Abstract
High myopia, which is extremely prevalent in the Chinese population, is one of the leading causes of blindness in the world. Genetic factors play a critical role in the development of the condition. To identify the genetic variants associated with high myopia in the Han Chinese, we conducted a genome-wide association study (GWAS) of 493,947 SNPs in 1088 individuals (419 cases and 669 controls) from a Han Chinese cohort and followed up on signals that were associated with p < 1.0 × 10−4 in three independent cohorts (combined, 2803 cases and 5642 controls). We identified a significant association between high myopia and a variant at 13q12.12 (rs9318086, combined p = 1.91 × 10−16, heterozygous odds ratio = 1.32, and homozygous odds ratio = 1.64). Furthermore, five additional SNPs (rs9510902, rs3794338, rs1886970, rs7325450, and rs7331047) in the same linkage disequilibrium (LD) block with rs9318086 also proved to be significantly associated with high myopia in the Han Chinese population; p values ranged from 5.46 × 10−11 to 6.16 × 10−16. This associated locus contains three genes—MIPEP, C1QTNF9B-AS1, and C1QTNF9B. MIPEP and C1QTNF9B were found to be expressed in the retina and retinal pigment epithelium (RPE) and are more likely than C1QTNF9B-AS1 to be associated with high myopia given the evidence of retinal signaling that controls eye growth. Our results suggest that the variants at 13q12.12 are associated with high myopia.
Main Text
People with myopia see near objects more clearly than objects far away because the images are focused on the vitreous inside the eye rather than on the retina. Myopia is the most common ocular disorder worldwide, and there is a high prevalence in populations of Asian (40%–70%)1–3 and European (20%–42%) descent.4,5 Myopia can be classified as low, medium, or high. High myopia, with a prevalence of 1%–2% in the general population,1–5 is commonly defined on the basis of a spherical equivalent refractive error equal to −6.00 diopter sphere (DS) or less. High myopia has long been known to pose a high risk for the development of sight-threatening eye diseases, including glaucoma, macular hemorrhage, choroidal neovascularization, and retinal detachment.6
Previous studies have indicated the involvement of genetic and environmental factors.7,8 Myopia can be inherited as a complex trait or in a monogenic form. Seventeen loci responsible for these complex traits or monogenic forms have been mapped by linkage analysis (MIM 160700).9,10 In recent genome-wide association studies (GWASs), Nakanish et al.11 and Li et al.12 identified risk variants for high myopia at 11q24.1 in a Japanese population and in catenin (cadherin-associated protein), delta 2 (CTNND2, MIM 604275) in Singapore Chinese and Japanese populations. At the same time, Solouki et al.13 and Hysi et al.14 performed GWASs in populations of European descent and identified loci at 15q14 and 15q25 responsible for common myopia and refractive error, respectively.
Here, in order to identify the genetic variants associated with high myopia in the Han Chinese, we performed a GWAS in a Shanghai Han Chinese dataset of 419 unrelated individuals with high myopia and 669 unrelated normal controls (Table 1; see also Figure S1, available online). These participants came from the Shanghai and Anhui region and were recruited at the Xinhua Hospital Ophthalmic Clinic, Shanghai Jiao Tong University and at the clinics of the hospital affiliated with Anhui Medical University in China. The diagnosis for high myopia in this study required the spherical equivalent to be less than or equal to −6.0 DS in at least one eye and the axial length of the eye globe to be greater than or equal to 26.0 mm (Figure S2). Individuals were excluded from the study if they had undergone ocular procedures that might alter refraction or if they had other symptoms besides high myopia (e.g.,in addition to eye problems, individuals with Stickler syndrome suffer from distinctive facial abnormalities, hearing loss, and joint phenotypes). Individuals with Retinopathy of Prematurity (ROP) were also excluded from the study if an individual was prematurely born according to the criteria of the International Classification of Retinopathy of Prematurity (ICROP). For the controls, the criteria were a spherical equivalent from −0.5 to +1.0 DS and no evidence of disease in either eye. The institutional review board of Xinhua Hospital, Shanghai Jiao Tong University and the institutional review board of Anhui Medical University, Anhui, China both approved this project. Informed consent was obtained from all participants of this cohort. The cases and controls of GWAS were randomly placed into two groups for genotyping. Group 1 consisted of 100 cases and 100 controls that were genotyped by deCODE genetics, Iceland. Group 2 consisted of 319 cases and 569 controls that were genotyped by the Key Laboratory of Dermatology at Anhui Medical University in China with Illumina Infinium HD Human 610-Quad BeadChips according to the manufacturer's instructions. SNPs with call rates <90% were eliminated from the analysis. SNPs were also excluded if they had a minor allele frequency (MAF) < 1% or if there was significant deviation from Hardy-Weinberg Equilibrium (HWE) in the controls (p < 10−7).15,16 SNPs on the X and Y chromosomes and mitochondrial as well as the copy number variant (CNV) probes were removed from further analysis. To eliminate uninformative SNPs, we excluded nonheterozygous SNPs. These exclusions were largely due to low call rates of <90%. We examined potential genetic relatedness on the basis of pairwise identity by state for all the successfully genotyped samples by using PLINK 1.06 software.17 Although the cases and controls were Han Chinese from the eastern part of China, population stratification might still have existed because the controls and the cases were from different regions. The remaining samples were therefore further assessed for population stratification with the software package EIGENSTRAT.18
Table 1.
Characteristics of Cases and Controls in the Study
| Dataseta |
GWAS Dataset |
FD1 (WZ) |
FD2 (CD) |
FD3 (GD-HK) |
Combined |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
| Number of samples | 419 | 669 | 843 | 2525 | 549 | 1890 | 1411 | 1227 | 3222 | 6311 |
| Female | 183 (43.7%) | 284 (42.5%) | 509 (60.4%) | 1462 (57.9%) | 336 (61.2%) | 1108 (58.6%) | 708 (50.2%) | 641 (52.2%) | 1736 (53.9%) | 3495 (55.4%) |
| Male | 236 (56.3%) | 385 (57.5%) | 334 (39.6%) | 1063 (42.1%) | 213 (38.8%) | 782 (41.4%) | 703 (49.8%) | 586 (47.8%) | 1486 (46.1%) | 2816 (44.6%) |
| Average age (years)b | 34.29 ± 14.12 | 32.68 ± 13.50 | 35.42 ± 15.44 | 44.21 ± 15.37 | 39.65 ± 15.72 | 57.44 ± 13.73 | 38.17 ± 16.43 | 46.55 ± 13.96 | 37.19 ± 15.58 | 47.41 ± 14.02 |
| Refractive errors (Diopters)b | ||||||||||
| ODc | −10.64 ± 4.27 | −13.13 ± 4.59 | −11.34 ± 4.33 | −12.89 ± 3.62 | −12.38 ± 4.12 | |||||
| OSd | −10.32 ± 4.11 | −12.32 ± 4.52 | −10.93 ± 4.07 | −12.57 ± 3.83 | −11.93 ± 4.06 | |||||
| Axial length (mm)b | ||||||||||
| OD c | 27.32 ± 1.71 | 28.74 ± 2.71 | 27.57 ± 1.77 | 28.38 ± 2.12 | 28.17 ± 2.15 | |||||
| OS d | 27.17 ± 1.44 | 28.26 ± 2.63 | 27.43 ± 1.52 | 28.31 ± 2.17 | 28.01 ± 2.08 | |||||
GWAS dataset: Shanghai cohort. FD1: follow-up dataset 1, Wenzhou cohort. FD2, follow-up dataset 2, Chengdu cohort. FD3, follow-up dataset 3, Guangdong-Hong Kong cohort.
± standard deviation.
OD, right eye.
OS, left eye.
We tested the association of 493,947 successfully genotyped SNPs in 419 cases and 669 controls. Principal component analysis (PCA, Figure S3) and genomic control (λgc = 1.07 for all 493,947 SNPs) indicated minimal inflation due to population stratification for the GWAS results. We carried out the Cochran-Armitage trend test to assess the genotype–phenotype association. We compared the distribution of the observed p values to the distribution expected under the null hypothesis. The quantile-quantile (Q-Q) plots of the logarithms of the p values showed a deviation from the expected distribution, suggesting the presence of significant genetic effects (Figure 1A).
Figure 1.
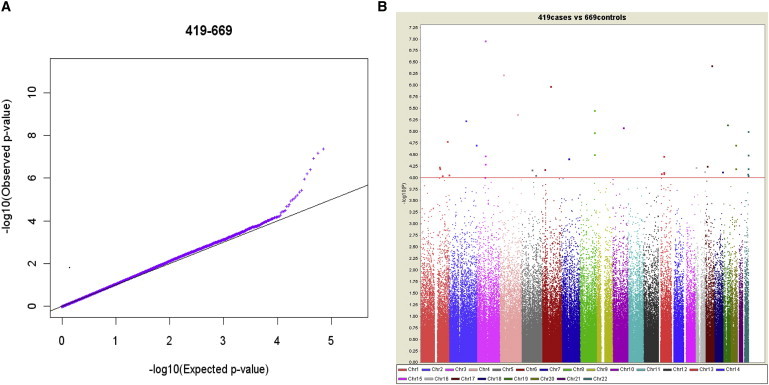
Genome-Wide-Association Results from the Initial GWAS
(A) Quantile-quantile plots of the observed p values (–log10P) for association.
(B) The genome-wide distribution of –log10 p values from the unadjusted Cochran-Armitage trend test is shown across the chromosomes. Values that take into account genetic matching and correction for the inflation factor of 1.07 are shown for 493,947 SNPs that were of sufficient quality, after quality-control filtering, in 419 unrelated Han Chinese patients with high myopia and in 699 unrelated Han Chinese controls. Each chromosome is depicted in a different color. –log10(UNADJ) refers to −log10 p value of the Cochran-Armitage trend test unadjusted by multiple testing. The dotted line indicates the genome-wide threshold for further follow-up studies (p < 1.0 × 10−4).
None of the SNPs were significantly associated after correction for multiple testing, i.e., nominal p < 2.5 × 10−7. However, 34 SNPs in 22 chromosomal regions were associated after adjustment for genomic control, gender, and age with a nominal p value < 1.0 × 10−4 (Figure 1B; Table S1). We tested these 34 SNPs in the first follow-up dataset (Wenzhou cohort), which was composed of 843 Han Chinese individuals with high myopia and 2525 controls (Table 1; Table S1) and obtained genotyping results for 34 SNPs that passed the genotyping quality control (Figure S1). Participants in the Wenzhou cohort were from the Wenzhou region of Zhejiang Province and were recruited at the School of Optometry and Ophthalmology and Eye Hospital Ophthalmic Clinic, Wenzhou Medical College, Zhejiang Province, China. The diagnosis criteria for the cases and controls were the same as those in the GWAS cohort. The institutional review board of Wenzhou Medical College approved this project. Informed consent was obtained from all participants of the Wenzhou cohort. Among these 34 SNPs, only one SNP, rs9318086 at 13q12.12, was significantly associated with high myopia after adjustment for gender and age (heterozygous odds ratio [ORhet] = 1.40, homozygous odds ratio [ORhom] = 1.68, allelic p = 2.28 × 10−6, p = 7.75 × 10−5 after Bonferroni adjustment; Table 2 and Table S1).
Table 2.
Association between Phenotype of High Myopia and Genetic Variants at 13q12.12 in the Han Chinese populations
| SNP | Position | Risk Allele | Dataseta | Number (Control/Case) | RAFb(Control/Case) | p_HWE (Control/Case) | p Valuec |
Odds ratio (95% CI)d |
|
|---|---|---|---|---|---|---|---|---|---|
| Homozygote | Heterozygote | ||||||||
| rs9510902 |
23328312 |
A |
GWAS (SH) | 0/419 | 0/0.520 | 0/0.344 | NA | NA | NA |
| FD1(WZ) | 2525/843 | 0.452/0.512 | 0.723/0.419 | 1.92 × 10−5 | 1.62 (1.29, 2.02) | 1.36 (1.13, 1.65) | |||
| FD2(CD) | 1890/549 | 0.454/0.520 | 0.455/0.546 | 1.06 × 10−4 | 1.71 (1.31, 2.23) | 1.20 (0.95, 1.52) | |||
| FD3(GD-HK) | 1227/1411 | 0.464/0.510 | 0.515/0.643 | 8.26 × 10−4 | 1.44 (1.16, 1.78) | 1.21 (1.01, 1.46) | |||
| Combined | 5642/3222 | 0.455/0.514 | 0.908/0.627 | 6.32 × 10−14 | 1.60 (1.41, 1.80) | 1.25 (1.12, 1.38) | |||
| rs9318086 |
23330467 |
A |
GWAS (SH) | 669/419 | 0.447/0.514 | 0.249/0.504 | 8.14 × 10−5 | 1.68 (1.19, 2.36) | 1.33 (0.99, 1.78) |
| FD1(WZ) | 2525/843 | 0.433/0.499 | 0.256/0.654 | 2.28 × 10−6 | 1.68 (1.35, 2.10) | 1.40 (1.16, 1.69) | |||
| FD2(CD) | 1890/549 | 0.435/0.513 | 0.963/0.532 | 5.09 × 10−6 | 1.86 (1.43, 2.44) | 1.30 (1.03, 1.63) | |||
| FD3(GD-HK) | 1227/1411 | 0.453/0.495 | 0.640/0.955 | 0.00217 | 1.40 (1.12, 1.74) | 1.22 (1.02, 1.46) | |||
| Combined | 6311/3222 | 0.439/0.502 | 0.178/0.806 | 1.91 × 10−16 | 1.64 (1.46, 1.85) | 1.32 (1.19, 1.46) | |||
| rs3794338 |
23331291 |
T |
GWAS (SH) | 0/419 | 0/0.419 | 0/0.176 | NA | NA | NA |
| FD1(WZ) | 2525/843 | 0.348/0.389 | 0.319/0.133 | 0.00227 | 1.45 (1.15, 1.84) | 1.13 (0.95, 1.34) | |||
| FD2(CD) | 1890/549 | 0.348/0.418 | 0.593/0.852 | 2.10 × 10−5 | 1.79 (1.35, 2.39) | 1.35 (1.10, 1.67) | |||
| FD3(GD-HK) | 1227/1411 | 0.363/0.395 | 0.288/0.066 | 0.0152 | 1.33 (1.05, 1.67) | 1.11 (0.94, 1.31) | |||
| Combined | 5642/3222 | 0.351/0.401 | 0.137/0.010 | 5.46 × 10−11 | 1.54 (1.35, 1.76) | 1.18 (1.07, 1.30) | |||
| rs1886970 |
23338498 |
G |
GWAS (SH) | 669/419 | 0.451/0.514 | 0.339/0.504 | 1.05 × 10−4 | 1.64 (1.16, 2.30) | 1.29 (0.96, 1.73) |
| FD1(WZ) | 2525/843 | 0.440/0.501 | 0.806/0.469 | 1.33 × 10−5 | 1.63 (1.30, 2.04) | 1.35 (1.12, 1.64) | |||
| FD2(CD) | 1890/549 | 0.438/0.512 | 0.766/0.711 | 1.67 × 10−5 | 1.81 (1.38, 2.37) | 1.28 (1.02, 1.62) | |||
| FD3(GD-HK) | 1227/1411 | 0.457/0.495 | 0.733/0.960 | 0.00512 | 1.36 (1.09, 1.69) | 1.19 (0.99, 1.42) | |||
| Combined | 6311/3222 | 0.444/0.502 | 0.636/0.945 | 2.31 × 10−14 | 1.59 (1.41, 1.80) | 1.27 (1.15, 1.41) | |||
| rs7325450 |
23365004 |
G |
GWAS (SH) | 0/419 | 0/0.504 | 0/0.558 | NA | NA | NA |
| FD1(WZ) | 2525/843 | 0.436/0.493 | 0.598/0.254 | 4.31 × 10−5 | 1.59 (1.27, 1.99) | 1.34 (1.11, 1.61) | |||
| FD2(CD) | 1890/549 | 0.427/0.492 | 0.767/0.373 | 1.32 × 10−4 | 1.70 (1.30, 2.22) | 1.19 (0.95, 1.49) | |||
| FD3(GD-HK) | 1227/1411 | 0.450/0.491 | 0.969/0.640 | 0.00274 | 1.39 (1.12, 1.73) | 1.15 (0.96, 1.38) | |||
| Combined | 5642/3222 | 0.436/0.493 | 0.603/0.758 | 1.49 × 10−13 | 1.59 (1.41, 1.80) | 1.23 (1.11, 1.36) | |||
| rs7331047 | 23365342 | C | GWAS (SH) | 0/419 | 0/0.499 | 0/0.696 | NA | NA | NA |
| FD1(WZ) | 2525/843 | 0.425/0.486 | 0.769/0.589 | 1.42 × 10−5 | 1.62 (1.30, 2.03) | 1.34 (1.11, 1.61) | |||
| FD2(CD) | 1890/549 | 0.425/0.505 | 0.655/0.767 | 2.05 × 10−6 | 1.93 (1.47, 2.53) | 1.33 (1.05, 1.67) | |||
| FD3(GD-HK) | 1227/1411 | 0.449/0.491 | 0.857/0.531 | 0.00245 | 1.40 (1.12, 1.74) | 1.16 (0.96, 1.38) | |||
| Combined | 5642/3222 | 0.430/0.493 | 0.957/0.680 | 6.16 × 10−16 | 1.66 (1.47, 1.88) | 1.27 (1.15, 1.41) | |||
GWAS cohort: Shanghai cohort. FD1: follow-up dataset 1, Wenzhou cohort. FD2, follow-up dataset 2, Chengdu cohort. FD3, follow-up dataset 3, Guangdong-Hong Kong cohort.
RAF, frequency of risk allele.
The allelic p value was corrected by age and gender, and genomic control was used for the GWAS cohort.
CI: confidence interval.
We then decided to look more closely at nine SNPs within 200 kb of rs9318086. Each of these was associated with a nominal p value of less than 0.05 in the initial GWAS, and five of these had a p value of less than 3.6 × 10−4 (The highest p value among the five SNPs; Table S2). These five SNPs are all within a linkage disequilibrium (LD) block spanning 47 kb from rs9507174 to rs9551019, as depicted in the Han Chinese data in the HapMap database (HapMap Public Release #24 on Genome Browser). One of these SNPs, rs1886970, is located about 8 kb downstream from rs9318086 and had a p value of 1.03 × 10−4 after correction for genomic control, gender, and age in the initial GWAS. rs9318086 and rs1886970 are in intron 10 and intron 14, respectively, of the mitochondrial intermediate peptide gene (MIPEP, MIM 602241). These two SNPs are in complete LD with each other in the Han Chinese Beijing of the HapMap database (r2 = 1.0, HapMap Public Release #24 on Genome Browser) and are in the same LD block with each other in our GWAS results (r2 = 0.991). We then genotyped rs1886970 in the Wenzhou following-up dataset by using the SNaPshot method (Table S3), and it was significantly associated with high myopia after correction for gender and age with an ORhet = 1.35, ORhom = 1.63, and p = 1.33 × 10−5 (Table 2). Therefore, this locus, including SNPs rs9318086 and rs1886970, is likely to be associated with high myopia.
To confirm this finding, we then genotyped these two SNPs (rs9318086 and rs1886970) in two additional follow-up datasets (Chengdu cohort and Guangdong-Hong Kong cohort; Figure S1). The Chengdu cohort was composed of 549 unrelated sporadic patients with high myopia and 1890 unrelated normal controls (Table 1). Participants in the Chengdu cohort were from the Chengdu region of Sichuan Province and were recruited at the Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital Ophthalmic Clinic, China. This project was approved by the Institutional Review Board of the Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital. The Guangzhou-Hong Kong cohort was composed of 1411 unrelated patients with high myopia and 1227 unrelated normal controls (Table 1). The participants came from the Guangzhou and Hong Kong regions and were recruited at the Zhongshan Eye Center, Zhongshan University and the Department of Ophthalmology and Visual Sciences, the Chinese University of Hong Kong, Hong Kong, China. The diagnosis criteria for the cases and controls were the same as those in the GWAS cohort. This project was approved by the institutional review board of the Zhongshan Eye Center, Zhongshan University and the institutional review board of the Chinese University of Hong Kong. Informed consent was obtained from all participants of both the Chengdu and Guangzhou-Hong Kong cohorts. We found that these two SNPs were also significantly associated with high myopia in these two cohorts after adjustment by gender and age (for rs9318086, ORhet = 1.30, ORhom = 1.86, p = 5.09 × 10−6 in the Chengdu cohort, ORhet = 1.22, ORhom = 1.40, p = 2.17 × 10−3 in the Guangdong-Hong Kong cohort; for rs1886970, ORhet = 1.28, ORhom = 1.81, p = 1.67 × 10−5 in the Chengdu cohort, ORhet = 1.19, ORhom = 1.36, p = 5.12 × 10−3 in the Guangdong-Hong Kong cohort; Table 2). The same risk alleles were found across the datasets (i.e., the direction of the effect was consistent in all cohorts being studied). The combined allelic p value for rs9318086 in all four datasets, including 3222 cases and 6311 controls, was 1.91 × 10−16; ORhet = 1.32 (95% confidence interval [CI]: 1.19, 1.46) and a ORhom = 1.64 (95% CI: 1.46, 1.85) (Figure 2B, Table 2) after adjustment for gender and age. With a p value of 2.31 × 10−14 after adjustment for gender and age in all combined samples, rs1886970 also proved to be highly significantly associated with high myopia (ORhet = 1.27, ORhom = 1.59, Table 2).
Figure 2.
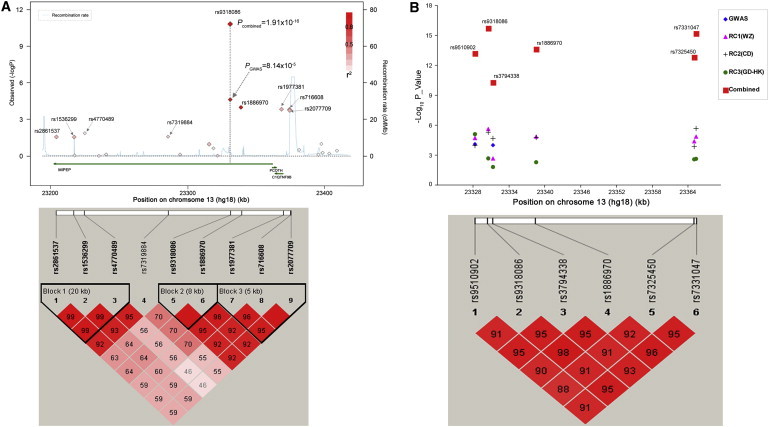
The 13q12.12 Region and Its Association with High Myopia in Han Chinese
(A) Results of the GWAS and replication identify 13q12.12 as a risk locus. Plot of –log10 p values of 18 SNPs genotyped in the GWAS within a 200 kb region surrounding this locus at 13q12.12. The color of each SNP spot reflects its r2 value: The top SNP (rs9318086) changes from red to white as the r2 value decreases. Estimated recombination rates were adapted from the University of California at Santa Cruz Genome Browser. The small diamonds indicate the p values of the initial GWAS (upper panel). The lower panel shows the LD block of nine SNPs (p values less than 0.05 in our GWAS data) surrounding the 13q12.12 region.
(B) The p values of rs9318086 and rs1886970 were 1.91 × 10−16 and 2.31 × 10−14, respectively, after all four datasets composed of 3222 cases and 6311 controls were combined (black circle). Four additional SNPs (rs9510902, rs3794338, rs7325450 and rs7331047) in the same LD block with rs9318086 and rs1886970 were shown to have a similar association with high myopia; p values ranged from 5.46 × 10−11 to 8.68 × 10−16 after all four datasets composed of 3222 cases and 5642 controls were combined (669 controls from the GWAS were absent because no DNA was available at this stage) (red square). The linkage disequilibrium block structure was examined with the program Haploview (Vision 4.0). The D′ values and r2 values for all pairs of SNPs were calculated, and the haplotype blocks were estimated with the program Haploview.
To verify that this locus (13q12.12) is a true high-myopia-responsible locus, we used the SNaPshot method to investigate four additional SNPs (rs9510902, rs3794338, rs7325450, and rs7331047) in the same LD block with rs9318086 and rs1886970 in the Han Chinese Beijing in the HapMap database in all samples available (Figure S1 and Table S3) in the four datasets (Figure S1). All four SNPs showed a significant association with the high myopia phenotype at p values ranging from 5.46 × 10−11 to 6.82 × 10−16 after adjustment for gender and age (Figure 2B, Table 2). The linkage disequilibrium block structure was examined with the program Haploview (Vision 4.0). The D′ values and r2 values for all pairs of SNPs were calculated, and the haplotype blocks were estimated with the program Haploview. All six SNPs genotyped in this study were in the same LD block in the samples studied and had D′ values from 0.88 to 0.98 (Figure 2B). Risk haplotype AATGGC generated from these six SNPs proved to be significantly different between the cases and controls (p = 8.77 × 10−20, Table 3). An individual with this risk haplotype has a 1.35-fold increase in susceptibility to high myopia.
Table 3.
The Haplotype Association with High Myopia in a Han Chinese Population at 13q12.12
| Haplotypea | Frequency | Case, Control Frequencies | Chi Square | p Value | Odds Ratio (95% CI)b |
|---|---|---|---|---|---|
| H1:GGCAAT | 0.490 | 0.462, 0.505 | 30.218 | 3.86 × 10−8 | 0.84 (0.79, 0.90) |
| H2:AATGGC | 0.338 | 0.381, 0.313 | 82.868 | 8.77 × 10−20 | 1.35 (1.27, 1.44) |
| H3:AACGGC | 0.082 | 0.087, 0.080 | 2.763 | 0.0965 | |
| H4:AGCAAT | 0.027 | 0.028, 0.026 | 0.519 | 0.4712 | |
| H5:GACGGC | 0.010 | 0.012, 0.008 | 4.513 | 0.0336 | 1.39 (1.02, 1.89) |
| H6:GGCAGT | 0.009 | 0.001, 0.014 | 70.779 | 4.00 × 10−17 | |
| H7:AATGAC | 0.008 | 0.003, 0.011 | 32.499 | 1.19 × 10−8 | |
| H8:AATGAT | 0.007 | 0.005, 0.009 | 7.765 | 0.0053 | |
| H9:GATGGC | 0.006 | 0.006, 0.007 | 1.259 | 0.2618 | |
| H10:AACGAT | 0.004 | 0.002, 0.005 | 11.479 | 0.0007 | |
| H11:AGTAGC | 0.004 | 0.002, 0.005 | 5.878 | 0.0153 | |
| H12:GGCAGC | 0.003 | 0.002, 0.004 | 4.45 | 0.0349 | |
| H13:GACGAT | 0.003 | 0.002, 0.003 | 0.253 | 0.6147 | |
| H14:AGTGGC | 0.003 | 0.001, 0.003 | 5.711 | 0.0169 | |
| H15:AATGGT | 0.002 | 0.003, 0.002 | 1.268 | 0.2601 | |
| H16:AACGAC | 0.002 | 0.002, 0.002 | 0.045 | 0.8324 | |
| H17:GGCGAT | 0.001 | 0.000, 0.002 | 14.93 | 0.0001 | |
| H18:AACGGT | 0.001 | 0.001, 0.001 | 0 | 0.9903 |
The haplotypes were generated from SNPs rs9510902, rs9318086, rs3794338, rs1886970, rs7325450, and rs7331047, in that order.
The odds ratio was not calculated when haplotype frequency was lower than 0.01.
Although SNPs in this locus did not reach genome-wide significance in our initial GWAS, we did replicate significant signals in our larger follow-up datasets. It is likely that we did not have enough power in our relatively small sample size to observe a significant association in the initial GWAS. Similar examples have been reported by previous studies.19,20 Raychaudhuri et al.19 identified a CDK6 (MIM 603368) variant responsible for rheumatoid arthritis at a GWAS p value of 5.5 × 10−5 at rs42041 (combined p = 4.0 × 10−6), and Barrett et al.20 identified a CDH1 (MIM 192090) variant responsible for ulcerative colitis at a GWAS p value of 1.8 × 10−5 at rs1728785 (combined p = 2.8 × 10−8). Furthermore, other groups successfully confirmed these findings in different populations in later studies.21–23 Here again, we showed that it is worth replicating SNPs with p values of greater than 2.5 × 10−7 in GWAS in complex-disease-identification studies.
The 200 kb sequence flanking rs9318086 contains three genes that might be involved in high myopia development at this locus: MIPEP, C1q, and tumor necrosis factor related protein 9B (C1QTNF9B, also called RP11-45B20.2), and C1QTNF9B antisense RNA 1 (non-protein coding) (C1QTNF9B-AS1) (Figure 2A). To investigate the expression of these three genes in ocular tissues, we examined the expression of the genes in different human tissues and human cell lines by using reverse-transcriptase polymerase chain reaction (RT-PCR) (Table S4). Human tissues were graciously donated by Han Chinese donors: the choroid and retina samples were from a deceased 55-year-old male, and the placenta and blood were from a 29-year-old female. Both MIPEP and C1QTNF9B genes were expressed in human retina and D047 cells (established from a primary culture of human retinal pigment epithelial cells) as well as in A2780 cells (established from a primary culture of human ovarian cancer cells), HEK293 cells (established from human embryonic kidney cell cultures), and human placenta (Figure 3). In addition, MIPEP was also expressed in human choroid and blood (Figure 3). These findings were consistent with previous studies that showed differential expressions of MIPEP and C1QTNF9B in human tissues.24,25 C1QTNF9B-AS1 was not expressed in either the retina or the D047 cells, but it was expressed in human blood, HEK293 cells, and placenta (Figure 3). These findings were consistent with previous studies that showed that C1QTNF9B-AS1 was not expressed in human eyes.
Figure 3.
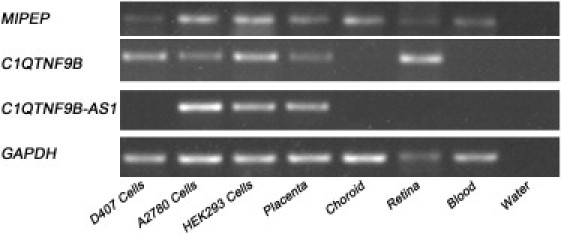
Expression Analysis with RT-PCR of MIPEP, C1QTNF9B, and C1QTNF9B-AS1 in Human Tissues and Cell Lines
We examined the expression of these three genes in different human tissues (human blood, choroid, placenta, and retina) and human cell lines (retinal pigment epithelium cell line [D407], kidney cell line [HEK293], and ovarian cell line [A2870]) by using RT-PCR. GAPDH was used as an internal control for cDNA quantification.
On the basis of expression in the human retina and RPE cells, both MIPEP and C1QTNF9B seem more likely to be high-myopia-associated genes within 13q12.12 than does C1QTNF9B-AS1, which was not expressed in human retina or RPE cells. The MIPEP protein is primarily involved in the maturation of oxidative phosphorylation (OXPHOS)-related proteins; the product of this gene performs the final step in processing a specific class of nuclear-encoded proteins targeted to the mitochondrial matrix or inner membrane.24 Given that the retina is the most energy-consuming tissue in the eye and that MIPEP is involved in the process of energy generation via oxidative metabolism, MIPEP is likely to play a role in normal eye development. Previous studies have also shown that mitochondrial protein might play an important role in the development of high myopia. A study by Andrew et al. demonstrated that two important mitochondrial genes, mitofusin 1 (MFN1, MIM 608506) and presenilin-associated, rhomboid-like (PARL, MIM 607858) on 3q26, might be involved in the development of myopia.10 Nakanishi et al. identified a susceptible locus for pathological myopia at 11q24.1, which contains BH3-like motif containing, cell death inducer (BLID, MIM 608853). BLID plays a proapoptotic role involving the BH3-like domain by inducing a caspase-dependent mitochondrial cell-death pathway.11,26 A recent study by Wojciechowski et al. suggested that matrix metallopeptidase 1 (MMP1, MIM 120353) and matrix metallopeptidase 2 (MMP2, MIM 120360) were involved in refractive variation in Old Order Amish, probably through insulin-like growth factors (IGFs). IGFs are fundamental cell regulators involved in cell adhesion and migration and the regulation of the cell cycle and apoptosis.27 The current study further suggested mitochondria-mediated cell death as a possible mechanism in the myopization. However, a mutation in complement C1q tumor necrosis factor5 (C1QTNF5, MIM 608752) (which, like C1QTNF9B, belongs to the complement C1q tumor necrosis factor family) can cause late-onset retinal degeneration. This is an autosomal-dominant disorder characterized by onset in the fifth to sixth decade, night blindness, and punctate yellow-white deposits in the retinal fundus; the disorder progresses to severe central and peripheral degeneration with choroidal neovascularization and chorioretinal atrophy.24 This suggests that a functional change in MIPEP or C1QTNF9B could, therefore, disrupt normal eye development and give rise to high myopia. However, because rs9318086, the SNP with the most significant association with high myopia at the locus, is located in an intron of MIPEP and 33 kb downstream of C1QTNF9B, rs9318086 might not directly affect the function of MIPEP or C1QTNF9B. The causal variation for high myopia at this locus might not have been identified yet. Because the underlying mechanism by which MIPEP or C1QTNF9B might cause high myopia has not yet been determined, C1QTNF9B-AS1 cannot be completely excluded as a candidate gene for causing high myopia at this stage at this locus. Further studies should address resequencing of this high-myopia-associated region to identify the true causal genetic variant(s). A functional dissection of the gene responsible for high myopia at this locus will provide insights into the pathogenesis of this serious eye condition.
Two loci, 15q14 and 15q25, have been recently reported to be associated with high myopia in European populations.13,14 A recent study in Japanese populations also found a significant association with 15q14 and a suggestive association with 15q25 when the same SNPs as those in the European populations were used.28 Here, in our GWAS, rs560766 at 15q14 (psmallest = 0.34) and rs939661 at 15q25 (psmallest = 0.017) were not significantly associated with high myopia. It is possible that larger datasets will be needed to allow observation of the association between 15q25 and 15q14 and high myopia in East Asian populations. Intriguingly, we found no association between high myopia and 11q24.1 (SNP, rs10892819, psmallest = 0.288 in our initial GWAS; Table S5), as was reported in the Japanese population.11 No association was found between high myopia and variants in CTNND2 either (SNP, rs6885224, psmallest = 0.496 in our initial GWAS; Table S5), as was reported in the Japanese and Singapore Chinese populations.12 The fact that previous reported loci were not replicated in our GWAS might be explained as follows: (1) the variation in association and effect sizes for high-myopia-susceptibility genes among different populations might suggest the genetic heterogeneity of high myopia among the populations; (2) it might simply be due to insufficient power or chance; (3) it could be due to different LD and haplotype patterns among the different populations; or (4) these loci might not be responsible for high myopia in Han Chinese in China; the p values of the combined GWAS and replication samples were only 2.2 × 10−7 and 1.14 × 10−5 in the previous studies, respectively.11,12 A recent study found no association between 11q42.1 and high myopia (SNP, rs11604461, psmallest = 0.073) in a Han Chinese cohort, either.29
In summary, we performed GWAS and replication studies of high myopia in a Han Chinese population and identified a genetic susceptibility locus for high myopia. This study not only helps us reveal the genetic basis of high myopia but also indicates the existence of genetic heterogeneity of disease susceptibility among different ethnic populations, which should provide valuable insights into the pathogenesis of high myopia.
Acknowledgments
We thank the patients and their families for their participation. We thank Kirk Thomas for his critical reading of the manuscript. Support for this work was obtained from the Natural Science Foundation of China (grants 30900809 [Y.S.], 81025006 [Z.Y.], 81070761 [F.L.], 30871350 [D.Z.], and 81070751 [J.Q.]), the National Basic Research Program of China (973 project, 2011CB504604 [Z.Y.]), the Department of Science and Technology of Sichuan Province, China (Z.Y.), and the Zhejiang Provincial Natural Science Foundation (grants R205739 and Z2100065 to X.Z.).
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
GenomeReference Consortium, http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/
Online Mendelian Inheritance in Man, http://www.ncbi.nlm.nih.gov/Omim/
UCSC Genome Browser, http://genome.ucsc.edu/
References
- 1.He M., Zheng Y., Xiang F. Prevalence of myopia in urban and rural children in mainland China. Optom. Vis. Sci. 2009;86:40–44. doi: 10.1097/OPX.0b013e3181940719. [DOI] [PubMed] [Google Scholar]
- 2.Sawada A., Tomidokoro A., Araie M., Iwase A., Yamamoto T., Tajimi Study Group Refractive errors in an elderly Japanese population: the Tajimi study. Ophthalmology. 2008;115:363–370, e3. doi: 10.1016/j.ophtha.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 3.Wong T.Y., Foster P.J., Hee J., Ng T.P., Tielsch J.M., Chew S.J., Johnson G.J., Seah S.K. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest. Ophthalmol. Vis. Sci. 2000;41:2486–2494. [PubMed] [Google Scholar]
- 4.Kempen J.H., Mitchell P., Lee K.E., Tielsch J.M., Broman A.T., Taylor H.R., Ikram M.K., Congdon N.G., O'Colmain B.J., Eye Diseases Prevalence Research Group The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Arch. Ophthalmol. 2004;122:495–505. doi: 10.1001/archopht.122.4.495. [DOI] [PubMed] [Google Scholar]
- 5.Vitale S., Sperduto R.D., Ferris F.L., 3rd Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch. Ophthalmol. 2009;127:1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 6.Banker A.S., Freeman W.R. Retinal detachment. Ophthalmol. Clin. North Am. 2001;14:695–704. doi: 10.1016/s0896-1549(05)70268-6. [DOI] [PubMed] [Google Scholar]
- 7.Dirani M., Chamberlain M., Shekar S.N., Islam A.F., Garoufalis P., Chen C.Y., Guymer R.H., Baird P.N. Heritability of refractive error and ocular biometrics: The Genes in Myopia (GEM) twin study. Invest. Ophthalmol. Vis. Sci. 2006;47:4756–4761. doi: 10.1167/iovs.06-0270. [DOI] [PubMed] [Google Scholar]
- 8.Lopes M.C., Andrew T., Carbonaro F., Spector T.D., Hammond C.J. Estimating heritability and shared environmental effects for refractive error in twin and family studies. Invest. Ophthalmol. Vis. Sci. 2009;50:126–131. doi: 10.1167/iovs.08-2385. [DOI] [PubMed] [Google Scholar]
- 9.Young T.L. Molecular genetics of human myopia: An update. Optom. Vis. Sci. 2009;86:E8–E22. doi: 10.1097/OPX.0b013e3181940655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrew T., Maniatis N., Carbonaro F., Liew S.H., Lau W., Spector T.D., Hammond C.J. Identification and replication of three novel myopia common susceptibility gene loci on chromosome 3q26 using linkage and linkage disequilibrium mapping. PLoS Genet. 2008;4:e1000220. doi: 10.1371/journal.pgen.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakanishi H., Yamada R., Gotoh N., Hayashi H., Yamashiro K., Shimada N., Ohno-Matsui K., Mochizuki M., Saito M., Iida T. A genome-wide association analysis identified a novel susceptible locus for pathological myopia at 11q24.1. PLoS Genet. 2009;5:e1000660. doi: 10.1371/journal.pgen.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y.J., Goh L., Khor C.C., Fan Q., Yu M., Han S., Sim X., Ong R.T., Wong T.Y., Vithana E.N. Genome-wide association studies reveal genetic variants in CTNND2 for high myopia in Singapore Chinese. Ophthalmology. 2011;118:368–375. doi: 10.1016/j.ophtha.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solouki A.M., Verhoeven V.J., van Duijn C.M., Verkerk A.J., Ikram M.K., Hysi P.G., Despriet D.D., van Koolwijk L.M., Ho L., Ramdas W.D. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat. Genet. 2010;42:897–901. doi: 10.1038/ng.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hysi P.G., Young T.L., Mackey D.A., Andrew T., Fernández-Medarde A., Solouki A.M., Hewitt A.W., Macgregor S., Vingerling J.R., Li Y.J. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat. Genet. 2010;42:902–905. doi: 10.1038/ng.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewan A., Liu M., Hartman S., Zhang S.S., Liu D.T., Zhao C., Tam P.O., Chan W.M., Lam D.S., Snyder M. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 16.Quan C., Ren Y.Q., Xiang L.H., Sun L.D., Xu A.E., Gao X.H., Chen H.D., Pu X.M., Wu R.N., Liang C.Z. Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat. Genet. 2010;42:614–618. doi: 10.1038/ng.603. [DOI] [PubMed] [Google Scholar]
- 17.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., Sham P.C. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price A.L., Patterson N.J., Plenge R.M., Weinblatt M.E., Shadick N.A., Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 19.Raychaudhuri S., Remmers E.F., Lee A.T., Hackett R., Guiducci C., Burtt N.P., Gianniny L., Korman B.D., Padyukov L., Kurreeman F.A. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat. Genet. 2008;40:1216–1223. doi: 10.1038/ng.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett J.C., Lee J.C., Lees C.W., Prescott N.J., Anderson C.A., Phillips A., Wesley E., Parnell K., Zhang H., Drummond H., UK IBD Genetics Consortium. Wellcome Trust Case Control Consortium 2 Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat. Genet. 2009;41:1330–1334. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Linden M.P., Feitsma A.L., le Cessie S., Kern M., Olsson L.M., Raychaudhuri S., Begovich A.B., Chang M., Catanese J.J., Kurreeman F.A. Association of a single-nucleotide polymorphism in CD40 with the rate of joint destruction in rheumatoid arthritis. Arthritis Rheum. 2009;60:2242–2247. doi: 10.1002/art.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Helm-van Mil A.H., Toes R.E., Huizinga T.W. Genetic variants in the prediction of rheumatoid arthritis. Ann. Rheum. Dis. 2010;69:1694–1696. doi: 10.1136/ard.2009.123828. [DOI] [PubMed] [Google Scholar]
- 23.van Sommeren S., Visschedijk M.C., Festen E.A., de Jong D.J., Ponsioen C.Y., Wijmenga C., Weersma R.K. HNF4α and CDH1 are associated with ulcerative colitis in a Dutch cohort. Inflamm. Bowel Dis. 2010 doi: 10.1002/ibd.21541. Published online November 28, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Chew A., Buck E.A., Peretz S., Sirugo G., Rinaldo P., Isaya G. Cloning, expression, and chromosomal assignment of the human mitochondrial intermediate peptidase gene (MIPEP) Genomics. 1997;40:493–496. doi: 10.1006/geno.1996.4586. [DOI] [PubMed] [Google Scholar]
- 25.Hayward C., Shu X., Cideciyan A.V., Lennon A., Barran P., Zareparsi S., Sawyer L., Hendry G., Dhillon B., Milam A.H. Mutation in a short-chain collagen gene, CTRP5, results in extracellular deposit formation in late-onset retinal degeneration: A genetic model for age-related macular degeneration. Hum. Mol. Genet. 2003;12:2657–2667. doi: 10.1093/hmg/ddg289. [DOI] [PubMed] [Google Scholar]
- 26.Broustas C.G., Gokhale P.C., Rahman A., Dritschilo A., Ahmad I., Kasid U. BRCC2, a novel BH3-like domain-containing protein, induces apoptosis in a caspase-dependent manner. J. Biol. Chem. 2004;279:26780–26788. doi: 10.1074/jbc.M400159200. [DOI] [PubMed] [Google Scholar]
- 27.Wojciechowski R., Bailey-Wilson J.E., Stambolian D. Association of matrix metalloproteinase gene polymorphisms with refractive error in Amish and Ashkenazi families. Invest. Ophthalmol. Vis. Sci. 2010;51:4989–4995. doi: 10.1167/iovs.10-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi H., Yamashiro K., Nakanishi H., Nakata I., Kurashige Y., Tsujikawa A., Moriyama M., Ohno-Matsui K., Mochizuki M., Ozaki M. Association of 15q14 and 15q25 with high myopia in Japanese. Invest. Ophthalmol. Vis. Sci. 2011 doi: 10.1167/iovs.11-7311. Published online March 24, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Zhao F., Bai J., Chen W., Xue A., Li C., Yan Z., Chen H., Lu F., Hu Y., Qu J. Evaluation of BLID and LOC399959 as candidate genes for high myopia in the Chinese Han population. Mol. Vis. 2010;16:1920–1927. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


