Abstract
Background
Point-of-care devices (POCDs) for monitoring long-term oral anticoagulation therapy (OAT) may be a useful alternative to laboratory-based international normalized ratio [INR] testing and clinical management.
Purpose
To determine clinical outcomes of the use of POCDs for OAT management by performing a meta-analysis. Previous meta-analyses on POCDs have serious limitations.
Data sources
PubMed, the Cochrane Library, DIALOG, MEDLINE, EMBASE, BIOSIS Previews and PASCAL databases.
Study selection
Randomized controlled trials of patients on long-term OAT, comparing anticoagulation monitoring by POCD with laboratory INR testing and clinical management.
Data extraction
1) rates of major hemorrhage; 2) rates of major thromboembolic events; 3) percentage of time that the patient is maintained within the therapeutic range; 4) deaths. Outcomes were compared using a random-effects model. Summary measures of rates were determined. The quality of studies was assessed using the Jadad scale.
Data synthesis
Seventeen articles (16 studies) were included. Data analysis showed that POCD INR testing reduced the risk of major thromboembolic events (odds ratio [OR] = 0.51; 95% confidence interval [CI] 0.35–0.74), was associated with fewer deaths (OR = 0.58; 95% CI = 0.38–0.89), and resulted in better INR control compared with laboratory INR testing. No significant difference between the two management modalities with respect to odds ratios for major hemorrhage was found.
Limitations
Quality scores varied from 1 to 3 (out of a maximum of 5). Only 3 studies defined how thromboembolic events would be diagnosed, casting doubt on the accuracy of the reporting of thromboembolic events. The studies suggest that only 24% of patients are good candidates for self-testing and self-management. Compared with patients managed with laboratory-based monitoring, POCD patients underwent INR testing at a much higher frequency and received much more intensive education on OAT management.
Conclusions
The use of POCDs is safe and may be more effective than laboratory-based monitoring. However, most patients are not good candidates for self-testing and self-management. Patient education and frequency of testing may be the most important factors in successful PODC management. Definitive conclusions about the clinical benefits provided by self-testing and self-management require more rigorously designed trials.
Oral anticoagulants in the form of vitamin K antagonists are widely used for the prevention and treatment of thromboembolic events in the presence of various clinical conditions. Long-term use is typically required for high-risk groups with particular conditions such as mechanical heart valves, chronic atrial fibrillation, venous thromboembolism, acute myocardial infarction, stroke, and peripheral arterial occlusion.1, 2 For many of these indications, a person must continue on oral anticoagulant therapy (OAT) for life.3, 4 In view of the aging of the population and an associated increase in the prevalence of atrial fibrillation and venous thromboembolism, it is expected that more patients will need OAT in the future. Evidence suggests that OAT reduces the incidence of thromboembolic complications (venous and arterial thrombosis) and associated mortality and morbidity in these patient populations.5
However, vitamin K antagonists have a narrow “therapeutic window,” or range of clinical effectiveness. Excessive anticoagulation confers an increased risk of bleeding, while sub-therapeutic anticoagulation is associated with an increased risk of stroke and other thromboembolic events.6, 7 Unfortunately, the biological effect of the vitamin K antagonists varies from one patient to another and within individual patients over time.5 For this reason, patients need regular monitoring of the international normalized ratio (INR), which is usually determined in a hospital or outpatient laboratory facility by a venipuncture sample processed in the lab. This can be inconvenient with respect to the blood sampling procedure and the time spent going for a laboratory test.1, 2 Point-of-care devices (POCDs) for monitoring long-term OAT were introduced in the 1990s. POCDs are portable and require only a drop of blood from a fingertip puncture. In some countries, such as Germany, self-testing and self-management with POCDs are widely employed, but in most countries uptake has been limited.8, 9
The POCD technology makes it possible for patients on long-term OAT to self-monitor and self-manage their OAT. Those who manage OAT programs need to know how POCDs compare in effectiveness and cost-effectiveness with standard laboratory tests. The objective of this meta-analysis was to assess the clinical implications of POCD use for OAT monitoring as well as any potential limitations of the available data. Our meta-analysis was intended to overcome weaknesses in previous studies, including the inclusion of few studies and the inclusion of studies that should have been excluded from a meta-analysis, and to present results by length of follow-up rather than by numbers of events per patient enrolled; this last consideration is important because the number of events per year gives physicians a better indication of the safety and effectiveness of point-of-care devices.
Literature search strategy
We obtained published literature by cross-searching the DIALOG, MEDLINE, EMBASE, BIOSIS Previews and PASCAL databases. There were no year or language restrictions. A broad search strategy with appropriate descriptors and keywords was used, in combination with a filter, to restrict results to controlled trials, meta-analyses and systematic reviews. We also ran parallel searches on PubMed and the Cochrane Library.
The original search was performed in July 2005. Regular alerts were established on the MEDLINE, BIOSIS Previews and EMBASE databases to capture new studies up to March 2007. Searches in the Cochrane Library were updated regularly. We obtained grey literature by searching the websites of regulatory agencies, health technology assessment agencies, and near-technology assessment agencies. Specialized databases such as the NHS Centre for Reviews and Dissemination at the University of York, England, and the Latin American and Caribbean Center on Health Sciences Information (LILACS), were also searched. The following professional associations’ web and conference sites were searched for additional information: Thrombosis Interest Group of Canada, Canadian Cardiovascular Society, American College of Cardiology, American Society of Hematology, and European Society of Cardiology. Non-randomized controlled trials were included in the literature search in view of their potential use in other sections of the report.
Selection criteria and method.
Studies that were included met the following selection criteria:
Study design: randomized controlled trial (RCT)
Population group: patients on long-term (at least 3 months) OAT (no a priori restrictions on age or mental capacity)
Interventions: anticoagulation monitoring by POCD; this could include POCD testing at an anticoagulation clinic, POCD self-testing by the patient, POCD self-testing plus self-management and control, or any other POCD management strategy
Comparators: usual care (venipuncture blood draw for an INR laboratory test, with management provided by an anticoagulation clinic or individual practitioner)
- Outcomes: studies must have reported on at least one of the following:
- Rates of major hemorrhage, where “major” was defined as resulting in death, or where hemorrhage was clinically overt and showed one of the following: critical site involvement (intra-cranial, retroperitoneal, intraocular, intraspinal, or pericardial); drop in hemoglobin of ≥ 2.0 g/dL; need for transfusion of > 2 units of packed red blood cells; or a bleeding index of > 2.0 (the number of units of packed red cells or whole blood transfused plus the hemoglobin values before the bleeding episode minus the hemoglobin values after the episode).
- Major thromboembolic event rates, noting whether the study required objective diagnostic tests for venous and arterial thromboembolic complications. Transient ischemic attacks were considered to be minor thromboembolic events and were included in a secondary analysis to evaluate all thromboembolic events.
- Percentage of time the patient’s blood was within the normal therapeutic INR range according to a method described by Rosendaal and colleagues.10 The Rosendaal algorithm is used to calculate the time that a patient stayed in a predetermined INR interval. The algorithm assumes a linear increase or decrease between 2 consecutive INR determinations.10
Reports were excluded if they were duplicate reports, preliminary reports of data presented in full, dose-finding studies, studies in which oral anticoagulants were combined with antiplatelet drugs, or studies that did not follow patients for more than 3 months. Although we had planned to exclude data based on patients who had not been on OAT for 3 months before entering the study, we dropped this criterion and performed analyses with and without these studies.
We assessed the retrieved references for possible inclusion by evaluating the title and the abstract according to the selection criteria. The reviewers pilot-tested the inclusion–exclusion criteria on 7 articles and performed a calibration exercise to ensure consistent application. Letters to the editor, review articles, editorials and commentaries were excluded. The remaining studies were fully assessed.
At least two reviewers independently reviewed each citation from the literature search. At the first stage, abstracts were selected independently by KC and LM. Consensus was reached by discussion. At the second stage, full-text articles were reviewed independently. Agreement on eligibility was achieved by discussion between the 2 reviewers.
Data extraction
A data extraction form was developed a priori. Two reviewers (PW, KC) independently extracted data from eligible articles and assessed their quality using a standard electronic form. PW and another reviewer (LM) then arrived at a consensus on the extracted data and quality values through discussion.
Strategy for quality assessment
Study quality was assessed using the criteria proposed by Jadad and colleagues, and the adequacy of allocation concealment was evaluated as appropriate or inappropriate according to the criteria proposed by Schulz and Grimes.11, 12 In the Jadad system, 1 point is scored for each of the following criteria, such that the total score can be anywhere from 0 to 5: randomization; appropriate method of randomization; double-blinding: appropriate method of double-blinding; and adequate description of withdrawals and dropouts. If information in the reports was insufficient, these issues were recorded as unclear or unstated. We successfully contacted authors when data were incomplete or missing.
Data analysis methods
To assess the outcomes of major hemorrhage, major thromboembolic events, and all thromboembolic events, we conducted a meta-analysis by calculating odds ratios and their 95% confidence intervals for the event rates, comparing the results for POCD testing and laboratory testing. All event rates were recorded with reference to intention to treat. A comparison of death rates was also performed. We used a random-effects model for all comparisons (according to the method described by DerSimonian and Laird13), recognizing that its use can reduce the effect of larger studies relative to a fixed-effects model. A random-effects model allows for between-study variation and was chosen as the more conservative option.
Differences between effects were tested using a Z test, and p values < 0.05 were considered to be significant. Given that a number of potential issues could influence the results of the meta-analysis, we planned a priori to evaluate how certain patient and trial characteristics would be associated with treatment effects, even in the absence of statistical heterogeneity in the primary analysis. For each comparison group, we estimated the between-study heterogeneity using the Q statistic in the Review Manager (RevMan) software package. Heterogeneity was considered significant for p < 0.05. The I2 statistic, indicating the proportion of total variation attributable to heterogeneity, was also calculated. For I2, the cut-off points were 25%, 50% and 75% for low, moderate and high heterogeneity, respectively.14 The summary measures of rates of major hemorrhage and thromboembolic events were determined using the inverse variance weighted averages. Forest plots were prepared. Funnel plots were generated to assess whether the magnitude of the observed association was related to the variance of each study and whether there was evidence of publication bias. We did a paired t-test of mean percentage time in the therapeutic range for the control and intervention groups.
Preplanned subgroup analysis
Although our primary analysis was to pool all studies, we also performed 4 subgroup analyses. The first subgroup included studies that required patients to be on OAT for more than 3 months before study entry. The second was to include only self-management studies; the third was to analyze only studies that described the requirement for objective tests to diagnose major thromboembolic events; and the fourth included only studies that scored ≥ 3 on the Jadad assessment tool.
Results
Quantity of research available
We identified 439 citations in our initial search. Routine updates yielded an additional 13 citations for a total of 452 (Figure 1). Of these 452 citations, 409 did not meet the selection criteria and were excluded, leaving 43 (39 from the initial search and 4 from the updated searches). Two were added for reconsideration after the study criteria were revised to include studies in which patients had been on OAT for < 3 months at the start of the study. We retrieved 46 potentially relevant articles for further review. Of these, we excluded 29 articles,15-43 leaving 17 relevant articles describing 16 unique RCTs.44-59, 60 One RCT was reported in 2 publications.50, 51 These articles by Koertke and colleagues were not duplicates because they reported different aspects of the RCT but informed the data extraction for the same study. The Gadisseur article provided 2 sets of data because the authors compared self-test plus self-management and self-test plus clinic management to routine care.47
Figure 1.
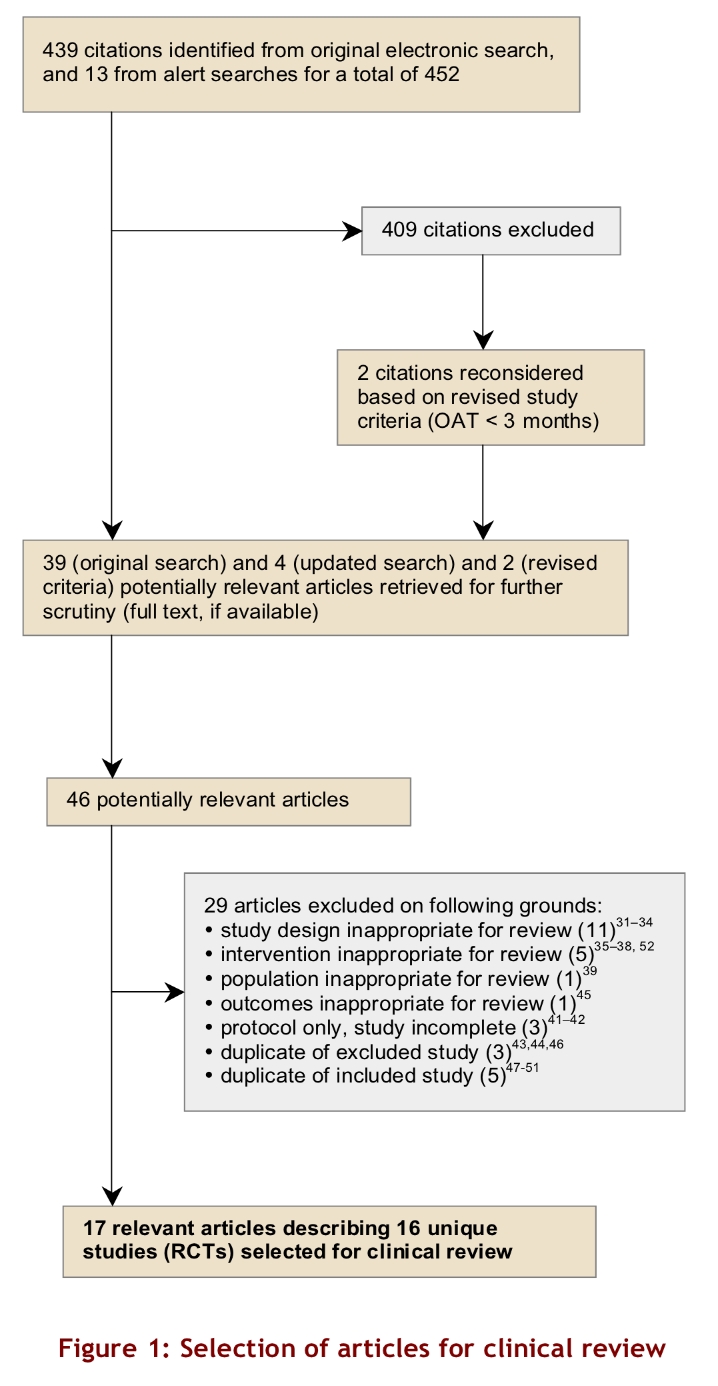
Selection of articles for clinical review
Of the 11 articles that were excluded on the basis of study design, 4 were reviews.22-25 Five articles were excluded because the intervention used was inappropriate for our review.26-29, 43 For example, although POCD testing was used in some studies, the patients were managed on the basis of results from laboratory testing, not POCD testing. As a result, no true comparison could be made with those patients in a group undergoing laboratory testing because this study design could miss results related to management based on POCD testing. One article was excluded on the basis of the study population,30 1 on the basis of outcome measures,36 and 3 because they were at the protocol stage.31-33 Three were duplicates of excluded articles,34, 35, 37 and 5 were duplicates of included articles, and so they were also excluded.38-42
Study characteristics
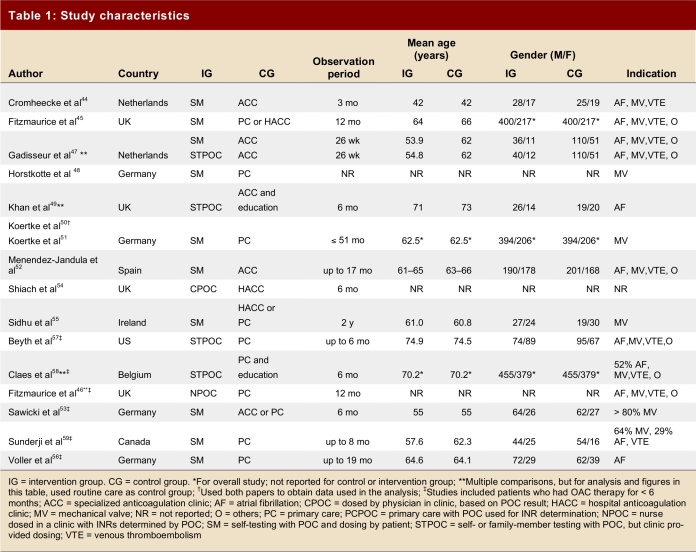
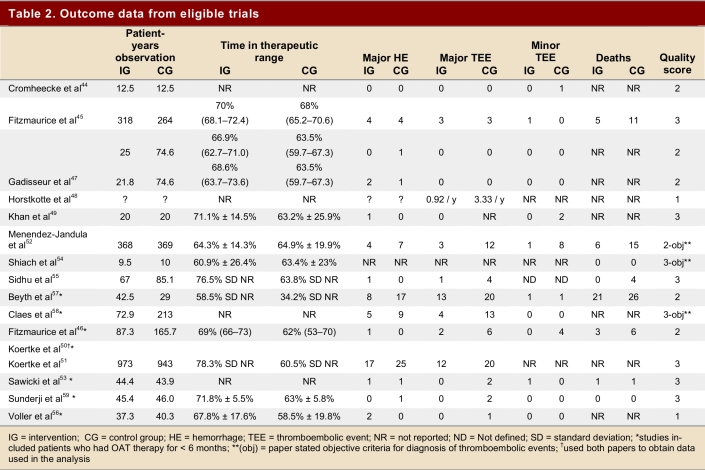
Table 1 summarizes the characteristics of the studies and demonstrates their variability with regard to observation periods, mean age of patients, and indication for anticoagulation. It was not possible to break down study outcomes according to the indication for anticoagulation. We found 12 studies that compared self-monitoring plus self-management to routine anticoagulation control. In 9 studies, only patients who had been on OAT for ≥ 3 months were enrolled; in 7 studies, patients were enrolled from the time of initiation of anticoagulation or the time could not be determined.46, 50, 51, 53, 56-59 The Coaguchek (Roche Diagnostics, Mannheim, Germany) was used in 14 studies, and the ProTime Microcoagulation System (International Technidyne Corporation, USA) in 2. Table 2 summarizes the outcome data from eligible trials.
Table 1.
Study characteristics
Table 2.
Outcome data from eligible trials
Data analysis and synthesis
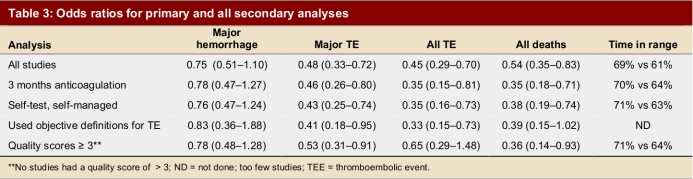
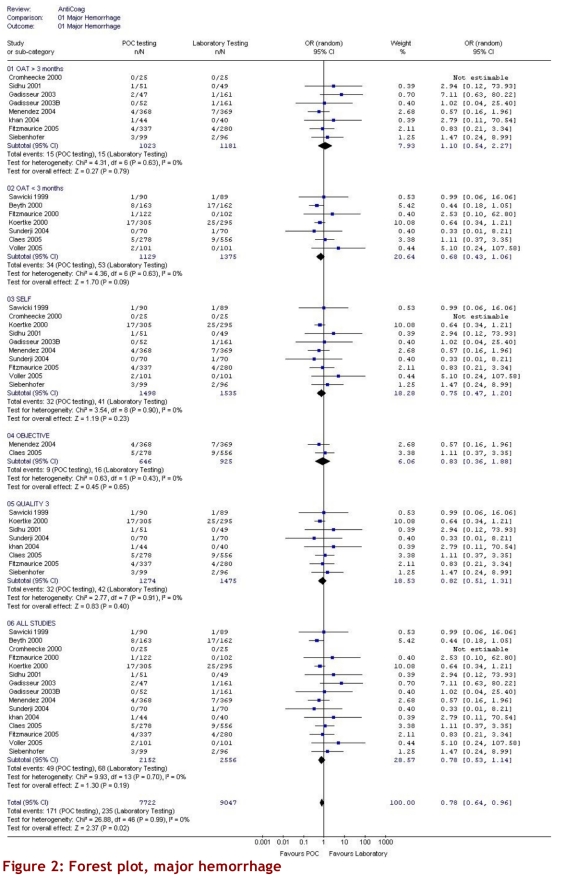
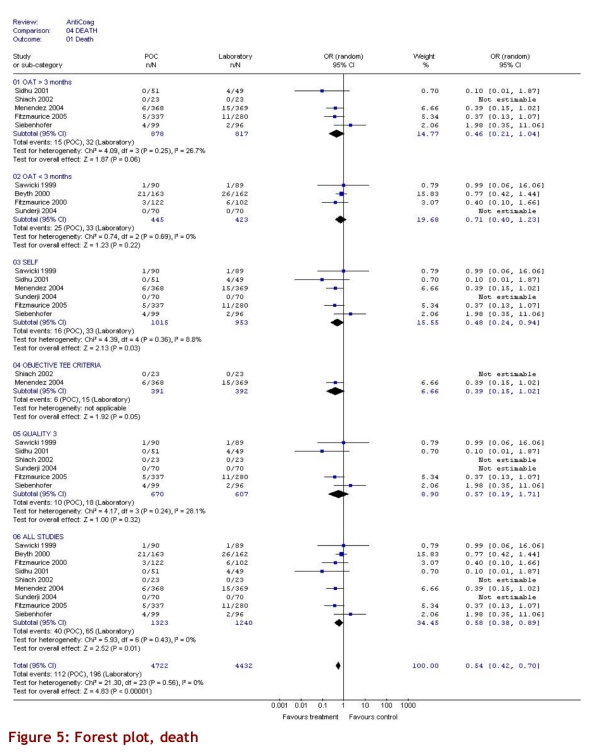
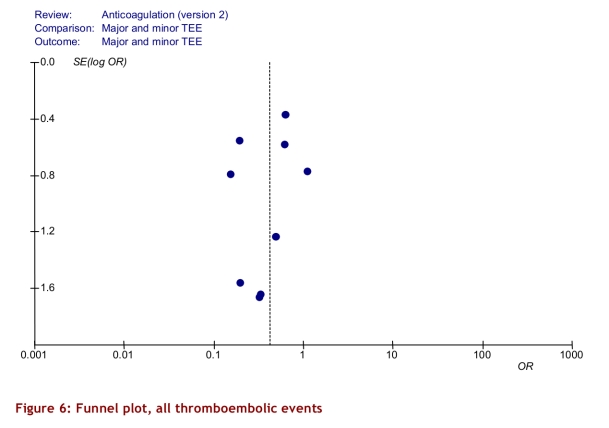
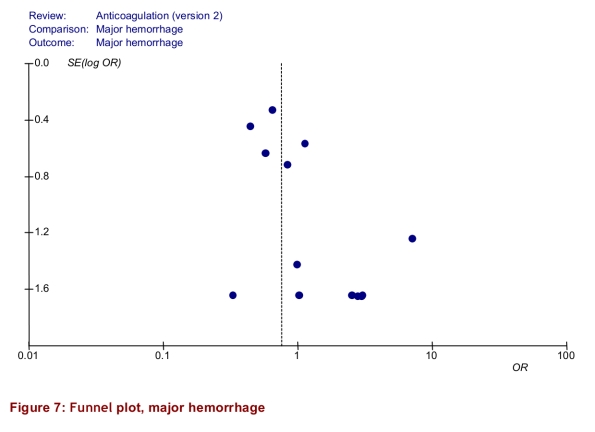
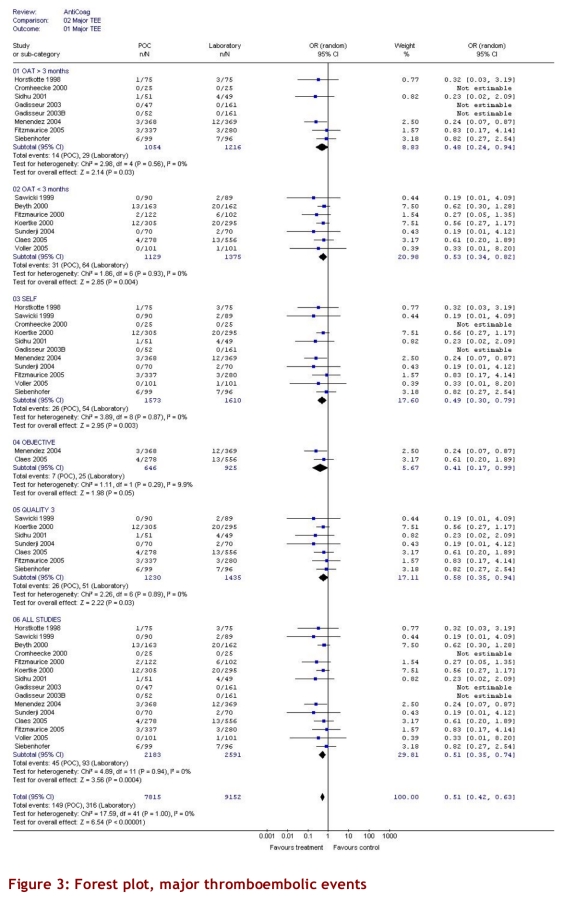
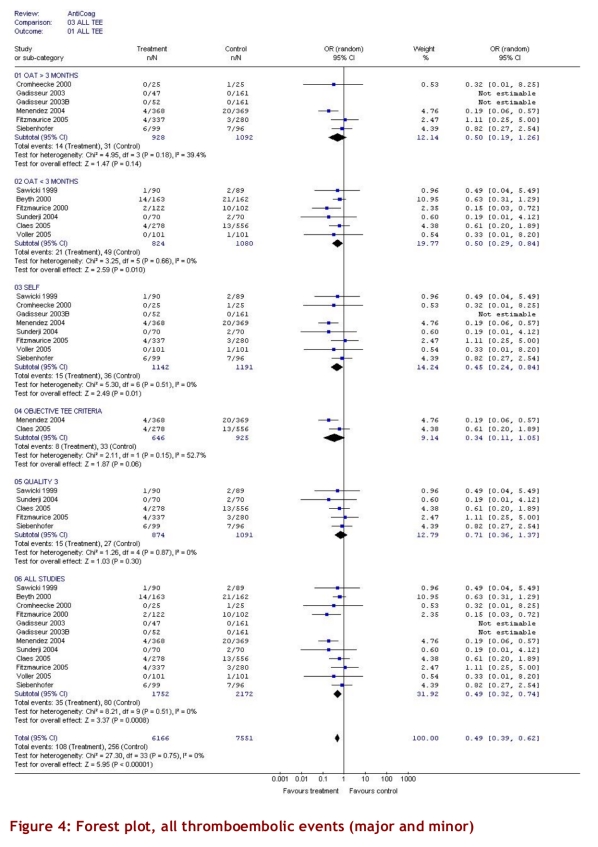
The intervention group comprised 2,144.6 patient-years of observation, while the control group comprised 2,316.1 patient-years. For all studies, there were significantly fewer major thromboembolic events in the POCD testing group than in the routine care group (OR = 0.51; 95% CI 0.35–0.74). This statistically significant difference was also observed in all 4 of the other subgroups (Table 3). The odds ratios for all thromboembolic events were similar. Death from any cause was significantly less likely in the POCD testing group (OR = 0.58; 95% CI 0.38–0.89) when all eligible studies were pooled, and this remained significant in all other analyses except those that included only 3 studies (i.e., those that defined the objective diagnostic criteria). For major hemorrhage, the odds ratio was not significantly different between the POCD testing group and the routine testing group in any of the analyses (OR = 0.78; 95% CI 0.53–1.14 for the analysis of all studies). The percentage time in therapeutic range was significantly better for the POCD group in all 4 relevant analyses (Table 3). For the “all studies” analysis, the mean percentage of time in range for the POCD testing group was 73% (95% CI 69%–76%) versus 62% (95% CI 59%–65%, p = 0.004) for the routine care group. Figures 2 to 5 show Forest plots for major hemorrhage, major thromboembolic events, all thromboembolic events, and death. Figure 6 shows the funnel plot for all thromboembolic events, which appears to be symmetrical and does not give an indication of publication bias. Figure 7 shows a funnel plot for major hemorrhage that suggests the possibility of publication bias. Figures 2 to 5 also provide information for assessing heterogeneity using Q and I2 statistics. This indicates a small effect of heterogeneity on the meta-analysis. The I2 values for major hemorrhage, major thromboembolism, all thromboembolism and death were 0%. When we analyzed the studies according to whether patients included had been on OAT for more than 3 months or for less than 3 months, these heterogeneity values did not change.
Table 3.
Odds ratios for primary and all secondary analyses
Figure 2.
Forest plot, major hemorrhage
Figure 5.
Forest plot, death
Figure 6.
Funnel plot, all thromboembolic events
Figure 7.
Funnel plot, major hemorrhage
We had hoped to be able to compare quality-of-life (QoL) scores between studies, but QoL was not uniformly measured, and when it was measured different tools were used. Five studies planned and performed formal evaluations. One used the EuroQol49 and reported no significant changes or differences between the study groups from study inception to completion. Two used the same 40-item structured questionnaire. Cromheeke44 demonstrated significant differences in 5 categories, in favour of the self-management group, and Sawicki53 demonstrated similar findings with the most pronounced improvements in general treatment satisfaction scores and distress scores. Two studies used locally developed satisfaction scales and demonstrated that patients were satisfied using POCDs, but the studies did not do any formal comparisons.54, 59
Subgroup analyses
For the subgroup analysis in which we analyzed separately the studies that had enrolled only patients who had been on OAT > 3 months, we found 9 studies. In 7 studies, patients were enrolled from the time of initiation of anticoagulation; of these, 2 studies (Sawicki53 and Völler56) did not state how long patients had been on anticoagulants, and so it was assumed that there was no 3-month minimum period. For arguably the most important subgroup analysis of self-testing and self-management there were 11 studies that compared self-testing and self-management to routine care (Table 2). Four of these studies compared self-testing and self-management with primary care as the routine management strategy, 3 compared it to hospital-based or specialized anticoagulation clinic care and 4 compared it to either primary care or anticoagulation clinic care. In this analysis the odds ratios were as follows: for major hemorrhage 0.75 (95% CI 0.47–1.20); for major thromboembolic events 0.49 (95% CI 0.30–0.79); for all thromboembolic events 0.45 (95% CI 0.24–0.84); and for deaths 0.48 (95% CI 0.24–0.94). For percentage time in range the means were 73% (95% CI 68%–78%) versus 62% (95% CI 60%–65%), p = 0.016, respectively. The quality score of the 16 studies varied from 1 to 3, with 9 attaining a score of 3 out of a maximum of 5 (Table 2). Two studies received a score of 1. Although no study was double-blinded (it could be argued that this is reasonable, and so the maximum quality score would be 3), the investigators could minimize potential bias by evaluating outcomes of hemorrhage and thromboembolic events without knowing whether patients underwent POCD testing or laboratory testing. This was done in 5 studies.47, 53, 57, 59, 60 Six studies used adequate allocation concealment.44, 45, 52, 58, 59, 60 Only 3 studies stated and defined how thromboembolic events would be diagnosed.52, 54, 58 Most studies did define a priori the criteria for major hemorrhage. All subgroup findings are illustrated in Figures 2 to 5.
To evaluate the potential scope for the use of POCDs, we considered whether they were well tolerated and easily employed. We looked for data on patient eligibility, agreement to consent, and withdrawal from studies. This is most relevant for the 11 studies in which patients were using the POCD for self-management. Three studies did not provide these data.48, 56, 58 The studies that did report these data showed the following:
With respect to the proportion of patients deemed eligible from a group of consecutive patients, in 9 studies 16% to 40% of patients were deemed unsuitable to use the POCD device, and most studies reported closer to 40% as unsuitable.
In 7 of the 11 studies, 10% to 28% of the patients dropped out after being randomly assigned to use the POCD and attempting the training program.
Seven studies reported that 8% to 19% abandoned the use of the POCD after the study began, compared with 0% to 6% who withdrew in the routine care groups.
Discussion
Our study reviewed 16 RCTs comparing POCD testing to routine anticoagulation monitoring care in a hospital or laboratory. Of those, 11 compared the use of POCDs for self-management and self-monitoring with routine anticoagulation monitoring. The latter comparison is the most relevant from the perspective of patient’s convenience of care, and the data in these trials suggest that POCDs result in significantly fewer major thromboembolic events. The odds ratio for major hemorrhage was 0.78, but the 95% confidence interval crossed 1 and therefore must be considered similar between the 2 groups. Insufficient data were provided in these studies to determine whether these differences were related to duration of time that the patient was over-anticoagulated or under-anticoagulated in respective groups. All results were unchanged regardless of the subgroup analysis performed. For all analyses, the comparison of percentage time in range between the POCD group and the control group demonstrated superiority with POCD use.
To test the robustness of the data we performed several subgroup analyses. One included only patients who had been on OAT for ≥ 3 months. We were initially concerned that including patients who were not yet stable on OAT could bias the results against the use of POCDs, given the known interactions with heparin and the difficulty in first achieving INR control.61 Our subgroup analysis showed that the results were essentially the same, regardless of the time that patients were on OAT at baseline. The odds ratios were also similar whether we included all studies (i.e., any POCD testing compared to routine INR testing) or just self-test plus self-management comparisons. The outcomes were unchanged regardless of who performed the dosing in the POCD groups. The summary data suggest that POCDs are advantageous. However, in all studies, the frequency of INR monitoring was higher in the POCD group than with routine anticoagulation monitoring. In most cases, the frequency of testing was dictated by the study protocol. It remains unknown whether similar frequent monitoring in routine care would eliminate these differences. It is also unknown whether this rigorous frequency of monitoring using POCDs would persist outside the study setting. It is possible that patients who self-manage may lose regular contact with their physician. The implications of this are unknown, but when assessed against the critical endpoint of death, it does not seem to be a disadvantage. It should be noted that we calculated odds ratios to approximate the relative risk, since the event rates were relatively “rare” (some take this as < 10%), and so the odds ratio approximation of the relative risk is good. The odds ratio will always be further from the neutral point of 1 than the relative risk (i.e., it is a less conservative measure), so the results should be interpreted with this in mind.
These findings are subject to certain limitations. First, the study methods were found to be less than ideal. The highest-quality score for any of the publications was 3, no study was double-blinded, and in many studies it was impossible to categorize what happened to the patients who withdrew. In all studies, the withdrawal rates of the POCD testing groups were higher than those of the routine testing groups. In most studies, thromboembolic events were not evaluated in a blinded and objective manner. This introduces the risk that the summary estimates may be biased. However, it is perhaps unreasonable to suggest in studies of this nature that double-blinding would be possible, and therefore in our analysis we categorized studies with a Jadad score of at least 3 as “high quality.”
Second, the criteria for patients’ eligibility for inclusion into the individual clinical trials and the high withdrawal rates are such that it is difficult to determine generalizability. It seems that the inclusion–exclusion criteria for the individual trials resulted in the exclusion of many patients deemed unsuitable for POCD self-testing before randomization, in addition to many such patients declining the invitation to participate. Perhaps more importantly, many patients failed to complete POCD training, and many who completed training subsequently dropped out. An upper bound of 24% of OAT patients could be eligible for self-testing or self-management with POCD.36 Consequently, the results of our meta-analysis may apply only to selected patients.
Third, the INR test frequency was much higher in the POCD testing strategies: INRs were performed approximately weekly versus monthly in the standard group. Furthermore, the POCD group underwent 3 2-hour, small-group education sessions in most studies, whereas no special education was provided to the standard care groups.
The final limitation of these studies is that there is no description of the frequency with which warfarin was withdrawn for surgical procedures or other interventions. As such, we have no way of knowing how many of the thromboembolic or hemorrhagic events could have been related to this phenomenon. It has been clearly documented that hospitalization creates the greatest risk of poor anticoagulation control, and there is a suggestion that the risk of thrombosis and hemorrhage is highest around the time of hospitalization of patients on oral anticoagulant therapy. Although these studies are all randomized, without this information it is difficult to know if there are discrepancies between the POCD group and the routine care group.64 These limitations make it impossible to determine what effects the frequency of monitoring, patient education, the POCD, or the patient selection may have on the outcomes.60
Although information on quality of life and patient satisfaction with the POCDs was collected, we were unable to provide quantitative summary measures of these factors. A qualitative analysis of the data suggest that, in general, patients were at least as satisfied with self-management using a POCD as with receiving care at an anticoagulation clinic, and that some preferred using a POCD. These patients were good candidates for using a POCD, having been self-selected or selected by a health care researcher.
This report is not the first systematic review to compare POCD testing with laboratory testing for the management of patients on OAT. Five systematic reviews have been published, but all have limitations.22, 24, 62, 63 The most recent analysis by Heneghan and colleagues suggests that POCD testing resulted in significantly fewer major hemorrhages, thromboembolic events, and deaths than conventional INR testing.61 The Heneghan analysis involved the examination of 14 studies, included 3 that we considered to be ineligible: one compared POCD testing only with other POCD testing (i.e., inappropriate comparison for their analysis), one did not use POCD test results for OAT management, and the third followed patients for only 8 weeks.21, 27, 43 Our report also included 5 articles that were either excluded or not yet published at the time of Heneghan’s analysis.45, 46, 54, 58 Finally, the summary data used by these authors reported events per patient enrolled and not by length of follow-up, as we did. As such, the rates we report are more accurate and more relevant. With the additional studies and analysis we used, we came to similar conclusions with respect to the rate of all thromboembolic events, deaths and time in therapeutic range. Heneghan did not report separately on the more relevant outcome of major thromboembolic events. Contrary to Heneghan’s study our analysis did not detect a significant difference for the outcome of major hemorrhage.
Our meta-analysis suggests that using POCDs to manage OAT results in significantly fewer thromboembolic events and better INR control than laboratory-based INR testing. Under usual care, warfarin therapy requires regular laboratory monitoring of the INR, coupled with frequent physician–patient contact for dosage adjustment to ensure efficacy and safety.9 The usual-care method can be cumbersome and inconvenient for the patient and the physician. There is also a potential for dosing errors resulting from misinterpretation of information conveyed by the physician or delays in contacting the patient.9 This, plus faster test results, greater convenience and more frequent testing, are plausible reasons for the incremental health benefits observed with POCD use.
Unfortunately, the studies designed to date do not allow a determination of why POCD care is superior. Further randomized controlled trials are needed to determine whether it is patient education or frequency of testing that provides superior outcomes. Widespread adoption of POCD monitoring at this time would be premature.
Figure 3.
Forest plot, major thromboembolic events
Figure 4.
Forest plot, all thromboembolic events (major and minor)
Biographies
Philip S. Wells is a professor in the departments of Medicine and of Epidemiology and Community Medicine at the University of Ottawa. He is the director of clinical research at The Ottawa Hospital and is the associate director of clinical research and a senior scientist with the Ottawa Health Research Institute, Ottawa, Ont.
Allan Brown is a health economist with the Canadian Agency for Drugs and Technologies in Health, Ottawa, Ont.
James Jaffey is a statistician with the Ottawa Health Research Institute, Ottawa, Ont.
Lynda McGahan is a research officer with the Canadian Agency for Drugs and Technologies in Health, Ottawa, Ont.
Man-Chiu Poon is a professor in the Department of Medicine, University of Calgary and Foothills Medical Centre, Calgary, Alta.
Karen Cimon is a research assistant with the Canadian Agency for Drugs and Technologies in Health, Ottawa, Ont.
Footnotes
Competing interests: None declared.
Funding source: Dr. Wells is the recipient of a Canada Research Chair Award.
References
- 1.Chai SJ, Macik BG. Improving the safety profile of warfarin. Semin Hematol. 2002;39(3):179–186. doi: 10.1053/shem.2002.34089. [DOI] [PubMed] [Google Scholar]
- 2.Ansell JE, Weitz JI, Comerota AJ. Advances in therapy and the management of antithrombotic drugs for venous thromboembolism. Hematology. 2000;(1):266–284. doi: 10.1182/asheducation-2000.1.266. http://asheducationbook.hematologylibrary.org/cgi/content/full/2000/1/266. [DOI] [PubMed] [Google Scholar]
- 3.Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):204S–233S. doi: 10.1378/chest.126.3_suppl.204S. [DOI] [PubMed] [Google Scholar]
- 1.Singer DE, Albers GW, Dalen JE, Go AS, Halperin JL, Manning WJ. Antithrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):429S–456S. doi: 10.1378/chest.126.3_suppl.429S. [DOI] [PubMed] [Google Scholar]
- 5.Schulman S. Oral anticoagulation. In: Beutler E, Coller BS, Lichtman MA, Kipps TJ, editors. Williams hematology. 6th ed. 2001. pp. 1777–1792. [Google Scholar]
- 6.Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):204S–233S. doi: 10.1378/chest.126.3_suppl.204S. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds MW, Fahrbach K, Hauch O, Wygant G, Estok R, Cella C, Nalysnyk L. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis. Chest. 2004 Dec;126(6):1938–1945. doi: 10.1378/chest.126.6.1938. [DOI] [PubMed] [Google Scholar]
- 8.Mörsdorf S, Erdlenbruch W, Taborski U, Schenk JF, Erdlenbruch K, Novotny-Reichert G, Krischek B, Wenzel E. Training of patients for self-management of oral anticoagulant therapy: standards, patient suitability, and clinical aspects. Semin Thromb Hemost. 1999;25(1):109–115. doi: 10.1055/s-2007-996433. [DOI] [PubMed] [Google Scholar]
- 9.Sunderji R, Fung A, Gin K, Shalansky K, Carter C. Patient self-management of oral anticoagulation: a review. Can J Cardiol. 2003 Jul;19(8):931–935. [PubMed] [Google Scholar]
- 10.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993 Mar 1;69(3):236–239. [PubMed] [Google Scholar]
- 11.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996 Feb;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 12.Schulz KF, Grimes DA. Unequal group sizes in randomised trials: guarding against guessing. Lancet. 2002 Mar 16;359(9310):966–970. doi: 10.1016/S0140-6736(02)07882-0. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 1.Takwale A, Tan E, Agarwal S, Barclay G, Ahmed I, Hotchkiss K, Thompson JR, Chapman T, Berth-Jones J. Efficacy and tolerability of borage oil in adults and children with atopic eczema: randomised, double blind, placebo controlled, parallel group trial. BMJ. 2003 Dec 13;327(7428):1385. doi: 10.1136/bmj.327.7428.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muir JM, Andrew M, Hirsh J, Weitz JI, Young E, Deschamps P, Shaughnessy SG. Histomorphometric analysis of the effects of standard heparin on trabecular bone in vivo. Blood. 1996 Aug 15;88(4):1314–1320. [PubMed] [Google Scholar]
- 16.Becker R, Andrew M, Ansell J. Multicenter trial of accurate home self-testing in oral anticoagulant patients with a novel whole blood system. J Am Coll Cardiol. 1997;29(2 Suppl A):525a. http://www.protimesystem.com/support/Multicenter_Trial_of_Accurate.pdf. [Google Scholar]
- 17.Biasiolo A, Rampazzo P, Furnari O, Filippi B, Pengo V. Comparison between routine laboratory prothrombin time measurements and fingerstick determinations using a near-patient testing device (Pro-Time) Thromb Res. 2000 Mar 15;97(6):495–498. doi: 10.1016/S0049-3848(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 18.Caliezi C, Niederer A, Wuillemin WA. [Patient self-management of oral anticoagulation] Ther Umsch Rev Ther. 2003;60:59–62. doi: 10.1024/0040-5930.60.1.59. [DOI] [PubMed] [Google Scholar]
- 19.Cosmi B, Palareti G, Moia M, Pengo V, Testa S. [Home use of an ambulatory monitor for the determination of prothrombin time (coaguchek) in patients treated with oral anticoagulants. Multicenter prospective study] Minerva Cardioangiol. 1999 Dec;47(12):598–599. [PubMed] [Google Scholar]
- 20.Watzke HH, Forberg E, Svolba G, Jimenez-Boj E, Krinninger B. A prospective controlled trial comparing weekly self-testing and self-dosing with the standard management of patients on stable oral anticoagulation. Thromb Haemost. 2000 May;83(5):661–665. [PubMed] [Google Scholar]
- 21.White RH, McCurdy SA, von Marensdorff H, Woodruff DE, Leftgoff L. Home prothrombin time monitoring after the initiation of warfarin therapy. A randomized, prospective study. Ann Intern Med. 1989 Nov 1;111(9):730–737. doi: 10.7326/0003-4819-111-9-730. [DOI] [PubMed] [Google Scholar]
- 22.de Solà-Morales Serra O, Elorza Ricart JM. [Portable coagulometers: a systematic review of the evidence on self-management of oral anticoagulant treatment] Med Clin (Barc) 2005 Mar 12;124(9):321–325. doi: 10.1157/13072418. http://www.elsevier.es/revistas/ctl_servlet?_f=7264&articuloid=13072418&revistaid=2. [DOI] [PubMed] [Google Scholar]
- 23.Ødegaard KJ. [Self-management in anticoagulation—a meta-analysis] Tidsskr Nor Laegeforen. 2004;124(22):2900–2903. http://www.tidsskriftet.no/index.php?seks_id=1100679. [PubMed] [Google Scholar]
- 24.Siebenhofer A, Berghold A, Sawicki PT. Systematic review of studies of self-management of oral anticoagulation. Thromb Haemost. 2004 Feb;91(2):225–232. doi: 10.1160/THR03-09-0598. [DOI] [PubMed] [Google Scholar]
- 25.Fitzmaurice DA, Gardiner C, Kitchen S, Mackie I, Murray ET, Machin SJ. An evidence-based review and guidelines for patient self-testing and management of oral anticoagulation. Br J Haematol. 2005 Oct;131(2):156–165. doi: 10.1111/j.1365-2141.2005.05739.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2141.2005.05739.x/full. [DOI] [PubMed] [Google Scholar]
- 26.Barreira R, Ribeiro J, Farinha M, Martins R, Rodrigues I, Mendes Z, Crespo F. [Monitoring therapy with oral anticoagulants. Anticoagulation clinics vs assistant physician] Acta Med Port. 2005 Jan 18;17(6):413–416. [PubMed] [Google Scholar]
- 27.Gardiner C, Pennaneac'h C, Walford C, Machin SJ, Mackie IJ. An evaluation of rapid D-dimer assays for the exclusion of deep vein thrombosis. Br J Haematol. 2005 Mar;128(6):842–848. doi: 10.1111/j.1365-2141.2005.05394.x. [DOI] [PubMed] [Google Scholar]
- 28.Koertke H, Minami K, Boethig D, Breymann T, Seifert D, Wagner O, Atmacha N, Krian A, Ennker J, Taborski U, Klövekorn WP, Moosdorf R, Saggau W, Koerfer R. INR self-management permits lower anticoagulation levels after mechanical heart valve replacement. Circulation. 2003 Sep 9;108(Suppl 1):II75–II78. doi: 10.1161/01.cir.0000089185.80318.3f. [DOI] [PubMed] [Google Scholar]
- 29.Jackson SL, Peterson GM, Vial JH, Jupe DML. Improving the outcomes of anticoagulation: an evaluation of home follow-up of warfarin initiation. J Intern Med. 2004 Aug;256(2):137–144. doi: 10.1111/j.1365-2796.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- 30.Caprini JA, Sehgal LR, Oyslender M. A novel system to manage anticoagulation in hip replacement patients employing patient self-testing and a telemonitoring system. Blood. 2003;102(11):325a. [Google Scholar]
- 31.McCahon D, Fitzmaurice DA, Murray ET, Fuller CJ, Hobbs RFD, Allan TF, Raftery JP. SMART: self-management of anticoagulation, a randomised trial [ISRCTN19313375] BMC Fam Pract. 2003 Sep 18;4:11. doi: 10.1186/1471-2296-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Völler H, Glatz J, Taborski U, Bernardo A, Dovifat C, Burkard G, Heidinger K. [Background and evaluation plan of a study on self-management of anticoagulation in patients with non-valvular atrial fibrillation (SMAAF Study)] Z Kardiol. 2000;89:284–288. doi: 10.1007/s003920050486. [DOI] [PubMed] [Google Scholar]
- 3.Matchar DB, Jacobson AK, Edson RG, Lavori PW, Ansell JE, Ezekowitz MD, Rickles F, Fiore L, Boardman K, Phibbs C, Fihn SD, Vertrees JE, Dolor R. The impact of patient self-testing of prothrombin time for managing anticoagulation: rationale and design of VA Cooperative Study #481--the Home INR Study (THINRS) J Thromb Thrombolysis. 2005 Jun;19(3):163–172. doi: 10.1007/s11239-005-1452-0. [DOI] [PubMed] [Google Scholar]
- 34.[Coagulation self-control after valve replacement. Stable anticoagulation permits lowering of the intensity] MMW Fortschr Med. 2004;146(14):44. [PubMed] [Google Scholar]
- 35.Williams E, Gardiner C, Mackie IJ. Preliminary data from the Medical Devices Agency (MDA) study of the CoaguCheck S in a patient self-testing environment. Br J Haematol. 2003;121(Suppl 1):7. [Google Scholar]
- 36.Murray R. Hit for six: BMJ has published pioneering work. BMJ. 2004 Jun 19;328(7454):1500. doi: 10.1136/bmj.328.7454.1500-b. discussion 1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzmaurice DA, Rose PE. The haematological primary/secondary care interface. A randomised controlled trial comparing primary care oral anticoagulant management utilising computerised decision support (DSS) and near patient testing (NPT), with traditional management. Br J Haematol. 1996;93(Suppl 2):9. [Google Scholar]
- 38.Fitzmaurice DA. A randomised controlled trial comparing primary care oral anticoagulant management utilising computerised decision support (DSS) and near patient testing (NPT) versus routine care: final results. Br J Haematol. 1997;97(Suppl 1):79. [Google Scholar]
- 39.Gadisseur APA, Kaptein AA, Breukink-Engbers WGM, van der Meer FJ, Rosendaal FR. Patient self-management of oral anticoagulant care vs. management by specialized anticoagulation clinics: positive effects on quality of life. J Thromb Haemost. 2004 Apr;2(4):584–591. doi: 10.1111/j.1538-7836.2004.00659.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1538-7836.2004.00659.x/full. [DOI] [PubMed] [Google Scholar]
- 40.Alain PA, Gadisseur AD, Kapstein M, Breukink-Engbers GM, van der Meer FJ, Rosendaal FR. Patient self-management of oral anticoagulant care versus management by specialized anticoagulation clinics: positive effects on quality of life [abstract] Blood. 2003 Nov 16;102(1):1152. doi: 10.1111/j.1538-7836.2004.00659.x. [DOI] [PubMed] [Google Scholar]
- 41.Körtke H, Minami K, Breymann T. [INR self-management after mechanical heart valve replacement: ESCAT (Early Self-Controlled Anticoagulation Trial)] Z Kardiol. 2001;90(Suppl 6):118–124. doi: 10.1007/s003920170019. [DOI] [PubMed] [Google Scholar]
- 42.Sawicki PT, Gläser B, Kleespies C, Stubbe J, Schmitz N, Kaiser T, Didjurgeit U. Long-term results of patients’ self-management of oral anticoagulation. J Clin Basic Cardiol. 2003;6:59–62. doi: 10.1046/j.1365-2796.2003.01215.x. http://www.kup.at/kup/pdf/3927.pdf. [DOI] [PubMed] [Google Scholar]
- 43.Fitzmaurice DA, Murray ET, Gee KM, Allan TF, Hobbs FDR. A randomised controlled trial of patient self management of oral anticoagulation treatment compared with primary care management. J Clin Pathol. 2002 Nov;55(11):845–849. doi: 10.1136/jcp.55.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cromheecke ME, Levi M, Colly LP, de Mol BJ, Prins MH, Hutten BA, Mak R, Keyzers KC, Büller HR. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomised cross-over comparison. Lancet. 2000 Jul 8;356(9224):97–102. doi: 10.1016/S0140-6736(00)02470-3. [DOI] [PubMed] [Google Scholar]
- 45.Fitzmaurice DA, Murray ET, McCahon D, Holder R, Raftery JP, Hussain S, Sandhar H. Self management of oral anticoagulation: randomised trial. BMJ. 2005;331(7524):1057. doi: 10.1136/bmj.38618.580903.AE. published erratum appears in BMJ 2005 Dec. 3;331;1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzmaurice DA, Hobbs FD, Murray ET, Holder RL, Allan TF, Rose PE. Oral anticoagulation management in primary care with the use of computerized decision support and near-patient testing: a randomized, controlled trial. Arch Intern Med. 2000;160(15):2343–2348. doi: 10.1001/archinte.160.15.2343. [DOI] [PubMed] [Google Scholar]
- 47.Gadisseur APA, Breukink-Engbers WGM, van der Meer FJ, van den Besselaar AM, Sturk A, Rosendaal FR. Comparison of the quality of oral anticoagulant therapy through patient self-management and management by specialized anticoagulation clinics in the Netherlands: a randomized clinical trial. Arch Intern Med. 2003 Nov 24;163(21):2639–2646. doi: 10.1001/archinte.163.21.2639. [DOI] [PubMed] [Google Scholar]
- 48.Neumayer U, Schmidt HK, Fassbender D, Mannebach H, Bogunovic N, Horstkotte D. Balloon mitral valvotomy after surgical commissurotomy: clinical and hemodynamic results of a large, single-center study. J Heart Valve Dis. 2004 Sep;13(5):760–765. [PubMed] [Google Scholar]
- 49.Khan TI, Kamali F, Kesteven P, Avery P, Wynne H. The value of education and self-monitoring in the management of warfarin therapy in older patients with unstable control of anticoagulation. Br J Haematol. 2004 Aug;126(4):557–564. doi: 10.1111/j.1365-2141.2004.05074.x. [DOI] [PubMed] [Google Scholar]
- 50.Koertke H, Minami K, Bairaktaris A, Wagner O, Koerfer R. INR self-management following mechanical heart valve replacement. J Thromb Thrombolysis. 2000;(Suppl 1):S41–S45. doi: 10.1023/a:1018712520472. [DOI] [PubMed] [Google Scholar]
- 51.Koertke H, Korfer R. International normalized ratio self-management after mechanical heart valve replacement: is an early start advantageous? Ann Thorac Surg. 2001;72:44–48. doi: 10.1016/s0003-4975(01)02656-x. [DOI] [PubMed] [Google Scholar]
- 52.Menéndez-Jándula B, Souto JC, Oliver A, Montserrat I, Quintana M, Gich I, Bonfill X, Fontcuberta J. Comparing self-management of oral anticoagulant therapy with clinic management: a randomized trial. Ann Intern Med. 2005;142(1):1–10. doi: 10.7326/0003-4819-142-1-200501040-00006. [DOI] [PubMed] [Google Scholar]
- 53.Sawicki PT Working Group for the Study of Patient Self-Management of Oral Anticoagulation. A structured teaching and self-management program for patients receiving oral anticoagulation: a randomized controlled trial. JAMA. 1999 Jan 13;281(2):145–150. doi: 10.1001/jama.281.2.145. http://jama.ama-assn.org/cgi/content/full/281/2/145. [DOI] [PubMed] [Google Scholar]
- 54.Shiach CR, Campbell B, Poller L, Keown M, Chauhan N. Reliability of point-of-care prothrombin time testing in a community clinic: a randomized crossover comparison with hospital laboratory testing. Br J Haematol. 2002 Nov;119(2):370–375. doi: 10.1046/j.1365-2141.2002.03888.x. [DOI] [PubMed] [Google Scholar]
- 55.Sidhu P, O'Kane HO. Self-managed anticoagulation: results from a two-year prospective randomized trial with heart valve patients. Ann Thorac Surg. 2001 Nov;72(5):1523–1527. doi: 10.1016/S0003-4975(01)03049-1. [DOI] [PubMed] [Google Scholar]
- 56.Völler H, Glatz J, Taborski U. [Self-management of oral anticoagulation in nonvalvular atrial fibrillation (SMAAF study)] Z Kardiol. 2005;94(3):182–186. doi: 10.1007/s00392-005-0199-0. [DOI] [PubMed] [Google Scholar]
- 57.Beyth RJ, Quinn L, Landefeld CS. A multicomponent intervention to prevent major bleeding complications in older patients receiving warfarin. A randomized, controlled trial. Ann Intern Med. 2000 Nov 7;133(9):687–695. doi: 10.7326/0003-4819-133-9-200011070-00010. [DOI] [PubMed] [Google Scholar]
- 58.Claes N, Buntinx F, Vijgen J, Arnout J, Vermylen J, Fieuws S, Van Loon H. The Belgian Improvement Study on Oral Anticoagulation Therapy: a randomized clinical trial. Eur Heart J. 2005 Oct;26(20):2159–2165. doi: 10.1093/eurheartj/ehi327. [DOI] [PubMed] [Google Scholar]
- 59.Sunderji R, Gin K, Shalansky K, Carter C, Chambers K, Davies C, Schwartz L, Fung A. A randomized trial of patient self-managed versus physician-managed oral anticoagulation. Can J Cardiol. 2004 Sep;20(11):1117–1123. [PubMed] [Google Scholar]
- 60.Siebenhofer A, Rakovac I, Kleespies C, Piso B, Didjurgeit U. Self-management of oral anticoagulation in the elderly: rationale, design, baselines and oral anticoagulation control after one year of follow-up. A randomized controlled trial. Thromb Haemost. 2007 Mar;97(3):408–416. [PubMed] [Google Scholar]
- 61.Phillips EM, Buchan DA, Newman N, Rajan A, Zia S. Low-molecular-weight heparin may alter point-of-care assay for international normalized ratio. Pharmacotherapy. 2005;25(10):1341–1347. doi: 10.1592/phco.2005.25.10.1341. [DOI] [PubMed] [Google Scholar]
- 62.Heneghan C, Alonso-Coello P, Garcia-Alamino JM, Perera R, Meats E, Glasziou P. Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet. 2006 Feb 4;367(9508):404–411. doi: 10.1016/S0140-6736(06)68139-7. [DOI] [PubMed] [Google Scholar]
- 63.Medical Science Advisory Committee, Department of Health and Aging. The use of INR point-of-care testing in general practice: assessment report [MSAC application 1071] 2005. http://nzhta.chmeds.ac.nz/publications/annotated.pdf.
- 64.van Walraven C, Austin PC, Oake N, Wells P, Mamdani M, Forster AJ. The effect of hospitalization on oral anticoagulation control: a population-based study. Thromb Res. 2007;119(6):705–714. doi: 10.1016/j.thromres.2006.05.017. [DOI] [PubMed] [Google Scholar]