Abstract
Understanding gene expression that is responsive to sensory stimulation is central to elucidate molecular mechanisms underlying neuronal plasticity. In this study we demonstrate two new methods of stimulating whiskers that provide major sensory input to rat neocortex. In the first paradigm, animals were placed on the top of a cylinder and their vibrissae were brushed by hand. In the second paradigm, animals were placed for a brief period of time into a new, wired cage resulting in vibrissae stimulation when they explored the new environment. Both approaches induced c-Fos expression in barrel cortex corresponding to the stimulated vibrissae, especially in layer IV. Layers II/III and V/VI also showed c-Fos induction, but there were no detectable changes in layer VIb. The majority of c-Fos-expressing cells are probably not inhibitory neurons, because they do not show parvalbumin staining. Both paradigms, in contrast to the previous methods, are simple to use and do not require anesthesia, restraint of animals, or elaborate experimental setups.
The rodent somatosensory cortex, representing mystacial vibrissae (large whiskers) of the snout, offers an exquisite system to study the effects of sensory stimulation on neocortical structure and function. Sensory input produced by deflection of the whiskers is conveyed via thalamus to layer IV of SmI, which contains discrete clusters of closely packed cells, termed barrels. There is one-to-one correspondence between each barrel and vibrissa. Each whisker in turn excites an entire cortical column. The whisker-to-barrel system is lateralized, so that the somatosensory cortex on one side represents vibrissae on the contralateral side of the snout. Removal of the whisker(s) in adults results in time-dependent modification of behavior, accompanied by neuronal alterations, and evokes plastic changes in cortical neurons (Kossut 1992; Diamond et al. 1993; Armstrong-James et al. 1994). The part of the barrel field containing the large barrels and representing the vibrissae of the mystacial pad has been named posteromedial barrel subfield (PMBSF; Woolsey and Van der Loos 1970).
Treatment of rats with apomorphine, a dopamine receptor agonist enhancing motor activity, results in the accumulation of c-fos and zif268 (the two best studied immediate early genes) mRNA in various brain regions including somatosensory cortex (Steiner and Gerfen 1994). This effect was apparently input specific, because clipping of the whiskers prevented the increase in the corresponding PMBSF. Furthermore, Melzer and Steiner (1997) showed that the expression of both genes was restricted largely to the radial columns across the barrels representing the stimulated whiskers, with the magnitude of expression being proportional to the intensity of stimulation. Previously, Mack and Mack (1992) achieved unilateral cortical elevation of c-Fos and Zif268 protein expression by selective mechanical stimulation of the whiskers on one side of the snout under anesthesia. All of these studies imply that sensory input is critical for c-fos and zif268 activation in the barrel cortex, similar to the visual cortex (Kaczmarek and Chaudhuri 1997).
These findings are important for two major reasons: (1) It is now well established that prolonged alteration of sensory stimulation initiates neuronal plasticity, i.e., a set of anatomical and physiological changes in the central nervous system that leads ultimately to the brain's ability to effectively adapt to the modified stimulus even in adulthood (for reviews, see Garraghty and Kaas 1992; Merzenich and Sameshima 1993; Daw 1994; O'Leary et al. 1994; Rauschecker 1995); (2) c-Fos and Zif268 are transcription factor proteins whose changes in gene expression are widely believed to underlay neuronal plasticity. Sensory stimulation also produces up-regulation of brain-derived neurotrophic factor (BDNF) mRNA expression in barrel cortex (Rocamora et al. 1996) but has no effect on nerve growth factor (NGF) and tyrosine hydroxylase mRNA levels (Schwarting et al. 1994).
At present, experimental models of vibrissae stimulation described in the literature require immobilization and pain (Jablonska et al. 1996; Lukasiuk et al. 1999), anesthesia (Mack and Mack 1992), other artificial situations like a Lausanne stimulator (an electromagnetic coil with magnetic field bursts moving metal pieces glued to whiskers, Welker 1964; Welker et al. 1989), or introduction of animals into a very elaborate environment (Montero 1997). We have developed simpler experimental systems to investigate the effects of tactile stimulation of vibrissae on gene expression. To further characterize the cells expressing c-Fos, we analyzed the expression pattern of a calcium-binding protein, parvalbumin, which is used to mark inhibitory interneurons (Celio 1986; Ren et al. 1992; Kubota and Jones 1993; Hiscock et al. 1996). Parvalbumin expression has been observed to be enriched in cortical regions of high cellular activity, such as those marked by elevated expression of cytochrome oxidase (Wong-Riley 1989; Seto-Ohshima 1994). These areas also show increases in c-Fos expression in response to sensory signal-driven neuronal stimulation (Kaczmarek et al. 1999).
RESULTS
The results described below are based on two experiments involving various means of vibrissae stimulation as outlined in Figures 1 and 2. See also Materials and Methods for details.
Figure 1.
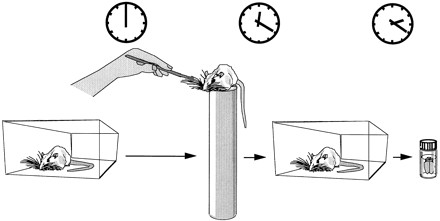
Design of experiment 1, in which rats that were pretrained to sit on the top of a copper cylinder had their vibrissae manually brushed on one side of the snout for 20 min. The animals were then transferred back to their home cage for 2 hr and perfused for immunocytochemistry.
Figure 2.
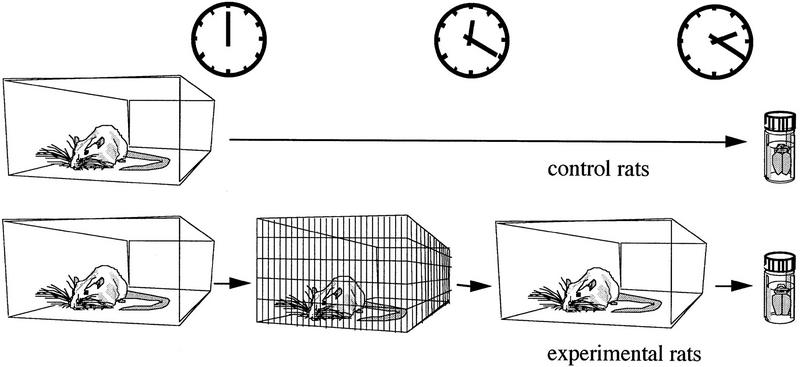
Design of experiment 2, in which rats were exposed to a new environment (the wired cage). After 20 min in a new cage the experimental animals were transferred back to their home cage for 2 hr. The experimental and naive rats were then perfused for immunocytochemistry.
Experiment 1
Behavior
The rats adjusted quickly to sitting on the top of the cylinder, refrained from jumping, and even slept during the habituation period. On the experimental day, during brushing, rats were active and whisking with all vibrissae on both sides of the snout. Even with these experimental manipulations, the rats remained on the platform.
Immunocytochemistry
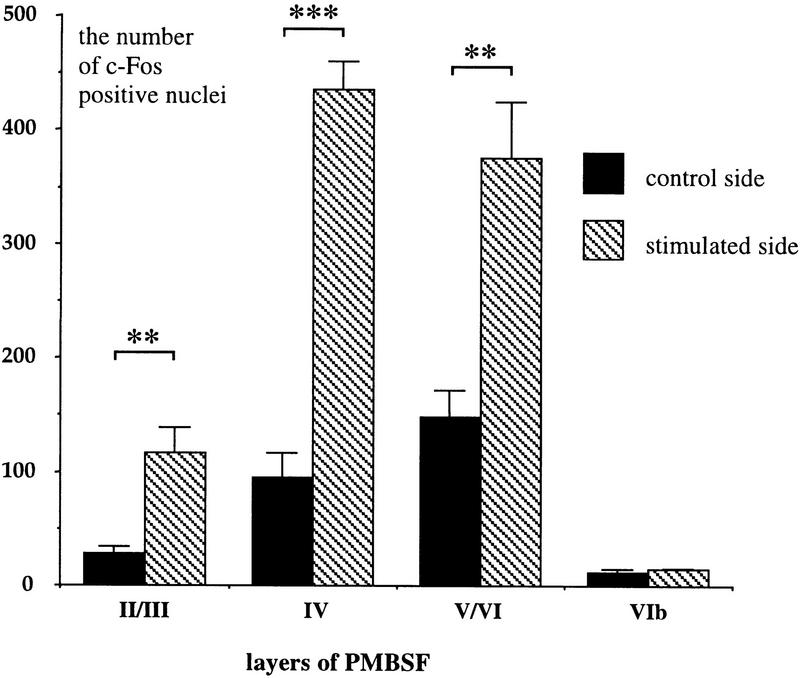
There was an increase in c-Fos expression in the PMBSF area corresponding to the brushed vibrissae (Fig. 3). This effect was most pronounced in layer IV (4.6-fold increase, 434.7 ± 25.0 vs. 95.2 ± 21.7), as well as in layers II/III (4.1-fold increase, 117.4 ± 21.6 vs. 28.6 ± 5.6) and V/VI (2.5-fold increase, 376 ± 48.5 vs. 148.2 ± 23.8) with significant up-regulation in all these layers (P < 0.01). No effect of the stimulation on c-Fos expression was observed in layer VIb, 1.2-fold increase, 15.3 ± 1.4 vs. 12.4 ± 2.7, P = 0.36 (Fig. 4).
Figure 3.
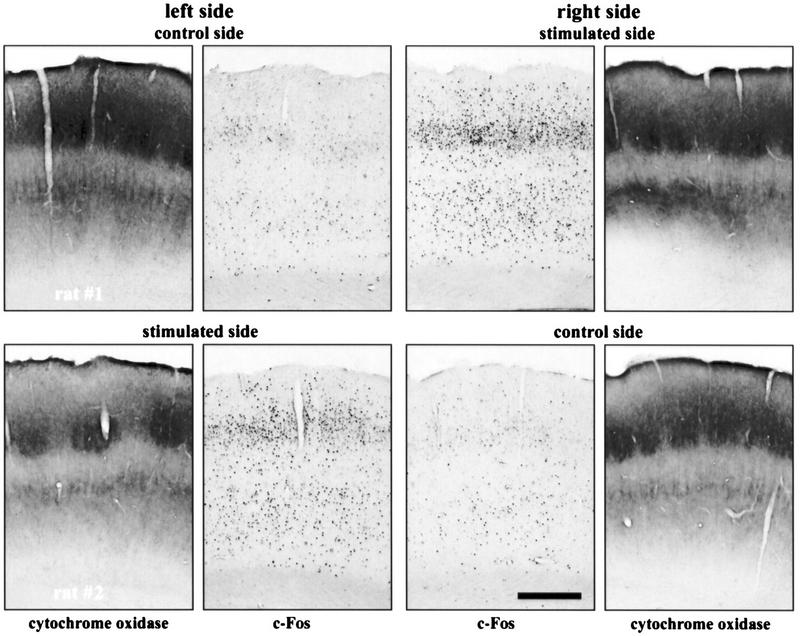
Immunocytochemistry showing an increase in c-Fos expression in the PMBSF corresponding to stimulated vibrissae (experiment 1). Shown are both left and right PMBSF of two rats that had their whiskers brushed on the left side (top) and the right side (bottom) of the snout. Pictures of cytochrome oxidase staining of neighboring sections are included. Calibration bar, 0.5 mm.
Figure 4.
Vibrissae brushing caused significant increase in c-Fos expression in the layers of the PMBSF (experiment 1). The results of immunocytochemistry were quantified in all layers on both control (n = 4, solid bars) and stimulated (n = 4, hatched bars) sides of the cortex (see Materials and Methods). Data are means and s.e.m. Statistically significant differences are marked with asterisks (**) for P < 0.01 and (***) for P < 0.001. According to one-way ANOVA analysis, these differences were most pronounced in layers IV [4.6-fold increase, F(1,6) = 105.39, P < 0.0001], II/III [4.1-fold increase, F(1,6) = 15.79, P < 0.01] and V/VI [2.5-fold increase, F(1,6) = 17.79, P < 0.01]. However, no significant difference in c-Fos expression was found in layer VIb following brushing [F(1,6) = 0.97, P = 0.36].
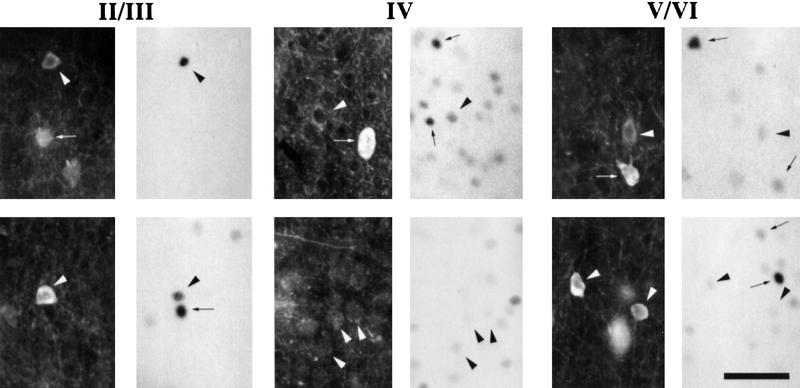
Counterstaining of the c-Fos immunostained sections with antibodies directed specifically against a calcium-binding protein, parvalbumin, revealed that the vast majority (i.e., >90%) of cells containing c-Fos in the PMBSF after stimulation did not show expression of parvalbumin. However, cells containing both c-Fos and parvalbumin were observed occasionally in all layers investigated (Fig. 5).
Figure 5.
Examples of cells containing parvalbumin (dark panels, left) and c-Fos (light panels, right) in II/III, IV, and V/VI layers of the PMBSF following stimulation (experiment 1). Cells containing one protein are marked with small arrows; cells with both proteins are marked with arrowheads. Photographs are placed such that their bottom and top edges are parallel to the surface of the cortex. Calibration, 50 μm.
Experiment 2
Behavior
To avoid any unnecessary stimulation, the exposure to the novel, wired cage was carried out in a dark, soundproof room. In preliminary experiments (in an illuminated environment), it was verified that exposure to such a cage caused an active exploratory behavior in the animals, involving intense sniffing and whisking.
Immunocytochemistry
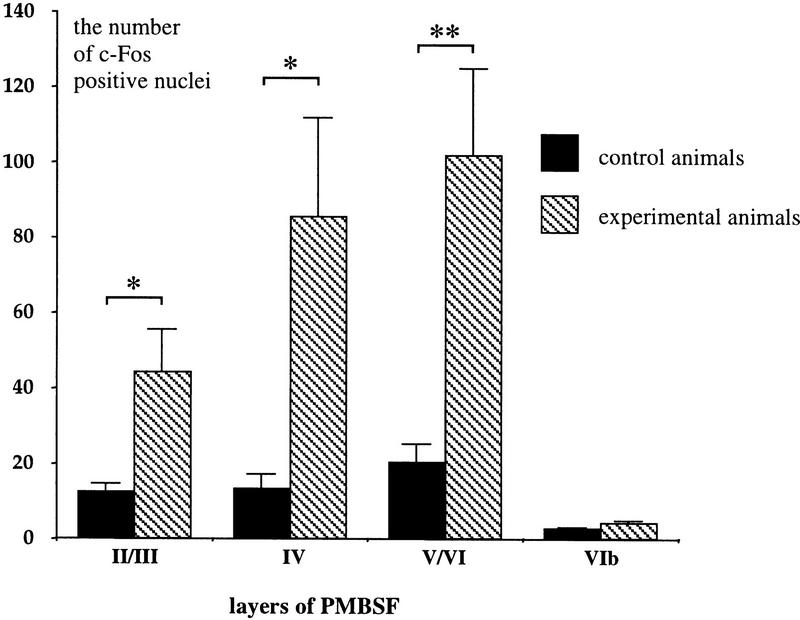
Little c-Fos expression was observed in control animals. An induction of c-Fos immunolabeling was observed following exposure of the rats to a new environment in the PMBSF area (Fig. 6). Figure 7 shows that c-Fos activation was strongest in layer IV (6.4-fold increase, 85.5 ± 26.2 vs. 13.3 ± 3.8), as well as layers II/III (3.5-fold increase, 44.15 ± 11.4 vs. 12.46 ± 2.1) and V/VI (5.0-fold increase, 101.9 ± 23.1 vs. 20.4 ± 4.9). One-way ANOVAs applied separately for each layer showed significant differences between control and experimental animals in layers II/III, IV, and V/VI (P < 0.05). Similarly, as in the previous experimental situation, the elevation of c-Fos expression was not significant in layer VIb (1.6-fold increase, 4.2 ± 0.6 vs. 2.7 ± 0.3, P = 0.06).
Figure 6.
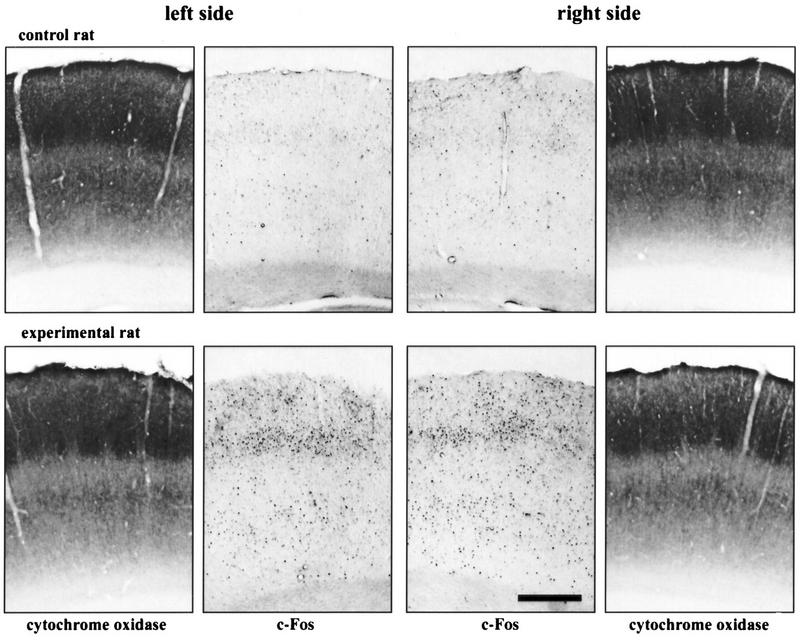
Immunocytochemistry showing an increase in c-Fos expression in the PMBSF after placing rats in a new environment (experiment 2). Shown are both left and right PMBSFs of two rats: The control (naive) rat (top panels) and the experimental rat (bottom panels). Pictures of cytochrome oxidase staining of neighboring sections are included. Calibration, 0.5 mm.
Figure 7.
Introducing rats to a new cage induced c-Fos expression in layers of the PMBSF (experiment 2). Data are means and s.e.m. The results of immunocytochemistry were quantified in all cortical layers of naive (n = 7, solid bars) and experimental (n = 8, hatched bars) rats (see Materials and Methods). Statistically significant differences are marked with asterisks (*) for P < 0.05 and (**) for P < 0.01 and were seen in layers II/III [3.5-fold increase, F(1,6) = 6.50, P = 0.02], IV [6.4-fold increase, F(1,6) = 6.48, P = 0.02] and V/VI [5-fold increase, F = 10.44, P < 0.01] in comparison to 1.6 induction in layer VIb that did not prove significant [F(1,6) = 4.41, P = 0.06].
DISCUSSION
This study presents two novel approaches to produce whisker stimulation-responsive changes in gene expression in the barrel cortex. We have focused on c-Fos, a transcription factor protein, as a prototypical marker for gene expression. Both of these approaches, mechanical whisker stimulation and exposure to a new environment, differ from those described previously. Notably, the previous experimental systems involved anesthesia (Mack and Mack 1992), artificial mode of stimulation (Welker 1964; Welker et al. 1989), or rather complicated experimental setup (Montero 1997).
Our experience shows that it is not just the presence of new objects, but their nature (especially the presence of holes between bars) that elicits whisker-dependent c-Fos expression (R. Filipkowski and L. Kaczmarek, unpubl.). This is in accordance with the findings of Lipp and Van der Loos (1991) who observed that mice appear to use their whiskers for detecting openings in their surroundings rather than for texture discrimination. In the case of the new environment, a natural tendency of rats is to explore (Eilam and Golani 1989) and this tendency was used in combination with a novelty element (completely new cage). The increase of c-fos expression after exposure to a new cage was also shown before in chicks (Anokhin et al. 1991).
Immunocytochemistry provides good spatial resolution. We have used the power of this approach to investigate laminar cortical distribution of c-Fos activation in detail. In both experiments, the highest induction of c-Fos was observed in the thalamorecipient layer IV, and slightly lesser c-Fos expression was observed in layers II/III and V/VI. All of these layers process sensory information (Waite and Tracey 1995). On the other hand, no changes were observed in sublayer VIb. This latter region is known for its specific role during the early phases of cortical development. Cells in layer VIb are among the first to differentiate. They show specific patterns of gene expression during development and throughout the adulthood (Valverde et al. 1989; Gaspar et al. 1995). Notably, expression of c-Fos as well as delayed/extended expression of Fras has been observed in subplate neurons between P1 and P15 (Alcantara and Greenough 1993).
It would be very difficult to directly compare the numerical results between experiments 1 and 2 for technical reasons as well as the inability to quantitate the amount of stimulation applied in each case. However, we noted that there was little difference in the overall levels of increases in c-Fos protein in any layer, with layer IV being the most responsive.
Exposure to a novel environment is an important model of plastic changes in the brain. This is especially true in the cortex, where major changes were documented in thickness and weight of the cortex; the size of neurons, their nuclei, cell bodies, synapses, dendritic branches, density of axons, dendrites, and synapses; glial cells; and blood vessels. Most of these results were obtained following long (usually 30–90 days) exposure to specific conditions (Rosenzweig 1996; Rosenzweig and Bennett 1996; Kolb and Whishaw 1998), although some of the aforementioned changes were seen after only 4 days (Wallace et al. 1992). In this study we show that even a very brief exposure of animals to a new environment results in an activation of expression of a protein forming transcription factor, thus suggesting a trigger of long-term neuronal changes (Kaczmarek 1993).
We have observed that only some of the PMBSF neurons show elevated c-Fos expression after mechanical stimulation of vibrissae. Similar findings have been presented by Melzer and Steiner (1997) who showed that not all of the barrel cortex neurons exhibit high level of c-fos mRNA expression after stimulation in a Lausanne apparatus. We have extended this observation to show that the majority of cells expressing c-Fos after mechanical stimulation turned out to be parvalbumin-negative. Parvalbumin has often been taken as a marker of certain inhibitory interneurons (Celio 1986; Ren et al. 1992; Kubota and Jones 1993; Hiscock et al. 1996). This finding is in agreement with the results obtained by Chaudhuri et al. (1995) who showed that the information-dependent expression of Zif268 in the cortex of monkeys was restricted to excitatory neurons lacking parvalbumin. Therefore, it remains to be clarified in further studies why excitatory neocortical neurons display increased c-Fos expression, while the large, fast-spiking, nonspiny interneurons on layer IV do not show this increase.
In conclusion, two new models of whisker stimulation are presented. They lead to the induction of c-Fos protein expression in the rat barrel cortex. This expression is observed in layers of the cortex known to receive and convey sensory stimulation, and in cells that are, presumably, excitatory neurons.
MATERIALS AND METHODS
Subjects
Results presented in this paper are based on material collected from a total of 19 adult Wistar male rats obtained from Nencki Institute Animal House. The animals were kept under natural light/dark cycle in Plexiglas cages (60 × 45 × 25 cm) with the cover made of 3-mm-thick iron wires, 1.5 cm spaced, with water and food provided ad libitum. To minimize animal suffering, the rules established by the Ethical Committee on Animal Research of Nencki Institute, based on disposition of the President of Polish Republic, were followed strictly in all experiments.
Experiment 1
To habituate nonspecific responses, four rats were placed on the top of a copper cylinder (50 cm high, 8 cm diam.) for 20 min each day for 20 days. If the rats jumped off, they were gently placed back on the top of the cylinder. On the experimental day, the rats were placed on the top of the cylinder and their whiskers were stimulated for 20 min (Fig. 1). Large whiskers were stimulated on either the left (two animals) or the right (two animals) side of the snout with three objects (a brush, a piece of Styrofoam with holes, and a piece of plastic). Two hours after the end of the brushing, the rat brains were processed for immunocytochemistry.
Experiment 2
The rats were divided into two groups. Experimental animals (eight rats) were put into a new (50 × 40 × 40 cm) cage made entirely of 1.5-mm-thick iron wires, 1 cm spaced, (Fig. 2) for 20 min. No manipulations were performed on the naive animals that served as controls (seven rats), and they were housed side by side with the experimental animals. Two hours after exposure to the new cage, the brains of experimental animals along with those of controls, were processed for immunocytochemistry.
Histology
All rats were anesthetized with chloral hydrate, perfused immediately with saline, followed by 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). The brains were removed and stored for 24 hr in the same fixative at 4°C and then transferred to 30% sucrose with sodium azide at 4°C until needed. The appropriate parts of the brain were slowly and gradually frozen in a heptane/dry ice bath. Coronal brain cryostat sections were cut at −20°C, 45 μm thick, 2.3–3.3 mm caudal to bregma (Paxinos and Watson 1986).
Nissl Staining
The sections were mounted on slides and air-dried (2–4 days), washed in PBS, followed by 70%, 100%, 70% ethanol and water, 0.5% cresyl violet solution (0.25 grams in 50 ml) with acetic acid (0.34 m) and sodium acetate (0.06 m), and finally dehydrated in ethanol (70% and 100%) and xylene, and embedded in Entellan (Merck).
Cytochrome Oxidase Staining
The sections were washed twice in PBS and incubated in staining solution (1% sucrose, 0.05% nickel sulfate, 0.025% DAB, 0.015% cytochrome c, 0.01% catalase, 2.5 μm, imidazole in 0.05 m phosphate buffer at pH 7.4) for several hours at 37°C. The stained sections were again washed in PBS, mounted on slides, and air-dried (2–4 days). The slides were then washed in PBS, dehydrated, and mounted as described above.
Immunocytochemistry
The expression of c-Fos was assessed as described by Kaminska et al. (1996). Briefly, the sections were washed three times in PBS (pH 7.4), incubated for 10 min in 0.3% H2O2 in PBS, washed twice in PBS, and incubated with polyclonal antibody (anti-c-Fos, 1:1000, Santa Cruz) for 48 hr at 4°C in PBS with sodium azide (0.01%) and normal goat serum (NGS, 3%). The sections were then washed three times in PBS with Triton X-100 (0.3%, Sigma), incubated with goat anti-rabbit biotinylated secondary antibody (1:1000, Vector) in PBS/Triton and NGS (3%) for 2 hr, washed three times in PBS/Triton, incubated with avidin–biotin complex (1:1000 in PBS/Triton, Vector) for 1 hr and washed three times in PBS. The immunostaining reaction was developed using the glucose oxidase/DAB/nickel method (Shu et al. 1988). The sections were incubated in PBS with DAB (0.05%), glucose (0.2%), ammonium chloride (0.04%), and ammonium nickel sulfate (0.1%) (all from Sigma) for 5 min; then 10% (vol/vol) glucose oxidase (Sigma, 10 U/ml in H2O) was added. The staining reaction was stopped by two to three washes with PBS. The sections were mounted on gelatin-covered slides, air-dried, dehydrated in ethanol solutions and xylene, and embedded in Entellan (Merck).
Some sections, after the last wash in PBS, were incubated for 2 hr in NGS (5%). For immunohistochemical detection of parvalbumin, monoclonal mouse antiserum (Sigma, 1:1000) was used. Following 3 days incubation with primary antibody (4°C), sections were washed in PBS and incubated in Cy3-conjugated goat anti-mouse secondary antibody (Jackson, 1:500). Primary and secondary antibodies were diluted in PBS containing 0.1% Triton X-100 and 1% normal goat serum. These sections were evaluated using the fluorescent microscope with appropriate filter set. Controls, in which primary antibody was replaced by 10% NGS, were negative.
Data Analysis
c-Fos-stained nuclei were counted in both hemispheres in the middle part of the PMBSF (1 cm wide), the region known to receive input from the mystacial vibrissae, using an image analysis system (MCID, IMAGING Research, Inc.). The number of c-Fos-stained nuclei in PMBSF areas were evaluated in cortical layers II/III, IV, V/VI (without the subplate), and VIb (layer VII, subplate, deep layer VI bordering the white matter). The position of the regions and layers was determined by Nissl and cytochrome oxidase staining. The nuclei were counted with the researcher being blind to the treatment. Averages from two to four slices per animal were used for statistical analysis. In both experiments, separate one-way ANOVAs were applied for each layer of PMBSF. Values in the text and figures are expressed as mean ±s.e.m. In the case of double-stained slices, the number of cells containing c-Fos, parvalbumin, or both proteins was estimated from four to five eye fields from all layers of rat PMBSF cortex after mechanical stimulation of vibrissae, under fluorescent microscope (magnification, 600×).
Acknowledgments
We thank Drs. Avi Chaudhuri, Malgorzata Kossut, Alena Savonenko, Karel Svoboda, and Jerry Yin for helpful comments and discussions. This work was supported by State Committee for Scientific Research (KBN, Poland) grants 6 P04A 064 10 and 6 P04A 008 08. RKF was a holder of the Young Scientist Award of the Foundation for Polish Science for 1995.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
REFERENCES
- Alcantara AA, Greenough WT. Developmental regulation of Fos and Fos-related antigens in cerebral cortex, striatum, hippocampus, and cerebellum of the rat. J Comp Neurol. 1993;334:75–85. doi: 10.1002/cne.903340106. [DOI] [PubMed] [Google Scholar]
- Anokhin KV, Mileusnic R, Shamakina IY, Rose SP. Effects of early experience on c-fos gene expression in the chick forebrain. Brain Res. 1991;544:101–107. doi: 10.1016/0006-8993(91)90890-8. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Diamond ME, Ebner FF. An innocuous bias in whisker use in adult rats modifies receptive fields of barrel cortex neurons. J Neurosci. 1994;14:6978–6991. doi: 10.1523/JNEUROSCI.14-11-06978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986;231:995–997. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Matsubara JA, Cynader MS. Neuronal activity in primate visual cortex assessed by immunostaining for the transcription factor Zif268. Vis Neurosci. 1995;12:35–50. doi: 10.1017/s095252380000729x. [DOI] [PubMed] [Google Scholar]
- Daw NW. Mechanisms of plasticity in the visual cortex. Invest Ophthalmol Vis Sci. 1994;35:4168–4179. [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D, Golani I. Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res. 1989;34:199–211. doi: 10.1016/s0166-4328(89)80102-0. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Kaas JH. Dynamic features of sensory and motor maps. Curr Opin Neurobiol. 1992;2:522–527. doi: 10.1016/0959-4388(92)90191-m. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: Cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Hiscock JJ, MacKenzie L, Willoughby JO. Fos induction in subtypes of cerebrocortical neurons following single picrotoxin-induced seizures. Brain Res. 1996;738:301–312. doi: 10.1016/s0006-8993(96)00806-2. [DOI] [PubMed] [Google Scholar]
- Jablonska B, Kossut M, Skangiel-Kramska J. Transient increase of AMPA and NMDA receptor binding in the barrel cortex of mice after tactile stimulation. Neurobiol Learn Mem. 1996;66:36–43. doi: 10.1006/nlme.1996.0041. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. Molecular biology of vertebrate learning: Is c-fos a new beginning? J Neurosci Res. 1993;34:377–381. doi: 10.1002/jnr.490340402. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L, Chaudhuri A. Sensory regulation of immediate-early gene expression in mammalian visual cortex: Implications for functional mapping and neural plasticity. Brain Res Rev. 1997;23:237–256. doi: 10.1016/s0165-0173(97)00005-2. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L, Zangenehpour S, Chaudhuri A. Sensory regulation of immediate-early genes c-fos and zif268 in monkey visual cortex at birth and throughout the critical period. Cereb Cortex. 1999;9:179–187. doi: 10.1093/cercor/9.2.179. [DOI] [PubMed] [Google Scholar]
- Kaminska B, Kaczmarek L, Chaudhuri A. Visual stimulation regulates the expression of transcription factors and modulates the composition of AP-1 in visual cortex. J Neurosci. 1996;16:3968–3978. doi: 10.1523/JNEUROSCI.16-12-03968.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Brain plasticity and behavior. Annu Rev Psychol. 1998;49:43–64. doi: 10.1146/annurev.psych.49.1.43. [DOI] [PubMed] [Google Scholar]
- Kossut M. Plasticity of the barrel cortex neurons. Prog Neurobiol. 1992;39:389–422. doi: 10.1016/0301-0082(92)90013-5. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Jones EG. Co-localization of two calcium binding proteins in GABA cells of rat piriform cortex. Brain Res. 1993;600:339–344. doi: 10.1016/0006-8993(93)91394-8. [DOI] [PubMed] [Google Scholar]
- Lipp HP, Van der Loos H. A computer-controlled Y-maze for the analysis of vibrissotactile discrimination learning in mice. Behav Brain Res. 1991;45:135–145. doi: 10.1016/s0166-4328(05)80079-8. [DOI] [PubMed] [Google Scholar]
- Lukasiuk K, Savonenko A, Nikolaev E, Rydz M, Kaczmarek L. Defensive conditioning-related increase in AP-1 transcription factor in the rat cortex. Mol Brain Res. 1999;67:64–73. doi: 10.1016/s0169-328x(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Mack KJ, Mack PA. Induction of transcription factors in somatosensory cortex after tactile stimulation. Mol Brain Res. 1992;12:141–147. doi: 10.1016/0169-328x(92)90077-o. [DOI] [PubMed] [Google Scholar]
- Melzer P, Steiner H. Stimulus-dependent expression of immediate-early genes in rat somatosensory cortex. J Comp Neurol. 1997;380:145–153. doi: 10.1002/(sici)1096-9861(19970331)380:1<145::aid-cne11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Sameshima K. Cortical plasticity and memory. Curr Opin Neurobiol. 1993;3:187–196. doi: 10.1016/0959-4388(93)90209-h. [DOI] [PubMed] [Google Scholar]
- Montero VM. c-fos induction in sensory pathways of rats exploring a novel complex environment: Shifts of active thalamic reticular sectors by predominant sensory cues. Neuroscience. 1997;76:1069–1081. doi: 10.1016/s0306-4522(96)00417-4. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Ruff NL, Dyck RH. Development, critical period plasticity, and adult reorganizations of mammalian somatosensory systems. Curr Opin Neurobiol. 1994;4:535–544. doi: 10.1016/0959-4388(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY: Academic Press; 1986. [Google Scholar]
- Rauschecker JP. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995;18:36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- Ren JQ, Aika Y, Heizmann CW, Kosaka T. Quantitative analysis of neurons and glial cells in the rat somatosensory cortex, with special reference to GABAergic neurons and parvalbumin-containing neurons. Exp Brain Res. 1992;92:1–14. doi: 10.1007/BF00230378. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Welker E, Pascual M, Soriano E. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR. Aspects of the search for neural mechanisms of memory. Annu Rev Psychol. 1996;47:1–32. doi: 10.1146/annurev.psych.47.1.1. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Pei G, Soderstrom S, Ebendal T, Huston JP. Unilateral stimulation or removal of rat vibrissae: Analysis of nerve growth factor and tyrosine hydroxylase mRNA in the brain. Behav Brain Res. 1994;60:63–71. doi: 10.1016/0166-4328(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Seto-Ohshima A. Calcium-binding proteins in the central nervous system. Acta Histochem Cytochem. 1994;27:93–106. [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Tactile sensory input regulates basal and apomorphine-induced immediate- early gene expression in rat barrel cortex. J Comp Neurol. 1994;344:297–304. doi: 10.1002/cne.903440210. [DOI] [PubMed] [Google Scholar]
- Valverde F, Facal-Valverde MV, Santacana M, Heredia M. Development and differentiation of early generated cells of sublayer VIb in the somatosensory cortex of the rat: A correlated Golgi and autoradiographic study. J Comp Neurol. 1989;290:118–140. doi: 10.1002/cne.902900108. [DOI] [PubMed] [Google Scholar]
- Waite PME, Tracey DJ. trigeminal sensory system. In: Paxinos G, editor. The rat nervous system. 2nd ed. San Diego, CA: Academic Press; 1995. pp. 705–724. [Google Scholar]
- Wallace CS, Kilman VL, Withers GS, Greenough WT. Increases in dendritic length in occipital cortex after 4 days of differential housing in weanling rats. Behav Neural Biol. 1992;58:64–68. doi: 10.1016/0163-1047(92)90937-y. [DOI] [PubMed] [Google Scholar]
- Welker E, Soriano E, Dorfl J, Van der Loos H. Plasticity in the barrel cortex of the adult mouse: Transient increase of GAD-immunoreactivity following sensory stimulation. Exp Brain Res. 1989;78:659–664. doi: 10.1007/BF00230256. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behaviour. 1964;22:223–244. [Google Scholar]
- Wong-Riley MT. Cytochrome oxidase: An endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]