Abstract
We have studied the effect of training conditions on hippocampal protein synthesis-dependent processes in consolidation of the inhibitory avoidance task. Adult male Wistar rats were trained and tested in a step-down inhibitory avoidance task (0.4 mA foot shock, 24 hr training–test interval). Fifteen minutes before or 0, 3, or 6 hr after training, animals received a 0.8-μl intrahippocampal infusion of the protein-synthesis inhibitor anisomycin (80 μg) or vehicle (PBS, pH 7.4). The infusion of anisomycin impaired retention test performance in animals injected 15 min before and 3 hr after the training session, but not at 0 or 6 h post-training. Pretraining with a low foot shock intensity (0.2 mA) 24 hr before training, prevented the amnestic effect of anisomycin injected at 15 min before or 3 hr after training. However, simple pre-exposure to the inhibitory avoidance apparatus did not alter the amestic effects of anisomycin. The results suggest that hippocampal protein synthesis is critical in two periods, around the time of, and 3 hr after training. A prior weak training session, however, which does not itself alter step-down latencies, is sufficient to prevent the amnestic effect of anisomycin, suggesting that even if not behaviorally detectable, weak training must be sufficient to produce some lasting cellular expression of the experience.
A distinguishing characteristic of long-term memory is its sensitivity to inhibitors of protein synthesis (Davis and Squire 1984). Earlier experiments, in many different paradigms and with a variety of species, demonstrated the importance of a single consolidation phase sensitive to inhibitors of protein synthesis at or around the time of training (Barraco and Stettner 1976; Davis and Squire 1984). One hr or more after the termination of the training protocol, memory was said to have entered a long-term, protein synthesis-independent phase (Gibbs et al. 1977). However, more recently it has become apparent that even beyond this early period, there are time windows during which later expression of memory is impaired by injection of protein-synthesis inhibitors. At least two such sensitive periods during which protein-synthesis inhibitors exert amnesic effects have been identified (Grecksch and Mathies 1980; Freeman et al. 1995; Chew et al. 1996). For example, two distinct time windows for the amnesic effect of protein-synthesis inhibitor anisomycin were reported for a passive avoidance task in chicks, one around the time of training and the other some 4 hr post-training (Freeman et al. 1995). The early phase was interpreted as being that during which transcription factors and immediate early genes were being expressed, the later phase that during which structural genes were being translated and their protein products inserted into synaptic structures during the remodeling believed to be required for longer term memory. At the molecular level, multiple waves of protein and gene induction have been observed during long-term facilitation in Aplysia (Barzilai et al. 1989) and long-term potentiation in the mammalian hippocampus (Abraham et al. 1993). Also shown was the activation of transcription factors and the induction of immediately early genes following training in different learning paradigms (for review, see Herdegen and Leah 1988). In addition, it was shown that following a single training trial in the step-down inhibitory avoidance, there are two periods of increased phospho-CREB immunoreactivity in the hippocampus, one immediately after, and another 3–6 hr after training (Bernabeu et al. 1997).
In a recent report, Bourtchouladze et al. (1998) demonstrated that weak training for contextual fear conditioning in mice shows two time periods of sensitivity to anisomycin, whereas stronger training exhibits only one. These studies suggest that different training protocols may recruit a common signaling pathway, albeit via different routes.
The involvement of biochemical events in the hippocampus related to long-term memory formation has been studied extensively in rats with a one trial step-down inhibitory avoidance task (for review, see Izquierdo and Medina 1997). As with many other tasks (Morris et al 1986; Burchuladze and Rose 1992), NMDA receptor antagonists such as AP5 are amnestic for the avoidance if injected into the hippocampus before and immediately after the training session. However, it was found recently that either pretraining or pre-exposure to the task apparatus could prevent the amnesia induced by intrahippocampal infusion of AP5 (Roesler et al. 1998). This resembles the finding that both nonspatial (Saucier and Cain 1995) and spatial (Bannerman et al. 1995) pretraining prevent the impairing effect of NMDA receptor antagonists on spatial recall of the Morris water maze, a task that depends on the hippocampus. This observation led Morris and colleagues to speculate that the amnestic effect of the NMDA blockers was more a response to novelty than to the specificity of the task per se. Could a similar effect account for the blockade of the AP5 effect by preexposure in the inhibitory avoidance task, and if so, might the same be the case for the effects of the protein-synthesis inhibitors? If so, the implications of the evidence for the universal involvement of protein-synthesis mechanisms in long-term memory consolidation (DeZazzo and Tully 1995) might need to be re-evaluated.
Therefore, the goal of the present experiments was to utilize the inhibitory avoidance task to evaluate the effects of pre- and multiple training on protein-synthesis-dependent mechanisms in the consolidation process. To do this, we explored the time-dependent interactions between experience of the task apparatus, training, and infusions of anisomycin on recall of the inhibitory avoidance.
Materials and Methods
SUBJECTS
A total of 220 male Wistar rats (age, 3–4 months; weight, 220–310 grams) were obtained from our breeding colony (Departmento de Bioquímica, ICBS, UFRGS, Porto Alegre, Brazil). They were housed five to a cage with food and water available ad libitum, and were maintained on a 12-hr light/dark cycle (lights on at 7 a.m.). Behavioral procedures were conducted between 1 and 4 p.m.
SURGERY
Animals were bilaterally implanted under thionembutal anesthesia (30 mg/kg, intraperitoneally) with a 9.0-mm guide cannulae aimed 1.0 mm above the dorsal CA1 region of hippocampus. Stereotaxic coordinates were according to the atlas of Paxinos and Watson (1986): A-4.3, L ± 4.0, V 3.4.
BEHAVIORAL PROCEDURES
Animals were trained 3–7 days after surgery. The inhibitory avoidance apparatus was a 50 × 25 × 25-cm acrylic box, whose floor consisted of parallel stainless-steel bars (1 mm diam.) spaced 1 cm apart. A 7-cm wide × 2.5-cm high platform was placed on the floor of the box against the left wall. Animals were placed on the platform and their latency to step down on the grid with all four paws was measured with an automatic device. In training sessions, immediately after stepping down on the grid, the animals received a 2.0-sec scrambled foot shock (Izquierdo et al. 1992, 1997; Jerusalinsky et al. 1992; Roesler et al. 1998). The shock intensity was 0.4 mA for animals given only one training session (experiments 1 and 3) and 0.2 mA for animals given two training sessions (experiment 2) (Roesler et al. 1998). In test sessions, no foot shock was administered and the step-down latency (maximum 180 sec) was used as a measure of retention (Izquierdo et al. 1992, 1997; Jerusalinsky et al. 1992; Roesler et al. 1998). In experiment 1, animals were given a single training session, followed by a retention test session 24 hr later. In experiment 2, the animals were given two training sessions separated by a 24-hr interval, followed by a retention test session carried out 24 h after the second training (Roesler et al. 1998). In experiment 3, the animals were pre-exposed to the task apparatus 24 hr before training. In this pre-exposure session, animals were placed on the platform and allowed to explore the box freely for 5 min without foot shock (Roesler et al. 1998). A training session was carried out 24 hr after pre-exposure, followed by a test session 24 hr after training.
DRUGS AND INFUSION PROCEDURES
Fifteen minutes before or 0, 3, or 6 hr after the inhibitory avoidance training session, an infusion cannula was fitted into the guide cannula. The tip of the infusion cannula protruded 1 mm beyond the guide cannula and was aimed at the CA1 area of the dorsal hippocampus. In experiment 1, infusions were made at 15 min pretraining, or 0, 3, and 6 hr post-training. In the other experiments, infusions were made only 15 min before or 3 hr after either the single (experiment 3) or the second (experiment 2) training session.
The animals received a bilateral 0.8-μl infusion of vehicle (PBS at pH 7.4) or anisomycin (80 μg/side) (Sigma) via the infusion cannula. Anisomycin was dissolved in a minimal volume of 3 n HCl and the solution adjusted to pH 7.2 and brought to a concentration of 100 μg/μl by addiction of 3n NaOH (Tiunova et al. 1996). The dose we used is likely to inhibit most protein synthesis in the hippocampus, as it is much higher than doses shown to inhibit protein synthesis in the chick brain (Freeman et al. 1995), in rat hippocampal slices (Frey and Morris 1997, 1998), and in the Hermissenda nervous system (Crow and Forrester 1990).
HISTOLOGY
Postmortem verification of cannulae placements was performed as described in previous papers (Izquierdo et al. 1992, 1997; Jerusalinsky et al. 1992). Briefly, animals were killed by decapitation, and 0.8 μl of a solution of 5% methylene blue in saline was infused through the cannulae. Brains were stored in formalin for at least 72 hr and cannulae placements were verified by histological examination. Cannulae were found to be correctly placed in the CA1 region of the dorsal hippocampus in 192 rats (Fig. 1). Only data from these animals were included in the final analysis.
Figure 1.

Schematic drawing of plane A—4.3 of the atlas of Paxinos and Watson (1986)—showing (stippled) the extent of the area reached by the infusion in the dorsal hippocampus.
STATISTICAL ANALYSIS
Data for inhibitory avoidance are shown as median (interquartile range) of step-down latencies. Comparisons of both training and test session step-down latencies between groups were performed with a Mann–Whitney U test. Comparisons between training and test sessions were done with a Wilcoxon test (Roesler et al. 1998).
Results
EXPERIMENT 1: two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats
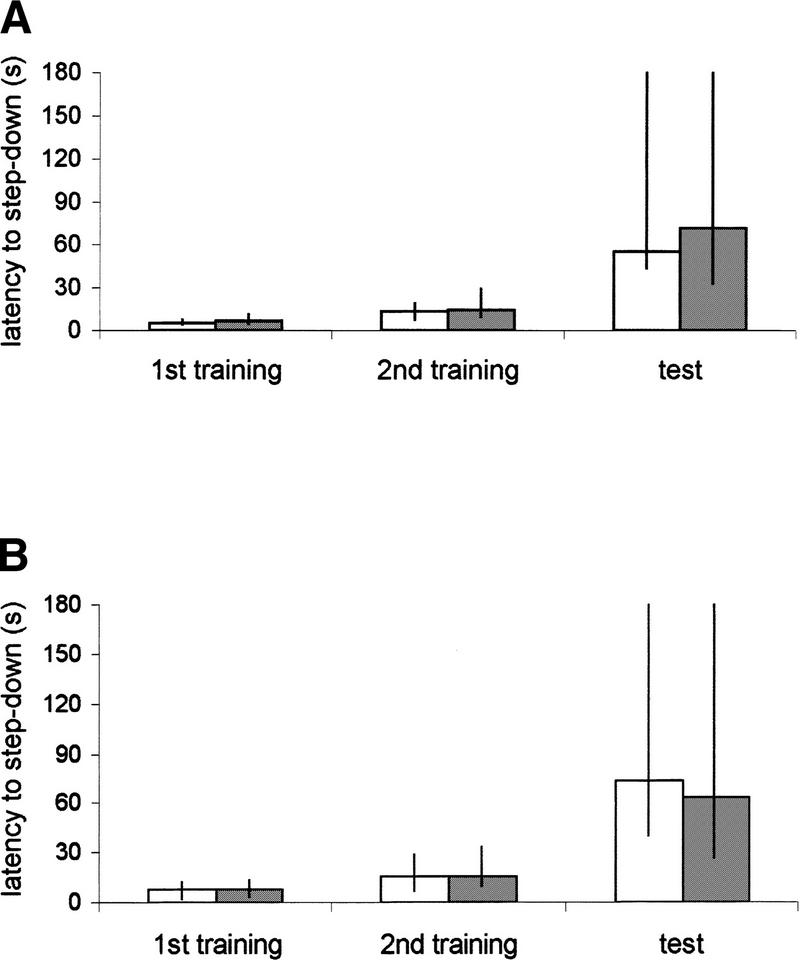
The first experiment was designed to evaluate the effects of pretraining (−15 min) or post-training (0, +3, or +6 hr) infusions of the protein-synthesis inhibitor anisomycin into the hippocampus on retention of an inhibitory avoidance response. The results are shown in Figure 2. There were no significant differences between groups in training performance in any group studied. However, anisomycin caused impairment of retention when injected at either −15 min or +3 hr (Mann–Whitney U test, P < 0.01), although not at 0 or +6 hr. In all groups, except that injected at −15 min, there were significant training-test differences (Wilcoxon test, P < 0.01). This result is consistent with previous studies showing two time windows of anisomycin-induced amnesia in passive avoidance for chicks (Freeman et al. 1995) and in contextual fear conditioning for mouse (Bourtchouladze et al. 1998).
Figure 2.
Effects of anisomycin on retention of one-trial step-down inhibitory avoidance task in rats (foot shock intensity, 0.4 ma; training-test interval, 24 hr). Data are expressed as median (interquartile range) training and test session latencies (in sec). Animals received bilateral 0.8-μl infusions of vehicle (□) or anisomycin (▪; 80 μg) in the CA1 region of the dorsal hippocampus at different times before or post-training. n = 10–13 animals per group. (*) Significant difference when compared with control group (Mann–Whitney U test, P < 0.01). All groups, except anisomycin −15 min, showed significant training-test differences (Wilcoxon test, P < 0.01).
EXPERIMENT 2: pretraining protects against anisomycin-induced amnesia
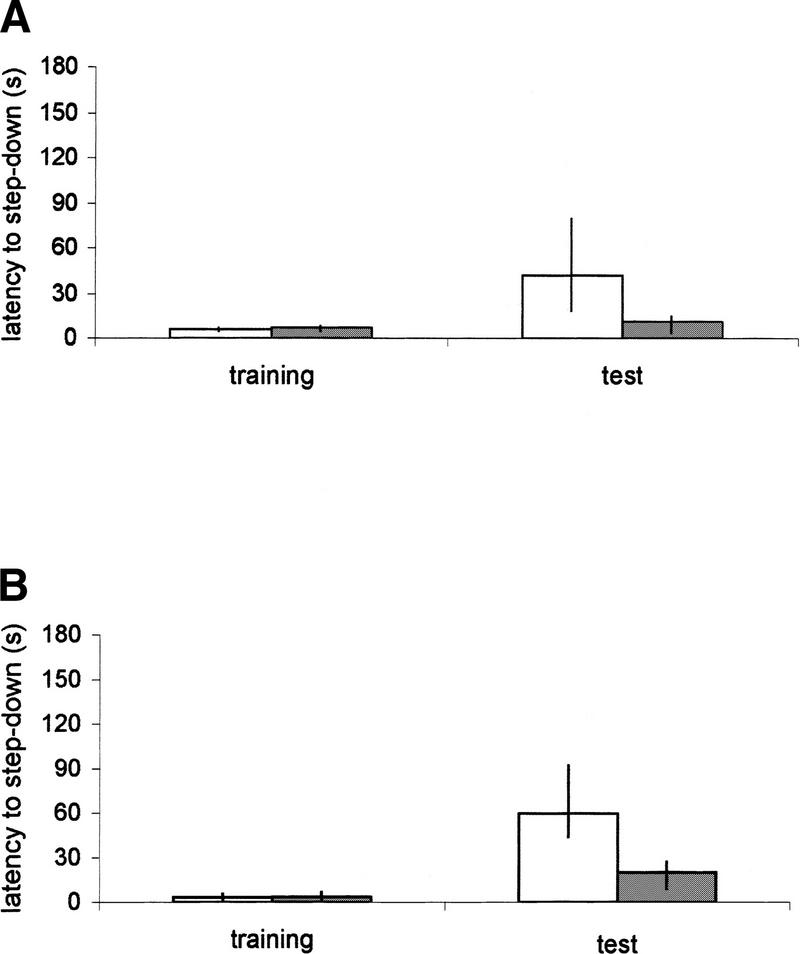
Experiment 2 was designed to determine whether pretraining of the rats on a weaker form of the task altered the amnestic effects of anisomycin. The results are shown in Figure 3A and B. Infusion of anisomycin 15 min before or 3 hr after the second training did not affect performance in any of the three sessions (Mann–Whitney U test, P >0.10). The difference between latencies in the second training compared with the test session was significant in all groups (Wilcoxon test, P < 0.01). This result indicates that pretraining prevents the anisomycin-induced amnesia.
Figure 3.
(A,B) Effects of anisomycin on enhancement of memory of step-down inhibitory avoidance task in rats pretrained in the same task (foot shock intensity, 0.2-ma; interval between sessions, 24 hr). Data of step-down inhibitory avoidance is expressed as median (interquartile range) session latencies (in seconds). Animals received bilateral 0.8-μl infusions of vehicle (□) or anisomycin (▪; 80 μg) in the dorsal hippocampus 15 min before (A) or 3 hr after (B) the second training session. n = 10–13 animals per group. There are no significant differences between groups in any of the three sessions (Mann–Whitney U test, P < 0.10). All groups showed significant difference between second training and test sessions (Wilcoxon test, P < 0.01).
EXPERIMENT 3: pre-exposure to the task apparatus does not prevent anisomycin-induced amnesia
To determine whether the prevention of anisomycin-induced amnesia by pretraining is due to the contextual or the aversive component of the inhibitory avoidance task in the next experiment, we merely pre-exposed the animals to the task apparatus without applying foot shock, followed 24 hr later by a standard training session. Anisomycin was injected 15 min before or 3 hr after the training session. The results are shown in Figure 4A and B. There were no significant differences between groups in training latencies (Mann–Whitney U test, p >0.10). However, anisomycin induced amnesia in both the −15 min and +3 hr groups (Mann–Whitney U test, P < 0.05). All groups, except anisomycin −15 min, showed significant training-test differences (Wilcoxon test, P <0.01). Thus, the abolition of the anisomycin-induced amnesia by pretraining is not a consequence simply of familiarity with the contextual component of the inhibitory avoidance task, but must relate to its aversive element.
Figure 4.
(A,B) Effects of anisomycin on retention of one trial step-down inhibitory avoidance task in rats preexposed to the task apparatus (foot shock intensity, 0.4 ma; training-test interval, 24 hr). Retention of step-down inhibitory avoidance is expressed as median (interquartile range) session latencies (in seconds). Animals received bilateral 0.8μl infusions of vehicle (□) or anisomycin (▪; 80 μg) in the dorsal hippocampus 15 min before (A) or 3 hr after (B) training session. n = 10–13 animals per group. (*) Significant difference when compared with control group (Mann–Whitney U test, P < 0.01). All groups, except anisomycin −15 min, showed significant training-test differences (Wilcoxon test, P < 0.01).
Discussion
These findings lead to three major conclusions. First, there are two time windows of anisomycin-induced amnesia for the one trial inhibitory avoidance task in rats. Second, pretraining, 24 hr previous to the training experience, blocks the amnestic effect of anisomycin whether applied just prior to or 3 hr post-training. Third, simple pre-exposure to the task apparatus does not prevent anisomycin-induced amnesia.
Several recent studies have demonstrated the importance of protein synthesis for long-term memory (Davis and Squire 1984; Freeman et al. 1995; Bourtchouladze et al. 1998). The two time windows for the amnestic effect of protein-synthesis blockade that we have found—the first around the time of training and the second ∼3 hr, but <6 hr, subsequently—are in accord with previous studies in both rats and chicks (Grecksch and Matthies 1980; Freeman et al. 1995; Bourtchouladze et al. 1998). Bourtchouladze et al. (1998), using contextual fear conditioning, have reported recently that the first period, around the time of training, is common to both weak and strong training protocols, whereas only in the weak protocol are there two sensitive periods, the first around, and the latter after, training. A similar conclusion has been arrived at by the one-trial passive avoidance task in chicks (Freeman et al. 1995; Scholey et al. 1995), and it has been suggested that whereas the first wave of protein synthesis is concerned with enhanced expression of immediately early genes and transcription factors, the second involves the structural proteins, including cell adhesion molecules, required for more lasting synaptic modulation (Anokhin et al. 1991; Rose 1995a,b; Bernabeu et al. 1997; Izquierdo and Medina 1997).
Surprisingly, anisomycin did not affect inhibitory avoidance retention when infused immediately after training. It has been shown that infusions of anisomycin into the chick hyperstriatum block inhibitory avoidance when given either pretraining (Freeman et al. 1995; Sojka et al. 1995) or up to 1.5 hr after training (Freeman et al. 1995). However, injections or anisomycin in mice impair inhibitory avoidance when given either at pretraining or immediately after training, but not shortly after (at 10, 20, or 30 min after training) (Davis et al. 1981). Thus, differences related to the species or route of drug administration used among different studies might explain the discrepancies in the results. Further studies that evaluate the dynamics of protein synthesis inhibition in the rat hippocampus are necessary to adequately address the finding that anisomycin had no effect when injected immediately after training in the rat hippocampus.
We have reported recently that both pretraining and pre-exposure to the task apparatus prevents the amnesia otherwise induced by intrahippocampal infusion of AP5 (Roesler et al. 1998). In the present paper, only pretraining on a weaker form of the task prevented the amnesia induced by intrahippocampal infusion of anisomycin. Even though the pretraining did not in itself produce a change in step-down latency, as shown in Figure 2, we must assume that some type of protein–synthesis-dependent trace was formed, sufficient to ensure that the second weak training session was enough to increase the step-down latency at the third test session. However, the retention induced by this second trial, as assessed by increased step-down latency in the retention test session apparently did not require further de novo hippocampal protein synthesis. There are a number of possible explanations for this independence. First, new protein synthesis might be occurring, but no longer in the hippocampus, as the injections were into a limited area. Second, relevant synapses could already have been modified—tagged, to use the term employed by Frey and Morris (1998) when they reinvented this original hypothesis introduced by Hyden (1973), and the subsequent modifications resulting from the second training session require only post-translational modification of these proteins, perhaps by glycosylation (Rose 1995). It is clear that the effects described in our experiments are training specific, because the single pre-exposure to the task apparatus was not able to prevent the anisomycin-induced amnesia, so we may conclude that such pre-exposure in itself does not result in recall-relevant new protein synthesis. Our results are biologically significant because they demonstrate an association between two consecutive events in a much longer time scale than previously shown in electrophysiological studies.
Together with our previous finding that intrahippocampal infusion of AP5 blocks retention of a new training trial but not of a second training, and with the early finding by Netto and Maltchik (1990) that opioid receptors modulate the first, but not the second training of inhibitory avoidance, the present results indicate that different neurochemical mechanisms are involved in the formation of memory of a new training and of a second training in a task in which the animal has been trained previously. Whereas the cascade of neurochemical events induced by a new, single-training trial in the step-down inhibitory avoidance task are now well known (Izquierdo and Medina 1997), the mechanisms involved in processing of a second training and their biological significance deserve further investigation.
In summary, we have shown that the normal recall of the step-down inhibitory avoidance task requires two waves of protein synthesis, but if a weaker version of the task is distributed across two training sessions, anisomycin given after the second session no longer impairs memory. The possible hypotheses to account for this observation can bet tested by further experiments.
Acknowledgments
This study was supported by Pronex (Brazil). J.Q., R.R., and F. de-P. are recipients of CAPES (Brazil) fellowships; M.R.M.V. is recipient of a CNPq (Brazil) fellowship.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Abraham WC, Mason SE, Demmer J, Williams JM, Richardson C, Tate W, Lawlor PA, Dragunow M. Correlation between immediate early gene induction and the persistence of long-term potentiation. Neuroscience. 1993;56:717–727. doi: 10.1016/0306-4522(93)90369-q. [DOI] [PubMed] [Google Scholar]
- Anokhin KV, Mileusnic R, Shamakina IY, Rose SP. Effects of early experience on c-fos gene expression in the chick forebrain. Brain Res. 1991;544:101–107. doi: 10.1016/0006-8993(91)90890-8. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RGM. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Barraco RA, Stettner LJ. Antibiotics and memory. Psychol Bull. 1976;83:242–302. [PubMed] [Google Scholar]
- Barzilai A, Kennedy TE, Sweat JD, Kandel ER. 5-HT modulates protein synthesis and the expression of specific proteins during long-term facilitation in Aplysia sensory neurons. Neuron. 1989;2:1577–1586. doi: 10.1016/0896-6273(89)90046-9. [DOI] [PubMed] [Google Scholar]
- Bernabeau R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathway in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Rose SPR. Memory formation in day-old chicks requires NMDA but non-NMDA glutamate receptors. Eur J Neurosci. 1992;4:533–538. doi: 10.1111/j.1460-9568.1992.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn & Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Chew S, Vicario D, Nottebohm F. Quantal duration of auditory memories. Science. 1996;274:1909–1914. doi: 10.1126/science.274.5294.1909. [DOI] [PubMed] [Google Scholar]
- Crow T, Forrester J. Inhibition of protein synthesis blocks long-term enhancement of generator potentials produced by one-trial in vivo conditioning in Hermissenda. Proc Natl Acad Sci. 1990;87:4490–4494. doi: 10.1073/pnas.87.12.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Rosenzweig MR, Kinkase PT, Benet EL. Effects of anisomycin on retention of the passive-avoidance habit as a function of age. Exp Aging Res. 1981;7:33–44. doi: 10.1080/03610738108259784. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire IR. Protein synthesis and memory: A review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- DeZazzo J, Tully T. Dissection of memory formation: From behavioral pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- Freeman F, Rose SPR, Scholey A. Two time windows of anisomycin-induced amnesia for passive avoidance training in the day-old chick. Neurobiol Learn Mem. 1995;63:291–295. doi: 10.1006/nlme.1995.1034. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- ————— Synaptic tagging: Implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Ng KT. Psychobiology of memory towards a model of memory formation. Behav Rev. 1977;1:113–136. [Google Scholar]
- Grecksch G, Matthies M. Two sensitive periods for the amnesic effect of anisomycin. Pharmacol Biochem Behav. 1980;12:663–665. doi: 10.1016/0091-3057(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox and CREB/ATF proteins. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hyden M. Changes in brain protein during learning. In: Ansele GB, Bradley PB, editors. Macromolecules and behaviour. London, UK: McMillan; 1973. pp. 3–50. [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MBC, Medina JH. Neurotransmitter receptors involved in memory processing by the amygdala, hippocampus and medial septum of rats. Behav Neural Biol. 1992;58:16–25. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Quillfeldt JA, Zanatta MS, Quevedo J, Schaeffer E, Schmitz PK, Medina JH. Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. Eur J Neurosci. 1997;9:786–793. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Jerusalinsky D, Ferreira MBC, Walz R, da Silva RC, Bianchin M, Ruschel AC, Zanatta MS, Medina JH, Izquierdo I. Amnesia by posttraining infusion of glutamate receptor antagonists into the amygdala, hippocampus, and entorhinal cortex. Behav Neural Biol. 1992;58:76–80. doi: 10.1016/0163-1047(92)90982-a. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockage of long-term potentiation by an N-methyl-D-aspartate agonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Netto CA, Maltchik M. Distinct mechanisms underlying memory modulation after the first and the second session of two avoidance tasks. Behav Neural Biol. 1990;53:29–38. doi: 10.1016/0163-1047(90)90763-v. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. San Diego, CA: Academic Press; 1986. [Google Scholar]
- Roesler R, Vianna M, Sant'Anna MK, Kuyven CR, Kruel AVS, Quevedo J, Ferreira MBC. Intrahippocampal infusion of the NMDA receptor antagonist AP5 impairs retention of an inhibitory avoidance task: Protection from impairment by pretraining or preexposure to the task apparatus. Neurobiol Learn Mem. 1998;69:87–91. doi: 10.1006/nlme.1997.3810. [DOI] [PubMed] [Google Scholar]
- Rose SP. Glycoproteins and memory formation. Behav Brain Res. 1995a;66:73–78. doi: 10.1016/0166-4328(94)00127-2. [DOI] [PubMed] [Google Scholar]
- ————— Cell-adhesion molecules, glucocorticoids and long-term memory formation. Trends Neurosci. 1995b;18:502–506. doi: 10.1016/0166-2236(95)92774-k. [DOI] [PubMed] [Google Scholar]
- Saucier D, Cain DP. Spatial learning without NMDA receptor-dependent long-term potentiation. Nature. 1995;378:186–189. doi: 10.1038/378186a0. [DOI] [PubMed] [Google Scholar]
- Scholey AB, Mileusnic R, Schachner M, Rose SP. A role for a chicken homolog of the neural cell adhesion molecule L1 in consolidation of memory for a passive avoidance task in the chick. Learn & Mem. 1995;2:17–25. doi: 10.1101/lm.2.1.17. [DOI] [PubMed] [Google Scholar]
- Sojka M, Davies HA, Harrison E, Stewart MG. Long-term increases in synaptic density in chicks CNS after passive avoidance training are blocked by an inhibitor of protein synthesis. Brain Res. 1995;684:209–214. doi: 10.1016/0006-8993(95)00403-d. [DOI] [PubMed] [Google Scholar]
- Tiunova A, Anokhin K, Rose SPR, Mileusnic R. Involvement of glutamate receptors, protein kinases, and protein synthesis in memory for visual discrimination in the young chick. Neurobiol Learn Mem. 1996;65:233–243. doi: 10.1006/nlme.1996.0028. [DOI] [PubMed] [Google Scholar]