Abstract
Purpose: Excessive daytime sleepiness is highly prevalent in the general population, is the hallmark of narcolepsy, and is linked to significant morbidity. Clinical assessment of sleepiness remains challenging and the common objective multiple sleep latency test (MSLT) and subjective Epworth sleepiness scale (ESS) methods correlate poorly. We examined the relative utility of pupillary unrest index (PUI) as an objective measure of sleepiness in a group of unmedicated narcoleptics and healthy controls in a prospective, observational pilot study. Methods: Narcolepsy (n = 20; untreated for >2 weeks) and control (n = 56) participants were tested under the same experimental conditions; overnight polysomnography was performed on all participants, followed by a daytime testing protocol including: MSLT, PUI, sleepiness visual analog scale (VAS), ESS, and the psychomotor vigilance test (PVT). Results: The narcolepsy and control groups differed significantly on psychomotor performance and each measure of objective and subjective sleepiness, including PUI. Across the entire sample, PUI correlated significantly with objective (mean sleep latency, SL) and subjective (ESS and VAS) sleepiness, but none of the sleepiness measures correlated with performance (PVT). Among narcoleptics, VAS correlated with PVT measures. Within the control group, mean PUI was the only objective sleepiness measure that correlated with subjective sleepiness. Finally, in an ANCOVA model, SL and ESS were significantly predictive of PUI as measure of sleepiness. Conclusion: The role of PUI in quantifying and distinguishing sleepiness of narcolepsy from sleep-satiated healthy controls merits further investigation as it is a portable, brief, and objective test.
Keywords: pupillary unrest index, narcolepsy, daytime sleepiness, vigilance-performance
Introduction
Comprehensive clinical evaluation of excessive daytime sleepiness (EDS) includes diagnostic testing to distinguish narcolepsy, 20% of the sleep clinic population, from other primary sleep disorders (Partinen and Hublin, 2000). Multiple sleep latency test (MSLT), the de facto gold standard for objective measurement of sleepiness and differential diagnosis of narcolepsy has several limitations (Chervin et al., 1995; Johns, 2000; Littner et al., 2005), making it unreliable as the sole indicator of manifest sleepiness in narcolepsy (Kotterba et al., 2004; Wise, 2006).
Pupillometry is a brief, non-invasive, physiologically based technique shown to concur with behavioral indicators of sleepiness in several populations (Lichstein et al., 1994; Wilhelm et al., 1998a, 2001, 2009; Wilhelm, 2008), including in narcolepsy, where its use dates back several decades (Yoss et al., 1969, 1970). The interpretation of pupillometry-derived measures of sleepiness is limited by a lack of normative data and a robust “single-best” measure. Several pupillometry-derived measures of sleepiness, e.g., pupillary diameter and stability, pupillary light reflex have been examined in narcolepsy with discrepant results (Kollarits et al., 1982; Pressman et al., 1984; Norman and Dyer, 1987; O’Neill et al., 1996).
Previous studies have investigated sleepiness-induced oscillations of pupil diameter pupillary unrest index (PUI) and its relationship to sleep deprivation (Hertz et al., 1988), circadian periodicity, and EEG (MSLT) parameters (Wilhelm et al., 1998a; Wilhelm and Ludtke, 1999; Merritt et al., 2004). The potential role of PUI in differentiating pathological sleepiness, particularly in narcolepsy, relative to sleepiness associated with societal factors in a normal control population has not been systematically evaluated.
The aims of this pilot study were (1) to evaluate the role of PUI as an objective measure of sleepiness in distinguishing narcolepsy from normal controls and (2) to understand the relationship of PUI to other measures of sleepiness and performance within these groups.
Materials and Methods
Participants
A total of 76 participants (41 females and 38 males) were recruited into this prospective observational study: 20 participants previously diagnosed with narcolepsy (14 females, 6 males, aged 33.8 ± 12.3 years, body mass index; BMI 24.3 ± 3.1 kg/m2, 13 with cataplexy, 7 without cataplexy); and 56 normal controls from the community (29 females, 27 males, aged 37.5 ± 11.1 years, BMI 23.0 ± 2.7 kg/m2). The study was reviewed and approved by the Institutional Research Board of the University of Illinois at Chicago, and all participants provided written consent for their participation and release of prior medical records. participants were 19–65 years of age; were negative on two urine drug screens (initial intake evaluation and first sleep laboratory visit); had unremarkable results on urinalysis, blood chemistry, thyroid tests, complete blood count, electrocardiogram (ECG), Beck depression inventory, current medical history, physical examination; and had normal pupil motility. A diagnosis of narcolepsy was made using the American Academy of Sleep Medicine published MSLT criteria: mean sleep latency (SL) < 8 min and at least two sleep onset rapid eye movement periods (SOREMP; Littner et al., 2005). All included participants reported attaining at least 7.5 h of sleep every night on a 7-day sleep log for the week prior to the clinic visit and for 1 week immediately prior to sleepiness assessment. Normal control participants that were included declared a usual daily caffeine intake of less than 500 mg, did not have a shift work schedule, and had no sleep complaints or primary sleep disorder identified by polysomnography (PSG). All participants who were undergoing treatment for any other conditions that might affect sleep (e.g., depression, antidepressant medication use) and participants with Apnea Hypopnea Index >5 on PSG were excluded.
Protocol
Narcolepsy participants already on treatment were required to discontinue treatment with stimulant medications for a period of at least 2 weeks prior to baseline measures, representing at least five pharmacokinetic half-lives of the medications. While most narcolepsy participants had been treated by modafinil alone (63%), fewer were taking methylphenidate (26%), or both medications (11%). A sleep assessment comprised of the following: participants reported to the sleep laboratory at 8:00 p.m. and overnight PSG was performed using a standard clinical montage: EEG, electrooculogram (EOG), electromyogram (EMG, chin and lower extremities), ECG, respiration, oxygen saturation, and snore microphone. Lights-out time was between 10:00 p.m. and 11:00 p.m. and lights-on the following morning was at about 7:00 a.m. Participants were served breakfast and lunch, but were not permitted to consume beverages or foods containing caffeine during the testing day following PSG.
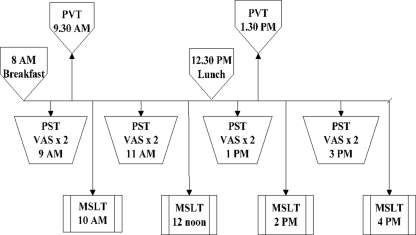
Figure 1 depicts the daytime testing timeline. Four 11-min PUI measurements during a pupillometry sleepiness test (PST) session, two 10-min psychomotor vigilance test (PVT) measurements (first in the morning and second in the afternoon), and four MSLT nap opportunities were provided per standard recommended clinical protocol across the day (Littner et al., 2005). A total of eight visual analog scale (VAS) tests were administered (before and after each of four PUI measures). The Epworth sleepiness scale (ESS) was administered once, after a randomly chosen PST session.
Figure 1.
Protocol for daytime tests.
A basic pupillary examination was conducted before the first PST, which was conducted in accordance with published recommendations (Wilhelm et al., 1996). The pupil image of the participant’s “dominant” eye (determined by the participant’s answer to the History Questionnaire) was recorded. Prior to PUI measurement, the participant wore infrared goggles for 8 min to facilitate dark adaptation. The participant was seated quietly in a comfortable chair with their head stabilized by a chinrest, and was instructed to relax and focus on a light-emitting diode directly in front of them. Pupil diameters were continuously recorded in real time with a portable electronic pupillometer (AMTech, Weinheim, Germany) for 11 min. Customized software enabled graphic display of pupil behavior in eight 82-s epochs and calculation of the power spectrum of up to 0.8 Hz as well as calculation of PUI for each epoch. To calculate the PUI the average of 16 consecutive values for the 25-Hz data was derived. Because PUI summarizes change in pupil size over time it is useful for inter-individual comparison. Details of this methodology have been published (Wilhelm et al., 1998b).
A 10-min PVT-192 for sustained attention was conducted two times (in the morning and afternoon). This was done on a hand-held device (Ambulatory Monitoring, Inc., Ardsley, NY, USA) with a 1-min practice exercise before each 10 min test session. The primary PVT measure variables assessed in the study were (1) the median reaction time (MRT) in each trial, and (2) the frequency of lapses (RT > 500 ms); instances where the participant failed to respond to in a timely manner to the stimulus.
The ESS is a self-administered questionnaire used to estimate sleepiness for eight different real-life situations and reflects on individual sleep propensity (Johns, 1991). The VAS for sleepiness (Schneider et al., 2004) is a subjective scale on a 10-cm line, with the left end or 0 being “asleep” and the right end or 10 being “as awake as I can be.”
Data Analysis
Data were analyzed using SAS (version 9.2, SAS Institute, Cary, NC, USA), and a p-value of <0.05 was considered significant. Outcome variables were examined for normality and appropriate transformations using Box–Cox method (Box and cox, 1964) were used to achieve normal distribution as applicable. A log transformation was used for PUI, a square transformation for VAS, and an inverse square root transformation for PVT MRT. All statistical analyses were performed on transformed variables when applicable (PUI, VAS, and PVT MRT). Tukey–Kramer multiple comparisons correction was performed for group differences. Pearson correlations and ANCOVA were used to assess the relationship of PUI to other measures of sleepiness and vigilance-performance, with each individual measure utilized separately as a covariate in addition to the group variable. Finally, receiver operating characteristic (ROC) analysis was performed to test the utility of PUI in distinguishing narcolepsy from controls and optimal point of PUI using Euclidian distances was obtained. We examined the time-of-day effects of the repeated measures (SL, PUI, and VAS), but in order to identify the inter-individual and between-group differences, we chose to conduct further analysis on mean scores.
Results
Descriptive statistics on all outcome measures in both groups are provided in Table 1. ANOVA analyses revealed that all performance and sleepiness measures, including PUI, in participants with narcolepsy were different from controls. Selected Pearson’s correlation coefficient’s for outcomes are noted in Table 2. Considering the entire participant sample (n = 76), all objective and subjective measures of sleepiness correlated significantly with each other while exhibiting no correlation with the vigilance-performance measures. The correlation coefficient’s between PUI and SL (r = −0.27, p = 0.04) and PUI and VAS (r = −0.27; p = 0.04) were significant in the control group. In contrast, SL did not exhibit any significant relationship with subjective sleepiness or vigilance-performance in controls. Within the narcolepsy group, neither PUI nor SL exhibited any significant correlation with subjective sleepiness, with vigilance-performance, or with each other. However subjective sleepiness (measured by VAS) was significantly correlated with vigilance-performance in narcolepsy.
Table 1.
Descriptive statistics and differences in measures between groups.
| Variable | Narcolepsy, n = 20 | Control, n = 56 | #p-value |
|---|---|---|---|
| *PUI | 11.48 ± 3.97 | 7.77 ± 2.66 | <0.0001 |
| SL | 4.74 ± 3.30 | 11.85 ± 5.22 | <0.0001 |
| ESS | 15.42 ± 20.70 | 5.73 ± 2.95 | <0.0001 |
| *VAS | 4.97 ± 1.87 | 7.55 ± 1.51 | <0.0001 |
| *PVT MRT | 244.58 ± 31.20 | 231.17 ± 38.31 | 0.02 |
| PVT lapse | 2.15 ± 3.13 | 1.20 ± 4.33 | 0.01 |
Data presented as mean ± SD (untransformed data).
*Transformation performed for non-normal distributions prior to all analyses.
#Parametric tests were performed on all outcomes and Tukey–Kramer method was used to correct for multiple comparisons.
*PUI, pupillary unrest index; SL, mean sleep latency; ESS, Epworth sleepiness scale, *VAS, visual analog sleepiness scale.
*PVT MRT: average PVT median reaction time.
PVT lapse: average PVT lapses.
Table 2.
Selected group-wise Pearson correlation coefficients and p-value’s.
| All study subjects; n = 76 | Narcolepsy; n = 20 | Controls; n = 56 |
|---|---|---|
| *PUI and SL | *PUI and SL | *PUI and SL |
| r = −0.47 | r = −0.40 | r = −0.27 |
| p = < 0.0001 | p = 0.07 | p = 0.04 |
| *PUI and ESS | *PUI and ESS | *PUI and ESS |
| r = 0.52 | r = 0.37 | r = 0.25 |
| p = < 0.0001 | p = 0.11 | p = 0.05 |
| *PUI and *VAS | *PUI and *VAS | *PUI and *VAS |
| r = −0.40 | r = −0.01 | r = −0.27 |
| p = 0.0003 | p = 0.95 | p = 0.04 |
| SL and ESS | ESS and *PVT MRT | ESS and *VAS |
| r = −0.50 | r = 0.39 | r = −0.31 |
| p = < 0.0001 | p = 0.09 | p = 0.01 |
| SL and *VAS | *VAS and *PVT MRT | |
| r = 0.38 | r = −0.64 | – |
| p = 0.0006 | p = 0.002 | – |
| ESS and *VAS | *VAS and PVT lapse | |
| r = −0.59 | r = −0.43 | – |
| p < 0.0001 | p = 0.05 | – |
All significant p-values are in highlighted in bold font.
*PUI, pupillary unrest index; SL, mean sleep latency; ESS, Epworth sleepiness scale; *VAS, visual analog sleepiness scale; *PVT MRT, average PVT median reaction time; PVT lapse, average PVT lapses.
*Analyses on transformed data.
To evaluate the relationship of PUI to other outcome measures, we performed multiple ANCOVA analyses (see Table 3); in each case with PUI as an outcome variable, participant group as predictor variable and each of the other measures individually as covariates (SL, ESS, VAS, PVT MRT, and PVT Lapse). The appropriateness of the ANCOVA model for these analyses was confirmed previously by testing any significant group and each outcome variable interaction effects with multiple linear regression. No significant group and outcome measure interactions were found for any of the five measures (covariates specified above). Overall, group effects on PUI remained significant when each measure was individually considered with the exception of ESS. When ESS was added to the model, the group effect on PUI was diminished and rendered insignificant. SL and ESS were the covariates noted to be significantly predictive of PUI.
Table 3.
Prediction of PUI by group and other measure.
| Predictor | Parameter estimate | p-Value |
|---|---|---|
| Group effect | 2.41 | 0.02 |
| SL | −0.18 | 0.004 |
| Group effect | 1.56 | 0.33 |
| ESS | 0.23 | 0.02 |
| Group effect | 0.32 | 0.004 |
| *VAS | −0.02 | 0.23 |
| Group effect | 3.64 | <0.0001 |
| *PVT MRT | 0.0013 | 0.80 |
| Group effect | 3.67 | <0.0001 |
| PVT lapse | −0.01 | 0.89 |
ANCOVA analyses: *PUI is dependent variable, group (referent group control’s vs. Narcoleptic’s) as predictor variable and each measure effect assessed individually as covariate. All significant p-values are in highlighted in bold font.
*PUI, pupillary unrest index; SL, mean sleep latency; ESS, Epworth sleepiness scale; *VAS, visual analog sleepiness scale; *PVT MRT, average PVT median reaction time; PVT lapse, average PVT lapses.
*Analyses on transformed data.
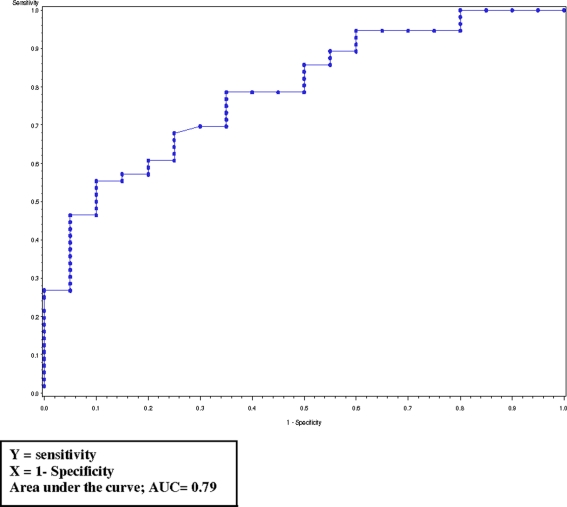
To understand the potential utility of PUI to discriminate narcolepsy participants from healthy control’s we performed ROC curve analyses. The area under the curve (AUC) for PUI was 0.79 (Figure A1 in Appendix), while consistent with previously published reports (Johns, 2000) AUC for ESS (0.94) and SL (0.86) was higher. PUI at 9.5 mm/min had a sensitivity of 0.65 and specificity of 0.78.
Discussion
Pupillometry is reproducible, devoid of motivational influences when performed under controlled circumstances (Wilhelm et al., 1996), and automated quantification permits elimination of inter-observer variability. However, previous investigations of pupillometry for assessment of narcolepsy have yielded conflicting results (Schmidt, 1982) and thus far a single-best pupillometry-derived index of pathologic sleepiness has not been identified. The potential utility of PUI has been suggested in narcolepsy (Newman and Broughton, 1991; Wilhelm et al., 1998b). The result of the present pilot study show that the PUI behaved in a manner equivalent to the current clinical standard objective sleepiness assessment tool: the MSLT. Further, PUI and SL were significantly correlated and showed similar patterns of correlation to measures of subjective sleepiness and vigilance-performance. These findings support the view that pupillary dynamics provide an objective biomarker of sleepiness in narcolepsy.
Within the overall sample (n = 76) the correlation between PUI and SL was similar to the value reported by McLaren et al. (2002) in a mixed group of controls and patients with narcolepsy, idiopathic hypersomnia, and poor sleep hygiene. However, because the mean values of each of these measures in our sample differed between narcolepsy and control participants, the overall correlations may be inflated.
Objective sleepiness quantified by SL was not associated with subjective sleepiness in either group considered separately. In contrast, PUI correlated SL, ESS, and VAS, indicating concordance with self-reported sleepiness in this presumably sleep-satiated, healthy control group. This discrepancy between objective sleepiness measures in the control group may be due to the differential sensitivity of PUI and SL to motivational influences or to a ceiling effect of SL, where the relationship of SL to subjective sleepiness degrades as SL increases (Chua et al., 1998). Among narcoleptic participants, PUI correlated with SL in a fashion similar to controls, but this did not achieve statistical significance likely due to sample size limitation and the poor sensitivity of SL to detect severe degrees of sleepiness (floor effect). Vigilance-performance was significantly correlated only to VAS within the narcolepsy group. Current clinical tests for objective quantification of sleepiness and wakefulness (MSLT and maintenance of wakefulness test; MWT, respectively) are known to be poor surrogates for performance prediction (Arand et al., 2005). Our results support no advantage of pupillometry in this regard either in the setting of narcolepsy or in the absence of pathologic sleepiness. Narcolepsy is associated with autonomic nervous system dysfunction (Hublin et al., 1994) and it is not known if the elevation of PUI among narcoleptics may be influenced by autonomic neuropathy in addition to alertness state. The narcolepsy participants in this study were previously diagnosed and treated (amphetamines and/or modafinil). Although, the treatment withdrawal was pharmacokinetically adequate (2 weeks or more), the long-term impact of narcolepsy with or without cataplexy, on pupillary function remains unknown and requires further investigation. A strong correlation between vigilance-performance and VAS has previously been shown in the setting of pathologic sleepiness, i.e., simulated shift work (Tremaine et al., 2010). Our study shows VAS may be a useful measure to predict performance in narcolepsy, particularly if this ability to self-monitor is maintained with treatment.
Notably, within the control group, 12/56 participants (21%) had a mean SL of ≤8 min and maybe considered “pathologically sleepy.” Only one participant in this sub-group had a single SOREMP at the third (1 p.m.) nap opportunity. With lack of objective habitual sleep time monitoring (Actigraphy), the reason for this pathologic sleepiness in the absence of any identifiable primary sleep disorder is uncertain. Possible explanations are first night effect leading to degraded sleep architecture on the preceding nocturnal in-laboratory study or chronic behavioral-induced insufficient sleep syndrome (BIISS). The high prevalence of BIISS (Komada et al., 2008) and its impact on mean SL has been previously reported (Janjua et al., 2003; Marti et al., 2009). We found a negative correlation between total sleep time (TST) on the preceding nocturnal PSG and mean SL on the following MSLT (r = −0.37; p = 0.005) in the control group as has been previously reported (Bonnet and Arand, 1995). This has been attributed to the “high and low sleepability” or individual ability to transition to sleep; by Arand et al. (2005), and may partly account for our observations. This “sleepy control” sub-group, defined by their low SL, was indistinguishable from “non-sleepy” normal controls on subjective sleepiness scores (ESS and VAS) and their PUI measurements were similar (see Table A1 in Appendix). Their vigilance-performance was intermediate (between narcolepsy and non-sleepy controls) and not significantly different from either. This suggests that PUI may be relatively devoid of trait “sleepability” influences, a potential advantage over mean SL alone in disease-free populations.
Pupillary unrest index was significantly predicted by SL and ESS (tests currently utilized in clinical practice), independent of group effects. However, the ROC analyses on ESS and SL confirmed their superiority over PUI as single screening and diagnostic tools for the diagnosis of suspected narcolepsy. Factors such as presence of cataplexy, preceding duration of disease, age, and treatment history may influence the diagnostic accuracy of the PUI in a fashion that cannot be determined in this small group of participants with narcolepsy.
Limitations
This study includes a heterogeneous group of narcoleptics (with and without cataplexy), where the underlying neurophysiologic substrates and mechanisms are distinct (Ahmed and Thorpy, 2010). Additionally, it has recently been shown that Orexin-A, primarily deficient in narcolepsy with cataplexy, modulates pupil size (Schreyer et al., 2009) and maybe a marker of hypocretin deficiency rather than a measure of alertness alone in narcolepsy with cataplexy (Plazzi et al., 2011). The presence of cataplexy, age of onset, durations of disease, prior treatment or lack thereof to account for potential direct and indirect effects of drugs on pupil size should be considered in future studies. Other limitations include a single baseline PSG for all participants (potential first night effect) and subjective assessment of habitual sleep time. We found significant time-of-day effects within individuals on all of the repeated measures: MSLT, PST, and VAS. The susceptibility of all of these tests to circadian effects has been previously reported (Kraemer et al., 2000; Danker-Hopfe et al., 2001). However, in order to examine the inter-individual trait differences and between-groups relationships we chose mean scores for further analysis. Examination and comparison of circadian effects on these instruments will better define the role of pupillometry in narcolepsy.
Conclusion
These results show that PUI distinguishes pathologic sleepiness of narcolepsy and is an accurate indicator of sleep propensity in controls. Pupillometry cannot substitute for the MSLT as a diagnostic tool in narcolepsy. However, given that the pupillometer is portable, the test protocol is short and the available metrics are reproducible without learning effects, tracking changes in PUI (or related indices) may prove to have value for longitudinal assessment in narcolepsy. It will be important for future studies to address the direct effects of modafinil and other approved drugs on pupillary dynamics and to directly examine longitudinal reliability and utility of these measures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by a grant from the National Institute of Nursing Research, National Institutes of Health, R01 NR4959. Additional support was provided by Mr. J. A. Piscopo, and Cephalon, Inc. The authors thank Kevin Grandfield for editorial assistance.
Appendix
Table A1.
Comparisons of sleepy control sub-group on outcomes.
| Outcome | Group | ||
|---|---|---|---|
| Sleepy control (n = 12) vs. Narcolepsy (n = 20) | Sleepy control (n = 12) vs. Non-sleepy control (n = 44) | Narcolepsy (n = 20) vs. Non-sleepy control (n = 44) | |
| *PUI | p = 0.08 | p = 0.30 | p < 0.0001 |
| SL | p = 0.90 | p < 0.0001 | p < 0.0001 |
| ESS | p < 0.0001 | p = 0.82 | p < 0.0001 |
| *VAS | p = 0.001 | p = 0.84 | p < 0.0001 |
| *PVT MRT | p = 0.10 | p = 0.90 | p = 0.08 |
| PVT lapse | p = 0.13 | p = 0.64 | p = 0.02 |
General linear model were performed; t-statistic with corresponding p-values are presented with Tukey–Kramer correction for multiple comparisons. Significant p-values are highlighted in bold font. SL data sub-group comparisons reflect the cut-off values chosen to define normal threshold value of 8 min or more.
*PUI, pupillary unrest index; SL, mean sleep latency; ESS, Epworth sleepiness scale; *VAS, visual analog sleepiness scale; *PVT MRT, average PVT median reaction time; PVT lapse, average PVT lapses.
*Analyses on transformed data.
Figure A1.
Receiver operating characteristic curve for log PUI.
Financial Support
Funding was provided by the National Institute of Nursing Research NR004959, Mr. J. A. Piscopo, and Cephalon, Inc.
References
- Ahmed I., Thorpy M. (2010). Clinical features, diagnosis and treatment of narcolepsy. Clin. Chest Med. 31, 371–381 10.1016/j.ccm.2010.02.014 [DOI] [PubMed] [Google Scholar]
- Arand D., Bonnet M., Hurwitz T., Mitler M., Rosa R., Sangal R. B. (2005). The clinical use of the MSLT and MWT. Sleep 28, 123–144 [DOI] [PubMed] [Google Scholar]
- Bonnet M. H., Arand D. L. (1995). 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep 18, 581–588 [DOI] [PubMed] [Google Scholar]
- Box G. E. P., Cox D. R. (1964). An analysis of transformations. J. R. Stat. Soc. Series B 26, 1–78 [Google Scholar]
- Chervin R. D., Kraemer H. C., Guilleminault C. (1995). Correlates of sleep latency on the multiple sleep latency test in a clinical population. Electroencephalogr. Clin. Neurophysiol. 95, 147–153 [DOI] [PubMed] [Google Scholar]
- Chua L. W. Y., Yu N. C., Golish J. A., Nelson D. R., Perry M. C., Foldvary N., Dinner D. S. (1998). Epworth sleepiness scale and the multiple sleep latency test: dilemma of the elusive link. Sleep 21(Suppl.), 184 [Google Scholar]
- Danker-Hopfe H., Kraemer S., Dorn H., Schmidt A., Ehlert I., Herrmann W. M. (2001). Time-of-day variations in different measures of sleepiness (MSLT, pupillography, and SSS) and their interrelations. Psychophysiology 38, 828–835 10.1111/1469-8986.3850828 [DOI] [PubMed] [Google Scholar]
- Hertz G., Spielman A. J., Hakerem G., Pressman M. (1988). Pupillometry and MSLT: the effects of napping on pupil indicators of sleepiness. Sleep Res. 17, 22 [Google Scholar]
- Hublin C., Matikainen E., Partinen M. (1994). Autonomic nervous system function in narcolepsy. J. Sleep Res. 3, 131–137 10.1111/j.1365-2869.1994.tb00119.x [DOI] [PubMed] [Google Scholar]
- Janjua T., Samp T., Cramer-Bornemann M., Hannon H., Mahowald M. W. (2003). Clinical caveat: prior sleep deprivation can affect the MSLT for days. Sleep Med. 4, 69–72 10.1016/s1389-9457(02)00065-5 [DOI] [PubMed] [Google Scholar]
- Johns M. W. (1991). A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545 [DOI] [PubMed] [Google Scholar]
- Johns M. W. (2000). Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J. Sleep Res. 9, 5–11 10.1046/j.1365-2869.2000.00177.x [DOI] [PubMed] [Google Scholar]
- Kollarits C. R., Lechman J., Kollarits F. J., Gillin J. C. (1982). The pupil dark response in narcolepsy. Curr. Eye Res. 2, 261–263 10.3109/02713688209011628 [DOI] [PubMed] [Google Scholar]
- Komada Y., Inoue Y., Hayashida K., Nakajima T., Honda M., Takahashi K. (2008). Clinical significance and correlates of behaviorally induced insufficient sleep syndrome. Sleep Med. 9, 851–856 10.1016/j.sleep.2007.08.018 [DOI] [PubMed] [Google Scholar]
- Kotterba S., Mueller N., Leidag M., Widdig W., Rasche K., Malin J. P., Schultze-Werninghaus G., Orth M. (2004). Comparison of driving simulator performance and neuropsychological testing in narcolepsy. Clin. Neurol. Neurosurg. 106, 275–279 [DOI] [PubMed] [Google Scholar]
- Kraemer S., Danker-Hopfe H., Dorn H., Schmidt A., Ehlert I., Herrmann W. M. (2000). Time-of-day variations of indicators of attention: performance, physiologic parameters, and self-assessment of sleepiness. Biol. Psychiatry 48, 1069–1080 [DOI] [PubMed] [Google Scholar]
- Lichstein K. L., Wilson N. M., Noe S. L., Aguillard R. N., Bellur S. N. (1994). Daytime sleepiness in insomnia: behavioral, biological and subjective indices. Sleep 17, 693–702 [DOI] [PubMed] [Google Scholar]
- Littner M. R., Kushida C., Wise M., Davila D. G., Morgenthaler T., Lee-Chiong T., Hirshkowitz M., Daniel L. L., Bailey D., Berry R. B., Kapen S., Kramer M. (2005). Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 28, 113–121 [DOI] [PubMed] [Google Scholar]
- Marti I., Valko P. O., Khatami R., Bassetti C. L., Baumann C. R. (2009). Multiple sleep latency measures in narcolepsy and behaviourally induced insufficient sleep syndrome. Sleep Med. 10, 1146–1150 10.1016/j.sleep.2009.03.008 [DOI] [PubMed] [Google Scholar]
- McLaren J. W., Hauri P. J., Lin S. C., Harris C. D. (2002). Pupillometry in clinically sleepy patients. Sleep Med. 3, 347–352 10.1016/S1389-9457(02)00017-5 [DOI] [PubMed] [Google Scholar]
- Merritt S. L., Schnyders H. C., Patel M., Basner R. C., O’Neill W. (2004). Pupil staging and EEG measurement of sleepiness. Int. J. Psychophysiol. 52, 97–112 [DOI] [PubMed] [Google Scholar]
- Newman J., Broughton R. (1991). Pupillometric assessment of excessive daytime sleepiness in narcolepsy-cataplexy. Sleep 14, 121–129 [DOI] [PubMed] [Google Scholar]
- Norman M. E., Dyer J. A. (1987). Ophthalmic manifestations of narcolepsy. Am. J. Ophthalmol. 103, 81–86 [DOI] [PubMed] [Google Scholar]
- O’Neill W. D., Oroujeh A. M., Keegan A. P., Merritt S. L. (1996). Neurological pupillary noise in narcolepsy. J. Sleep Res. 5, 265–271 [DOI] [PubMed] [Google Scholar]
- Partinen M., Hublin C. (2000). “Epidemiology of sleep disorders,” in Principles, and Practices of Sleep Medicine, 3rd Edn, eds Kryger M. H., Roth T., Dement T. (Philadelphia: WB Saunders Company; ), 558–579 [Google Scholar]
- Plazzi G., Moghadam K. K., Maggi L. S., Donadio V., Vetrugno R., Liguori R., Zoccoli G., Poli F., Pizza F., Pagotto U., Ferri R. (2011). Autonomic disturbances in narcolepsy. Sleep Med. Rev. 15, 187–196 [DOI] [PubMed] [Google Scholar]
- Pressman M. R., Spielman A. J., Korczyn A. D., Rubenstein A. E., Pollak C. P., Weitzman E. D. (1984). Patterns of daytime sleepiness in narcoleptics and normals: a pupillometric study. Electroencephalogr. Clin. Neurophysiol. 57, 129–133 [DOI] [PubMed] [Google Scholar]
- Schmidt H. S. (1982). Pupillometric assessment of disorders of arousal. Sleep 5(Suppl. 2), S157–S164 [DOI] [PubMed] [Google Scholar]
- Schneider C., Fulda S., Schulz H. (2004). Daytime variation in performance and tiredness/sleepiness ratings in patients with insomnia, narcolepsy, sleep apnea and normal controls. J. Sleep Res. 13, 373–383 10.1111/j.1365-2869.2004.00427.x [DOI] [PubMed] [Google Scholar]
- Schreyer S., Buttner-Ennever J. A., Tang X., Mustari M. J., Horn A. K. (2009). Orexin-A inputs onto visuomotor cell groups in the monkey brainstem. Neuroscience 164, 629–640 10.1016/j.neuroscience.2009.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaine R., Dorrian J., Lack L., Lovato N., Ferguson S., Zhou X., Roach G. (2010). The relationship between subjective and objective sleepiness and performance during a simulated night-shift with a nap countermeasure. Appl. Ergon. 42, 52–61 10.1016/j.apergo.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Wilhelm B., Giedke H., Ludtke H., Bittner E., Hofmann A., Wilhelm H. (2001). Daytime variations in central nervous system activation measured by a pupillographic sleepiness test. J. Sleep Res. 10, 1–7 10.1046/j.1365-2869.2001.00239.x [DOI] [PubMed] [Google Scholar]
- Wilhelm B., Wilhelm H., Ludtke H., Adler M., Streicher P. (1996). Pupillography for objective vigilance assessment. Methodological problems and possible solutions. Ophthalmologe 93, 446–450 [PubMed] [Google Scholar]
- Wilhelm B., Wilhelm H., Ludtke H., Streicher P., Adler M. (1998a). Pupillographic assessment of sleepiness in sleep-deprived healthy subjects. Sleep 21, 258–265 [PubMed] [Google Scholar]
- Wilhelm H., Ludtke H., Wilhelm B. (1998b). Pupillographic sleepiness testing in hypersomniacs and normals. Graefes Arch. Clin. Exp. Ophthalmol. 236, 725–729 10.1007/s004170050149 [DOI] [PubMed] [Google Scholar]
- Wilhelm B. J. (2008). Pupillography for the assessment of driver sleepiness. Klin. Monbl. Augenheilkd. 225, 791–798 10.1055/s-2008-1027453 [DOI] [PubMed] [Google Scholar]
- Wilhelm B.J., Widmann A., Durst W., Heine C., Otto G. (2009). Objective and quantitative analysis of daytime sleepiness in physicians after night duties. Int. J. Psychophysiol. 72, 307–313 10.1016/j.ijpsycho.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Wilhelm H., Ludtke H. (1999). “Spontaneous pupillary oscillations,” in Pupillography: Principles, Methods and Applications (Clinical pharmacology), eds Kuhlmann J., Böttcher M. (Muenchen: W. Zuckschwerdt Verlag; ), 27–36 [Google Scholar]
- Wise M. S. (2006). Objective measures of sleepiness and wakefulness: application to the real world? J. Clin. Neurophysiol. 23, 39–49 10.1097/01.wnp.0000190416.62482.42 [DOI] [PubMed] [Google Scholar]
- Yoss R. E., Moyer N. J., Hollenhorst R. W. (1970). Pupil size and spontaneous pupillary waves associated with alertness, drowsiness, and sleep. Neurology 20, 545–554 [DOI] [PubMed] [Google Scholar]
- Yoss R. E., Moyer N. J., Ogle K. N. (1969). The pupillogram and narcolepsy. A method to measure decreased levels of wakefulness. Neurology 19, 921–928 [DOI] [PubMed] [Google Scholar]