Abstract
Exogenous recombinant human transforming growth factor β-1 (TGF-β1) induced long-term facilitation of Aplysia sensory-motor synapses. In addition, 5-HT-induced facilitation was blocked by application of a soluble fragment of the extracellular portion of the TGF-β1 type II receptor (TβR-II), which presumably acted by scavenging an endogenous TGF-β1-like molecule. Because TβR-II is essential for transmembrane signaling by TGF-β, we sought to determine whether Aplysia tissues contained TβR-II and specifically, whether neurons expressed the receptor. Western blot analysis of Aplysia tissue extracts demonstrated the presence of a TβR-II-immunoreactive protein in several tissue types. The expression and distribution of TβR-II-immunoreactive proteins in the central nervous system was examined by immunohistochemistry to elucidate sites that may be responsive to TGF-β1 and thus may play a role in synaptic plasticity. Sensory neurons in the ventral–caudal cluster of the pleural ganglion were immunoreactive for TβR-II, as well as many neurons in the pedal, abdominal, buccal, and cerebral ganglia. Sensory neurons cultured in isolation and cocultured sensory and motor neurons were also immunoreactive. TGF-β1 affected the biophysical properties of cultured sensory neurons, inducing an increase of excitability that persisted for at least 48 hr. Furthermore, exposure to TGF-β1 resulted in a reduction in the firing threshold of sensory neurons. These results provide further support for the hypothesis that TGF-β1 plays a role in long-term synaptic plasticity in Aplysia.
The transforming growth factor β (TGF-β) superfamily consists of ∼30 structurally related growth factors that regulate an extensive array of cellular processes responsible for development, growth, homeostasis, extracellular matrix (ECM) production and regulation, and wound repair (for review, see Hogan 1996; Massagué 1998). TGF-β family members mediate these cellular events in a range of organisms from fruit flies to humans, sharing not only structural similarities but also mechanisms of receptor activation and signaling pathways (for review, see Massagué 1996; Massagué and Weis-Garcia 1996). In addition to the TGF-β isoforms, this superfamily includes subfamilies of bone morphogenetic proteins (BMP), the Drosophila homolog Decapentaplegic (Dpp), activins, growth and differentiation factors (GDF), and the Xenopus homolog Vestigial (Vg1). So similar are many of these growth factors and their receptors that there seems to be very little species specificity in the ligand-receptor interactions. Dpp receptors bind human BMPs (Estevez et al. 1993; Brummel et al. 1994; Penton et al. 1994), Dpp induces bone formation in mammals (Sampath et al. 1993), and dpp phenotypes are rescued by a human BMP4 transgene (Padgett et al. 1993).
With the exception of the distantly related glial cell-derived neurotrophic factor (GDNF), which signals through the receptor tyrosine kinase Ret (Massagué 1996a), all other members of the TGF-β superfamily appear to signal through a family of transmembrane protein serine/threonine kinase receptors (Massagué and Weis-Garcia 1996). On the basis of structural and functional properties, this receptor family is divided into type-I and type-II receptors. The mechanisms of receptor activation are similar for each member of the superfamily, although specific receptors exist for each factor. For example, TGF-β1 first binds the TGF-β1 type-II receptor (TβR-II), enabling it to recruit the TGF-β1 type-I receptor (TβR-I) to form a heteromeric complex. On TβR-II-mediated phosphorylation of the GS-domain of TβR-I (Wrana et al. 1994), TβR-I propagates the signal downstream. TGF-β signals are most often propagated downstream by activated type-I receptors through the direct activation of the Smad family of transcription factors (for review, see Heldin et al. 1997; Derynck et al. 1998; Whitman 1998), although several other pathways have been described that also modulate TGF-β signaling (for review, see Massagué 1998), such as the mitogen-activated protein kinase (MAPK) cascade. TGF-β modulation of MAPK is extremely interesting in light of work in Aplysia showing that MAPK is essential for long-term synaptic facilitation (Martin et al. 1997; Michael et al. 1998). A third class of accessory receptors (TβR-III) has been described that consists of the cell surface proteins betaglycan and endoglin that function primarily to aid in the presentation of the ligand to TβR-II (for review, see Massagué 1998). These receptors do not appear to have an intrinsic signaling function.
An important role for TGF-β1 in long-term synaptic plasticity in Aplysia has been shown previously (Zhang et al. 1997). Application of TGF-β1 induced a long-term increase in the synaptic efficacy of sensory-motor connections in isolated ganglia that persisted for at least 48 hr. In addition, serotonin (5-HT)-induced synaptic facilitation was blocked by the application of a soluble portion of TβR-II representing the ligand-binding extracellular domain. On the basis of these findings, TGF-β1 appears to be both necessary and sufficient for the induction of long-term synaptic facilitation. However, it is not known whether components of the TGF-β1 system exist in this preparation. At the present time, no homolog of the TGF-β1 receptor type II is known to be present in invertebrates. However, the finding that TGF-β1 plays a critical role in long-term synaptic plasticity in Aplysia makes it likely that there is a TGF-β1 receptor type-II-like molecule that transduces these effects. It is also not clear whether TGF-β1 exerts its effects directly by acting on the sensory and/or motor neurons or indirectly by modulating other cells that may affect the sensory-motor synapse. Finally, it is not known whether TGF-β1 affects processes other than synaptic efficacy.
Because TβR-II is necessary for transmembrane signaling by TGF-β1 (Wrana et al. 1992), we sought to clarify these points by examining sections of Aplysia ganglia for TβR-II immunoreactivity. The localization of TβR-II will aid in the elucidation of sites that are responsive to TGF-β1 and undergo electrophysiological and morphological changes associated with long-term plasticity. Also, such localization may help determine which cells produce TGF-β1, because one of TGF-β1’s actions is to upregulate its own transcription (Kim et al. 1989). We found that TβR-II immunoreactivity is present in pleural, pedal, abdominal, buccal, and cerebral ganglia as well as in both cultured mechanoafferent sensory neurons and the identified motor neuron L7. It is also expressed in many peripheral tissues, as found by Western blot analysis of a variety of Aplysia tissues. Furthermore, we show that TGF-β1 also mediates another form of long-term plasticity, an increase in sensory neuron excitability. In isolated sensory neurons in culture, this effect persists for at least 48 hr. We also found that exposure to TGF-β1 resulted in a decrease in the firing threshold of isolated sensory neurons that persisted for at least 48 hr.
Materials and Methods
TISSUE EXTRACTION, SDS-PAGE, AND WESTERN BLOT ANALYSIS
Aplysia californica (150–200 grams) were supplied by Alacrity Marine Biological (Redondo Beach, CA) and Marinus (Long Beach, CA) and maintained in circulating artificial seawater (Instant Ocean) at 15°C. Animals were anesthetized by injection of isotonic MgCl2 equal to approximately one half of the body volume. Samples (0.5 grams wet weight) were taken from the central nervous system (CNS; desheathed ganglia from two animals combined), body wall, buccal mass, foot muscle, heart, kidney, and ovotestis. Hemocytes were obtained by centrifuging hemolymph at 3400 rpm for 15 min. Tissue samples, excluding hemocytes, were frozen on dry ice and crushed with a mortar and pestle. All samples were then homogenized individually with a Kontes homogenizer with an extraction buffer containing 1% SDS, 10 mm EDTA, 10 mm EGTA, 20 mm Tris (pH 7.5) 1 mm PMSF, and 1 mm leupeptin. Protein concentration of each extract was determined by Bradford assay (Bio-Rad). Total protein (50 μg from each extract was resolved on a 12% SDS–polyacrylamide gel and electrotransferred to a nitrocellulose membrane. The membrane was probed by an affinity-purified polyclonal goat IgG raised against the extracellular domain of the human TGF-β type-II receptor (10 μg/ml anti-TβR-II, R&D Systems, Inc.). The membrane was then exposed to a horseradish peroxidase-conjugated rabbit-anti-goat IgG (1:5000, Pierce) and immunoreactive bands were visualized with a chemiluminescent substrate (Pierce).
PREPARATION AND WESTERN BLOT ANALYSIS OF MEMBRANE FRACTIONS
Membrane fractions were prepared according to a procedure in which plasma membrane fractions are enriched (Bawab et al. 1992). Briefly, tissue samples (1 gram wet weight) from CNS, hemocytes, and kidney were homogenized at 4°C with a polytron homogenizer (5 bursts, 10 sec each) in a homogenization buffer of 10 mm sodium phosphate buffer with 30 mm NaCl, 1 mm MgCl2, 0.02% NaN3 (pH 7.5). The homogenate was filtered through cotton gauze before layering over a 41% sucrose cushion and subsequent centrifugation at 95,000g for 1 hr at 4°C. The interfacial bands of membranes were collected, diluted with homogenization buffer, and centrifuged at 95,000g for 30 min at 4°C to rinse and pellet the membranes. The pellets were collected and resuspended in 200 μl of homogenization buffer and PMSF was added to a final concentration of 1 mm. Protein concentration was determined by Bradford assay. Total protein (40 μg) from each extract was resolved on a 12% SDS–polyacrylamide gel and electrotransferred to a nitrocellulose membrane. The membrane was probed with anti-TβR-II. The membrane was then exposed to a horseradish peroxidase-conjugated rabbit–anti-goat IgG (1:5000, Pierce), and immunoreactive bands were visualized with a chemiluminescent substrate (Pierce).
IMMUNOHISTOCHEMISTRY
Pleural–pedal, abdominal, buccal, and cerebral ganglia were removed from anesthetized animals, pinned to a Sylgard (Dow-Corning)-coated dish and fixed by immersion in 4% paraformaldehyde in PBS containing 30% sucrose overnight at 4°C. Ganglia were rinsed overnight in PBS and sectioned on a cryostat at a nominal thickness of 16 μm. Slides were rinsed in PBS containing 0.1% Triton X-100 and permeabilized by serial dehydration through 10%, 20%, 30%, and 40% ethanol and rehydration to PBS. Endogenous peroxidase activity was blocked by 0.3% hydrogen peroxide in PBS for 10 min. After incubation in 2% normal rabbit serum (Vector Laboratories) for 2 hr at room temperature, anti-TβR-II IgG was applied at 0.5 μg/ml overnight at 4°C. The concentration of anti-TβR-II used for these experiments represents a 10-fold decrease from the concentration used for the staining of cultured neurons (described below) and a 20-fold decrease from the concentration used for Western blotting due to the amplification of signal provided by the Vectastain reagents. Control sections were incubated in 2% normal rabbit serum. All slides were then rinsed, incubated for 1 hr at room temperature in biotinylated rabbit–anti-goat IgG (1:200 dilution, Vector Laboratories) followed by incubation in ABC-HRP (1:100 dilution, Vector Laboratories). After rinsing in PBS, slides were exposed to 0.05% DAB (Sigma), 0.01% H2O2, and 250 μm NiCl in 100 mm Tris (pH 7.5), after which they were rinsed in PBS, dehydrated in increasing concentrations of ethanol, cleared in xylenes, and mounted in a xylene-based mounting medium. Stained sections were viewed and photographed with bright field light microscopy with a Zeiss Axiophot. Control sections were processed and photographed under identical conditions with the exception of exposure to the primary antibody.
Culturing procedures followed those described by Schacher and Proshanksy (1983), and Rayport and Schacher (1986). Briefly, pleural–pedal ganglia from 80- to 150-gram animals (Alacrity) and abdominal ganglia from 1- to 3-gram juveniles (National Institutes of Health-Aplysia Resource Facility, University of Miami, FL) were incubated in 1% protease type IX (Sigma) at 34.5°C and then manually desheathed. Neurons were removed individually by microelectrodes with fine tips and plated on poly-L-lysine coated glass slides in large petri dishes of culture medium containing 50% hemolymph and 50% isotonic L15 (Sigma). Staining procedures for cultured neurons were as follows. Neurons were allowed to grow for 5 days before fixation with 4% paraformaldehyde in PBS containing 7.5% sucrose for 30 min at room temperature. Cells were rinsed with PBS and incubated in 2% normal rabbit serum in PBS containing 0.1% Triton X-100 for 45 min at room temperature before incubation with anti-TβR-II (5 μg/ml, on the basis of the recommendation of R&D Systems, Inc.) overnight at 4°C. Cultures were rinsed with PBS and incubated with rabbit-anti-goat IgG conjugated to tetramethylrhodamine (ICN Pharmaceuticals) for 45 min at room temperature in the dark. After rinsing with PBS, cells were mounted in Prolong (Molecular Probes). Immunofluorescence was viewed with epifluorescence microscopy with filter set 14 on a Zeiss Axiophot. Control cultures were photographed under identical conditions.
ELECTROPHYSIOLOGY
Pleural sensory neurons from the ventral–caudal cluster were isolated as described above and plated on poly-L-lysine (Sigma) coated petri dishes. Three to five neurons were plated per dish and allowed to grow for 5 days, with a medium change on the third day. Culture medium was exchanged for 50% L15/50% artificial seawater (450 mm NaCl, 10 mm KCl, 11 mm CaCl2, 29 mm MgCl2, 10 mm HEPES at pH 7.6) prior to recording. Neurons were impaled with a single microelectrode (10–20 MΩ resistance) and held at −45 mV. Input resistance was measured by applying 0.1 nA of hyperpolarizing current for 2 sec. Firing threshold was measured by applying 2 seconds of depolarizing current in increasing increments of 0.1 nA until an action potential was triggered. The lowest current intensity necessary to fire one action potential was considered the firing threshold. Excitability was measured by counting the number of action potentials triggered by applying 0.5 nA of depolarizing current for 2 sec. When the firing threshold of sensory neurons was at or above 0.5 nA, 1 nA of depolarizing current was used to measure excitability. This was the case for one BSA-treated dish and one TGF-β1-treated dish. Measurements from all cells in one dish were averaged. Cells were included if their resting potential was at least −35 mV. After baseline measurements, culture dishes were treated for 6 hr with either TGF-β1 (final concentration 1 ng/ml, R&D Systems, Inc.) in 10 μg/ml BSA (Pentex) or BSA alone. A 6-hr incubation period was chosen on the basis of past evidence that the induction of some RNAs, such as that of a variety of extracellular matrix proteins, generally occurs within 3–5 hr of TGF-β1 treatment (for review, see Massagué 1998). All treatments were performed blind. Cultures were returned to culture medium after treatment, and 24 and 48 hr later, resting potential, input resistance, firing threshold, and excitability were assessed in the same manner as baseline measurements. Data were analyzed by two-way ANOVA with repeated measures with SigmaStat software (Jandel Scientific). Post-hoc tests were not included in the analysis because of the fact that the ANOVAs indicated that the only significant effect was due to treatment with TGF-β1. There was no effect of time, nor was there an interaction between time and treatment, indicating that the effect of treatment was significant at both time points.
Results
APLYSIA TISSUES EXPRESS A TβR-II-IMMUNOREACTIVE PROTEIN
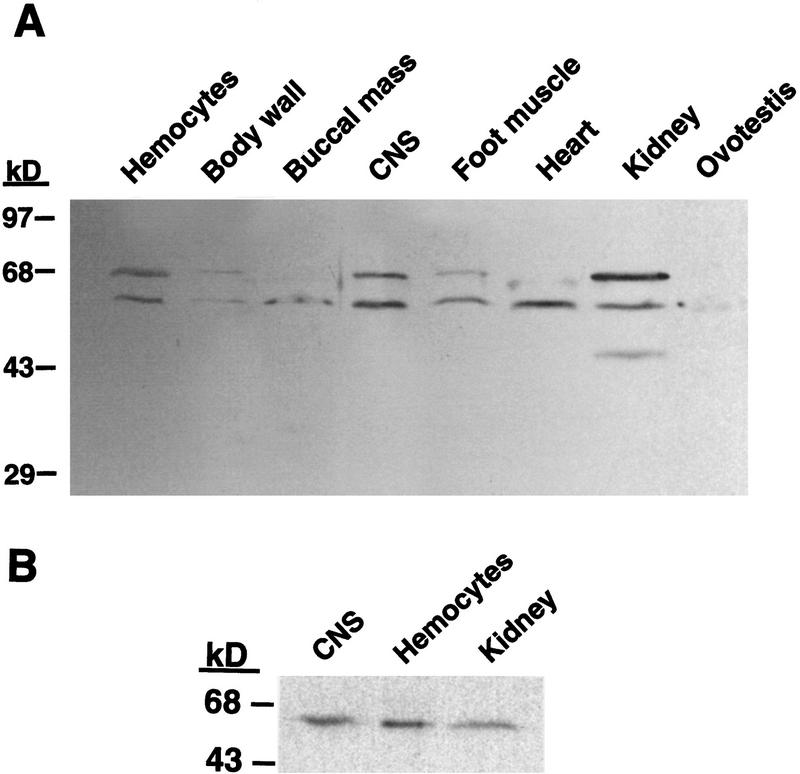
TGF-β signals through heteromeric complexes of type I and type II high-affinity receptors, and the type-II receptor is essential for transmembrane signaling (Wrana et al. 1994). By use of an affinity-purified antibody raised against the extracellular domain of human TβR-II, Western blot analysis revealed the presence of two immunoreactive bands with apparent molecular weights of ∼60 and ∼68 kD in most of the tissue types examined, although relative intensities of the bands varied between tissues (Fig. 1A). Central nervous system, hemocytes, and kidney extracts exhibited the greatest amounts of the protein, whereas only a faint band at 60 kD was seen in the ovotestis extract. The presence of these two bands is consistent with results from studies of mink lung epithelial cells, in which it was shown that a 60-kD precursor form of the receptor can be found as well as a 70-kD mature form of the receptor, which is sensitive to deglycosylation by a mixture of enzymes that removes N- and O-linked oligosaccharides (Koli and Arteaga 1997).
Figure 1.
Distribution of TβR-II in Aplysia tissues. Tissue samples were frozen on dry ice, crushed, and subsequently homogenized in an extraction buffer containing EDTA, EGTA, and protease inhibitors. A total of 50 μg of protein from each sample was loaded and resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with an affinity-purified polyclonal goat IgG raised against the extracellular domain of the human TGF-β type-II receptor and exposed to a HRP-conjugated rabbit–anti-goat IgG. Immunoreactive bands were visualized with a chemiluminescent substrate. (A) Two bands of protein (60 and 68 kD) are found in almost all Aplysia tissues examined, which may represent precursor and mature forms of the receptor. (B) One band of protein is detected in a membrane fraction.
To better understand the functional relationship of the two immunoreactive bands, a subcellular fractionation procedure, which had been shown to enrich plasma membrane constituents (Bawab et al. 1992), was used. In membrane fractions prepared from CNS, hemocytes and kidney, we detected only one major band of protein by Western blot analysis (Fig. 1B). This band was of an apparent molecular mass of ∼62 kD, and presumably corresponds to the 68-kD band observed in crude homogenates. In some experiments, a second light band was observed at ∼55 kD, which may represent the presence of the precursor form. The difference in apparent molecular weights from the previous experiments (Fig. 1A) may be due to differences in the ionic composition of the buffers in which the extracts were prepared.
TβR-II IMMUNOREACTIVITY IS FOUND IN SECTIONS OF APLYSIA GANGLIA
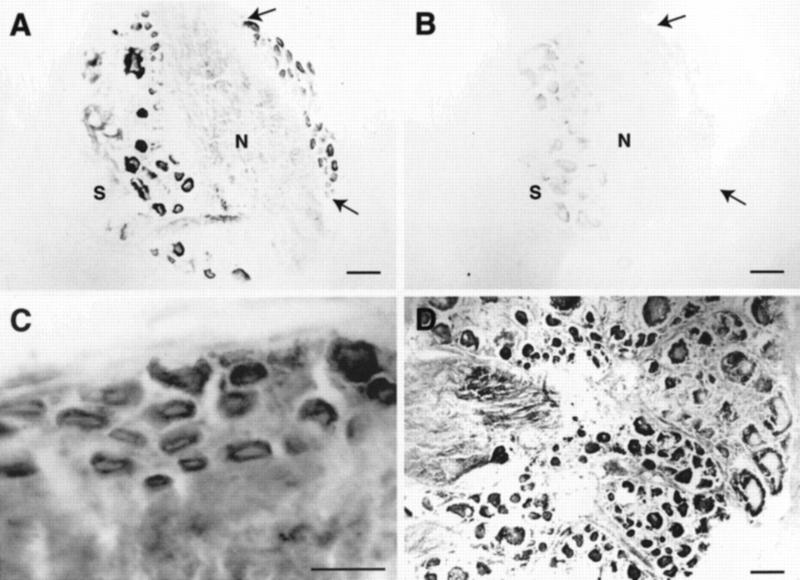
TGF-β produces trophic and protective effects and is an important regulator of cell growth in the nervous system of many species (for review, see Flanders et al. 1998) as well as a modulator of extracellular matrix production and remodeling (for review, see Roberts and Sporn 1996). Because recombinant human TGF-β1 applied exogenously to isolated Aplysia pleural–pedal ganglia was found to induce long-term facilitation of sensory-motor synapses (Zhang et al. 1997), we examined sections of Aplysia ganglia to determine which cells may be responsible for this plasticity. We found immunoreactive neurons in pleural, pedal, abdominal, buccal, and cerebral ganglia. Most immunoreactivity was found in the cytoplasm of cell bodies, although some immunoreactivity was also found in the neuropil, which likely represents staining along neuronal processes. In the pleural ganglion, many neurons were immunoreactive (Fig. 2A) including the small cluster of tail sensory neurons in the ventral–caudal region (Fig. 2A,C). Control sections that were exposed to secondary, but not primary, antibody showed no staining (Fig. 2B). Many unidentified neurons throughout the pedal ganglion were also immunoreactive (Fig. 2D).
Figure 2.
TβR-II immunoreactivity in sections of pleural and pedal ganglia. (A) Staining is present in neuronal cell bodies in the pleural ganglia as well as in the neuropil, which may represent staining along neuronal processes. The cluster of mechanoafferent sensory neurons in the ventral–caudal cluster also exhibit immunoreactivity and can be identified by size and position (area between arrows). (S) Sheath; (N) neuropil. (B) Control section adjacent to that in A shows little staining. (C) Higher magnification view of mechanoafferent sensory neurons from a different section than that shown in A. (D) Immunoreactivity is also present in many cells of the pedal ganglion, particularly in the caudal region, shown in this section. Scale bars, 100 μm (A,B,D) and 50 μm (inset).
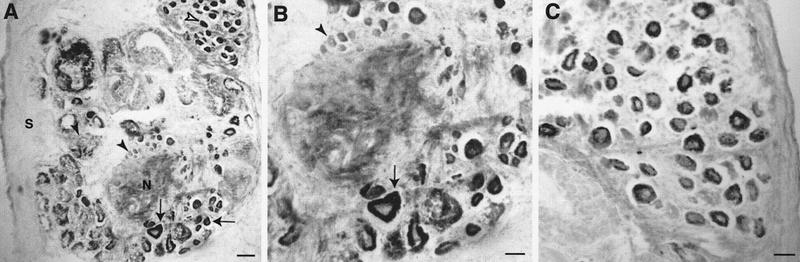
In abdominal ganglia, many neurons throughout the ganglia were immunoreactive, particularly in the caudal regions (Fig. 3A,B, arrows). Unstained neurons were present as well (Fig. 3A,B, solid arrowheads). Bag cells were also highly immunoreactive (Fig. 3A,C). In the buccal ganglion, the S1/S2 cluster of sensory neurons exhibited high immunoreactivity (Fig. 4A) as well as many other neurons in the ganglia (Fig. 4A,B). On the basis of size and position, it appears that two of the labeled cells may be B8a and B8b (Fig. 4A; E. Kabotyanski, pers. comm.). Many neurons in the cerebral ganglion were also immunoreactive (data not shown).
Figure 3.
TβR-II immunoreactivity in sections of the abdominal ganglion. (A) Low-magnification view of the abdominal ganglion showing distribution of immunoreactivity in the ganglion. Some neurons in the caudal region are heavily stained (arrows), whereas other neurons show little immunoreactivity (solid arrowheads). Bag cells are also darkly stained (open arrowhead). (S) Sheath; (N) neuropil. (B) Higher magnification view of the neurons in the caudal region of the section shown in A. Arrow shows a positively stained neuron and arrowhead marks an unstained neuron. (C) Higher magnification view of the bag cell cluster from a different section than that in A. Scale bars, 200 μm in A and 100 μm in B and C.
Figure 4.
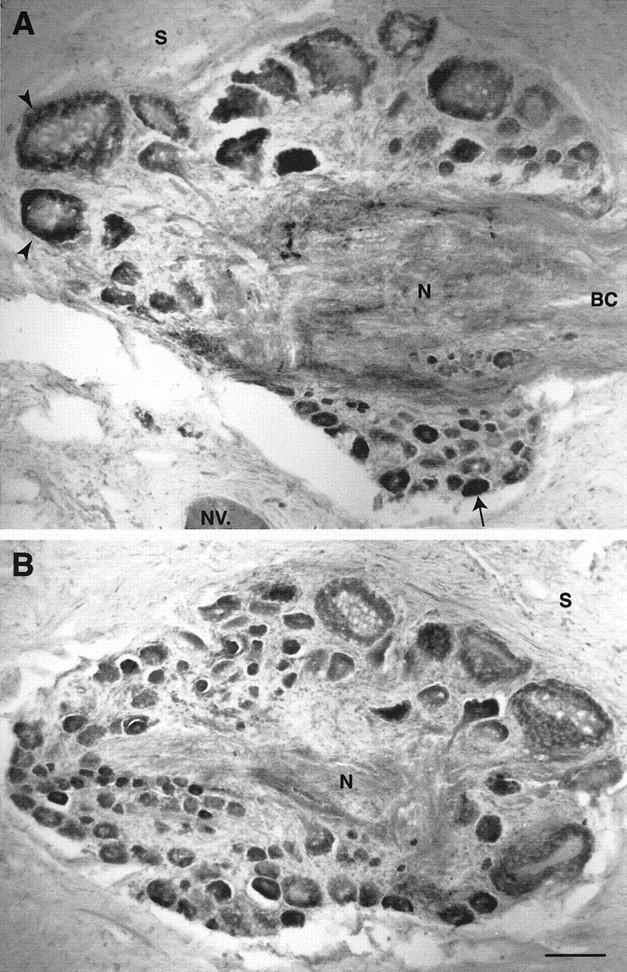
TβR-II immunoreactivity in sections of the buccal ganglion. (A) Many neurons in the S1/S2 cluster exhibit immunoreactivity (arrow) as well as putative B8 neurons (arrowheads). Immunoreactivity is also present along tracts in the neuropil. (S) Sheath; (N) neuropil; (NV) nerve; (BC) buccal commissure. (B) Section from a more caudal region of the buccal ganglion than that in A showing more immunoreactive neurons. Scale bar, 100 μm.
TβR-II-LIKE MOLECULES ARE EXPRESSED IN CULTURED NEURONS
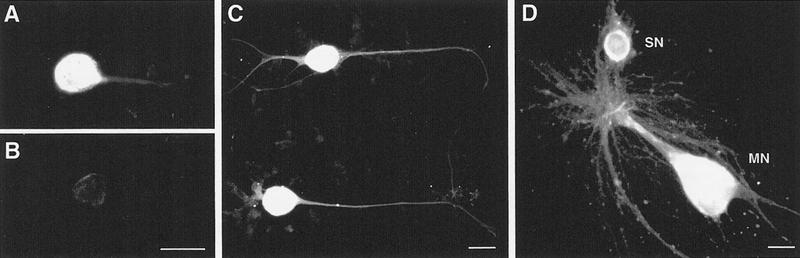
Cultures of mechanoafferent sensory neurons from the ventral–caudal cluster of the pleural ganglion also exhibited immunoreactivity (Fig. 5A,C). Immunoreactivity was found on the somata as well as throughout the extent of the neurites, similar to TβR-II staining in cultures of chick dorsal root ganglion neurons (Unsicker et al. 1995). Not all neurons exhibited the same levels of immunoreactivity, as shown in Figure 5, A and C. Cocultures of sensory neurons and the identified motor neuron L7 also exhibited immunoreactivity both on cell bodies as well as neuronal processes (Fig. 5D). Both sensory neuron and motor neuron were immunoreactive.
Figure 5.
Cultured neurons exhibit TβR-II immunoreactivity. Neurons were isolated and allowed to grow for 5 days before fixation. Immunoreactivity was visualized with a rhodamine-conjugated rabbit-anti-goat IgG. (A) Sensory neurons from the ventral–caudal cluster of the pleural ganglion grown in culture with no postsynaptic target exhibit immunoreactivity along the cell body and neurites. (B) Control cultures show little staining. (C) Not all sensory neurons exhibit the same intensity of immunoreactivity. Note that these sensory neurons exhibit higher levels of immunoreactivity than that in A. (D) Cocultured sensory neuron and L7, an identified motor neuron. The synaptic connection was verified by extracellularly stimulating the sensory neuron and recording an EPSP in the motor neuron (not shown). Both cell bodies are immunostained as well as the major axons of each neuron and finer neuronal processes. Scale bars, 30 μm.
TGF-β1 INDUCES A LONG-TERM INCREASE IN EXCITABILITY OF CULTURED SENSORY NEURONS
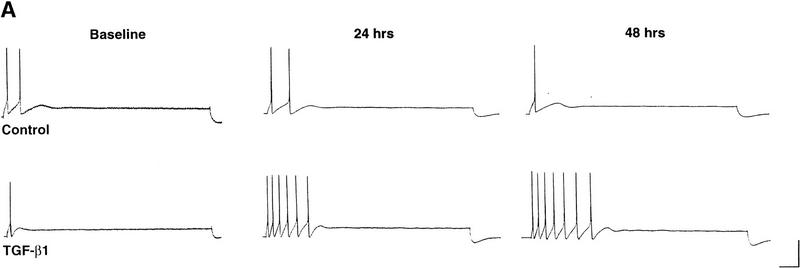
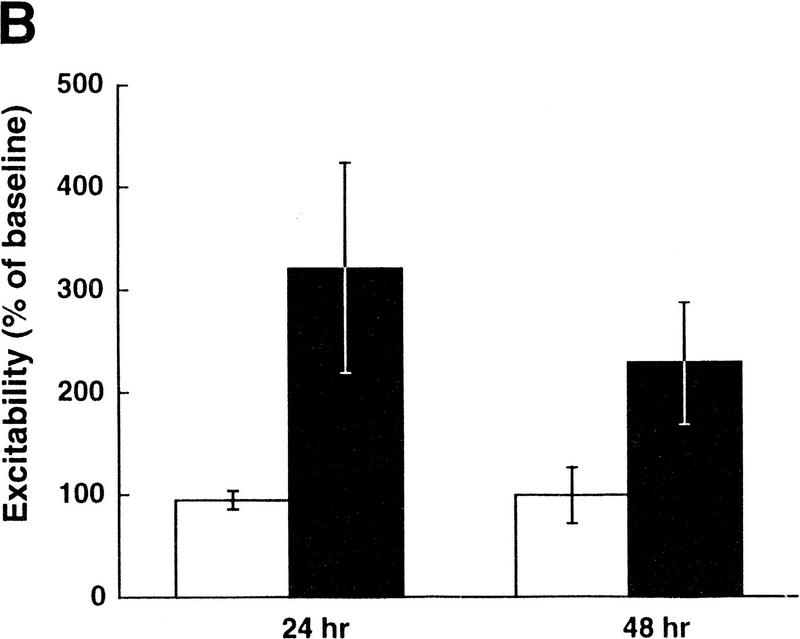
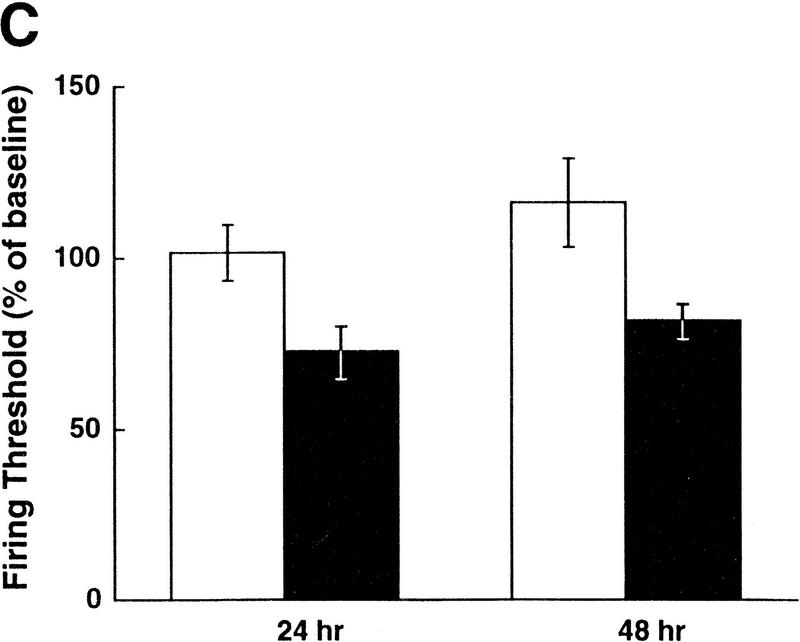
5-HT is a known neuromodulator that induces long-term facilitation of Aplysia synapses as well as long-term increases in excitability of sensory neurons (for review, see Montarolo et al. 1986; Dale et al. 1987; Emptage and Carew 1993; Byrne and Kandel 1996; Zhang et al. 1997). The effects of 5-HT on excitability are seen even in cultures of isolated sensory neurons with no postsynaptic target, and this increase in excitability is dependent on protein synthesis (Dale et al. 1987). Because TGF-β1 appears to mediate long-term synaptic facilitation downstream of 5-HT (Zhang et al. 1997), we sought to determine whether TGF-β1 also plays a role in the increase in excitability of sensory neurons. Application of TGF-β1 (1 ng/ml) to isolated sensory neurons for 6 hr induced an increase in excitability that lasted for at least 48 hr (Fig. 6A). On average, TGF-β1 exposure resulted in a 210% increase in excitability tested 24 hr after application, and a 115% increase in excitability tested 48 hr after application (n = 5 for both BSA- and TGFβ1-treated groups, representing measurements from 15 and 22 neurons, respectively (Fig. 6B). Two-way ANOVA with repeated measures revealed a significant difference between the treatments of TGF-β1 and the control (F1,8 = 5.47, P < 0.05). In addition, TGF-β1 treatment decreased the firing threshold of sensory neurons by ∼30% at both the 24-hr and 48-hr time points (F1,8 = 8.59, P < 0.05; Fig. 6C). There was a trend for an increase in input resistance of the sensory neurons as a result of TGFβ1 treatment, but this difference was not significantly different (F1,8 = 0.224, P > 0.05; data not shown). Resting membrane potential was not affected by TGF-β1 treatment (F1,8 = 0.001, P > 0.05).
Figure 6.
TGF-β1 induces a long-term increase in sensory neuron excitability. (A) Examples of the spike trains evoked by current injection before, 24- and 48-hr after treatment with TGF-β1 or BSA (control) for 6 hr. Calibration bar, 100 msec, 20 mV. (B) Summary data of excitability changes at the 24- and 48-hr time points. Bars, means ± s.e.m. of the normalized excitability. Two-way ANOVA with repeated measures revealed a significant difference between the treatments of TGF-β1 (solid bars) and the control (open bars) (F1,8 = 5.47, P < 0.05). (C) Summary data showing that TGF-β1 induces a long-term decrease in sensory neuron firing threshold. Bars, means ± s.e.m. of the normalized current necessary to evoke one action potential (F1,8 = 8.59, P < 0.05).
Discussion
Members of the TGF-β superfamily regulate cellular processes in a range of organisms including flies, frogs, mice, and humans. They have important functions in development, wound healing, and synaptic plasticity. The widespread use of TGF-β members reflects not only the multifunctional nature of these factors, but also similarities in the cellular processes that are regulated in each case. Cell growth, motility, adhesion, and ECM remodeling are all regulated by TGF-βs and are common processes that are important both in development and synaptic plasticity.
DISTRIBUTION OF TβR-II IMMUNOREACTIVITY IN APLYSIA
An important role for TGF-β1 in long-term synaptic plasticity in Aplysia was shown recently, and accordingly, we now show that the ligand-binding receptor necessary for signaling appears to be present in most Aplysia tissues, as visualized by TβR-II immunoreactivity. The antibody used in these studies was an affinity-purified polyclonal antibody against the extracellular domain of the human TGF-β1 type II receptor. Because a TGF-β1 type II receptor in Aplysia has not been sequenced, we chose to use this antibody because previous experiments indicated functional similarities between human and Aplysia forms. For example, the human form of TGF-β1 induced long-term facilitation in isolated ganglia of Aplysia (Zhang et al. 1997). Moreover, the soluble fragment of the human form of the type-II receptor was able to block 5-HT-induced facilitation. Because the sequence of the Aplysia homolog of the TGF-β1 type-II receptor is as yet unknown, we cannot definitively demonstrate that the immunoreactivity we observed was due to the presence of the TGF-β1 type-II receptor. Such a demonstration will require sequencing of the molecules in Aplysia bound by the human TβR-II antibody.
The two proteins found by Western blot analysis may represent differentially glycosylated forms similar to that found in cultures of mink lung epithelial cells (Koli and Arteaga 1997). In those studies, two forms of the TGF-β1 type-II receptor were also found with apparent molecular weights of 60 and 70 kD. The 60-kD form was found to be sensitive to endoglycosidase H, which cleaves only high mannose oligosaccharides, but not more complex structures. This suggests that this protein represents an endoplasmic reticulum, pre-Golgi precursor form of the receptor. Furthermore, deglycosylation by enzymes that remove all N- and O-linked oligosaccharides eliminated the 70-kD band, indicating that it represented the mature type-II receptor. Our results also suggest that the two proteins recognized by anti-TβR-II in Western blot analysis of crude homogenates represent mature and precursor forms, as we were able to detect only a single major band in a membrane fraction. The presence of these two forms of the receptor may underlie the present finding that TβR-II immunoreactivity is observed throughout the cytoplasm of stained cells, rather than simply around the perimeter of the cell membrane as might be expected for the distribution of a receptor. This cellular staining pattern is similar to that found in other cells such as corneal epithelial cells as well as astrocytes (Obata et al. 1996; Ata et al. 1997). TβR-II immunoreactivity was also observed in the neuropil, although with less intensity, suggesting that the receptor is also localized in neuronal processes.
TGF-β1-MEDIATED PLASTICITY IN APLYSIA
TβR-II levels have been shown to correlate with TGF-β responsiveness (Sun et al. 1994). The presence of TβR-II immunoreactivity in certain Aplysia neurons may indicate that those cells are modulated by TGF-β1 to express the electrophysiological and morphological changes that underlie long-term plasticity. TβR-II immunoreactivity may also indicate which cells are important for the production of the TGF-β1 that mediates long-term plasticity, as TGF-β1 is known to potently stimulate its own transcription (Kim et al. 1989). It is also possible that the cells that undergo electrophysiological and morphological changes in synaptic plasticity also produce TGF-β1 that acts back on the cell in an autocrine fashion. In this study, we showed that cocultured sensory neurons and the identified motor neuron L7 both exhibited TβR-II immunoreactivity in the cell body region as well as neuronal processes. This reduced system is capable of exhibiting two forms of long-term plasticity in response to the neuromodulator 5-HT synaptic facilitation and increased excitability in the sensory neuron (Montarolo et al. 1986; Dale et al. 1987; Bailey et al. 1992; Emptage and Carew 1993). The presence of TβR-II immunoreactivity on both the sensory and motor neuron suggests that TGF-β1 may act on both of these cells to bring about these plastic changes. Exposure of isolated sensory neurons to TGF-β1 was sufficient to induce a long-term increase in excitability. Similar results were found with application of 5-HT (Dale et al. 1987).
Preliminary results show that the increase in excitability induced by TGF-β1 is blocked by simultaneous application of anti-TβR-II, the same antibody used in the immunohistochemical experiments (unpubl.). This result contrasts with previously published results showing that application of TGF-β1 did not mimic the effects of 5-HT on sensory neuron excitability (Zhang et al. 1997). Although there was no statistically significant difference in sensory neuron excitability between control and TGF-β1-treated ganglia, there was a trend for increased excitability after TGF-β1 treatment (Zhang 1997). The large amount of variability in that study may have masked a significant difference between the two groups. Application of SRII, a soluble receptor fragment that presumably acts by scavenging endogenous TGF-β1, blocked 5-HT-induced long-term synaptic facilitation, but did not block the 5-HT-induced long-term increase in excitability (Zhang et al. 1997). The ability of SRII to block facilitation, but not changes in excitability, may be due to different thresholds for inducing these changes in excitability and facilitation (Bunge et al. 1997). Thus, it is possible that SRII was able to scavenge enough TGF-β1 to block the induction of synaptic facilitation but not the increase in sensory neuron excitability, which may have a lower threshold for the induction. It is not likely that the difference in results between this study and the previous one resulted from inherent differences between cultured cells and the intact ganglion. Recent studies showed that application of TGF-β1 to isolated ganglia produced increased excitability of sensory neurons 24 hr after treatment (Povelones et al. 1998; Farr et al. 1999). Thus, TGF-β1 acts directly on sensory neurons, and appears to play an important role in two forms of plasticity (synaptic facilitation and increased sensory neuron excitability) that are correlated with, and may underlie, long-term sensitization of defensive withdrawal reflexes in Aplysia (Frost et al. 1985; Walters 1987; Cleary et al. 1998).
We found that the pleural, pedal, abdominal, buccal, and cerebral ganglia all contain neurons that are immunoreactive for TGF-β1. Various forms of plasticity have been described in all of these ganglia (for review, see Byrne et al. 1991, 1993), leaving open the possibility that TGF-β1 is also involved in the changes found with these forms of plasticity. Although we were able to identify some of the immunoreactive cells as being the mechanoafferent sensory neurons in the ventral–caudal cluster of the pleural ganglion on the basis of size and position, we have not confirmed that other immunoreactive cells have been identified as being involved in plasticity.
The presence of TβR-II immunoreactivity on neurons does not necessarily imply that they are involved in TGF-β1-mediated plasticity. An alternative explanation for the finding that many neurons in all Aplysia ganglia exhibit immunoreactivity is simply that like its relatives in many other systems (for review, see Hogan 1996), TGF-β1 plays an important role in the development of the nervous system of Aplysia as well as the development of other tissues. Thus, although some cells such as the mechanoafferent sensory neurons in the pleural ventral–caudal cluster exhibit TβR-II immunoreactivity and are implicated as having an important role in TGF-β1-mediated plasticity, other cells that exhibit immunoreactivity may not be involved in the synaptic plasticity associated with learning but retain receptors for TGF-β1 from developmental stages and/or continued growth and maturation of the nervous system.
MODULATION OF TGF-β1 ACTIVITY: IMPLICATIONS FOR PLASTICITYDRIVEN REGULATION
An important point of regulation of TGF-β1 activity is the activation of the growth factor. TGF-β1 is secreted from cells in an active form that is rendered latent by noncovalent interactions with its propeptide region, known as the latency-associated peptide, or LAP. Activation of TGF-β1 refers to its dissociation from LAP or the induction of a conformational change such that TGF-β1 is free to bind its receptor (for review, see Flaumenhaft et al. 1993). The activation of TGF-β1 has been extensively studied and several means of activation have been found. Proteolytic cleavage of LAP by serine proteases such as plasmin or thrombin (Sato and Rifkin 1989; Barnard et al. 1990; Lyons et al. 1990), acidification (Jullien et al. 1989; Brown et al. 1990; Bonewald et al. 1991), interaction with the extracellular matrix molecule thrombospondin (Crawford et al. 1998; Schultz-Cherry and Murphy-Ullrich 1993), and recently shown binding to the integrin α5β6 (Munger et al. 1998) all result in activation of TGF-β1. The latter mechanism of activation is interesting in light of several recent studies that show a role for integrins in learning-related plasticity. In Drosophila, Volado is a memory mutant that is deficient in a specific α-integrin that is preferentially expressed in mushroom body cells, neurons known to mediate olfactory learning in insects (Grotewiel et al. 1998). Volado mutants demonstrate impaired olfactory memories within a few minutes of training, suggesting that the α-integrin is important for short-term memory processes. A role for integrins in long-term plasticity in the hippocampus has also been demonstrated (Bahr et al. 1997; Staubli et al. 1998). These studies showed that although integrins do not play an important role in the induction of long-term potentiation (LTP), they are required for the maintenance of LTP. In addition, recent studies in Aplysia have shown changes in integrin expression in response to both behavioral sensitization and in vitro analogs such as treatment with 5-HT to isolated ganglia (Ren et al. 1997). Together these studies implicate two roles for integrins in long-term plasticity, that is, stabilization of synaptic contacts and activation of TGF-β1.
In developmental systems, an increase in signaling by TGF-β family members relies on protein–protein interactions with members of the astacin family of zinc metalloproteases such as Drosophila tolloid or human BMP-1 (Wozney et al. 1988; Shimell et al. 1991; Childs and O’Conner 1994; Finelli et al. 1994; Sarras 1996). Although these proteases have been found to cleave inhibitory proteins from TGF-β family members that results in increased signaling (Piccolo et al. 1996, 1997; Marquez et al. 1997), it is not clear whether they perform further functions. An Aplysia homolog of tolloid and BMP-1, apTBL-1, has been cloned recently (Liu et al. 1997). Interestingly, both behavioral sensitization and treatment with 5-HT result in an increase of apTBL-1 mRNA levels. Furthermore, protein levels have been found to increase in sensory neuron cell bodies by 3 hr after 5-HT treatment (R. Zwartjes, pers. comm.). This plasticity-modulated increase suggests that interaction with apTBL-1 may also be a mechanism for regulation of TGF-β action.
The effects of TGF-β1 in long-term synaptic facilitation and long-term increases in sensory neuron excitability in Aplysia add to the large, and ever growing amount of evidence that developmental plasticity and learning-related plasticity share many common mechanisms (Carew et al. 1998). Growth factors first found to mediate growth and development of the nervous system are now known to also mediate synaptic plasticity in adult animals. The neurotrophins neurotrophin-4/5 (NT-4/5), neurotrophin-3 (NT-3), nerve growth factor (NGF) and in particular, brain-derived neurotrophic factor (BDNF) have been studied extensively for their roles in modulating synaptic transmission in developmental systems as well as adult animals (for review, see Fitzsimonds and Poo 1998; Schuman 1999). In cultured neurons and hippocampal slices, these neurotrophins have been found to enhance both spontaneous and evoked release, and may involve modulation of both pre- and postsynaptic mechanisms (for review, see Levine and Black 1997). Neurotrophins also act similarly in vivo to induce plasticity. Intracerebroventricular infusions of NT-3, NT-4/5, or NGF can improve age-related declines in water-maze performance (Fischer et al. 1994). Infusion of BDNF into the hippocampus of adult rats induces long-lasting enhancement of synaptic transmission in the dentate gyrus (Messaoudi et al. 1998). Recent analysis of the ataxic mutant mouse stargazer demonstrated that BDNF levels in the cerebellum of these mutant mice are reduced by 70%, and that these mice fail to learn the conditioned eyeblink response when a tone and a shock to the eye are paired (Qiao et al. 1998). These studies and others provide evidence that growth factors play important roles in both developmental and learning-related synaptic plasticity.
Increasing evidence now demonstrates that TGF-β1 also plays a role in neuronal plasticity. The role for TGF-β1 in synaptic plasticity has only been shown recently, thus little is known about the mechanisms that regulate TGF-β1 activity or how TGF-β1 transduces its signal to the nucleus to bring about the electrophysiological and morphological changes underlying plasticity. Further work is required to determine the mechanisms governing the activity of TGF-β1 and to compare the effects of TGF-β1 in Aplysia with that of growth factors in other systems, such as those described above.
Acknowledgments
We thank Drs. E. Kabotyanski and G. Phares for helpful discussions and comments on earlier versions of the manuscript and R. Grenda and H. Zhang for assistance with the illustrations. This work was supported by National Institutes of Health (NIH) predoctoral fellowship F31MH1210701 to J.C. and NIH grants NS19895 to J.H.B, NS28462 to A.E and NS38100 to L.J.C.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Ata AK, Funa K, Olsson Y. Expression of various TGF-β isoforms and type I receptor in necrotizing human brain lesions. Acta Neuropathol. 1997;93:326–333. doi: 10.1007/s004010050623. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Staubli U, Xiao P, Chun D, Ji Z, Esteban E, Lynch G. Arg-Gly-Asp-Ser-selective adhesion and the stabilization of long-term potentiation: Pharmacological studies and the characterization of a candidate matrix receptor. J Neurosci. 1997;17:1320–1329. doi: 10.1523/JNEUROSCI.17-04-01320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Montarolo PG, Chen M, Kandel ER, Schacher S. Inhibitors of protein and RNA synthesis block structural changes that accompany long-term synaptic plasticity in Aplysia. Neuron. 1992;9:749–758. doi: 10.1016/0896-6273(92)90037-e. [DOI] [PubMed] [Google Scholar]
- Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor β. Biochim Biophys Acta. 1990;1032:79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Bawab W, Querido E, Crine P, DesGroseillers L. Identification and characterization of aminopeptidases from Aplysia californica. Biochem J. 1992;286:967–975. doi: 10.1042/bj2860967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald L, Oreffo R, Seyedin S, Mundy G. Isolated osteoclasts activate latent TGF-β. J Bone Min Res. 1988;120:158. doi: 10.1016/0006-291x(89)92795-2. [DOI] [PubMed] [Google Scholar]
- Brown PD, Wakefield LM, Levinson AD, Sporn MB. Physicochemical activation of recombinant latent transforming growth factor-beta’s 1, 2, and 3. Growth Factors. 1990;3:35–44. doi: 10.3109/08977199009037500. [DOI] [PubMed] [Google Scholar]
- Brummel TJ, Twombly V, Marques G, Wrana J, Newfield SJ. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell. 1994;78:251–261. doi: 10.1016/0092-8674(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Mauelshagen J, Carew TJ. Reversal of relative thresholds for synaptic facilitation and increased excitability induced by serotonin and tail nerve stimulation in Aplysia sensory neurons. Neurobiol Learn & Mem. 1997;67:259–263. doi: 10.1006/nlme.1996.3764. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: State- and time-dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JH, Baxter DA, Buonamano DV, Cleary LJ, Eskin A, Goldsmith JR, McClendon E, Nazif FA, Noel F, Scholz KP. Neural and molecular bases of nonassociative and associative learning in Aplysia. Ann New York Acad Sci. 1991;627:124–149. doi: 10.1111/j.1749-6632.1991.tb25918.x. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Zwartjes R, Homayouni R, Critz S, Eskin A. Roles of second messenger pathways in neuronal plasticity and in learning and memory: Insights gained from Aplysia. In: In: Nairn AC, Shenolikar S, editors. Advances in second messenger and phosphoprotein research. New York, NY: Raven Press; 1993. pp. 47–108. [PubMed] [Google Scholar]
- Carew TJ, Menzel R, Shatz C. Mechanistic relationships between development and learning. West Sussex, NJ: John Wiley & Sons Ltd.; 1998. [Google Scholar]
- Castellucci VF, Blumenfeld H, Goelet P, Kandel ER. Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J Neurobiol. 1989;20:1–9. doi: 10.1002/neu.480200102. [DOI] [PubMed] [Google Scholar]
- Childs SR, O’Connor MB. Two domains of the tolloid protein contribute to its unusual genetic interaction with decapentaplegic. Dev Biol. 1994;162:209–220. doi: 10.1006/dbio.1994.1079. [DOI] [PubMed] [Google Scholar]
- Cleary LJ, Lee WL, Byrne JH. Cellular correlates of long-term sensitization in Aplysia. J Neurosci. 1998;18:5988–5998. doi: 10.1523/JNEUROSCI.18-15-05988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Dale N, Kandel ER, Schacher S. Serotonin produces long-term changes in the excitability of Aplysia sensory neurons in culture that depend on new protein synthesis. J Neurosci. 1987;7:2232–2238. doi: 10.1523/JNEUROSCI.07-07-02232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng X-H. Smads: Transcriptional activators of TGF-β responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- Emptage NT, Carew TJ. Long-term facilitation in the absence of short-term facilitation in Aplysia neurons. Science. 1993;262:253–255. doi: 10.1126/science.8211146. [DOI] [PubMed] [Google Scholar]
- Estevez M, Attisano L, Wrana J, Albert PS, Massagué J, Riddle DL. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- Farr, M., J. Mathews, D.-F. Zhu, and R. Ambron. 1999. Inflammation causes a long-term hyperexcitability in the nociceptive sensory neurons of Aplysia. Learn. Mem. (this issue). [PMC free article] [PubMed]
- Finelli A, Bossie C, Xie T, Padgett R. Mutational analysis of the Drosophila tolloid gene, a human BMP-1 homologue. Development. 1994;120:861–870. doi: 10.1242/dev.120.4.861. [DOI] [PubMed] [Google Scholar]
- Fischer W, Sirevaag A, Wiegand SJ, Lindsay RM, Bjorkland A. Reversal of spatial memory impairments in aged rats by nerve growth factor and neurotrophin-3 and neurotrophin-4/5 but not by brain-derived neurotrophic factor. Proc Natl Acad Sci. 1994;91:8607–8611. doi: 10.1073/pnas.91.18.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimonds RM, Poo MM. Retrograde signaling in the development and modification of synapses. Physiol Rev. 1998;78:143–170. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Ren RF, Lippa CF. Transforming growth factor-βs in neurodegenerative diseases. Prog Neurobiol. 1998;54:71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R, Kojima S, Abe A, Rifkin D. Activation of latent transforming growth factor β. Adv Pharmacol. 1993;24:51–76. doi: 10.1016/s1054-3589(08)60933-3. [DOI] [PubMed] [Google Scholar]
- Frost WN, Castellucci VF, Hawkins RD, Kandel ER. Monosynaptic connections made by the sensory neurons of the gill- and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Nat Acad Sci. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotoweil MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Heldin C-H, Miyazano K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hogan B. Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes & Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Jullien P, Berg TM, Lawrence DA. Acidic cellular environments: Activation of latent TGF-β and sensitization of cellular responses to TGF-β and EGF. Int J Cancer. 1989;43:886–891. doi: 10.1002/ijc.2910430525. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jeang KT, Glick AB, Sporn MB, Roberts AB. Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. J Biol Chem. 1989;264:7041–7045. [PubMed] [Google Scholar]
- Koli K, Arteaga CL. Processing of the transforming growth factor β type I and II receptors. J Biol Chem. 1997;272:6423–6427. doi: 10.1074/jbc.272.10.6423. [DOI] [PubMed] [Google Scholar]
- Levine ES, Black I. Trophic factors, synaptic plasticity, and memory. Ann NY Acad Sci. 1997;835:12–19. doi: 10.1111/j.1749-6632.1997.tb48614.x. [DOI] [PubMed] [Google Scholar]
- Liu, Q.R., S. Endo, S. Hattar, K. MacPhee, H. Zhang, L. Cleary, J.H. Byrne, and A. Eskin. 1997. A developmental gene (Tolloid/BMP-1) is regulated in Aplysia neurons by treatments that induce long-term sensitization. J. Neurosci. 17: No.2. [DOI] [PMC free article] [PubMed]
- Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent transforming growth factor β1 by plasmin. J Cell Biol. 1990;110:1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques G, Musacchio M, Shimell M, Wunnunberg-Stapleton K, Cho K, O’Connor MB. Production of a DPP gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- Martin KC, Michael D, Rose JC, Barad M, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- Massagué J. Crossing receptor boundaries. Nature. 1996a;382:29–30. doi: 10.1038/382029a0. [DOI] [PubMed] [Google Scholar]
- ————— TGF-β signaling: Receptors, transducers, and mad proteins. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- ————— TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massagué J, Weis-Garcia F. Serine/threonine kinase receptors: Mediators of transforming growth factor beta family signals. Cancer Surv. 1996;27:41–64. [PubMed] [Google Scholar]
- Massagué J, Hata A, Liu F. TGF-β signaling through the Smad pathway. Trends Cell Biol. 1997;7:187–192. doi: 10.1016/S0962-8924(97)01036-2. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Bardsen K, Srebo B, Bramham CR. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 1998;79:496–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- Michael D, Martin KC, Seger R, Ning MM, Baston R, Kandel ER. Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia. Proc Natl Acad Sci. 1998;95:1864–1869. doi: 10.1073/pnas.95.4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER. A critical period of macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Munger J, Huang X, Kawakatsu H, Griffiths M, Dalton SL, Wu J, Pittet J-F, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin α5β6 binds and activates latent TGF-β1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Obata H, Kabuaki T, Kato M, Hamayashi H. Expression of TGF-β type I and type II receptors in rat eyes. Curr Eye Res. 1996;15:335–340. doi: 10.3109/02713689609007629. [DOI] [PubMed] [Google Scholar]
- Padgett RW, Wozney J, Gelbart WM. Human BMP sequences can confer normal dorsal-ventral patterning in the Drosophila embryo. Proc Natl Acad Sci. 1993;90:2905–2909. doi: 10.1073/pnas.90.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton A, Chen YJ, Staehling-Hampton K, Wrana J, Attisano L, Szidonya J, Cassill JA, Massagué J, Hoffman FM. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell. 1994;78:239–250. doi: 10.1016/0092-8674(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis E. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis E. Cleavage of chordin by xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann Organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M, Farr J, Zhu D, Zhang XP, Lewin M, Ambron RT. Inflammation induces hyperexcitability in Aplysia nociceptive neurons: Putative roles for TGF-β and amebocyte-neuron recognition. Soc Neurosci. 1998;24:555. [Google Scholar]
- Qiao X, Chen L, Gao G, Bao S, Heft F, Thompson RF, Knusel B. Cerebellar brain-derived neurotrophic factor-TrB defect associated with impairment of eyeblink conditioning in Stargazer mutant mice. J Neurosci. 1998;18:6990–6999. doi: 10.1523/JNEUROSCI.18-17-06990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayport SG, Schacher S. Synaptic plasticity in vitro: Cell culture of identified Aplysia neurons mediating short-term habituation and sensitization. J Neurosci. 1986;6:759–763. doi: 10.1523/JNEUROSCI.06-03-00759.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Endo S, Sherry DM, Eskin A. Identification of extracellular matrix proteins in the pleural-pedal ganglia of Aplysia californica. Soc Neurosci Abst. 1997;23:524.18. [Google Scholar]
- Roberts AB, Sporn MB. Transforming growth factor-β. In: Clark RA, editor. The molecular and cellular biology of wound repair. New York, NY: Plenum Press; 1996. pp. 275–307. [Google Scholar]
- Sampath T, Rashka KE, Doctor JS, Tucker RF, Hoffman FM. Drosophila transforming growth factor beta superfamily proteins induce endochondral bone formation in mammals. Proc Natl Acad Sci. 1993;9:6004–6008. doi: 10.1073/pnas.90.13.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarras MP. BMP-1 and the astacin family of metalloproteases: A potential link between the extracellular matrix, growth factors and pattern formation. BioEssays. 1996;18:439–442. doi: 10.1002/bies.950180604. [DOI] [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: Activation of a latent transforming growth factor-β1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S, Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: Modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983;3:2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman E. Neurotrophin regulation of synaptic transmission. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Shimell MJ, Ferguson EL, Childs S, O’Connor MB. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein-1. Cell. 1991;67:469–481. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- Staubli U, Chun D, Lynch G. Time dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu G, Willson JKV, Zborowska E, Yang J, Rajkarunanayake I, Wang J, Gentry LE, Wang X-F, Brattain MG. Expression of transforming growth factor type II receptor leads to reduced malignancy in human breast cancer MCF-7 cells. J Biol Chem. 1994;269:26449–26445. [PubMed] [Google Scholar]
- Unsicker K, Meier C, Krieglstein K, Sartor BM, Flanders K. Expression, localization and function of transforming growth factor-βs in embryonic chick spinal cord, hindbrain, and dorsal root ganglia. J Neurobiol. 1996;29:262–276. doi: 10.1002/(SICI)1097-4695(199602)29:2<262::AID-NEU10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Walters ET. Multiple sensory neuronal correlates of site-specific sensitization in Aplysia. J Neurosci. 1987;7:408–417. doi: 10.1523/JNEUROSCI.07-02-00408.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M. Smads and early developmental signaling by the TGF-β superfamily. Genes & Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- Wozney J, Rosen V, Celeste A, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang X-F, Massagué J. TGF-β signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wiser R, Ventura F, Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Zhang F. “Long-term synaptic facilitation of sensory-motor connections in Aplysia.” Ph.D. thesis. Houston, TX: University of Texas-Houston Graduate School of Biomedical Sciences; 1997. [Google Scholar]
- Zhang F, Endo S, Cleary L, Eskin A, Byrne JH. Role of transforming growth factor beta in long-term synaptic facilitation in Aplysia. Science. 1997;275:1318–1320. doi: 10.1126/science.275.5304.1318. [DOI] [PubMed] [Google Scholar]