Abstract
Congenital heart defects that have a component of right ventricular outflow tract obstruction, such as tetralogy of Fallot, are frequently palliated in childhood by disruption of the pulmonary valve. Although this can provide an initial improvement in quality of life, these patients are often left with severe pulmonary valve insufficiency. Over time, this insufficiency can lead to enlargement of the right ventricle and to the deterioration of right ventricular systolic and diastolic function. Pulmonary valve replacement in these patients decreases right ventricular volume overload and improves right ventricular performance. To date, few studies have examined the effects of pulmonary valve replacement on left ventricular function in patients with biventricular dysfunction. We sought to perform such an evaluation.
Records of adult patients who had undergone pulmonary valve replacement from January 2003 through November 2006 were analyzed retrospectively. We reviewed preoperative and postoperative echocardiograms and calculated left ventricular function in 38 patients.
In the entire cohort, the mean left ventricular ejection fraction increased by a mean of 0.07 after pulmonary valve replacement, which was a statistically significant change (P < 0.01). In patients with preoperative ejection fractions of less than 0.50, mean ejection fractions increased by 0.10.
We conclude that pulmonary valve replacement in patients with biventricular dysfunction arising from severe pulmonary insufficiency and right ventricular enlargement can improve left ventricular function. Prospective studies are needed to verify this finding.
Key words: Adult; cardiac output; cardiac volume; heart valve prosthesis; hemodynamics; pulmonary valve insufficiency/complications/surgery; reoperation; retrospective studies; stroke volume; tetralogy of Fallot/surgery; time factors; treatment outcome; ventricular dysfunction, left; ventricular dysfunction, right
Congenital heart defects that have a component of right ventricular (RV) outflow tract obstruction, such as tetralogy of Fallot, are frequently palliated in childhood by disruption of the pulmonary valve. Although this can provide an initial improvement in quality of life, these patients are often left with severe pulmonary valve insufficiency. Over time, this insufficiency can lead to enlargement of the RV1,2 and to worsening RV systolic and diastolic function.3 Pulmonary valve replacement (PVR) in these patients has been shown to decrease RV volume overload and improve RV performance.4 Although the optimal timing of PVR has yet to be determined, the improvements in RV health are well documented.
Much has been written about the RV after PVR, but data regarding the effect of PVR on the left ventricle (LV) are relatively few. Previous studies have suggested that there is an interaction between right and left ventricular function and that RV dysfunction can result in an unfavorable RV–LV interaction.5
Conversely, because of this known interaction, improvement in RV performance after PVR could translate into a favorable effect on the LV in patients who have preoperative biventricular dysfunction.
Patients and Methods
After approval from the institutional review board, we retrospectively identified adult patients from our surgical database who had undergone PVR from January 2003 through November 2006, late after repair of tetralogy of Fallot or other surgery for pulmonary valve disruption. Sixty patients were initially identified. Preoperative and postoperative echocardiograms were reviewed; 22 patients were excluded due to inability to obtain preoperative echocardiograms (3 patients), poor echocardiographic images that made measurement of the LV dimensions unreliable (18), and intraoperative death (1).
Patient Population
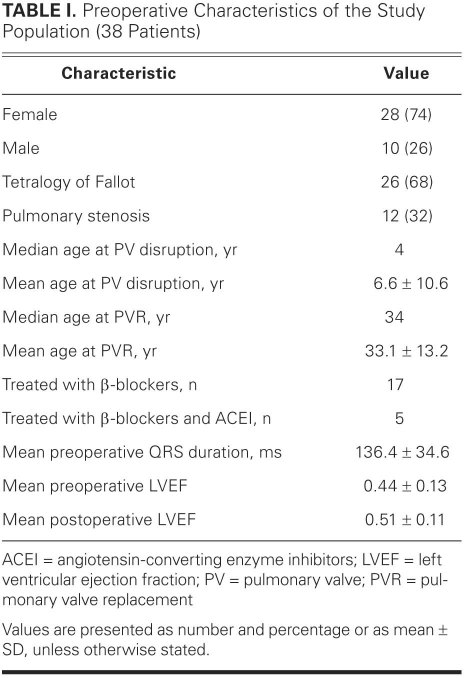
Thirty-eight patients were included in the final analysis. Data on these patients' preoperative characteristics appear in Table I. There were 28 females and 10 males. Twenty-six patients had a diagnosis of tetralogy of Fallot, and 12 patients had a diagnosis of pulmonary stenosis. The median age at the time of pulmonary valve disruption for the entire study population was 4 years, ranging from less than 1 year to 56 years. The mean age at the time of pulmonary valve disruption was 6.6 ± 10.6 years. Thirty-six patients had surgical disruption of the pulmonary valve at an age of less than 18 years. One patient required pulmonary valve disruption surgery a second time because of restenosis. The median age at PVR for the entire study population was 34 years, and the mean age was 33.1 ± 13.2 years. Six patients in our study underwent a second PVR, and in those cases we followed the results of that procedure.
TABLE I. Preoperative Characteristics of the Study Population (38 Patients)
Echocardiographic Measurements
Transthoracic echocardiograms were reviewed after our final identification of patients who had undergone PVR late after repair of tetralogy of Fallot or other surgery for pulmonary valve disruption. We chose as the preoperative echocardiogram the one performed closest to the time of surgery. The median time from PVR surgery to the postoperative echocardiogram was 55 days (range, 5–840 d). Data from the preoperative and postoperative echocardiograms were obtained by retrospective analysis. Left ventricular ejection fractions (LVEFs) were calculated by means of Simpson's method, using both the 2- and 4-chamber views. For those patients with 2-chamber views of insufficient quality to allow for accurate measurements, LVEFs were calculated using only the 4-chamber view in both the preoperative and postoperative studies.
Statistical Analysis
Preoperative and postoperative results were compared via a simple Student paired t test using Microsoft Excel 2007. A P value of less than 0.05 was considered statistically significant.
Results
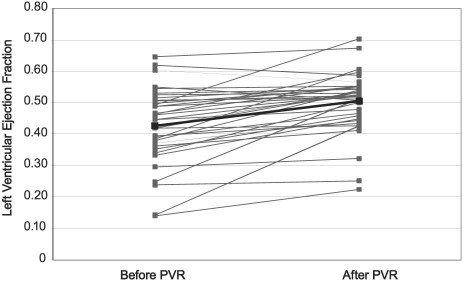
For the entire study population, there was a significant increase in LVEF between the preoperative echocardiogram (mean, 0.44 ± 0.13) and the postoperative echocardiogram (mean, 0.51 ± 0.11) (P <0.01). The mean increase was 0.07 (Fig. 1). Twenty-six patients had a preoperative LVEF of less than 0.50. For these patients, there was also a significant postoperative increase in the LVEF (mean, 0.48 ± 0.11), compared with the preoperative LVEF (mean, 0.38 ± 0.10) (P <0.01). The mean increase was 0.10. Thirty-three of the 38 patients (87%) had an improvement in LV function after PVR.
Fig. 1 Mean left ventricular ejection fraction change before and after pulmonary valve replacement surgery (PVR).
Two patients underwent pulmonary valve disruption surgery as adults, at ages 40 and 56 years. After PVR, the 1st patient had a slight increase in LVEF of 0.03. The 2nd patient had a decrease in LVEF of 0.04.
All told, 5 patients showed a slight decline in LVEF after PVR. The LVEF was reduced by 0.04 in 3 patients, 0.03 in 1, and 0.02 in 1. Despite these reductions, the postoperative LVEF was greater than 0.50 in 4 of the 5 patients.
Preoperative Use of Heart-Failure Medications
Twenty-one patients were not taking cardiovascular medications before surgery. The mean LVEF in these 21 patients increased by 0.07, from 0.47 to 0.54. Seventeen patients were taking a β-blocker preoperatively. In the β-blocker group, the mean LVEF increased by 0.07, from 0.41 to 0.48. Five patients in the β-blocker group were also treated preoperatively with angiotensin-converting enzyme inhibitors. Their mean LVEF increased by 0.05, from 0.40 to 0.45. The improvements in LVEF in patients taking β-blockers and those taking both β-blockers and angiotensin-converting enzyme inhibitors preoperatively were not significantly different from those of patients who were taking no cardiovascular medications (P = 0.4 and P = 0.3, respectively).
Discussion
Repair of tetralogy of Fallot in early childhood has low operative mortality rates and excellent long-term results.2,6 Many investigators have examined in depth the causal relationships between disruption of the pulmonary valve annulus and subsequent pulmonary insufficiency, deleterious effects on the RV, and increased risk of sudden death.7–10 Improvement in RV function after PVR has also been well documented in the medical literature.11,12 It has been suggested that the reduced volume load on the RV after PVR results in the rapid improvement of RV systolic function and in a more gradual recovery of RV diastolic function.3 Our study provides evidence of improvement in LV systolic function after PVR in patients with a history of pulmonary valve disruption surgery.
Overall, in our study, patients had a mean increase in LVEF of 0.07 (Fig. 1). Those patients who started with an LVEF of less than 0.50 experienced a greater mean increase of 0.10.
The exact cause of this favorable result is unclear. One likely cause is improved RV–LV interaction resulting from a decrease in RV volume. An unfavorable RV–LV interaction has been shown in patients with repaired tetralogy of Fallot.5 Another possibility is improved synchrony accompanied by a decrease in QRS duration.
Medications
The use of heart-failure medications preoperatively did not correlate with significant postoperative improvement in LVEF.
Ventricular Interaction
The interdependence of the RV and LV is well accepted. Previous studies have suggested that RV dysfunction can result in an unfavorable RV–LV interaction, which leads ultimately to LV dysfunction in the preoperative period.5 Because of the same interaction, improvement in RV performance after PVR could have a favorable effect on the LV in patients who are experiencing biventricular dysfunction.
Other mechanical mechanisms of decreased LV function late after pulmonary valve disruption to palliate tetralogy of Fallot or pulmonary stenosis can include abnormalities of septal motion due to patching of a ventricular septal defect or to septal fibrosis or myocardial injury in association with the initial repair. These abnormalities are not likely to improve after PVR. Improvement of LV function after PVR most probably involves improvement in the unfavorable interaction between a volume-overloaded, dilated RV and the LV.
Results for the 2 patients in our study who underwent pulmonary valve disruption at older ages did not show a meaningful trend toward improvement in LVEF. Undergoing pulmonary valve disruption and PVR therapy late in life raises the possibility of irreversible ventricular dysfunction. Although the optimal time for PVR in patients who have a RV that is beginning to dilate has not yet been established, it is probable that the passage of a certain period of time diminishes the improvement that can be expected in LV function consequent to improved RV–LV interaction.
Limitations
A major limitation of our study is the retrospective nature of the data collection. High-quality echocardiographic 2-chamber views were not available for all patients in our population. This necessitated the use of single-plane (4-chamber) images for analysis. While the absolute measurements of LV end-diastolic and end-systolic volumes might be affected by this limitation, changes in LVEF over time can still be evaluated. Clearly, better volumetric analysis, such as that available with the use of cardiovascular magnetic resonance, would have been ideal. Many of the patients in our study, however, received care before cardiovascular magnetic resonance was readily available.
Another possible limitation of this study lies in our placing patients with tetralogy of Fallot in the same category as those with pulmonary stenosis. Tetralogy of Fallot is of course a different disease entity, which raises other pathophysiologic concerns. In comparing the effects of pulmonary valve disruption, however, we believe that similarities between the 2 groups of patients can be used to evaluate the effects of PVR on LV systolic function.
Conclusion
Despite some limitations, this study clearly shows that LV function can improve, dramatically at times, after PVR in patients who manifest biventricular dysfunction some years after surgery for pulmonary valve disruption. Prospective studies are necessary to evaluate this finding and to identify those features that are associated with minimal or no improvement in LV function and those that are associated with favorable outcomes.
Footnotes
Address for reprints: Colin Kane, MD, Division of Pediatric Cardiology, 1935 Medical District Dr., Dallas, TX 75235
E-mail: colin.kane@utsouthwestern.edu
References
- 1.Gatzoulis MA, Elliott JT, Guru V, Siu SC, Warsi MA, Webb GD, et al. Right and left ventricular systolic function late after repair of tetralogy of Fallot. Am J Cardiol 2000;86(12):1352–7. [DOI] [PubMed]
- 2.Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med 1993;329 (9):593–9. [DOI] [PubMed]
- 3.van Straten A, Vliegen HW, Lamb HJ, Roes SD, van der Wall EE, Hazekamp MG, de Roos A. Time course of diastolic and systolic function improvement after pulmonary valve replacement in adult patients with tetralogy of Fallot. J Am Coll Cardiol 2005;46(8):1559–64. [DOI] [PubMed]
- 4.Vliegen HW, van Straten A, de Roos A, Roest AA, Schoof PH, Zwinderman AH, et al. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of Fallot. Circulation 2002;106(13):1703–7. [DOI] [PubMed]
- 5.Davlouros PA, Kilner PJ, Hornung TS, Li W, Francis JM, Moon JC, et al. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: detrimental role of right ventricular outflow aneurysms or akinesia and adverse right-to-left ventricular interaction. J Am Coll Cardiol 2002;40(11):2044–52. [DOI] [PubMed]
- 6.Horneffer PJ, Zahka KG, Rowe SA, Manolio TA, Gott VL, Reitz BA, Gardner TJ. Long-term results of total repair of tetralogy of Fallot in childhood. Ann Thorac Surg 1990;50(2): 179–85. [DOI] [PubMed]
- 7.Bove EL, Byrum CJ, Thomas FD, Kavey RE, Sondheimer HM, Blackman MS, Parker FB Jr. The influence of pulmonary insufficiency on ventricular function following repair of tetralogy of Fallot. Evaluation using radionuclide ventriculography. J Thorac Cardiovasc Surg 1983;85(5):691–6. [PubMed]
- 8.Ilbawi MN, Idriss FS, DeLeon SY, Muster AJ, Gidding SS, Berry TE, Paul MH. Factors that exaggerate the deleterious effects of pulmonary insufficiency on the right ventricle after tetralogy repair. Surgical implications. J Thorac Cardiovasc Surg 1987;93(1):36–44. [PubMed]
- 9.Zahka KG, Horneffer PJ, Rowe SA, Neill CA, Manolio TA, Kidd L, Gardner TJ. Long-term valvular function after total repair of tetralogy of Fallot. Relation to ventricular arrhythmias. Circulation 1988;78(5 Pt 2):III14–9. [PubMed]
- 10.Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation 1995;92(2):231–7. [DOI] [PubMed]
- 11.Bove EL, Kavey RE, Byrum CJ, Sondheimer HM, Blackman MS, Thomas FD. Improved right ventricular function following late pulmonary valve replacement for residual pulmonary insufficiency or stenosis. J Thorac Cardiovasc Surg 1985;90 (1):50–5. [PubMed]
- 12.Warner KG, Anderson JE, Fulton DR, Payne DD, Geggel RL, Marx GR. Restoration of the pulmonary valve reduces right ventricular volume overload after previous repair of tetralogy of Fallot. Circulation 1993;88(5 Pt 2):II189–97. [PubMed]