Abstract
In classical fear conditioning, a neutral sensory stimulus (CS) acquires the ability to elicit fear responses after pairing to a noxious unconditioned stimulus (US). As amygdala lesions prevent the acquisition of fear responses and the lateral amygdaloid (LA) nucleus is the main input station of the amygdala for auditory afferents, the effect of auditory fear conditioning on the sensory responsiveness of LA neurons has been examined. Although conditioning was shown to increase CS-evoked LA responses, the specificity of the changes in responsiveness was not tested. Because conditioning might induce nonspecific increases in LA responses to auditory afferents, we re-examined this issue in conscious, head-restrained cats using a differential conditioning paradigm where only one of two tones (CS+ but not CS−) was paired to the US. Differential conditioning increased unit and field responses to the CS+, whereas responses to the CS− decreased. Such changes have never been observed in the amygdala except in cases where the CS− had been paired to the US before and fear responses not extinguished. This suggests that fear conditioning is not only accompanied by potentiation of amygdalopetal pathways conveying the CS+ but also by the depression of sensory inputs unpaired to noxious stimuli.
The amygdaloid complex is a nucleated structure of the temporal lobe receiving sensory inputs of all modalities and projecting to functionally diverse brain structures, from the highest computational levels of the brain to hypothalamic and brainstem autonomic nuclei involved in cardiovascular control and the expression of species-specific fear responses (Hopkins and Holstege 1978; Price and Amaral 1981; Bandler 1982; Veening et al. 1984; Russchen 1986; Amaral et al. 1992).
Much evidence implicates the amygdala in fear learning and expression. For instance, stimulation of the central amygdaloid nucleus elicits increases in heart rate and blood pressure (Kaada 1972; Iwata et al. 1987). Moreover, amygdala lesions prevent the acquisition of classically conditioned fear responses (Kapp et al. 1979; Gentile et al. 1986; Iwata et al. 1986; Hitchcock and Davis 1987; LeDoux et al. 1990b; Killcross et al. 1997). These and other findings (for review, see Davis 1992; LeDoux 1995) have prompted investigators to search for the cellular correlates of fear learning in the amygdala.
As the lateral amygdaloid (LA) nucleus is the main input station of the amygdala for auditory afferents (LeDoux et al. 1990a,b; Romanski and LeDoux 1992), a few studies have examined how auditory fear conditioning affects tone-evoked LA responses (Quirk et al. 1995; Rogan et al. 1997). These studies reported that fear conditioning increases auditory-evoked responses in the LA. These changes in responsiveness were not a simple consequence of the arousal produced by the unconditioned stimulus (US) as they were not observed when the auditory conditioned stimulus (CS) and the US were presented in an unpaired manner. However, the possibility that the CS–US pairings induced nonspecific increases in LA responses to auditory afferents was not investigated.
Determining whether the changes in sensory responsiveness are specific to the conditioned tone is critical to test the idea that plastic events, taking place in the LA or one of its afferents, underlie the acquisition of fear responses. Indeed, if this is the case, the change in sensory responsiveness displayed by LA neurons should be specifically associated to the CS and not to other stimuli, much like conditioned fear responses. Thus, we re-examined this issue using a differential fear conditioning paradigm where only one of two tones (CS+ but not CS−) was paired to a US.
RESULTS
Conscious, head-restrained cats, chronically implanted with multiple high-impedance microelectrodes in the LA, were subjected to a discriminative fear conditioning paradigm (see Materials and Methods). To assess the acquisition of conditioned fear responses, in 20 conditioning sessions, we compared changes in electromyographic (EMG) activity evoked by the CS+ and the CS− before pairing (last 10 Control CS), after pairing (first 10 Extinction CS), and after extinction (last 10 Extinction CS). EMG activity was monitored by means of electrodes implanted in the posterior neck muscles.
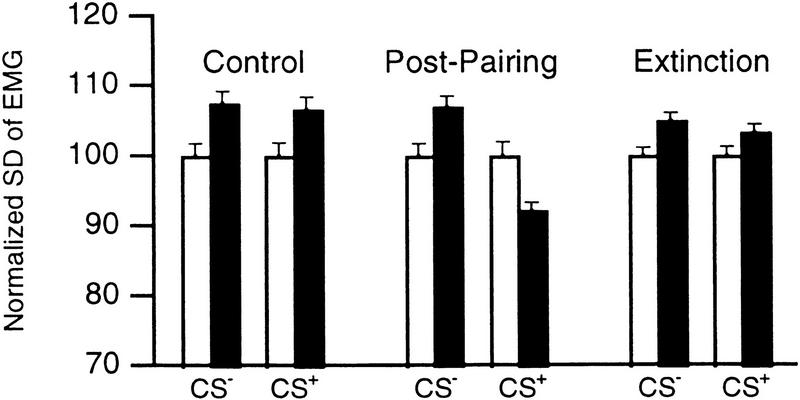
Changes in EMG activity were assessed using a repeated-measures analysis of variance (ANOVA). A significant interaction was found between the stimulus (CS+, CS−) and the difference in EMG activity before versus during the tones (F = 21.52; P < 0.05). Two-tailed paired t-tests df = 19) revealed that the CS+ and CS− evoked an increase in EMG activity in the Control period (CS+, 6.5 ± 1.06%, t = 6.13; CS−, 7.3 ± 1.24%, t = 7.29; P < 0.05). In contrast, after pairing, the CS+, but not the CS−, elicited a reduction in EMG activity (8.22 ± 1.39%; t = −5.91; P < 0.05). Repeated unpaired presentations of the CS+ abolished the conditioned response (Fig. 1, Extinction).
Figure 1.
Conditioning produces reversible CS+-evoked decreases in EMG activity. We computed the standard deviations (s.d.) of the EMG signal (filtered 100–1000 Hz; normalized to pretone values) during epochs of 500 msec obtained before (open bars) and 500 msec after (solid bars) tone onsets. Bars show Control (left), Post-pairing (middle), and Extinction (right) values.
To analyze how fear conditioning influenced the auditory responsiveness of LA neurons, auditory-evoked unit discharges and field potentials were recorded simultaneously through the same LA microelectrodes. The histologically determined location of recorded sites is shown in Figure 2 (dots). In control conditions, the two tones (5 and 10 kHz) elicited a multiphasic field potential (Fig. 3, thick lines). The latency to peak of the first negative wave evoked by the two tones was not statistically different (43.0 ± 0.52 msec and 42.3 ± 0.41 msec for 5 and 10 kHz, respectively; paired t-test, df = 34, t = 1.15, P > 0.05). The latency to peak of tone-evoked fields is longer than that observed in rats (see, for example, Rogan and LeDoux 1995), presumably because of the larger size of cat brains.
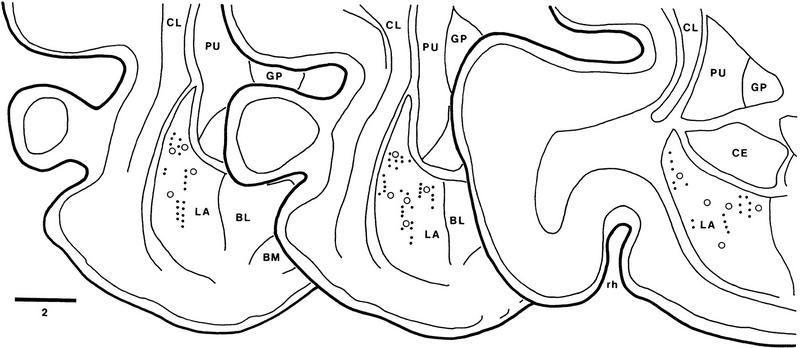
Figure 2.
Location of recorded cells in the LA nucleus. Scheme of three coronal sections arranged from rostral (left) to caudal (right). Dots indicate the location of cells recorded in conditioning experiments. (○) Cells recorded in experiments where tones were presented without US to determine whether their tone responses habituated. The location of recorded cells was determined from thionin-stained coronal sections, by combining micrometric readings with the placement of electrolytic lesions (see Materials and Methods for details). (BL) Basolateral nucleus of the amygdala; (BM) basomedial nucleus of the amygdala; (CE) central nucleus of the amygdala; (CL) claustrum; (GP) globus pallidus; (LA) lateral nucleus of the amygdala; (PU) putamen; (rh) rhinal sulcus. Bar, 2 mm.
Figure 3.
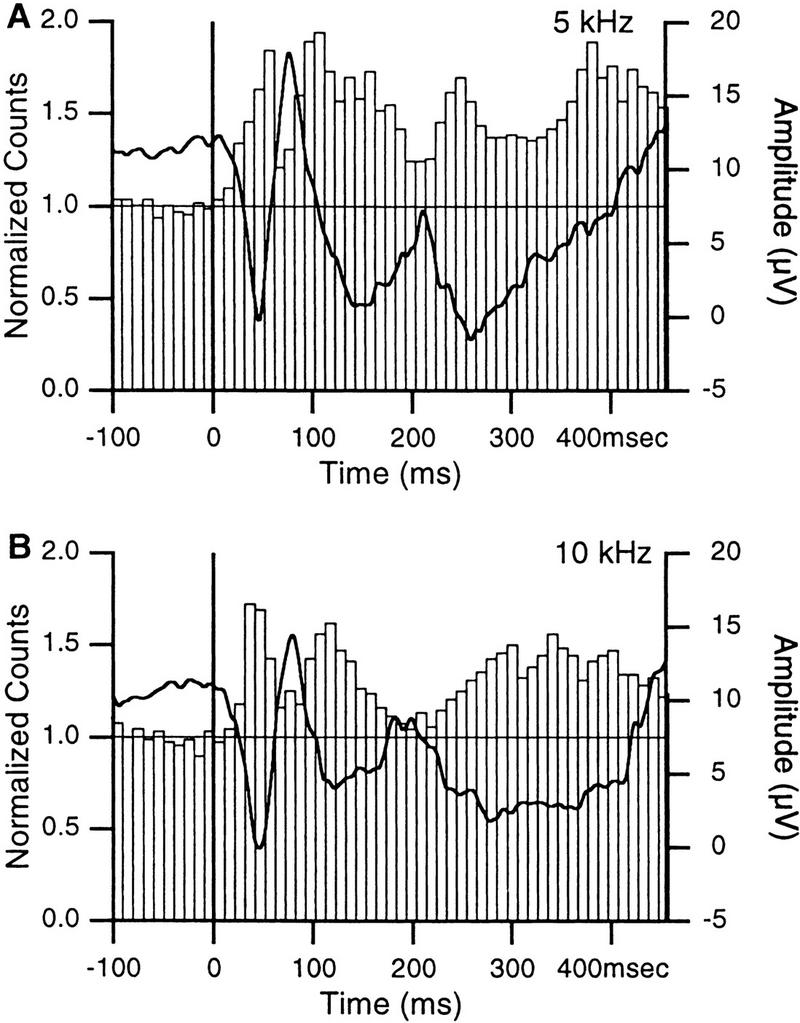
Auditory-evoked field potentials and unit activity in the LA. Responses to 1-sec tones of 5 (A) and 10 (B) kHz. Vertical lines indicate tone onsets. Focal waves were digitally filtered (3–300 Hz, thick lines). Each tone was presented 20 times and the responses of 35 LA sites averaged. Superimposed on the focal waves are peristimulus histograms showing the summed activity of 72 LA neurons. Bins of 10 msec.
The initial components of these auditory-evoked field potentials consisted of negative-, positive-, and negative-going waves which, in LA neurons, were associated to increasing, decreasing, and increasing firing probabilities, respectively (Fig. 3). Auditory-evoked changes in firing probability were assessed by verifying whether the first peak increase in spike counts exceeded the value required to reach statistical significance (P < 0.05) in a one-tailed t-test (average prestimulus value ± 1.64 times the corresponding standard deviation, s.d.). In Figure 3, A, B, the peak exceeded average prestimulus values by 24.1 and 14.9 times the corresponding s.d., respectively.
The close temporal correspondence between these fluctuations in firing probability and the initial components of auditory-evoked field potentials indicate that they were not volume conducted from neighboring structures. Rather, it suggests that they reflect local extracellular currents associated with synaptic activity in the LA, as recently argued (Rogan and LeDoux 1995). In the following, we will focus on the short-latency component of this response, as much evidence suggests that it results from direct inputs originating in the auditory thalamus (Rogan and LeDoux 1995).
To measure conditioned changes in auditory responsiveness of the LA, we first compared the average amplitude of the field potentials evoked by the CS+ and the CS− in the Control, Post-pairing, and Extinction phases (Fig. 4), as was done for the EMG activity (Fig. 1). To control for possible inter-sessions effects, this was performed after dividing the conditioning sessions into two groups (termed New CS+ and Repeated CS+). The first group included sessions where a different CS+ (5 kHz or 10 kHz) had been paired to foot shocks on the previous day (9 sessions for a total of 20 recording sites; Fig. 4A). The second group was comprised of sessions where the same CS+ had been paired on the previous day (8 sessions for a total of 15 recording sites; Fig. 4B). See Materials and Methods (Recording and Analysis) for the method used to measure the amplitude of the field potentials.
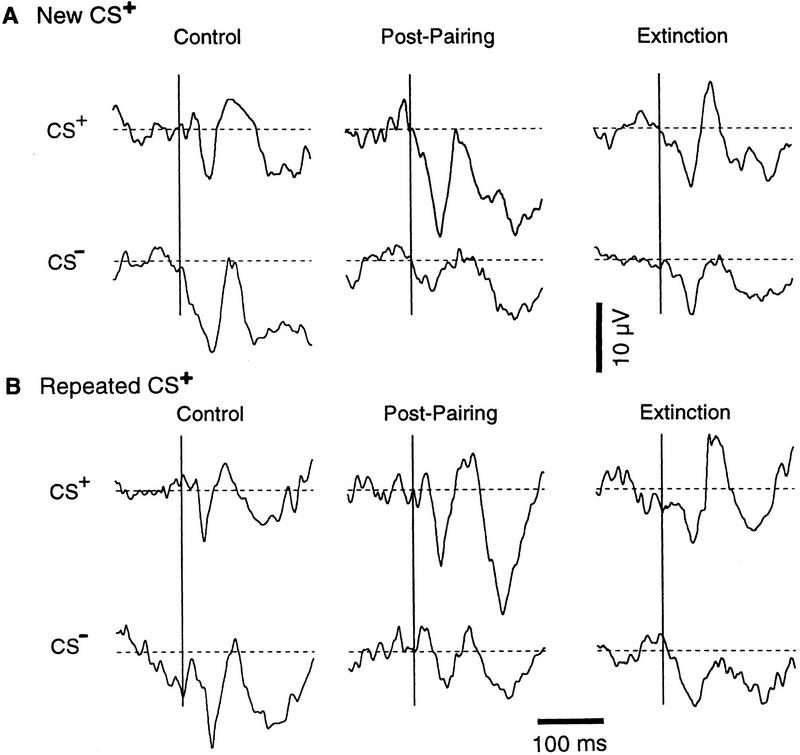
Figure 4.
Conditioning causes opposite changes in focal LA responses to the CS+ and CS−. Average auditory-evoked responses in sessions where a different (A) or the same (B) CS+ was used in the previous session. Broken lines indicate average voltage values before the tone onsets. The average amplitude of the field potentials (in μV) in A are, for the CS+: Control, 7.5 ± 2.9; Post-Pairing, 15.8 ± 3.79; Extinction, 8.6 ± 2.33. For the CS−, Control, 13.3 ± 3.19; Post-Pairing, 4.8 ± 2.05; Extinction, 7.9 ± 3.09. In B, for the CS+, Control, 7.4 ± 2.11; Post-Pairing, 12.1 ± 3.4 ; Extinction, 8.5 ± 2.26. For the CS−, they are Control, 13.9 ± 2.48; Post-Pairing, 5.4 ± 1.66; Extinction, 8.2 ± 2.17. Vertical bar, 10 μV; horizontal bar 100 msec.
Figure 4 depicts the average field potentials elicited by the CS+ and CS− in the LA for the two groups of sessions, in the three phases. See the legend of Figure 4 for the amplitude of the field potentials. Changes in field potential amplitude were assessed using a repeated-measures ANOVA. A significant interaction was found between the stimulus (CS+, CS−) and experimental phase (Control, Post-pairing, Extinction; F = 16.75; P < 0.05) but not with the session order (New CS+, Repeated CS+; F = 0.38). The effect of the stimulus, experimental phase and session order variables did not reach significance (F = 0.26, 1.59, 0.07, respectively).
Two-tailed paired t-tests revealed that, irrespective of the order in which the CS+ was presented on consecutive days, the differential conditioning paradigm significantly increased focal LA responses to the CS+ (New CS+, by 111 ± 49.7%, t = 2.28; Repeated CS+, 64 ± 14.2%, t = 3.63; P < 0.05) and decreased those to the CS− (New CS−, by 41 ± 30.3%, t = −3.22; Repeated CS−, 61 ± 18.7%, t = −3.16; P < 0.05). Auditory-evoked field potentials returned toward control values following extinction. However, CS−-evoked responses remained significantly lower than control ones (t = −3.19 and −2.31 for sessions with new and repeated CS+, respectively).
Differences in the amplitude of field responses evoked by the CS+ and CS− in the Control phase did not reach significance for the New CS+ group of sessions (df = 19, t = 1.21, P > 0.05), but did for the Repeated CS+ group (df = 14, t = 3.002, P < 0.05). This difference results from the fact that 10 kHz tones tended to evoke smaller field responses (see Fig. 3B) and that, by chance, more 10 kHz tones happened to be used as the CS+.
We then turned our attention to the changes in auditory-evoked unit activity induced by fear conditioning. One obstacle in carrying out such analyses is that most LA neurons fire at low rates (Paré and Gaudreau 1996; Collins and Paré 1999). Thus, to maximize spike counts, and because the above analysis revealed that the session order did not affect the directions of the changes, all LA units (n = 72) were pooled together (Fig. 5).
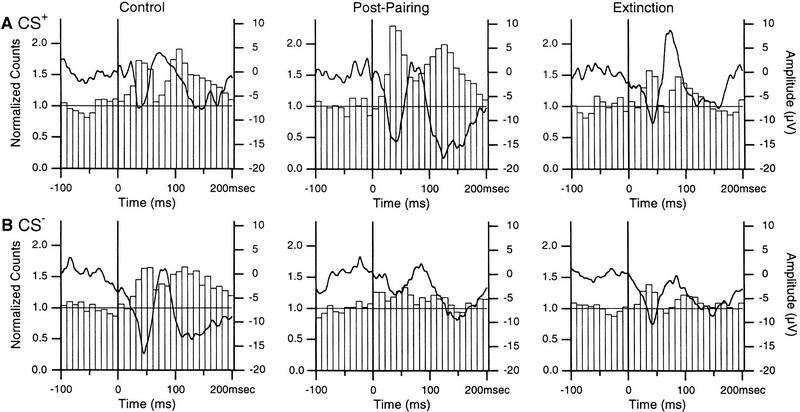
Figure 5.
Conditioning causes inverse changes in unit responses to the CS+ (A) and CS− (B) in the LA. The responses of 72 neurons were summed and normalized to the average prestimulus bin height and superimposed on average focal waves (thick lines). Using the criteria of Fig. 3, short-latency increases in firing probability were significant (P < 0.05) in all conditions.
Modifications in auditory-evoked firing were analyzed as follows. For each condition, the difference between the two bins coinciding with the peak of the first negative focal wave and the prestimulus bins was computed, resulting in six arrays of 20 values. Using a repeated-measures ANOVA, a significant interaction was found between the stimulus and experimental phase variables (F = 251.03; P < 0.05). A significant effect of the stimulus and experimental phase variables was also found (F = 412.57 and 152.79, respectively).
Consistent with the changes in auditory-evoked field potentials, two-tailed paired t-tests (df = 19) revealed that although responsiveness to the two stimuli was not different in control conditions (t = 2.003), pairing increased (P < 0.05) responses to the CS+ (by 54 ± 2.7%, t = 20.09) and decreased (P < 0.05) those to the CS− (by 33 ± 2.1%, t = 10.85). After extinction, responses returned toward control values, but remained significantly different (CS+, t = 5.3; CS−, t = 7.3).
To determine whether the depression of CS−-evoked activity reflected habituation of responses, we analyzed the auditory responsiveness of 14 additional LA cells recorded in the course of previous extracellular studies on the activity of amygdala neurons (Collins and Paré 1999; D. Paré, unpubl.). These cells (empty circles in Fig. 2) were selected because they were clearly tone responsive and the animals had never been presented the tones (3–10 kHz) prior to the recordings. The averaged unit and field response to tone presentations 1–10 (Fig. 6A) and 31–40 (Fig. 6B) was analyzed. Note that in contrast with the previous experiments, tones 21–30 were not paired to foot shocks.
Figure 6.
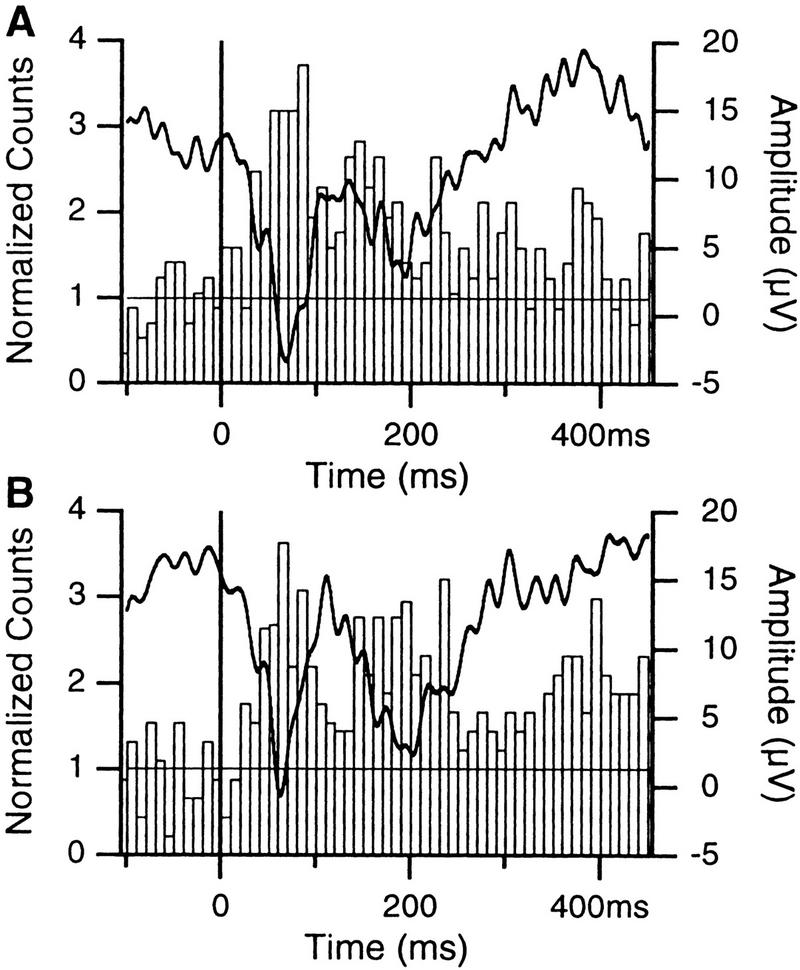
Effect of multiple presentations of the same tones on the responsiveness of LA cells. Averaged field (thick lines, right axis) and unit responses (histograms, left axis; n = 14) to presentations 1–10 (A) and 31–40 (B) of the same tones (3–10 kHz). Bins of ten msec. When the cells were recorded, the animals had never been presented these tones before and no foot shock had been administered in conjunction to auditory stimuli. Note that the spike counts were normalized to the average pretone activity.
The amplitude of field responses averaged 16.4 ± 3.3 μV and 16.2 ± 2.7 μV for tone presentations 1–10 (Fig. 6A) and 31–40 (Fig. 6B), respectively. The difference was not statistically significant (df = 13, t = 0.07, P > 0.05). To determine whether the auditory responsiveness of the cells changed from tones 1–10 to tones 31–40, the difference between the two bins coinciding with the peak of the first negative focal wave and the prestimulus bins was computed, resulting in two arrays of 20 values. However, the difference did not reach significance (df = 19, t = 0.058, P > 0.05).
DISCUSSION
Nature of the Conditioned EMG Responses
In this study, conditioned fear reactions were assessed by comparing the changes in EMG activity evoked by the CS+ and CS− before and after conditioning. In the control period, the CS+ and CS− evoked an increase in EMG activity of the posterior neck muscles whereas after pairing, the CS+, but not the CS−, elicited a reduction in EMG activity.
This result might seem surprising as, following fear conditioning, freely moving rats were reported to exhibit increased CS-evoked EMG responses associated with freezing (Hennevin et al. 1998). We speculate that our counter-intuitive finding is a consequence of the head restraint imposed on the animals. The animals quickly learned that release from the head restraint involves downward head movements. Presumably, the opposite result would have been obtained had we recorded from muscles assisting these movements.
Nevertheless, irrespective of the underlying mechanisms, the specificity of the conditioned EMG response suggests that it resulted from the differential pairing of the CS+ to the shocks, not from a nonspecific arousal produced by the shocks or the auditory stimuli.
Modifications in Auditory Responsiveness
Previous studies examining the effect of fear conditioning on the auditory responsiveness of LA neurons (Quirk et al. 1995; Rogan et al. 1997; Hennevin et al. 1998) can be criticized for two reasons (Malenka and Nicoll 1997). First, the increase in auditory-evoked LA responses caused by fear conditioning was not shown to be specific to the conditioned tone. Second, the possibility that conditioned increases in responsiveness reflected alterations in auditory relays located upstream from the LA could not be excluded.
With regards to the first criticism, our results demonstrate that fear conditioning does not provoke a nonspecific increase in auditory responsiveness. Rather, fear learning is accompanied by a potentiation of responses to the CS+ and a depression of those to the CS−. In fact, our study is the first to show that the changes in auditory responsiveness produced by fear conditioning in the LA are frequency specific.
Several studies using discriminative fear conditioning have reported specific increases in the responsiveness of other amygdala neurons to the CS+ (see, for example, Pascoe and Kapp 1985; Maren et al. 1991; McEchron et al. 1995). However, in contrast with the present findings, decreases in responsiveness to the CS− were not observed in the amygdala except in cases where the CS− had been paired previously to the US and the fear responses not extinguished, an approach known as reversal. This is in contrast with the paradigm used here, where the two CS were presented 60 times each to extinguish fear responses prior to the next conditioning session.
Several factors suggest that the decreased LA responses to the CS− induced by fear conditioning do not reflect a simple habituation. First, previous work has shown that explicitly unpaired presentations of the US and one auditory CS do not provoke significant decreases in evoked responses (Rogan et al. 1997). Second, in our study, CS−-evoked responses returned toward control values following repeated unpaired presentation of the two tones. Last, repeated presentations of the tones without the noxious stimuli did not depress significantly tone-evoked unit and field responses in the LA. These considerations suggest that the differential association of the two tones to the US is essential for decreases in CS−-evoked responses to be observed.
Regarding the second criticism, previous studies examining the effects of fear conditioning on the auditory responsiveness of thalamic and cortical auditory neurons (Oleson et al. 1975; Disterhoft and Stuart 1976; Ryugo and Weinberger 1978; Bakin and Weinberger 1990; Edeline and Weinberger 1992) reported that conditioning increased responses to the CS+. Thus, potentiation of CS+-evoked LA responses observed in this and previous studies (Quirk et al. 1995; Rogan et al. 1997) could reflect, in part or totality, alterations in auditory relays located upstream from the LA. However, the reportedly late development of conditioned responses in the auditory cortex compared to the LA (Quirk et al. 1997) suggests that thalamic inputs play a predominant role in this respect.
It is unclear whether the pronounced depression of CS−-evoked LA responses observed here can be explained by changes occurring at the thalamic or cortical levels. In studies comparable to ours, responses to unpaired tones were described as unchanged (medial geniculate, Ryugo and Weinberger 1978), slightly increased (auditory cortex, Oleson et al. 1975), or decreased by an average of 12–16% (auditory cortex, Bakin and Weinberger 1990; medial geniculate, Edeline and Weinberger 1992). The reduction observed in the latter studies represents roughly half the effect observed here.
Although this difference suggests that the decreased responsiveness of LA neurons to the CS− cannot be ascribed solely to changes in thalamic or cortical afferents, this point awaits confirmation using simultaneous recordings in these various sites. If true, this finding would constitute strong evidence that the amygdala does more than relay sensory inputs to brain structures mediating fear responses, a much debated issue (Cahill et al. 1999; Fanselow and LeDoux 1999).
MATERIALS AND METHODS
Surgery
Experiments were conducted in four adult cats in agreement with the guidelines of the Canadian Council on Animal Care. Research protocols were approved by the local ethics committee of University Laval. We chose this species because the large size of cat brains facilitate the placement of multiple microelectrodes in different nuclei of the amygdala.
The anesthesia was induced with ketamine (15 mg/kg, i.m.) and atropine sulfate (0.05 mg/kg, i.m.) was administered to prevent secretions. Then, sodium pentobarbital was injected gradually (Somnotol, 15 mg/kg, i.v.). The bone overlying the amygdaloid complex was removed on one side and the dura mater removed. Then, an array of 21 tungsten microelectrodes (3 rows of 7 electrodes) was lowered until the electrodes reached the dorsal aspect of the amygdala. These electrodes (FHC, Brunswick, ME) had a maximal outer diameter of 80 μm and an impedance of 2–6 m at 1 KHz. To construct the array, small holes were drilled in a circular delrin block and the electrodes inserted into them. The delrin block was inserted in a tightly fitting sleeve that was cemented to the bone. During the recording sessions, the electrodes could be lowered by means of a screw pushing on the delrin block. To monitor the muscle tone, two Teflon-insulated wires were inserted in the posterior neck muscles. Finally, four screws were cemented to the skull to fix the cat's head in a stereotaxic position without pain or pressure. Recording sessions began six days later. Bicillin (i.m. daily for 3 days) and buprenorphine (0.03 mg/kg, i.m. every 12 hr for 24 hr) were administered postoperatively. Recording sessions began six to eight days after the surgery. In between experimental sessions, the animals slept, ate, and drank ad libitum. At the end of the experiments, the animals were deeply anesthetized (40 mg/kg) and selected recording sites were marked with electrolytic lesions (0.5 mA for 5 sec). Then, they were perfused with saline followed by a fixative. The brains were sectioned at 80 μm and stained with thionin. The microelectrode tracks were reconstructed by combining micrometer readings with the histological controls, as shown in Collins and Paré (1999).
Recording and Analysis
The activity of LA neurons was observed on a digital oscilloscope, printed on a chart recorder, digitized, and stored on tape. Analyses were performed off-line with the software IGOR (Wavemetrics, Oregon) and homemade software running on Macintosh microcomputers. Field potentials and unit activity were recorded simultaneously with the same electrodes and isolated off-line using digital filtering (action potentials, 0.3–10 kHz; field potentials, 3–300 Hz). The average amplitude of tone-evoked field potentials was measured by subtracting the voltage 42.7 msec after the tone onset from the average voltage value (broken lines in Fig. 4) of the 100 msec preceding the tone onset. The interval of 42.7 msec corresponds to the average latency to peak of the field potentials evoked by the 5 and 10 kHz tones (see Results). Spikes were detected with a window discriminator after digital filtering of the data. Peristimulus histograms were smoothed with a moving average of 3.
The LA contains two main cell types (see McDonald 1992). Most cells (85%) are spiny glutamatergic projection neurons and a minority of cells are aspiny local-circuit neurons immunopositive for γ-aminobutyric acid (GABA; McDonald 1985; McDonald and Augustine 1993; Paré and Smith 1993). Thus, our sample of LA neurons should be mainly comprised of projection cells. Nevertheless, to ensure that we studied an homogeneous population of neurons, we attempted to further restrict our analyses to projection cells. We distinguished projection cells from interneurons on the basis of criteria obtained in a previous electrophysiological investigation of LA neurons in behaving cats (Paré and Gaudreau 1996). In this study, all LA neurons that could be backfired from known projection fields of the LA fired spontaneously at low rates (generally <1 Hz) whereas none of the cells with high spontaneous firing rates (>10 Hz) could be antidromically invaded. Consequently, they were presumed to be local-circuit cells. This idea is supported by the results of intracellular studies where the physiological and morphological properties of LA neurons were correlated (Paré et al. 1995; Lang and Paré 1998), revealing that aspiny neurons of the LA can sustain high firing rates for prolonged periods of time (Paré et al. 1995; Lang and Paré 1998). Thus, in the present study, all cells of the LA with high spontaneous firing rates (>5 Hz) were excluded from the analyses.
Conditioning
Auditory stimuli consisted of 1-sec tones of 5 or 10 kHz at 60 db. The US were electrical shocks (0.5 sec, 1.5 mA) delivered to the front paws by means of surface electrodes 0.5 sec after the CS+ onset. Inter-tone intervals ranged between 2 sec and 5 min. Each recording session the electrode array was lowered 80–200 μm and the electrodes scanned for units with a signal to noise ratio ≥ 3. Thirty minutes later, the cats were presented two different tones (5 and 10 kHz), 20 times, in a random order. Subsequently, one of the two tones (CS+) was presented 10 times, paired to a foot shock, with an equal number of randomly interspersed CS− presentations. To extinguish conditioned fear responses, 60 unpaired presentations of the two tones followed. Hereafter, these three phases of the conditioning paradigm will be referred to as the Control, Pairing, and Extinction phases, respectively. On subsequent days, the same protocol was carried out with the proviso that the CS+ could remain the same or change, randomly. Fewer than six conditioning sessions were performed in each cat.
Acknowledgments
This work was supported by the Canadian Medical Research Council. We would like to thank Drs. M. Davis, É. Audinat, S. Charpak, and E.J. Lang for useful comments on an earlier version of this manuscript.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL Denis.Pare@phs.Ulaval.CA; FAX (418) 656-7898.
REFERENCES
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bandler R. Induction of “rage” following microinjections of glutamate into midbrain but not hypothalamus of cats. Neurosci Lett. 1982;5:183–188. doi: 10.1016/0304-3940(82)90294-4. [DOI] [PubMed] [Google Scholar]
- Cahill L, Weinberger NM, Roozendaal B, McGaugh JL. Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- Collins DR, Paré D. Reciprocal changes in the firing probability of lateral and central medial amygdala neurons. J Neurosci. 1999;19:836–844. doi: 10.1523/JNEUROSCI.19-02-00836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Stuart DK. Trial sequence of changed unit activity in auditory system of alert rat during conditioned response acquisition and extinction. J Neurophysiol. 1976;39:266–281. doi: 10.1152/jn.1976.39.2.266. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: Receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Gentile CG, Jarrell TW, Teich AH, McCabe PM, Schneiderman N. The role of amygdaloid central nucleus in differential Pavlovian conditioning of bradycardia in rabbits. Behav Brain Res. 1986;20:263–276. doi: 10.1016/0166-4328(86)90226-3. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Maho C, Hars B. Neuronal plasticity induced by fear conditioning is expressed during paradoxical sleep: Evidence from simultaneous recordings in the lateral amygdala and the medial geniculate in rats. Behav Neurosci. 1998;112:839–862. doi: 10.1037//0735-7044.112.4.839. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Fear-potentiated startle using an auditory conditioned stimulus: effect of lesions of the amygdala. Physiol Behav. 1987;39:403–408. doi: 10.1016/0031-9384(87)90242-3. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Meeley MP, Arneric S, Reis DJ. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 1986;383:195–214. doi: 10.1016/0006-8993(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Iwata J, Chida K, LeDoux JE. Cardiovascular responses by stimulation of neurons in the central nucleus of the amygdala in awake but not anesthetized rats resemble conditioned emotional responses. Brain Res. 1987;418:183–188. doi: 10.1016/0006-8993(87)90978-4. [DOI] [PubMed] [Google Scholar]
- Kaada BR. Stimulation and regional ablation of the amygdaloid complex with reference to functional representations. In: In: Elefther BE, editor. The neurobiology of the amygdala. New York, NY: Plenum; 1972. pp. 205–281. [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: Effects on heart rate conditioning in the rabbit. Physiol Behav. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Paré D. Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience. 1998;83:877–889. doi: 10.1016/s0306-4522(97)00420-x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990a;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990b;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Learning and memory—Never fear, LTP is hear. Nature. 1997;390:552–553. doi: 10.1038/37472. [DOI] [PubMed] [Google Scholar]
- Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Res. 1991;549:311–316. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Immunohistochemical identification of gamma-aminobutyric acid-containing neurons in the rat basolateral amygdala. Neurosci Lett. 1985;53:203–207. doi: 10.1016/0304-3940(85)90186-7. [DOI] [PubMed] [Google Scholar]
- ————— . Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss; 1992. pp. 67–96. [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52:281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- McEchron MD, McCabe PM, Green EJ, Llabre MM, Schneiderman N. Simultaneous single unit recordings in the medial nucleus of the medial geniculate nucleus and amygdaloid central nucleus throughout habituation, acquisition, and extinction of the rabbit's classically conditioned heart rate. Brain Res. 1995;682:157–166. doi: 10.1016/0006-8993(95)00331-j. [DOI] [PubMed] [Google Scholar]
- Oleson TD, Ashe JH, Weinberger NM. Modification of auditory and somatosensory system activity during pupillary conditioning in the paralyzed cat. J Neurophysiol. 1975;38:1114–1139. doi: 10.1152/jn.1975.38.5.1114. [DOI] [PubMed] [Google Scholar]
- Paré D, Smith Y. Distribution of GABA immunoreactivity in the amygdaloid complex of the cat. Neuroscience. 1993;57:1061–1076. doi: 10.1016/0306-4522(93)90049-l. [DOI] [PubMed] [Google Scholar]
- Paré D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: Distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Pape HC, Dong JM. Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: Electrophysiological properties and morphological features. J Neurophysiol. 1995;74:1179–1191. doi: 10.1152/jn.1995.74.3.1179. [DOI] [PubMed] [Google Scholar]
- Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res. 1985;16:117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT. Cortical and subcortical afferents of the amygdaloid complex. In: Schwarz R, Ben-Ari Y, editors. Excitatory amino acids and epilepsy. New York, NY: Plenum; 1986. pp. 35–52. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Weinberger NM. Differential plasticity of morphologically distinct neuron populations in the medial geniculate body of the cat during classical conditioning. Behav Biol. 1978;22:275–301. doi: 10.1016/s0091-6773(78)92351-9. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: A combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]