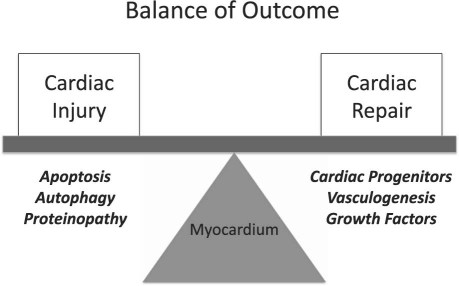
Cancer chemotherapy aims to induce rapid apoptosis or necrosis in proliferating cancer cells, often in union with growth deprivation, suppression of angiogenesis, or both. When these mechanisms are enacted in the heart, the result can be a terminally differentiated organ of limited proliferative potential, cell death, and organ dysfunction. This last develops when there is cumulative myocardial injury and a compromise in the endogenous cardiac repair capacity, which suggest a “multi-hit” hypothesis for the development of cancer-therapy–induced cardiac failure (Fig. 1).
Fig. 1 The balance of outcome in the pathogenesis of cardiotoxicity.
Novel Mechanisms of Cancer-Therapy–Induced Cardiomyocyte Injury
Anthracyclines such as doxorubicin are known to induce both double-stranded DNA breaks (possibly through their effect on topoisomerase 2-beta) and free-radical formation (through iron catalysis and mitochondrial activation). Both of these effects can lead to intracellular accumulation of mutated or oxidatively modified proteins, which in turn induces endoplasmic reticulum (ER) stress. The latter can accelerate the ubiquitin-proteasome system for protein removal but, when excessive, can induce activation of the cascade of caspases, thereby leading to cell death.
Anthracyclines can also lead to cardiomyocyte injury via the induction of autophagy. It has been known for 30 years that lysosomal pathways are activated both in models of heart disease and in samples from failing human hearts. Recent efforts from our laboratory and others have focused on pathways upstream of the lysosome, and specifically on cardiomyocyte autophagy.1 Autophagy is an evolutionarily conserved mechanism whereby damaged proteins and organelles are removed and recycled, a process that supports cell survival during times of stress. Within the context of certain developmental and disease states, however, autophagy promotes cell death.
A number of findings point to autophagy as playing a central role in doxorubicin-induced cardiomyopathy. First, doxorubicin exposure results in robust induction of autophagic activity in cardiomyocytes. Stimulation of autophagy in this setting increases cytotoxicity, whereas inhibition of autophagy decreases it. In animals exposed to doxorubicin, the mammalian target of rapamycin (mTOR, an established repressor of autophagy) is inhibited. Further, cardiomyocyte-restricted activation of mTOR protects against doxorubicin-induced cardiomyopathy. Interestingly, this cardiotoxicity is independent of apoptosis, which is consistent with a role for autophagy in the pathogenesis.
Given this, we have proposed that maladaptive cardiomyocyte autophagy is a mechanism underlying doxorubicin-induced heart failure, and work is under way in our laboratories to test this hypothesis. Coupled with this, we have strong evidence that histone deacetylase inhibition (HDACi), an emerging treatment option in oncology, blunts stress-induced maladaptive autophagy in cardiac myocytes. This finding is particularly striking, given that HDACi promotes autophagy in tumor cells.
Impaired Progenitor-Cell–Induced Repair May Contribute to Cancer-Therapy–Induced Cardiotoxicity
It is now also clear that the heart has intrinsic regenerative potential, albeit too low to regenerate effective myocardium after an injury such as myocardial infarction. Many of the new tyrosine kinase inhibitors specifically target repair-cell mobilization signals, such as c-kit (stem cell factor receptor). C-kit signaling turns out to be important in the mobilization of repair cells, such as mobilization from the bone marrow to the heart under conditions of injury. Even partial loss of c-kit signaling can lead to very significant impairment of repair-cell mobilization, and to defective cardiac remodeling and functional recovery.2 Furthermore, recent work has shown that anthracyclines deplete endogenous cardiac progenitors, which predisposes the heart to stress-induced damage.3 Importantly, recent studies suggest that the epicardium can encompass the niche in which endogenous cardiac progenitors reside, and that these epicardial progenitors significantly contribute to cardiac repair after ischemic injury.4 Taken together, these studies suggest that strategies designed to protect endogenous cardiac progenitors may be effective in preventing cancer-therapy–induced cardiotoxicity.
Reengineering Targeted Anti-Cancer Agents: A Way Forward
Although targeted cancer therapies such as tyrosine kinase inhibitors are typically aimed at molecules that are overexpressed in cancer cells, the fact remains that many receptor tyrosine kinases are expressed in normal tissues, and these molecules can play a role in the normal physiology of many organ systems, including the cardiovascular system. Furthermore, none of the small-molecule tyrosine kinase inhibitors are truly selective. Therefore, determining the mechanisms of toxicity requires the identification of the specific target responsible. Such target identification can drive the development of a new generation of highly effective anti-cancer agents that will have minimal effects on the cardiovascular system.
Our work with imatinib mesylate provides proof of the conceptual strategy of rational drug redesign to minimize cardiotoxic effects. Imatinib has revolutionized the treatment of chronic myelogenous leukemia and gastrointestinal stromal tumor (GIST), but a subset of imatinib-treated patients develops cardiac dysfunction and heart failure. Using preclinical in vitro and in vivo models, we found that imatinib administered at clinically relevant dosages exerts a direct toxic effect on cardiomyocytes, which is associated with activation of the ER stress response.5 Furthermore, overexpression of an imatinib-resistant isoform of Abl kinase (an established imatinib target) significantly reduced imatinib-induced cardiomyocyte death.
On the basis of these findings, we modified imatinib to hamper its inhibition of Abl kinase while preserving its effects on c-kit kinase, the imatinib target presumed to account for the drug's therapeutic efficacy in GIST patients. We found that the structurally modified imatinib had significantly diminished toxic effects on the heart, but maintained therapeutic efficacy in a preclinical GIST model.6 These findings show that reengineered imatinib maintains its efficacy in the treatment of GIST with minimal cardiotoxicity; and they reveal, furthermore, a rational approach to control drug specificity.
Cardiac Toxicity as a “Discovery Platform”
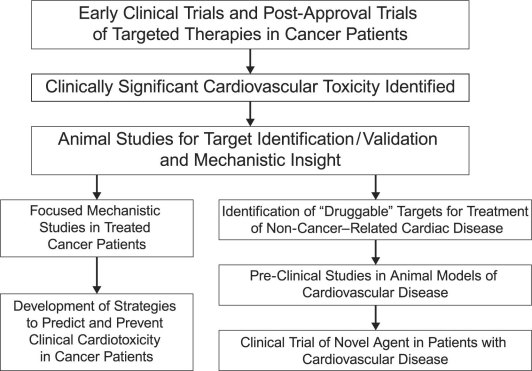
In addition to providing clues to the prevention of cancer-therapy–induced cardiotoxicity, a thorough understanding of the mechanics of that toxicity can reveal novel molecular-signaling pathways that are relevant to non-cancer-therapy–induced heart failure (Fig. 2). For example, insights gained from cardiac toxicity due to trastuzumab—a monoclonal antibody directed against ErbB2—gave rise to elegant studies demonstrating a key role of ErbB2 signaling in cardiac biology. Subsequently, preclinical work showed a potent cardioprotective effect of neuregulin-1, an ErbB2 agonist, in diverse models of cardiac failure. Indeed, neuregulin-1 is now in clinical trials for the treatment of patients with chronic left ventricular (LV) dysfunction and heart failure.7
Fig. 2 Cardiotoxicity as a discovery platform.
Recently, probing the mechanism of cardiac toxicity of sunitinib malate has led to novel insights in the role of platelet-derived growth factor receptor (PDGFR) signaling in the heart. Sunitinib malate, a receptor tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR), PDGFR, and c-kit, is used for the treatment of metastatic renal cell carcinoma. We and others have reported that sunitinib results in hypertension, LV dysfunction, and heart failure in subsets of treated patients. We postulated that cardiac toxicity due to sunitinib and other receptor tyrosine kinases targeting PDGFR (for example, sorafenib) may be indicative of a previously undescribed role of PDGFR signaling in the cardiac stress response. Using PDGFR-β cardiac-specific knockout mice, we showed that PDGFR-β is a regulator of the paracrine angiogenic capacity of cardiac myocytes and a critical modulator of the cardiac response to vascular stress.8 These findings suggest that manipulation of PDGFR expression or activation in the heart might be a rational therapeutic strategy for patients with chronic heart failure, and in particular for the treatment of subsets of heart failure patients with coronary microvascular disease.
Can Aerobic Exercise Increase the Cardiovascular Reserve of Doxorubicin-Treated Cancer Patients?
Much of the work in the field of cancer-therapy–induced heart failure has focused on drug-induced effects on the myocardium. However, cardiac function is only one component of a highly integrative unit (the lung/heart/vascular muscle axis) that collectively is responsible for oxygen transport, which is a fundamental mammalian requirement. Remarkably, the aerobic capacity in women who have been treated with doxorubicin is ∼30% below that of age-matched sedentary women without a history of breast cancer, despite normal LV ejection fraction (≤ 0.50),9 which suggests that doxorubicin-induced injury extends beyond the heart to affect other oxygen-transport organs. Marked impairment in aerobic capacity is probably the consequence of the direct as well as secondary (for example, lifestyle perturbations) effects of cytotoxic therapy on the organ components of oxygen transport and use. Whether doxorubicin-induced injury extends beyond the heart in cases of early breast cancer remains poorly understood. Finally, effective countermeasures will be needed to offset the acute and long-term consequences of doxorubicin on the heart and other oxygen-transport organs. To this end, aerobic exercise might be particularly effective, because it is one of the few interventions that can simultaneously increase the reserve capacity of multiple oxygen-transport organs, thereby leading to improvements in aerobic capacity.
Summary
The convergence of novel molecular techniques for the study of cardiovascular disease—together with the development of an armamentarium of molecular-targeted anticancer therapies—is fueling the interest of molecular cardiologists in probing the mechanisms of cancer-therapy–induced cardiac toxicity. Such studies have the potential to develop strategies that will prevent cardiac toxicity from becoming a barrier to effective anticancer therapy. They might also provide novel insights into the pathogenesis of non-cancer-therapy–induced human heart disease.
Footnotes
Address for reprints: Aarif Y. Khakoo, MD, The University of Texas MD Anderson Cancer Center, Unit 1451, 1515 Holcombe Blvd., Houston, TX 77030
E-mail: aykhakoo@mdanderson.org
Presented at the First International Conference on Cancer and the Heart; from The University of Texas MD Anderson Cancer Center and the Texas Heart Institute at St. Luke's Episcopal Hospital; Houston, 3–4 November 2010.
References
- 1.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest 2007;117(7):1782–93. [DOI] [PMC free article] [PubMed]
- 2.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci U S A 2006;103(7): 2304–9. [DOI] [PMC free article] [PubMed]
- 3.Huang C, Zhang X, Ramil JM, Rikka S, Kim L, Lee Y, et al. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation 2010;121(5):675–83. [DOI] [PMC free article] [PubMed]
- 4.Russell JL, Goetsch SC, Gaiano NR, Hill JA, Olson EN, Schneider JW. A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res 2011;108 (1):51–9. [DOI] [PMC free article] [PubMed]
- 5.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 2006;12(8):908–16. [DOI] [PubMed]
- 6.Fernandez A, Sanguino A, Peng Z, Ozturk E, Chen J, Crespo A, et al. An anticancer C-Kit kinase inhibitor is reengineered to make it more active and less cardiotoxic. J Clin Invest 2007;117(12):4044–54. [DOI] [PMC free article] [PubMed]
- 7.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, et al. A phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol 2010;55(18):1907–14. [DOI] [PubMed]
- 8.Chintalgattu V, Ai D, Langley RR, Zhang J, Bankson JA, Shih TL, et al. Cardiomyocyte PDGFR-beta signaling is an essential component of the mouse cardiac response to load-induced stress. J Clin Invest 2010;120(2):472–84. [DOI] [PMC free article] [PubMed]
- 9.Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 2009;10(6):598–605. [DOI] [PubMed]