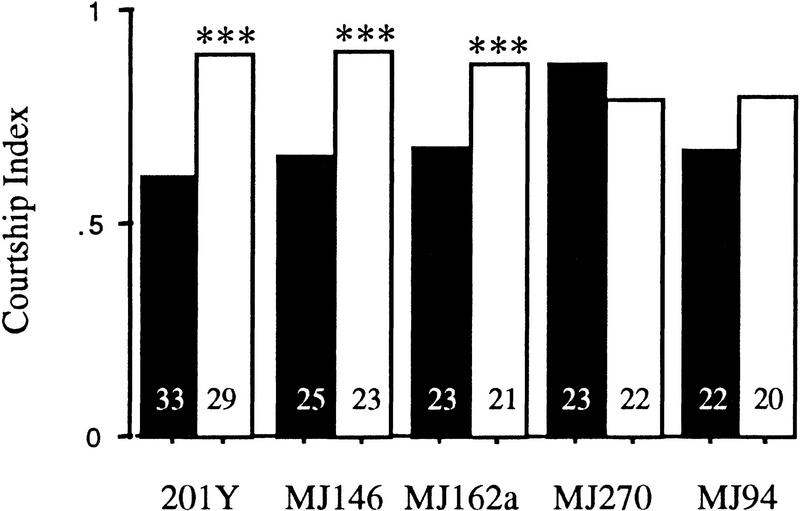
Memory is disrupted severely when CaM kinase activity is reduced by two different inserts expressing ala in the lateral protocerebrum (MJ286c and MJ63, P < 0.005; Fig. 5B; Table 1). In only one line was the response to the mated female during the conditioning period affected (MJ146, P < 0.05; Fig. 5A). This line has no statistically significant memory phenotype (P > 0.1; Fig. 5B).
Figure 5.
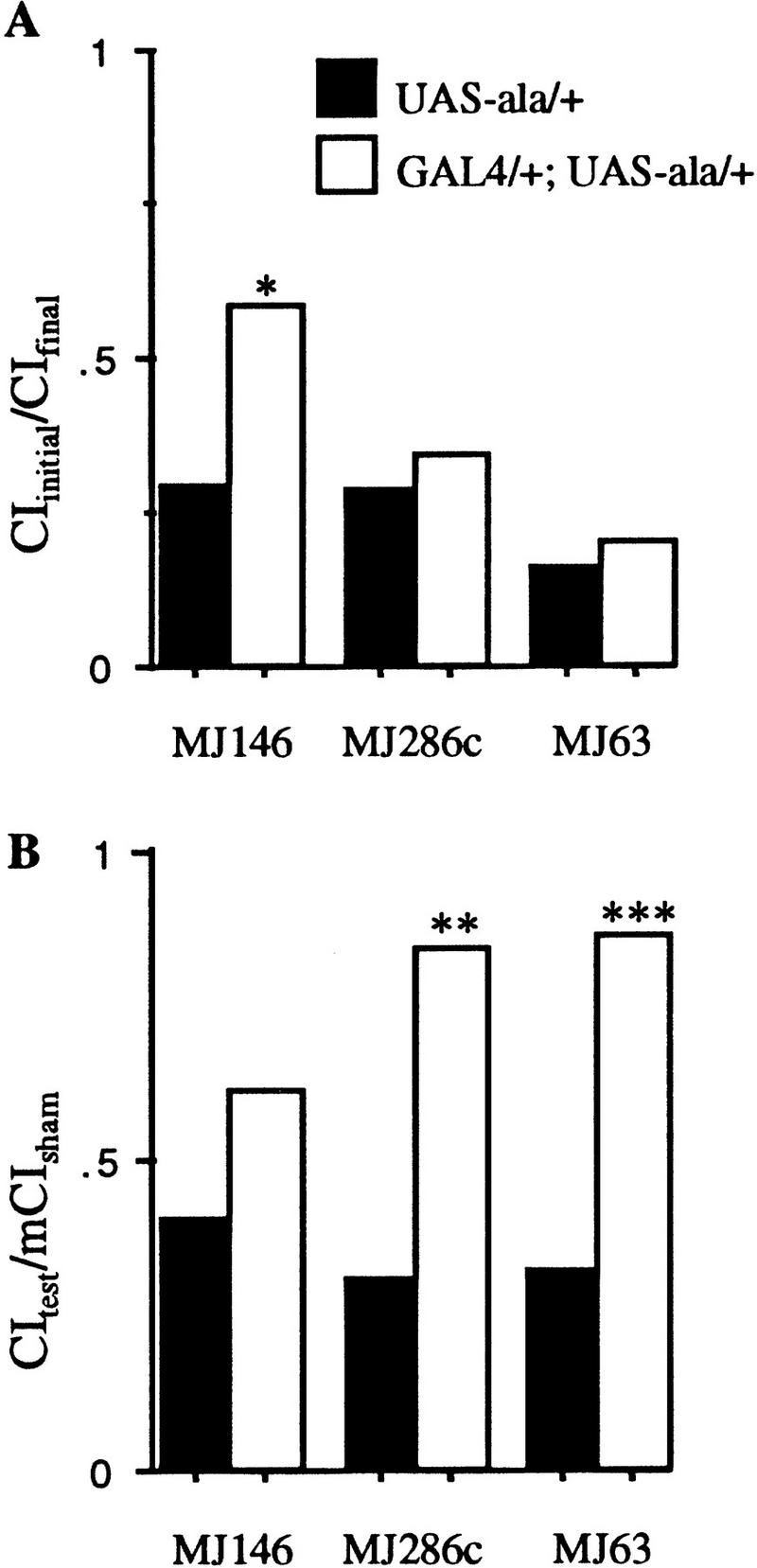
Response to the mated female during conditioning, and memory for flies with CaM kinase activity primarily reduced in the lateral protocerebrum. Solid bars represent data for P[GAL4] hemizygotes or heterozygotes; open bars represent data for P[GAL4] transheterozygous with UAS–ala. (See Fig. 1 legend for details.)
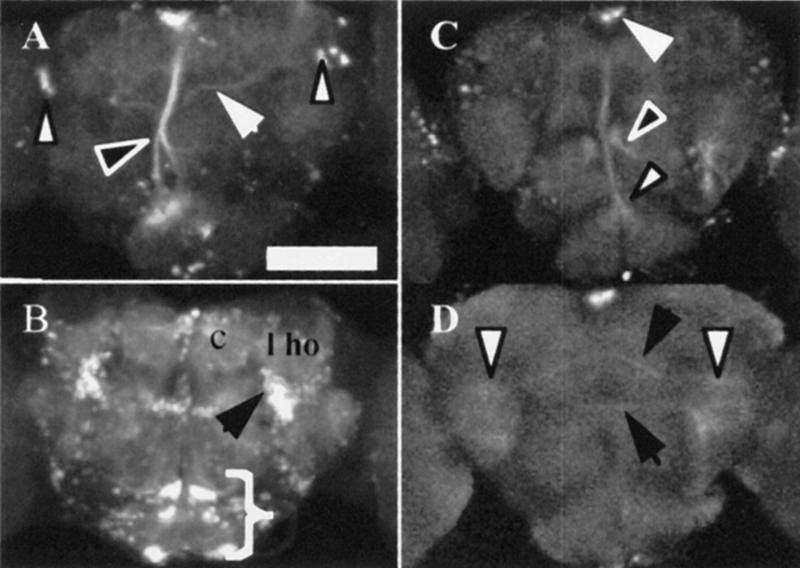
MJ146 expression is limited to the pars intercerebralis, a small subset of α- and β-lobe mushroom body neurons, a group of about five cells in the lateral protocerebrum and a few cells in the antennal lobes (Fig. 6A). Dendritic trees of the median bundle can be observed around the pars intercerebralis, within the antennal lobes and in the dorsal subesophageal ganglion. The expression pattern of the median bundle for this line therefore consists of both ascending and descending axons. When rotated, the median bundle appears bistratified (data not shown). MJ286c expresses in neurons ventromedial to the lateral horn, commissures that link the two lateral protocerebra, commissures in the anterior region, and the commissure that links the two antennal lobes (Fig. 6B). MJ63 expresses in a select group of cells including some of the pars intercerebralis and two connected lateral groups of cells (Fig. 6C,D). This P[GAL4] insert expresses in very few cells in the adult brain and yet had a significant effect on memory when transheterozygous with UAS–ala.
Figure 6.
Adult brain expression patterns of lateral protocerebral P[GAL4] lines. (A–D) Confocal reconstruction of P[GAL4] transheterozygous with UAS–GFP. Dorsal is up in all images. (A) 30 × 3.24-μm sections of MJ146 show expression in a set of paired lateral cells (white arrows), cell bodies of the median bundle and processes branching at two points (black arrowheads) and a narrow core of α- and β-lobes of the mushroom bodies (large white arrow). (B) 25 × 3.06-μm sections of MJ286 show prominent expression ventral (small white arrowhead) to the lateral horn (l ho) and calyx (c), and in three paired sets of cells in the anterior brain (bracket) extending processes laterally. (C,D) 6 × 2.7-μm sections of MJ63 C are posterior to D. (C) Expression in the median bundle cell bodies (arrow), terminating in the ventral brain (white arrowhead) and extrinsic projections to the median bundle (black arrowhead). D shows expression in two commissures that traverse the central brain (black arrows) and terminate in the lateral protocerebrum (small white arrowheads). Scale bar = 50 μm.
Two P[GAL4] lines (MJ286c and MJ63) with expression in different subsets of lateral protocerebrum cells both have some effect on memory. The median bundle contains descending axons that project from cell bodies of the pars intercerebralis to the subesophageal ganglion. The pars intercerebralis and median bundle are labeled in two other lines that have no memory defect (40B and MJ162a). Interestingly, 11 of the 13 P[GAL4] inserts with strong memory defects show expression in this group of neurons (OK348 and J183 do not express in these cells) and therefore this commissure cannot be ruled out as having a role in information processing for memory.
INHIBITING CaM KINASE IN THE ANTENNAL LOBES AFFECTS CONDITIONING
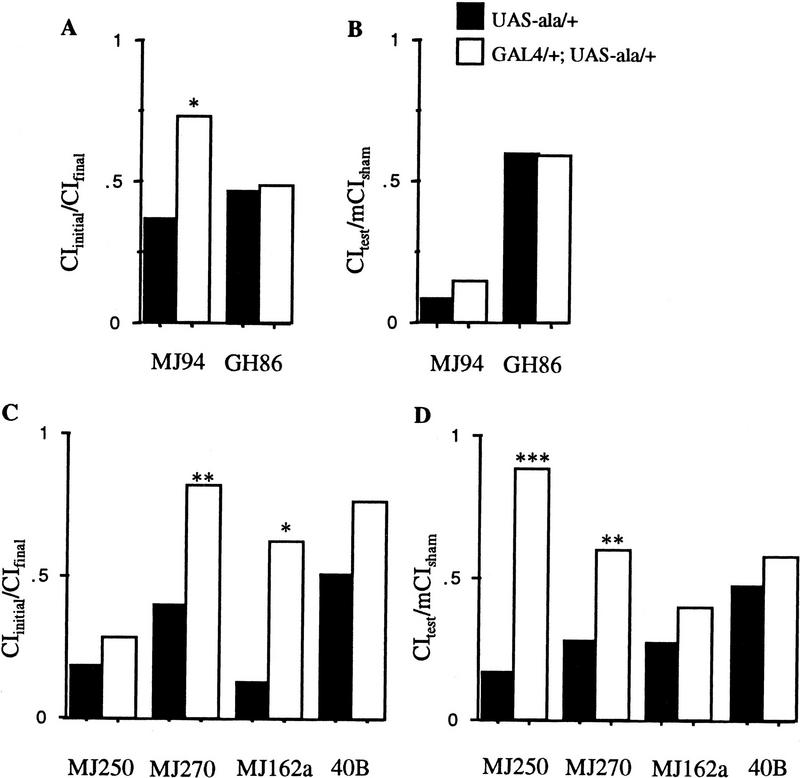
In two lines tested, expression of ala is restricted to the antennal lobes (MJ94 and GH86). Conditioning is affected (P < 0.05; Fig. 7A; Table 1) but not memory (P > 0.1) for MJ94;UAS–ala/+ animals. Both conditioning and memory are intact for GH86;UAS–ala/+ animals (P > 0.7; Fig. 7A,B; Table 1). The expression pattern for these two P[GAL4] inserts is very similar; both stain fairly specifically in antennal lobe neurons of the central nervous system (Fig. 8; Heimbeck et al. 1999). The expression pattern of the two inserts may overlap in some neurons of the antennal lobes, but it appears that they express in a different set of antennal glomeruli (Fig. 8, cf. A and B). The antennal nerve is composed of two types of axons and projects dorsally after entering the head from the anteriorally located antenna. One type of axon that carries chemosensory information arborizes and projects dorsally to the antennal lobes in the front of the head. The second set of motor and mechanosensory neurons continues ventrolaterally forming a loose set of glomeruli (Power 1946; Tissot et al. 1997). Axons carrying chemosensory cues project from sensilla basiconica on both the third antennal segment and the maxillary palps to three glomeruli of the antennal lobes (Singh and Nayak 1985). The maxillary palps express GFP in MJ94;UAS–GFP/+ flies, whereas GH86 does not express in these sensory structures (Heimbeck et al. 1999).
Figure 7.
Response to the mated female during conditioning, and memory for flies with CaM kinase activity primarily reduced in the antennal lobes. Solid bars represent data for P[GAL4] hemizygotes or heterozygotes; open bars represent data for P[GAL4] transheterozygous with UAS–ala. (A,C) Data for the response to the mated female; (B,D) data from the memory test. (See Fig. 1 legend for details.)
Figure 8.
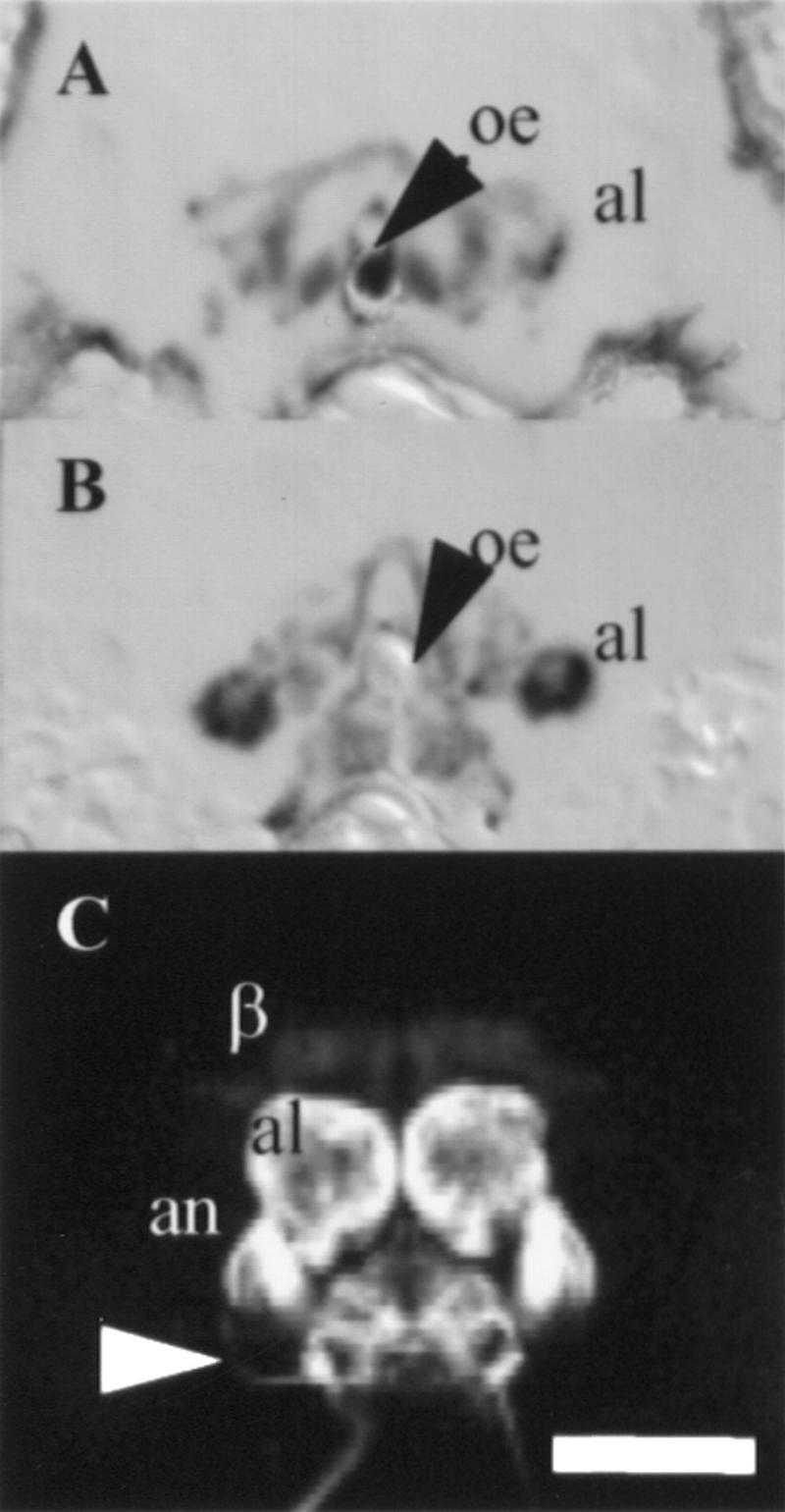
Adult brain expression patterns of antennal lobe P[GAL4] lines. (A,B) β-Gal staining of P[GAL4] lines transheterozygous with UAS–lacZ. (C) Confocal reconstruction P[GAL4] transheterozygous with UAS–GFP. Dorsal is up in all images. (A) Frontal cryostat section (12 μm) of GH86 shows predominant β-Gal staining in parts of the antennal lobe (al). (B) Frontal cryostat section (12 μm) of MJ94, at approximately the same level as A, shows predominant β-Gal staining in parts of the antennal lobe, different from GH86 staining. (C) Sections of MJ94 (10 × 2.23 μm) show β-Gal expression in the antennal lobe, antennal nerve (an) and sensory projections from the subesophageal ganglion (white arrowhead). Light staining is seen in the β-lobes in this view. Scale bar, 50 μm. (oe) Esophagus.
In MJ94 we observed a wider array of neurons expressing the reporter gene when UAS–GFP was used rather than UAS–lacZ. For instance, with GFP expression a faint set of mushroom body neurons were observed that was not seen with lacZ as the reporter, even after overnight staining of the head sections in the β-Gal buffer (Fig. 8B,C). GFP may be better able to mark neurons with minimal GAL4 expression. This result has two implications. First, as the low level of mushroom body expression in MJ94 was not sufficient to cause memory defects, this suggests that there are dosage thresholds for the actions of the ala peptide. Second, the lacZ patterns should be taken as minimal expression patterns, as the detection of GFP appears to be more sensitive in some cases. It is worth noting, however, that no changes in expression pattern were observed for 11 other lines (201Y, 29BD, 30Y, 40B, MJ126a, MJ146, MJ162a, MJ250, MJ270, MJ286c, and MJ63) in which both lacZ and GFP expression were used to describe the P[GAL4] lines. lacZ expression patterns of 7 of the 18 lines used in this study (201Y, 30Y, c309, c232, c739, GH86, and OK348) have also been published previously (Yang et al. 1995; Connolly et al. 1996). In general, the lacZ pattern is likely to give the regions of highest expression for a particular GAL4 line. For the lines with identical lacZ and GFP patterns there may simply not be a population of cells with very low GAL4 expression. The properties of the protein expressed under GAL4 control are therefore relevant to the final expression pattern of that protein. The ala peptide is very unstable in vivo (Griffith et al. 1993) and levels of peptide sufficient to inhibit CaM kinase are likely only be attained in the cells expressing the highest levels of GAL4.
INHIBITION OF CaM KINASE IN BOTH ANTENNAL LOBES AND MUSHROOM BODIES AFFECTS CONDITIONING AND MEMORY
Inhibition of CaM kinase concurrently in the mushroom bodies and the antennal lobes using four P[GAL4] lines produced both conditioning and memory defects. When ala was expressed in the antennal lobes under control of the P[GAL4] lines MJ270 and MJ162a, conditioning was affected (P > 0.05; Fig. 7C; Table 1). Lines 40B and MJ250 produced statistically insignificant effects on conditioning (Table 1). Memory was severely affected by expression of ala under control of MJ250 and MJ270 (P < 0.005; Fig. 7D; Table 1). The memory effects of lines MJ162a and 40B were not statistically significant.
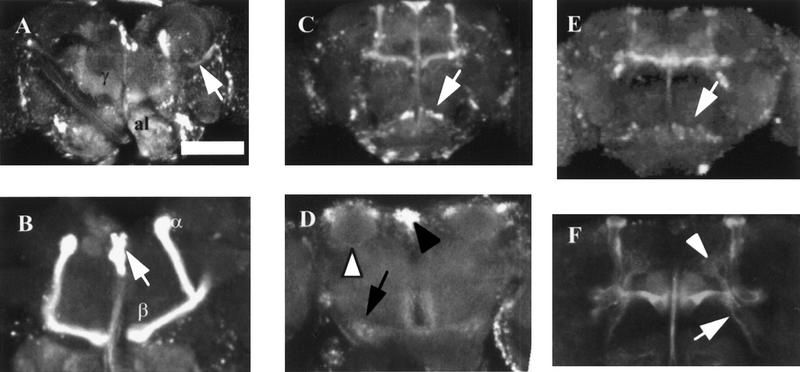
MJ270 expresses in the α-, β-, and γ-lobes of the mushroom bodies, heavily in the pars intercerebralis, and in both mechanosensory and chemosensory antennal lobes. When the GFP expression of MJ270 is rotated, two layers of the mushroom bodies and pars intercerebralis are detected (data not shown). MJ162a is expressed in a subset of mushroom body and pars intercerebralis neurons, in ventral antennal lobes neurons, and in some neurons that connect the antennal lobes to the calyces (Fig. 9E,F). GAL4 is expressed in the maxillary palps of this line (data not shown). 40B expresses in the β-lobe and a different subset of medial antennal glomeruli than MJ162a (Fig. 9, cf. C and E). The pars intercerebralis is stained heavily in 40B and some staining is observed in the lamina of the optic lobes (Fig. 9C). MJ250 is expressed in α-, β-, and γ-lobes of the mushroom bodies, calyces, in the ventral subesophageal ganglion and in the lateroposterior antennal mechanosensory and motor center lateral set of antennal glomeruli (Fig. 9D). Data from these lines that express both in the mushroom body and antennal lobes support the results obtained with P[GAL4] lines that express either in the mushroom bodies or the antennal lobes. Expression of ala in the chemosensory antennal lobes, and not the mechanosensory lobes, affects the response to the mated female during the conditioning period and the γ-lobe mushroom body expression of ala in these lines correlates with the memory defect.
Figure 9.
Adult brain expression patterns of antennal lobe and mushroom body P[GAL4] lines. (A–F) Confocal reconstruction of P[GAL4] transheterozygous with UAS–GFP. Dorsal is up in all images. (A) 21 × 2.05-μm sections of MJ270 shows GFP expression in both the mushroom bodies and antennal lobe (al). Arrow indicates an antennocerebral tract. (B) Mushroom body sections (30 × 1.98 μm) show expression of MJ270 is limited to the α- and β-lobes. Arrow indicates strong expression in some of the pars intercerebralis cell bodies. (C) 40B sections (25 × 3.06 μm) show weak expression in the mushroom bodies and pars intercerebralis and strong expression in the ventral antennal lobes (arrow). (D) MJ250 sections (25 × 3.06). This posterior view shows expression in the calyx (white arrowhead), pars intercerebralis (black arrowhead) and lateroposterior antennal mechanosensory and motor center (arrow). (E) MJ162 sections (25 × 3.06 μm) show expression in the mushroom bodies, pars intercerebralis and ventral antennal lobe (arrow). (F) MJ162a sections (15 × 1.98 μm) show weak expression within the α-, β-, and γ-lobes of the mushroom bodies. A neural tract originating from the antennal lobe extends processes dorsally to the mushroom bodies (arrow) that terminates near the anterior–medial calyces (arrowhead). Scale bar, 50 μm for A, C, D, and E; 20 μm for B and F.
LOCOMOTION AND CHEMOSENSORY ACUITY ARE UNAFFECTED BY INHIBITION OF CaM KINASE
The progeny of each of the P[GAL4] lines crossed to UAS–ala flies were tested for locomotion in the courtship-assay chambers. None of the genotypes tested showed a significant difference in locomotion in the line-crossing test when compared to wild-type Canton-S flies (P > 0.01, Dunnett’s test with Canton-S as the control, Table 2). To account for the possibility of fatigue over the hour conditioning period, 29BD/+;UAS–ala/+ flies were tested for locomotion after being with mated females for 1 hr. MJ85b, a line that expresses throughout the brain (Joiner and Griffith 1997), was also tested. Again, no significant difference was observed for locomotion of 29BD or MJ85b flies before or after conditioning (P > 0.01, Dunnett’s test with Canton-S as the control, Table 2).
Table 2.
Locomotor activity
| Genotype | Line crossing |
|---|---|
| Canton-S wild type | 116 ± 7 |
| 201Y/+; UAS–ala/+ | 135 ± 7 |
| 30Y/+; UAS–ala/+ | 108 ± 11 |
| c309/+; UAS–ala/+ | 137 ± 22 |
| c739/+; UAS–ala/+ | 90 ± 4 |
| c232/+; UAS–ala/+ | 112 ± 10 |
| MJ126a/+; UAS–ala/+ | 130 ± 6 |
| OK348/+; UAS–ala/+ | 151 ± 16 |
| J183; UAS–ala/+ | 113 ± 9 |
| MJ94; UAS–ala/+ | 148 ± 16 |
| GH86; UAS–ala/+ | 103 ± 9 |
| MJ250; UAS–ala/+ | 113 ± 3 |
| MJ270; UAS–ala/+ | 115 ± 7 |
| MJ162a/+; UAS–ala/+ | 119 ± 7 |
| 40B/+; UAS–ala/+ | 129 ± 5 |
| MJ146; UAS–ala/+ | 107 ± 11 |
| MJ286c/+; UAS–ala/+ | 99 ± 7 |
| MJ63/+; UAS–ala/+ | 138 ± 8 |
| 29BD/+; UAS–ala/+ | 127 ± 8 |
| 29BD/+; UAS–ala/+ after 1 hr | 106 ± 6 |
| MJ85b | 99 ± 9 |
| MJ85b after 1 hr | 87 ± 6 |
| MJ85b; UAS–ala/+ | 130 ± 5 |
| MJ85b; UAS–ala/+ after 1 hr | 89 ± 9 |
Locomotor activity was measured by counting spontaneous line crossings in the courtship conditioning chamber for 4 min. For lines 29BD and MJ85b, locomotor activity of males that had been paired with a fertilized female for 1 hr was also measured. MJ85b expresses throughout the fly brain (Joiner and Griffith 1997). Assay was performed in dim red light; n = 10 for all genotypes. Data are presented as mean ± s.e.m.
In animals expressing the ala-inhibitory peptide under control of the heat-shock-70 promoter, no chemosensory defects were detected using a food trap assay (Griffith et al. 1993). This test of olfaction, however, does not indicate if the flies can sense pheromones. Analysis of the initial response of these males to virgin females indicates that are all competent to sense the attractive pheromones as each of the P[GAL4] lines expressing ala performs normal initial courtship, a behavior that depends on sensing attractive pheromones. To test whether the aversive pheromone was being sensed, we compared initial levels of courtship of mated and virgin females for all the lines that showed a defect in modification of the response to the mated female. Initial courtship values of these lines with virgin females were not significantly different from Canton-S (P > 0.08), with the exception of MJ94 and MJ270 (P < 0.005), which have a high level of initial courtship. Three of the five genotypes that showed abnormal results in the first part of the courtship conditioning test also show ability to discriminate between fertilized and virgin females (Fig. 10). This would be expected if the male were sensing both the stimulatory and aversive pheromones (Siegel and Hall 1979; Tompkins et al. 1983; Gailey et al. 1986). It is possible that MJ270 and MJ94 flies, which do not show discrimination when expressing ala may not be able to show courtship modification by mated females because they cannot sense the aversive pheromone (Fig. 10). As the assay was done in red light and the female is anesthetized, the behavior of the female is moot and the male is simply responding to olfactory and tactile cues. These data suggest that two of the lines tested are potentially insensitive to the pheromones pertinent to the courtship assay. The ability of three of the lines to clearly sense the aversive pheromone supports the idea that defects in the modification of behavior toward the mated female in this assay can also result from a deficiency in neural plasticity rather than sensory perception.
Figure 10.
Ability of experience-dependent courtship conditioning deficient flies expressing ala with P[GAL4] inserts indicated, to show discrimination. CI index for the initial 10 minutes with a mated female (black bars) and with a virgin female (open bars) for males with the indicated P[GAL4] insert expressing the ala-inhibitor peptide. Statistical significance was assessed by Wilcoxon’s signed-rank test. (*) Significantly different from the genotype control with level of significance as indicated: (*) P < 0.05; (**) P < 0.005; (***) P < 0.0005.
Discussion
NEURAL PLASTICITY MAPPING
We have mapped the anatomical location of CaM kinase-requiring neurons that are used for courtship conditioning using a total of 18 different P[GAL4] lines. In some of the lines we use, the expression pattern of GAL4 appears to be isolated within a single neural structure. In other lines, expression is predominantly localized to one or two structures with less intensive expression in other parts of the neuropil. By using, for example, five lines that predominantly express in the mushroom bodies we can rule out other areas of nonoverlapping expression between the P[GAL4] lines as having a role in the observed phenotypes. We can also control for developmental effects using this approach, as the early temporal and spatial patterns of expression of independent lines are different. The use of multiple lines has also allowed us to determine that there are thresholds of inhibition below which we see few effects, e.g., with the low level of MJ94 mushroom body expression.
These experiments suggest that behavior during the conditioning period is determined by information processing at early synapses in the circuit in the antennal lobes. The main antennal lobes consist of primary sensory neurons that project from the olfactory and gustatory sense organs, as well as second-order intrinsic neurons. Cooling experiments in the honey bee have shown that initial processing of external stimuli occurs in the antennal glomeruli (Hammer 1997). This study shows that incoming information is processed in the first- and second-order neurons of the antennal nerve and lobes and neurons from the maxillary palps. This processing is required to modulate behavior toward the mated female during conditioning.
Memory formation and modification of behavior toward subsequently presented females is determined by information processing deeper in the brain at higher-order synapses. Direct connections from the antennal nerve to the lateral protocerebrum have been described (Power 1946; Stocker et al. 1983) and P[GAL4] inserts (MJ146 and MJ286c) expressing ala in the lateral protocerebrum show that second- or third-order processing of this information occurs here. Memory is formed beyond the initial sensory processing centers in the brain at a number of different sites including the mushroom bodies, central complex, and lateral protocerebrum.
THE COURTSHIP CONDITIONING CIRCUIT
The environmental inputs that drive this behavior in the absence of visual input are largely chemosensory (Joiner and Griffith 1997). The neuronal circuit begins with chemosensory inputs (Power 1946; Singh and Nayak 1985; Stocker and Gendre 1989), which send their processes to the antennal lobes where local inhibitory neurons and projection neurons interact (Fig. 11; Stocker et al. 1983). Of the two tracts from the antenna, mechanosensory and chemosensory, only a subset of the chemosensory input is used in this behavior. From select antennal glomeruli and directly from the antennal nerve, information is transferred to both the lateral protocerebrum and calyces of the mushroom bodies via unilateral connectives (Power 1946; Stocker et al. 1997). Although the antennal nerve sends projections to both the lateral protocerebrum and indirectly to the central complex (Power 1946), our results imply that processing of conditioning only occurs in the lateral protocerebrum.
Figure 11.
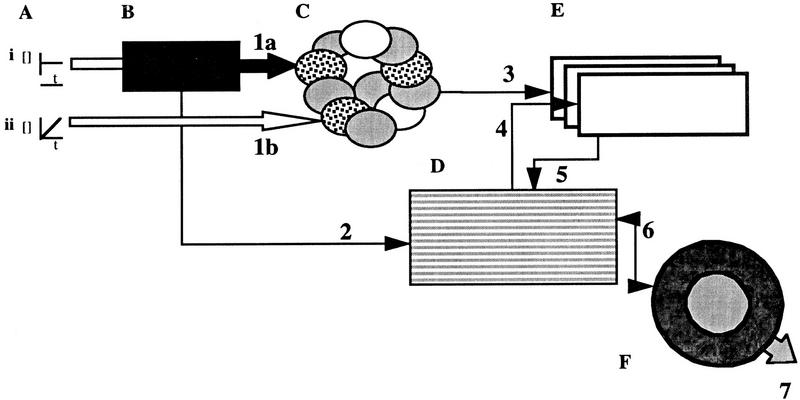
Schematic of courtship conditioning information flow based on courtship conditioning results of 18 P[GAL4] insert lines and anatomical data of the fly brain. (A) Sensory input (aphrodisiacs) for the courtship conditioning assay through the third (i) antennal segment and (ii) maxillary palps (antiaphrodisiacs). (B) The antennal nerve and maxillary nerve carry sensory information (1a and 1b) to the same set of antennal glomeruli and (2) the lateral protocerebrum. (C,D) Modification of the males behavior to the mated female during conditioning occurs in the antennal glomeruli and lateral protocerebrum. (E) Information about (4) courting and (3,4) olfactory cues are assembled in the mushroom bodies. Olfactory information is primarily sent from a subset of the (3) antennal glomeruli to the lateral horn and calyces of the mushroom bodies. Extrinsic (4) mushroom body neurons synapse onto the mushroom body pedunculi and lobes. (F) Further processing of stimuli and output directives occur in the (5) lateral protocerebrum and (6) central complex. Letters represent neural structures and numbers represent connections between groups of neuropil. Information about appropriate behavior is sent to the (7) thoracic ganglia. (t) Time; ([]) concentration.
The mushroom bodies are used exclusively for memory in CaM kinase-dependent courtship conditioning and send projections to the lateral protocerebrum (Ito et al. 1998). This is underscored by the results of the line 201Y, the only mushroom body line that shows a defect in response to the mated female. The most striking difference between the pattern of expression for 201Y and the other four mushroom-body expressing P[GAL4] lines are two pairs of cells, one in the lateral ventral protocerebrum and the other in the lateral dorsal region. The ventral pair resemble cellular expression of MJ146 (Fig. 6A) and the dorsal pair resemble those of MJ162a (Fig. 9E). Both of these lines are defective for modulation of the response to the mated female.
Unlike the lateral protocerebrum, the central complex, a neuropil involved in behavior output (Bouhouche et al. 1993; Strauss and Heisenberg 1993) is used solely for memory. Although no prominent neural tracts project directly to the central complex, it receives inputs from most areas of the brain, including the antennal nerve, the lateral protocerebrum, and the optic lobes, but not the mushroom bodies (Power 1946; Hanesch et al. 1989; Ito et al. 1998; see http://flybrain.org). The central complex is also known to be involved with motor output programs (Strauss and Heisenberg 1993), and it is possible that inhibition of CaM kinase in this structure could impair output pathways. A strong argument against this is that global inhibition of CaM kinase has been shown to affect memory formation but not retrieval. In addition, we show that there are no gross motor defects induced by CaM kinase inhibition; in fact failure to remember is associated with an increase in courtship behavior. These results would argue that the memory problems we document here are caused by impairment of formation, not retrieval.
These experiments also demonstrate a difference between courtship conditioning and classical conditioning of odor avoidance in the circuitry and/or biochemistry underlying learning. Expression of activated Gαs in the mushroom bodies, but not in the central complex, using a subset of the lines used in this study, disrupted learning of odor avoidance (Connolly et al. 1996). Memory was not tested in this study. In particular, OK348, which expresses in the fan-shaped body disrupts memory formation in the courtship-conditioning assay, but not learning in the odor avoidance assay. c232, which expresses in the ellipsoid body, does not affect either behavior. An additional difference is that the relative magnitudes of disruption caused by mushroom body expression varies between studies. 201Y, for example, was the least disruptive for classical conditioning, but the most disruptive for courtship conditioning.
This biochemically defined circuit is not the equivalent of a connectivity circuit as defined electrophysiologically. The genetic manipulation of signal transduction pathways in discrete areas of the brain that are known to be connected directly or indirectly gives us insight into the flow of information, but cannot rule out parallel pathways.
COURTSHIP CONDITIONING PRODUCES TWO INDEPENDENT BEHAVIORS
The courtship-conditioning assay is divided into two distinct parts. The first is the training period during which the male is in contact with a mated female. The male normally modifies his behavior toward the trainer female during this period. The second part is the test period in which the male is presented with a virgin female. His response to this female is a measure of his memory of associations made during the training period. Previous studies have demonstrated that an association between attractive and aversive cues and courtship is required for the male to show intact memory in the test period. In flies in which CaM kinase activity is blocked in the mushroom bodies or parts of the central complex we observe a memory defect with no change in the male’s modification of his behavior during the training period. In other recent studies (Joiner and Griffith 1997; Kane et al. 1997) the decrement in courtship shown by wild-type males during the conditioning hour has been shown to be distinct from, and not necessary for, associative memory formation as assayed during the test period. One conclusion that can be drawn from these studies is that these two behaviors are not interdependent. This idea is supported strongly by the anatomical separation of these two responses demonstrated in this study. The decrement in courtship of the mated female is dependent upon intact CaM kinase in antennal lobes, whereas memory formation is dependent upon other brain structures. It is possible that this initial response to the mated female may even represent a form of nonassociative learning. The neural network model proposed by Joiner and Griffith (1997), however, accounts for the differences between the phases of the assay in a more integrative way than by simply assigning nonassociative or associative labels to the behaviors.
Acknowledgments
We would like to thank Cahir O’Kane and Vivian Budnik for providing GAL4 lines, Dan Eberl for help with dissections, and Michael Dailey for the use of his confocal. Ed Dougherty provided expert assistance with microscopy and graphics. This study was supported by P01 GM 33205 (L.C.G.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Ackerman SL, Siegel RW. Chemically reinforced conditioned courtship in Drosophila: Responses of wild-type and the dunce, amnesiac and don giovanni mutants. J Neurogenet. 1986;3:111–123. doi: 10.3109/01677068609106898. [DOI] [PubMed] [Google Scholar]
- Bouhouche A, Choulli MK. Study of acquired sexual inhibition in a neurologic mutant, no-bridgeKS49, of Drosophila melanogaster. Can J Zool. 1994;72:1376–1382. [Google Scholar]
- Bouhouche A, Vaysse G, Corbiere M. Immunocytochemical and learning studies of a Drosophila melanogaster neurological mutant, no-bridgeKS49 as an approach to the possible role of the central complex. J Neurogenet. 1993;9:105–121. doi: 10.3109/01677069309083453. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brand AH, Dormand EL. The GAL4 system as a tool for unraveling the mysteries of the Drosophila nervous system. Curr Opin Neurobiol. 1995;5:572–578. doi: 10.1016/0959-4388(95)80061-1. [DOI] [PubMed] [Google Scholar]
- Buchner E, Buchner S, Burg MG, Hofbauer A, Pak WL, Pollack I. Histamine is a major mechanosensory neurotransmitter candidate in Drosophila melanogaster. Cell Tissue Res. 1993;273:119–125. doi: 10.1007/BF00304618. [DOI] [PubMed] [Google Scholar]
- Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, Tully T, O’Kane CJ. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han K-A, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learn & Mem. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Han K-A. Neuroanatomy: Mushrooming mushroom bodies. Curr Biol. 1996;6:146–148. doi: 10.1016/s0960-9822(02)00447-5. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- ————— Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: A case study of the mushroom body miniature gene (mbm) Proc Natl Acad Sci. 1996;93:9875–9880. doi: 10.1073/pnas.93.18.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Tully T. Gene discovery in Drosophila: New insights for learning and memory. Annu Rev Neurosci. 1998;21:407–444. doi: 10.1146/annurev.neuro.21.1.407. [DOI] [PubMed] [Google Scholar]
- Fischer JA, Giniger E, Maniatis T, Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988;332:853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- Gailey DA, Lacaillade RC, Hall JC. Chemosensory elements of courtship in normal and mutant, olfaction-deficient Drosophila melanogaster. Behav Genet. 1986;16:375–405. doi: 10.1007/BF01071319. [DOI] [PubMed] [Google Scholar]
- Gailey DA, Villella A, Tully T. Reassessment of the effect of biological rhythm mutations on learning in Drosophila melanogaster. J Comp Physiol A. 1991;169:685–697. doi: 10.1007/BF00194897. [DOI] [PubMed] [Google Scholar]
- Griffith LC, Verselis LM, Aitken KM, Kyriacou CP, Danho W, Greenspan RJ. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- Griffith LC, Wang J, Zhong Y, Wu CF, Greenspan RJ. Calcium/calmodulin-dependent protein kinase II and potassium channel subunit eag similarly affect plasticity in Drosophila. Proc Natl Acad Sci. 1994;91:10044–10048. doi: 10.1073/pnas.91.21.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC. Control of male reproductive behavior by the central nervous system of Drosophila: Dissection of a courtship pathway by genetic mosaics. Genetics. 1979;92:437–457. doi: 10.1093/genetics/92.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Hammer M. The neural basis of associative reward learning in honeybees. Trends Neurosci. 1997;20:245–252. doi: 10.1016/s0166-2236(96)01019-3. [DOI] [PubMed] [Google Scholar]
- Han PL, Levin LR, Reed RR, Davis RL. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron. 1992;9:619–627. doi: 10.1016/0896-6273(92)90026-a. [DOI] [PubMed] [Google Scholar]
- Han PL, Meller V, Davis RL. The Drosophila brain revisited by enhancer detection. J Neurobiol. 1996;31:88–102. doi: 10.1002/(SICI)1097-4695(199609)31:1<88::AID-NEU8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Hanesch U, Fischbach K-F, Heisenberg M. Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res. 1989;37:3–45. [Google Scholar]
- Heimbeck, G., V. Bugnon, N. Gendre, C. Häberlin, and R. Stocker. 1999. Smell and taste perception in D. melanogaster larva: Toxin expression studies in chemosensory neurons. J. Neurosci. (in press). [DOI] [PMC free article] [PubMed]
- Heisenberg M. Mutants of brain structure and function: What is the significance of the mushroom bodies for behavior? In: Siddiqi O, Babu P, Hall LM, Hall JC, editors. Development and biology of Drosophila. New York, NY: Plenum; 1980. pp. 337–390. [DOI] [PubMed] [Google Scholar]
- Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki K, Estes P, Ramaswami M, Yamamoto D, Strausfeld NJ. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster meigen. Learn & Mem. 1998;5:52–77. [PMC free article] [PubMed] [Google Scholar]
- Jäger RF, Fischbach K-F. Some improvements on the Heisenberg-Bohl method for mass histology of Drosophila heads. Drosophila Inf Svc. 1987;17:162–165. [Google Scholar]
- Joiner MA, Griffith LC. CaM kinase II and visual input modulate memory formation in the neuronal circuit controlling courtship conditioning. J Neurosci. 1997;17:9384–9391. doi: 10.1523/JNEUROSCI.17-23-09384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane NS, Robichon A, Dickinson JA, Greenspan RJ. Learning without performance in PKC-deficient Drosophila. Neuron. 1997;18:307–314. doi: 10.1016/s0896-6273(00)80270-6. [DOI] [PubMed] [Google Scholar]
- Kelly P. Calmodulin-dependent protein kinase II: Multifunctional roles in neuronal differentiation and synaptic plasticity. Mol Neurobiol. 1991;5:153–177. doi: 10.1007/BF02935544. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. New York, NY: John Wiley and Sons; 1950. pp. 481–490. [Google Scholar]
- Neckameyer WS. Dopamine and mushroom bodies in Drosophila: Experience-dependent and -independent aspects of sexual behavior. Learn & Mem. 1998;5:157–165. [PMC free article] [PubMed] [Google Scholar]
- Nighorn A, Healy MJ, Davis RL. The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron. 1991;6:455–467. doi: 10.1016/0896-6273(91)90253-v. [DOI] [PubMed] [Google Scholar]
- Power ME. The brain of Drosophila melanogaster. J Morphol. 1943;72:287–300. [Google Scholar]
- ————— The antennal centers and their connections within the brain of Drosophila melanogaster. J Comp Neurol. 1946;85:485–517. doi: 10.1002/cne.900850307. [DOI] [PubMed] [Google Scholar]
- Prokop A, Technau GM. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev Biol. 1993;161:321–337. doi: 10.1006/dbio.1994.1034. [DOI] [PubMed] [Google Scholar]
- Siegel RW, Hall JC. Conditioned response in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci. 1979;76:565–578. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RW, Hall JC, Gailey DA, Kyriacou CP. Genetic elements of courtship in Drosophila: Mosaics and learning mutants. Behav Genet. 1984;14:383–410. doi: 10.1007/BF01065442. [DOI] [PubMed] [Google Scholar]
- Singh NR, Nayak SV. Fine structure and primary sensory projections of sensilla on the maxillary palp of Drosophila melanogaster meigen. Int J Insect Morphol Embryol. 1985;14:291–306. [Google Scholar]
- Skoulakis EM, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron. 1993;11:197–208. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Gendre N. Courtship behavior of Drosophila genetically or surgically deprived of basiconic sensilla. Behav Genet. 1989;19:371–385. doi: 10.1007/BF01066165. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Singh RN, Schorderet M, Siddiqi O. Projection patterns of different types of antennal sensilla in the antennal glomeruli of Drosophila melanogaster. Cell Tissue Res. 1983;232:237–248. doi: 10.1007/BF00213783. [DOI] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor F, Zhu XR, Kaltenbach E, Ackermann A, Baumann A, Canal I, Heisenberg M, Fischbach K-F, Pongs O. minibrain: A new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron. 1995;14:287–301. doi: 10.1016/0896-6273(95)90286-4. [DOI] [PubMed] [Google Scholar]
- Tissot M, Gendre N, Hawken A, Stortkuhl KF, Stocker RF. Larval chemosensory projections and invasion of adult afferents in the antennal lobe of Drosophila. J Neurobiol. 1997;32:281–297. doi: 10.1002/(sici)1097-4695(199703)32:3<281::aid-neu3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Tompkins L. Genetic analysis of sex appeal in Drosophila. Behav Genet. 1984;14:411–440. doi: 10.1007/BF01065443. [DOI] [PubMed] [Google Scholar]
- Tompkins L, Siegel RW, Gailey DA, Hall JC. Conditioned courtship in Drosophila and its mediation by association of chemical cues. Behav Genet. 1983;13:565–578. doi: 10.1007/BF01076402. [DOI] [PubMed] [Google Scholar]
- Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]