Abstract
Double-chambered right ventricle is a congenital anomaly in which the right ventricle is divided into 2 portions by anomalous muscle bundles. These cases often present in children, but rarely in adults.
We discuss 2 cases of double-chambered right ventricle, in patients aged 42 and 35 years. When cases remain asymptomatic until adulthood, they can present with unusual symptoms that lead to incorrect diagnosis. Our cases represent 2 very different manifestations of double-chambered right ventricle, which differ in presentation, in the site of abnormal obstructive muscle bundles, and in the presence of associated lesions. Both of our patients underwent successful surgical resection of the obstruction. One patient also underwent closure of a ventricular septal defect.
We also review the literature on the various mechanisms that have been proposed to account for the complex morphology of the abnormal muscle bundles.
Key words: Double outlet right ventricle/complications/diagnosis/surgery; echocardiography, transesophageal; echocardiography, transthoracic; heart catheterization; heart defects, congenital/pathophysiology/surgery; heart septal defects, ventricular/complications; heart ventricles/abnormalities; ventricular outflow tract obstruction/diagnosis
Double-chambered right ventricle (DCRV) is a form of right ventricular (RV) outflow tract obstruction caused by anomalous muscular or fibromuscular bundles that divide the right ventricle into proximal high-pressure and distal low-pressure chambers.1 These cases most often present in children, and in 80% to 90% of patients DCRV is associated with other congenital anomalies.2 Rarely, a patient remains asymptomatic while the obstruction progresses gradually, until it presents in adulthood.3,4 Under these circumstances, the presentation can be difficult to diagnose.
We present the cases of 2 adult patients who varied in their symptomatic presentations, in the sites of their anomalous obstructive bundles, and in their associated lesions. Both cases were managed successfully by surgical intervention.
Case Reports
Patient 1
In November 2009, a 42-year-old man presented with chest pain and dyspnea on exertion. The patient's medical history revealed that at age 6 years he had been diagnosed with ventricular septal defect. At 26 years of age, he had reported limitation of activity, dyspnea, and dizziness, followed by chest pain and palpitations.
Upon admission, the patient was in sinus rhythm with normal blood pressure. His neck veins were distended, and there was slight bilateral edema of the lower limbs. Abdominal palpation showed enlargement of the liver. Cardiac palpation revealed a right ventricular heave, and auscultation revealed a harsh 4/6 systolic ejection murmur along the left sternal border, loudest at the 3rd left intercostal space. Electrocardiography revealed RV hypertrophy and right atrial enlargement. Transthoracic echocardiography (TTE) showed RV hypertrophy, severe obstruction of the outflow tract, and moderate-to-severe tricuspid regurgitation. The pulmonary artery was 2.8 cm in diameter, with no evidence of valvular stenosis or incompetence. The left ventricle was of normal size and function.
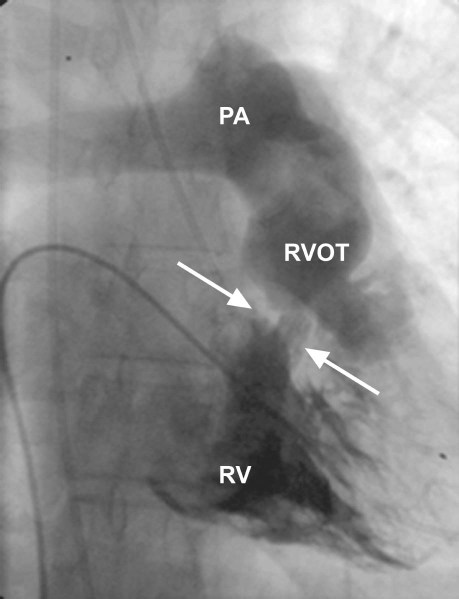
Cardiac catheterization confirmed the presence of DCRV, with an 80-mmHg pressure gradient between the proximal and distal segments (Fig. 1). The coronary angiographic results were normal.
Fig. 1 Right ventriculography. Arrows indicate the fibromuscular obstruction.
PA = pulmonary artery; RV = right ventricle; RVOT = right ventricular outflow tract
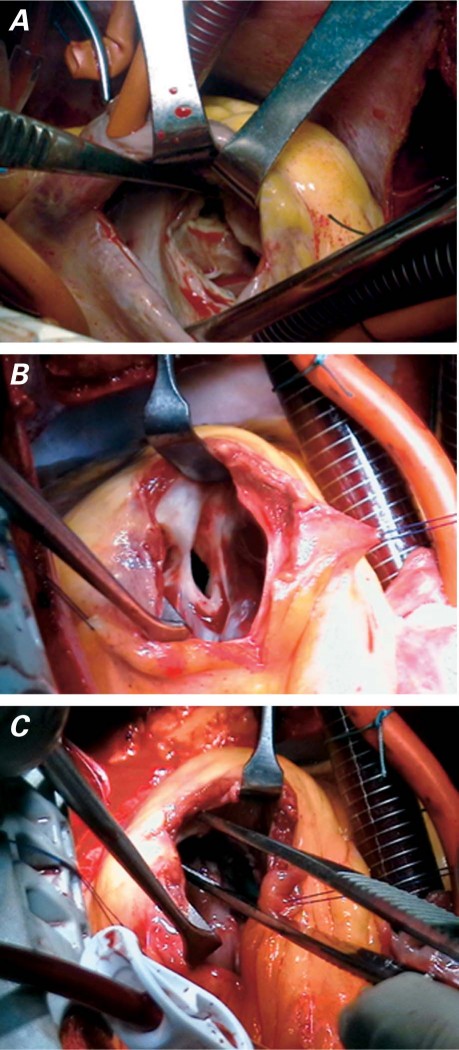
Surgery was undertaken using standard cardiopulmonary bypass (CPB) with moderate hypothermia and blood cardioplegic solution to protect the myocardium. A right atriotomy, followed by retraction of the tricuspid valve, revealed a fibromuscular band below the infundibulum that obstructed the RV (Fig. 2A). A small right ventriculotomy showed, below the normal pulmonary valve, a well-formed chamber that connected with the RV cavity via a small opening (Fig. 2B). We excised both the fibrous membrane and the surrounding hypertrophied muscle (Fig. 2C). There was no evidence of an associated cardiac anomaly. The tricuspid valve was repaired by means of a DeVega annuloplasty. The patient had an uneventful recovery and was discharged from the hospital after 4 days.
Fig. 2 A) Right atriotomy. The obstruction can be seen after retraction of the tricuspid valve. B) Right ventriculotomy. The incision shows a well-formed chamber below the normal pulmonary valve, connected to the right ventricular cavity by a small opening. C) Fibromuscular bundles have now been excised.
Postoperative TTE showed a residual gradient of 10 to 15 mmHg across the RV outflow tract.
Patient 2
In August 2004, a 35-year-old woman presented for the investigation of a heart murmur that had been discovered post partum, when she was 29 years of age. Transthoracic echocardiography revealed a 9-mm perimembranous ventricular septal defect (VSD) with a 55-mmHg gradient. Cardiac catheterization confirmed the presence of the VSD, together with evidence of mild obstruction between the RV inlet and the infundibulum (estimated pressure gradient, 10 mmHg), which had been caused by hypertrophied trabeculae. The pulmonary trunk was minimally dilated, with normal branches and a normal pulmonary valve. The estimated pressure measurements were RV, 50/4 mmHg; left ventricle, 110/8 mmHg; aorta, 95/55/74 mmHg; and pulmonary artery, 38/20/28 mmHg. The patient refused surgery and was given prophylaxis against endocarditis, then monitored closely by means of TTE.
Six years later, follow-up TTE showed an increase in the gradient between the 2 chambers of the RV to 43 mmHg. The size of the VSD had decreased to 6 mm, and the gradient across the VSD had decreased to 50 mmHg. Clinically, the patient was completely asymptomatic. She had a normal blood pressure and a regular pulse of 87. The only clinical sign of DCRV was the presence of a 3/6 systolic ejection murmur at the left lower sternal border. Right ventricular hypertrophy was seen upon electrocardiography. Elective surgery was undertaken using standard CPB. A right atrial approach enabled resection of the hypertrophied muscle bundles that obstructed the mid ventricle. The VSD was closed by means of a GORE-TEX® patch (W.L. Gore & Associates, Inc.; Flagstaff, Ariz). Postoperative TTE revealed no residual VSD or obstruction across the RV outflow tract.
Discussion
Various mechanisms have been proposed to account for the complex morphology of the abnormal muscle bundles in DCRV. Earlier work by Pongiglione and coworkers5 and Maron and colleagues6 proposed that the obstruction is the result of an acquired process whereby increased blood flow among patients with VSD causes hypertrophy of the supraventricular crest. Wong's group7 proposed that the obstruction is caused initially by the superior displacement of the septomarginal trabecula (moderator band), which then undergoes hypertrophy over time. Alva and coworkers1 based their work on the hearts of 26 surgical patients and 10 normal subjects aged 6 months to 24 years. In opposition to the above theories, they found that the abnormal muscle bundles originated from the accentuation of the septoparietal trabeculations. A muscular shelf extends from these trabeculations towards the trabecular component of the apical RV. They maintained that these prominent septoparietal trabeculations, in combination with acquired factors, form the causal basis of DCRV.
The incidence of DCRV varies from 0.5% to 2%.8 In 80% to 90% of cases, it is associated with VSD. Other coexisting lesions include subaortic stenosis, pulmonary valve stenosis, double-outlet RV, tetralogy of Fallot, anomalous pulmonary venous drainage, complete or corrected transposition of the great vessels, pulmonary atresia with intact septum and Ebstein anomaly,1 and (rarely) anomalous coronary vessels.9,10
Most cases of DCRV become manifest during childhood. The age of presentation usually varies from 4 months to 20 years.11 Most patients are asymptomatic and are referred for evaluation of a cardiac murmur. In a study of 52 patients, Cil and colleagues11 found that 40% were asymptomatic, 35% presented with fatigue, and 17% exhibited exertional dyspnea; congestive heart failure, cyanosis, and palpitation were present in 10% to 12% of the same group.
Rarely, cases remain asymptomatic or undiagnosed until adulthood.3,8–13 These patients can present with unusual symptoms and be diagnosed incorrectly.3 The commonest presentation is dyspnea and decreased exercise tolerance.12 However, syncope, dizziness, chest pain, and endocarditis have also been reported.3
Our cases represent 2 very different manifestations of DCRV. They differ in presentation, in the site of abnormal obstructing muscle bundles, and in the presence of associated lesions. In patient 1, the main presenting symptom was chest pain, although he had other symptoms of congestive heart failure. The abnormal muscle bundle was fibromuscular, discrete, high, and horizontal. The patient had no associated cardiac lesion, which was confirmed intraoperatively. The initial murmur was discovered during childhood and was thought to be a VSD, but was actually an isolated DCRV from the start. This explains our patient's severe symptoms. Our patient 2 was asymptomatic, which is a rare presentation in this age group. This absence of symptoms is explained by the low pressure gradient and the small VSD. This case supports the hypothesis that obstruction by anomalous muscle bundles might be an acquired phenomenon in patients with VSD, as was proposed by Pongiglione and colleagues.5 There was progression of the obstruction over a period of 6 years, which increased the pressure gradient 4-fold.
The variation of such cases—in presentation, in severity, and in location of the obstructing bundles—renders diagnosis difficult in adults. But it must be added that the inaccuracy of transthoracic echocardiography contributes to this misdiagnosis. Although TTE is an important tool for diagnosing congenital heart disease in children, it does not show DCRV well in adult patients. Visualization of the RV is limited by its irregular shape and retrosternal location.14 Previous studies have shown that TTE can yield an accurate diagnosis of DCRV in only 8% to 17% of adult patients.8,10 Lascano and colleagues10 in their study showed that transesophageal echocardiography provides better visualization of the RV than does TTE. They recommended the use of right-sided cardiac catheterization in evaluating adult patients with suspected RV outflow tract obstruction and VSD. In our patients, the diagnosis of DCRV was suggested by TTE imaging, but was confirmed only by means of right-sided cardiac catheterization with pressure measurements.
Noninvasive imaging, such as contrast computed tomography and magnetic resonance imaging, has been used in the diagnosis of DCRV. Cardiac magnetic resonance has the ability to visualize RV anatomy, movement, and flow.15 Visualization of the obstructing muscular bundles and sites of attachment can be accurately obtained, together with a determination of the pressure gradient.16 Such techniques replace the use of invasive right-sided cardiac catheterization.
All symptomatic adult patients should undergo surgery to resect the obstructive muscle bundles and to repair any associated lesions. This also applies to asymptomatic patients whose gradients exceed 40 mmHg,3 because the obstruction is rapidly progressive among adults. Resection of the anomalous muscle can be approached through a right atriotomy, a right ventriculotomy, or a combined transatrial–transpulmonary incision.3,10,12 The right atriotomy and the combined transatrial–transpulmonary incision are the most commonly used. Right ventriculotomy is rarely used, because it can cause such side effects as ventricular arrhythmias or impaired RV function. However, it can still be used in patients whose obstructions are severe and too bulky to be excised via a right atriotomy.13 We used a combined approach (transatrial and transventricular) in our 1st patient, because the anomalous bundles would have been difficult to resect through the right atrium. However, in the 2nd patient, the resection was easily performed through the right atrium. Postoperatively, both techniques proved effective.
Double-chambered RV should be suspected in adults when there is a RV outflow tract obstruction with unusual symptoms. Cases of DCRV should in general be treated surgically, because the obstruction is progressive and ends in heart failure.
Footnotes
Address for reprints: Ahmad K. Darwazah, PhD, FRCS, Department of Cardiac Surgery, Makassed Hospital, P.O. Box 19482, Jerusalem 91194, Israel
E-mail: darwaz30@hotmail.com
References
- 1.Alva C, Ho SY, Lincoln CR, Rigby ML, Wright A, Anderson RH. The nature of the obstructive muscular bundles in double-chambered right ventricle. J Thorac Cardiovasc Surg 1999;117(6):1180–9. [DOI] [PubMed]
- 2.Restivo A, Cameron AH, Anderson RH, Allwork SP. Divided right ventricle: a review of its anatomical varieties. Pediatr Cardiol 1984;5(3):197–204. [DOI] [PubMed]
- 3.McElhinney DB, Chatterjee KM, Reddy VM. Double-chambered right ventricle presenting in adulthood. Ann Thorac Surg 2000;70(1):124–7. [DOI] [PubMed]
- 4.Osborn RC Jr, Taylor J, Soto B, Burnum JF. Double chambered right ventricle in a 70-year-old woman. Ala J Med Sci 1984;21(1):73–7. [PubMed]
- 5.Pongiglione G, Freedom RM, Cook D, Rowe RD. Mechanism of acquired right ventricular outflow tract obstruction in patients with ventricular septal defect: an angiocardiographic study. Am J Cardiol 1982;50(4):776–80. [DOI] [PubMed]
- 6.Maron BJ, Ferrans VJ, White RI Jr. Unusual evolution of acquired infundibular stenosis in patients with ventricular septal defect. Clinical and morphologic observations. Circulation 1973;48(5):1092–103. [DOI] [PubMed]
- 7.Wong PC, Sanders SP, Jonas RA, Colan SD, Parness IA, Geva T, et al. Pulmonary valve-moderator band distance and association with development of double-chambered right ventricle. Am J Cardiol 1991;68(17):1681–6. [DOI] [PubMed]
- 8.Hoffman P, Wojcik AW, Rozanski J, Siudalska H, Jakubowska E, Wlodarska EK, Kowalski M. The role of echocardiography in diagnosing double chambered right ventricle in adults. Heart 2004;90(7):789–93. [DOI] [PMC free article] [PubMed]
- 9.Joseph T, Raccuglia S, Kort S, Oviasu F, Mangion JR. Anomalous right coronary artery from the main pulmonary artery in a patient with double-chambered right ventricle. Echocardiography 2002;19(8):687–90. [DOI] [PubMed]
- 10.Lascano ME, Schaad MS, Moodie DS, Murphy D Jr. Difficulty in diagnosing double-chambered right ventricle in adults. Am J Cardiol 2001;88(7):816–9. [DOI] [PubMed]
- 11.Cil E, Saraclar M, Ozkutlu S, Ozme S, Bilgic A, Ozer S, et al. Double-chambered right ventricle: experience with 52 cases. Int J Cardiol 1995;50(1):19–29. [DOI] [PubMed]
- 12.Nagashima M, Tomino T, Satoh H, Nakata T, Ohtani T, Saito H. Double-chambered right ventricle in adulthood. Asian Cardiovasc Thorac Ann 2005;13(2):127–30. [DOI] [PubMed]
- 13.Hachiro Y, Takagi N, Koyanagi T, Morikawa M, Abe T. Repair of double-chambered right ventricle: surgical results and long-term follow-up. Ann Thorac Surg 2001;72(5):1520–2. [DOI] [PubMed]
- 14.Chang RY, Kuo CH, Rim RS, Chou YS, Tsai CH. Transesophageal echocardiographic image of double-chambered right ventricle. J Am Soc Echocardiogr 1996;9(3):347–52. [DOI] [PubMed]
- 15.Kilner PJ, Sievers B, Meyer GP, Ho SY. Double-chambered right ventricle or sub-infundibular stenosis assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2002;4(3):373–9. [DOI] [PubMed]
- 16.Masci PG, Gewillig M, Bogaert J. Double-chambered right ventricle. Eur Heart J 2007;28(18):2237. [DOI] [PubMed]