Abstract
Thoracic spine manipulation has been shown to be effective for the management of neck pain. The purpose of this study was to investigate the immediate effect of a T3–T4 spinal thrust manipulation on autonomic nervous system activity in subjects with chronic cervical pain. An additional aim was to determine if the manipulation resulted in an immediate pain relief in patients with chronic neck pain when compared to a placebo intervention. One hundred subjects with chronic neck pain were randomly assigned to receive either a thoracic thrust manipulation or a placebo intervention. The Friedman’s test was used to evaluate the change in pupil diameter within both groups. The Wilcoxen signed-ranks test was used to explore pupil changes over time and to make paired comparisons of the pupil change between the groups. The Mann–Whitney U test was used to compare the change in pain perception for the chronic cervical pain group subjects receiving either the thrust manipulation or the placebo intervention. The results demonstrated that manipulation did not result in a change in sympathetic activity. Additionally, there was no significant difference in the subject’s pain perception (P = 0.961) when comparing the effects of the thrust manipulation to the placebo intervention within this group of subjects with chronic neck pain. The clinical impression of this study is that manipulation of the thoracic spine may not be effective in immediately reducing pain in patients with chronic neck pain.
Keywords: Chronic cervical pain, Manipulation, Sympathetic response, Pupillometry
Introduction
The occurrence of chronic spinal pain has been reported to be as high as 54–80%,1,2 resulting in substantial disability and economic burden. Health care costs related to neck disorders includes visits to health care providers, the inability to work, and loss of work-related productivity.3–5 Approximately 10% of the adult population will experience neck pain at any one time and up to 60% of patients continue to report chronic pain 5 years after the initial onset of symptoms.1,6–8 The development of chronic pain has been associated with a state of increased excitability of spinal inter-neurons.9,10–13 This phenomenon is known as central sensitization. The increased spinal neuron activity can lead to the development of hyperalgesia and allodynia9 in the peripheral tissues and can cause increased muscle tone or the subjective sensation of pain.14,15
Physical therapists frequently use manipulation as an intervention in the management of patients with neck pain16–20 as it has been shown to be effective.21–23 Although the exact physiological mechanisms underlying spinal manipulative techniques is still unknown, a few hypotheses have been proposed offering mechanical, neurophysiological, and psychological rationales.11,24 Mechanical force used during manipulation has a direct effect on the central nervous system, creating positive neurophysiological responses resulting in a reduced overall central sensitization.11,12,15 The effects of manipulation is beyond biomechanical changes only;15 however, in the current literature, there is no clear explanation for some of the effects of manipulation.11
Within the nervous system, both the somatic and autonomic nervous system functions as one unit, with interaction between both systems, and their functioning can be influenced by each other.14,25,26 Somatic-nociceptive and autonomic regulatory regions in the central nervous system often respond to the same type of somatic or visceral afferent input. They receive convergent nociceptive and viscerosensory information. The central system contains regions that initiate autonomic, anti-nociceptive, and behavioral responses to the somatic and visceral afferent input.27 Several areas of interaction between the somatic and autonomic nervous system have been identified in the periphery, dorsal horn of the spinal cord, brainstem, and forebrain.28,29 Bialosky et al.11 have presented a model which identifies several pathways within the peripheral and central nervous system that could explain the effects of manipulation. Within this model, it was suggested that the effects of manipulation either could influence or be influenced by the autonomic nervous system. During a state of central sensitization, there is a corresponding over-activity in the lateral gray matter and an expected increase in sympathetic activity will be present.30–32
Recently, studies have demonstrated that manipulation targeting the thoracic spine results in positive benefits for patients with acute/subacute mechanical neck pain resulting in increased cervical range of motion, reduction in pain, and a decreased level of self-reported disability.21,23,33–36 Manipulation techniques used in the clinical prediction rules are less segmental and tissue specific,37,38 and clinical research has shown that they result in measurable objective improvements.38–40 To date, little is known about the direct effect of thoracic manipulation in the chronic neck pain population. However, findings in the acute and subacute populations support the notion that manipulation, in general, achieves neurophysiological changes in both the peripheral and central nervous system. The primary mechanism responsible for maintaining normal quality of tissues is the autonomic nervous system.41–43 Therefore, it seems a plausible hypothesis that both the more classical tissue-specific mechanical approaches and the more indirect approaches can affect the autonomic nervous system functioning and, therefore, reduce the state of central sensitization and achieve positive outcomes.
A close relationship exists between pain and the autonomic nervous system; therefore, its parameters are often regarded as objective measures of pain in humans.44 There have been several methods used to assess the activity of the autonomic nervous system and its components. Traditionally, the autonomic symptoms consisting of vasomotor, sudomotor disturbances, and trophic changes have been observed and measured in the periphery.45,46 The pupil is an example of an organ that receives innervation from the autonomic nervous system exclusively. Both the sympathetic and parasympathetic components influence the pupil.43,47–49 The innervation of the constrictor pupillae muscle is parasympathetic50 and the dilator pupillae muscle is sympathetically innervated.49 Based on a negative feedback mechanism within the autonomic nervous system, the pupil diameter is based on the functional balance between the sympathetic and parasympathetic nervous system.43,47,51 Pfeifer et al.52 demonstrated that pupil size increased by stimulation of the sympathetic nervous system and decreased during stimulation of the parasympathetic system. Additionally, it has been demonstrated that sympathetic activation through the upper thoracic spine causes pupil dilation.14 Owing to the pupils exclusive autonomic innervation, more recently an interest has emerged to measure the pupil diameter to obtain a direct impression of autonomic function.53–56 A dilation of the pupil is a result of simultaneous increasing activity of the sympathetic system and decreasing parasympathetic activity.45,47,49
There is a lack in the literature accounting for some of the neurophysiological effects that seem to take place following manipulation techniques.11 Vicenzino et al. demonstrated that a manipulation of the cervical spine resulted in an increase in pain threshold in the forearm and a sympathoexcitatory effect.57 Bialosky et al. have demonstrated hypoalgesic effects following manipulation in segmental related areas.58,59 These studies seem to provide evidence that neurophysiological changes occur following manipulation. To further validate the use of manipulation techniques in clinical practice, continued investigation into the neurophysiological effects is necessary. Based on the relationship between the autonomic nervous system, in the upper thoracic spine, and the cervical spine, it seems a plausible hypothesis that thoracic manipulation may directly affect the autonomic nervous system functioning. Hence, the purpose of this study was to investigate the effect of a manipulation directed at the upper thoracic spine on the autonomic nervous system function in patients with chronic neck pain and compare this to non-specific placebo effects in a control group. Furthermore, studies investigating the effects of thoracic spine manipulation on patients with neck pain to date have primarily used a sample of patients with acute/subacute symptoms.23,60 Therefore, a secondary aim of this study was to investigate if there was an immediate reduction in pain following thoracic manipulation in subjects with chronic neck pain.
Material and Methods
Subjects
Consecutive patients between May and August of 2008 with chronic cervical pain were recruited from five outpatient physical therapy clinics in Indiana. For the purpose of this study, cervical pain was operationally defined as the presence of non-specific pain in the cervical and cervicothoracic region down to T4, which was provoked with neck movements.21,23 Chronicity was operationally defined as the presence of pain that had not subjectively changed in intensity and had been present for at least 3 months.61
All available patients were screened for eligibility criteria. To participate, patients had to be between the ages of 18 and 65 years and able to fluently speak and read the English language. Patients were instructed not to take any medication that could alter the functioning of the autonomic nervous system for at least 24 hours before participating in the study. If physician approval could not be obtained, the patients were not eligible to participate in the study. All patients were instructed not to consume caffeinated drinks, smoke, or eat anything for at least 12 hours before the study. Patients were also excluded from this study if they were previously diagnosed with autonomic diseases such as the Horner’s syndrome; had a history of current neurological, ocular, and/or retinal disease; used two or more alcoholic beverages daily; or were trained for endurance sports. This study received IRB and HPD approval from NOVA Southeastern University. All patients provided written consent before participating in the study.
Self-reported measures
The visual analog scale (VAS) was used to assess pain before treatment intervention and immediately after all pupil measurements were taken. The VAS consists of a 100 mm line with an anchor at each end. The left anchor indicated ‘no pain’ and the right anchor indicated ‘the worst pain imaginable’.62,63 The validity and reliability of the VAS have been previously reported for patients with acute and chronic pain.62,64–68
Automated measures
To obtain a measure of autonomic nervous system activity, the pupil diameter can be measured directly.49,52,54,56,69,70 Fully automated pupillometry devices have been used previously in a number of studies investigating autonomic nervous system activity.53,56,71–76 The human eye has difficulties detecting differences of the pupil that are smaller than 1.0 mm.76,77 The automated pupillometric measurement will be able to detect differences smaller than 0.2 mm and will, therefore, be much closer to the ‘true’ measure than any direct manual measure can ever be.78 A fully automated pupillometry device was used during this study, thereby eliminating researcher bias during the measurement phase and minimizing rater reliability issues.
The measurement error has been shown to be minimal and pupil changes smaller than 0.2 mm will be detected.74,76,77,79 It has been previously demonstrated that the intra-rater repeatability of automatic pupillometric devices is good, with coefficient of repeatability ranging from 0.6 to 1.4 mm.71,72 The diameter of the pupil can be considered as a direct reflection of the ‘live’ balance between the parasympathetic and the sympathetic nervous systems. Several studies have evaluated the correlation between autonomic function tests and pupillometry and have shown that there is a significant correlation.80,81 Other studies have shown that pupillometry is sensitive enough to identify autonomic differences.53,79,82,83
The pupil responses during this study were measured with the fully automated Vorteq® system. The Vorteq system for recording of the pupil reaction was developed by Micromedical Technologies, Inc. It includes goggles, which the subject wears during the measurement, creating a completely dark environment for both eyes. An infrared camera is attached to the goggles, allowing measurement of the pupil diameter of the right eye (Fig. 1).
Figure 1.

Goggles, with the infrared camera attached to the right eye, manufactured by Micromedical Technologies.
Study protocol
To consistently identify the localization of the T3–T4 level for this study, each patient was tested by the same researcher. In all patients, C7 was identified as that vertebrae that has the largest spinous process, and C6 was identified as that spinous process that would relatively disappear upon extension motion of the cervical spine.84,85 Passive neck flexion was used to identify inter-segmental motion to determine the T3–T4 level.16 A clear mark was placed on the skin identifying the T3–T4 inter-spinous space and the T4 transverse process allowing for easy identification during the intervention.
During the measurement phase, all patients were placed in the supine position with the knees slightly flexed over a bolster, and the head, with the goggles in place, was placed on a pillow in a neutral position of the cervical spine. This standardized position was assumed by all patients. During the manipulation or placebo maneuver, the patients had the arms crossed over the chest (Fig. 2). All patients were in complete darkness during the pupillometric measurements. The darkness achieved by the goggles allowed for a constant maximum pupil diameter during the pupil measurement without the influences of light.
Figure 2.
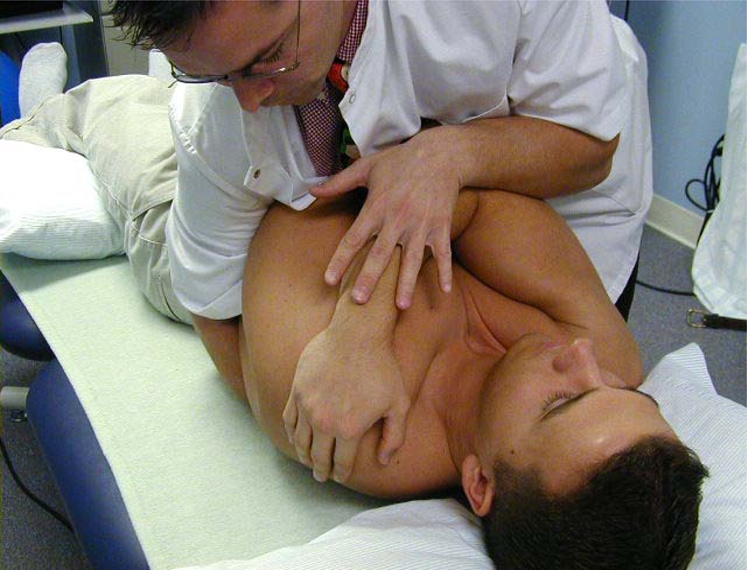
Standardized position during the manipulation maneuver.
After 3 minutes of accommodation to the dark environment, the pupil of the right eye was measured continuously for a 60-second duration. During this 3-minute period, patients were randomly assigned to receive thrust manipulation or a placebo directed at T3–T4. Concealed allocation was performed by using a computer-generated randomized table of numbers created before the beginning of the study. Individual, sequentially numbered index cards with the random assignment were prepared and placed in sealed opaque envelopes.
Directly following the baseline measurement, the patient received either the manipulation or the placebo intervention. The physical therapeutic manipulation consisted of a high velocity, mid-range, and posterior-to-anterior force to the upper thoracic spine targeting the T3–T4 segment in the supine position. Before the manipulation application, the right thenar eminence of the researcher was placed on the marked transverse process of T4. In order to create capsular tension at the T3–T4 segment before the manipulation, added components of segmental flexion, rotation, side bending, and some slight compression of the patient’s chest were introduced using the research’s own chest and left arm. The right hand simultaneously performed a pronation of the right forearm and a slight distraction toward the pelvis, thereby further tightening the capsule. When the tissue barrier was felt, the manipulation maneuver was achieved by pushing the patient’s elbows toward the shoulders (Fig. 2). This manipulation technique was previously described by Hartman.86
The placebo treatment consisted of an open-hand placement in such a way that it should not have resulted in a manipulation at the T3–T4 level on the right side similar to the previously used sham technique described by Cleland.87 The researcher’s right hand was placed with a flat hand contact under the T4 segment. The patient crossed the arms over the chest, and in this position with minimal mobilization and without manipulation effect, the patient was asked to take a deep inhalation followed by an exhalation at which time a light 3-second compression of the arms into the chest was achieved. All manipulation and placebo treatments were provided by the same experienced physical therapist who has more than 14 years of experience in manual therapy.
Directly following the intervention, a continuous measurement of the right pupil was taken for 60 seconds. Four minutes after the intervention modality, there was a final 60-second measure of the right eye, after which the pupil measurement was concluded.
Statistical analysis
Data analyses were performed using the statistical software package SPSS (version 14.0; SPSS Inc., Chicago, IL, USA). The assumptions for the use of parametric statistics was not satisfied, based on analysis of skewness and kurtosis for both groups in regard to the gender distribution, the pre-intervention pupil diameter, and the post-intervention pupil diameters; therefore, nonparametric analysis was necessary. The chi-square test for categorical data was used to analyze the gender distribution amongst both groups. For each group, the Friedman’s test was used to evaluate the change in pupil diameter from the pre-intervention to the post-intervention measures and the Cohen’s d was used to determine the effect size. The Wilcoxen signed-ranks test was used to explore how the pupil changed over time in the placebo group and to make paired comparisons of the pupil change between the groups. And finally, the Mann–Whitney U test was used to compare the change in pain perception for the chronic cervical pain group patients receiving either the thrust manipulation or the placebo intervention.
Results
Baseline characteristics for both groups
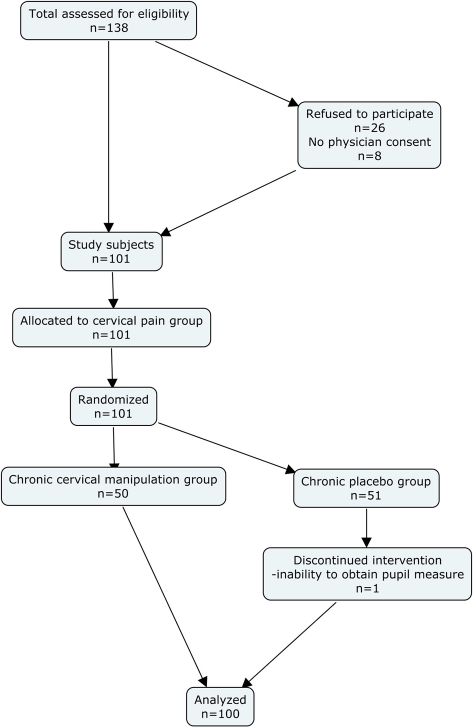
A total of 135 consecutive patients with chronic neck pain were assessed for eligibility. Twenty-six patients refused to participate and eight did not have physician approval. The remaining 101 patients were enrolled in the study. One patient was removed during the measurement phase because the patient was unable to keep their eye in a position that the infrared camera could measure the pupil. Hence, 100 patients completed the testing protocol (Fig. 3). Baseline demographics for both groups can be found in Table 1. No significant differences in the gender distribution (chi-square(2) = 0.66; P = 0.72) and pre-intervention VAS (P = 0.17) existed between the groups.88
Figure 3.
Consort flow diagram.
Table 1. Baseline demographic.
| Group | Gender |
Mean age (years) | Mean VAS pre-test (mm) | Mean duration of symptoms (month) | Pre-test pupil diameter measured in computer pixels | |
| Female | Male | |||||
| Manipulation | 40 | 10 | 42.7 | 38 | 23.3 | 152.49 |
| Placebo | 37 | 13 | 46.84 | 33 | 25.3 | 142.79 |
| P value | 0.72 | 0.18 | 0.17 | 1.0 | 0.63 | |
Change in pupil diameter
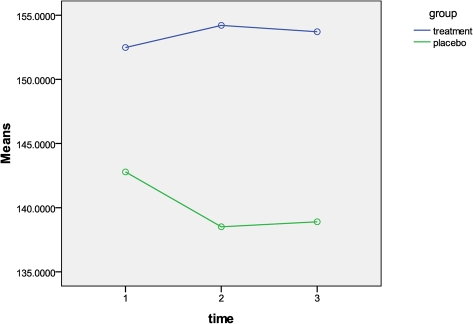
The Friedman’s test was used to evaluate the change in pupil diameter from pre-intervention measure to post-intervention measure 1 (post 1) and post-intervention measure 2 (post 2), separately for each treatment group. Table 2 demonstrates the mean pupil diameter between the three time points for either group. When comparing the pupil diameter at the three measurement points for the manipulation group, there was a slight increase in pupil diameter (Fig. 4). However, the Friedman’s test showed that there was no statistical significant change in the mean pupil diameter (P = 1.0). Additionally, the Cohen’s d effect size was 0.01 indicating no trivial effect from the manipulation on the pupil diameter. There was a statistical significant difference in the mean pupil diameter among the three time points within the placebo group (P<0.001). This indicates that the pupil diameter decreased over time for the placebo group.
Table 2. Mean pupil diameters, in computer pixels, and standard deviations for both groups before intervention (pre-test), directly after the intervention (post 1), and 5 minutes following the intervention (post 2).
| Group | Pre-test | Post 1 | Post 2 | Change pre-post1 | Change pre-post 2 |
| Manipulation | 152.49 (SD = 27.54) | 154.21 (SD = 27.17) | 153.71 (SD = 27.75) | 1.68 | 1.23 |
| Placebo | 142.79 (SD = 27.40) | 138.51 (SD = 23.58) | 138.90 (SD = 25.42) | −4.28 | −3.89 |
Note: Change scores identify the pupil diameter change; a negative change score represents a constriction of the pupil; a positive change score represents a dilation of the pupil.
Figure 4.
Mean pupil diameter, measured in pixels, at the three measurement points for both groups.
To further explore how the mean pupil diameter changed over time within the placebo group, the non-parametric Wilcoxon signed-ranks test was used to compare pre-intervention measure versus post 1, pre-intervention measure versus post 2, and post 1 versus post 2.89 There was a statistical significant difference between pre-intervention measure and post 1 (P = 0.002), and between pre-intervention measure and post 2, (P<0.001), but there was not a statistical significant difference between post 1 and post 2 (P = 0.076). Therefore, it was concluded that the pupil in the placebo group was significantly more dilated before intervention compared to both post intervention measures.
Effect of manipulation on pain
To determine the effect of the thrust manipulation on the pain perception of the subjects with chronic cervical pain, the VAS change score was compared for the subjects that underwent the manipulation and the subjects that underwent the placebo intervention (Table 3). The assumptions to use parametric statistics were not satisfied for the pupil diameter; therefore, the Mann–Whitney U test was used to compare the VAS change score for both groups.88 The within-group comparison demonstrated a significant reduction of pain for both groups. However, there was no a statistical significant difference in VAS change score between both groups following the intervention (P = 0.961). Therefore, it was concluded that the thrust manipulation did not result in immediate pain reduction in the patients with chronic neck pain.
Table 3. Mean VAS values of pre-test and post-test, P values, and VAS change scores for both groups.
| Group | Mean pre-test VAS (mm) | Mean post-test VAS (mm) | P value within group difference | Mean change score pre-post VAS (mm) |
| Manipulation | 38 | 32 | 0.06 | −5.3 |
| Placebo | 33 | 28 | 0.03 | −4.3 |
| P value | 0.17 | 0.076 | 0.961 |
Note: a negative value represents a decrease in pain report.
Discussion
The sympathetic and parasympathetic nervous systems are functionally opposite of each other and a balance between them is necessary to have a state of homeostasis in the tissues.14,47,90,91 In order to obtain an impression about the autonomic system, the pupil diameter and its response to stimuli can be measured.49,52,54,56,69,70 A method of automated pupillometry was used in this study to capture pupil responsiveness. Pupillometry has been previously used to measure the pupil and has been shown to be an easy, valid, and reliable method of assessing the nervous system without the presence of much examiner bias.52–54,71,72,74,77–79,92 It has been shown that the pupil dilates as a result of noxious stimulation and that the pathway through which the sympathetic systems creates dilation passes through the midbrain and the hypothalamus, which indicates a central supraspinal mechanism affecting the pupil diameter.93 However, the diameter itself is by no means only pain specific.54,56 It does appear that the pupil diameter is an indication for general arousal, stress, anxiety, and noxious stimulation.94,95
The normal pupil size ranges between 10 and 85 mm.71,96–98 There have been reports that the pupil diameter decreases with age.54,76,97 Each pupil has a slightly different curvature; therefore, the distance between the infrared camera and the eye is not the same for each subject. Twa et al.77 have reported a similar concern during their assessment of pupil diameter using a digital camera. Both the age difference between both groups and the camera–pupil distance might account for the pre-intervention pupil diameter difference between both groups. During the statistical analysis, change scores between pre-intervention, post 1, and post 2 were used. Hence, this difference between pre-intervention pupil diameter should not have affected the outcomes of this study. The pupil diameter is not static, it is the reflection of the direct ‘live’ balance between the two components of the autonomic nervous system.47,99 Therefore, pupil diameter measurement will show continued fluctuation in size. This pupillary fluctuation, or hippus, is synchronous in both eyes and has been reported previously.71,72,97,100 Considering this normal fluctuation in pupil diameter, it is necessary to capture the pupil for a longer period of time so the risk of capturing the pupil at a moment of relative constriction is minimized. In this study, the duration of the pupil measurement was 1 minute, after which the mean pupil diameter was determined. Using this methodology should have minimized the direct effect of pupillary hippus and minimize this threat to the internal validity of this study.
Pfeifer et al.52 demonstrated that there is no statistical significant difference between the pupil diameter of the right versus the left eye in both the dark and the light environments. For this reason, only one pupil was assessed for change during the measurement phase of this study. There is agreement that the pupil diameter in the dark is a direct representation of the balance between the parasympathetic and sympathetic nervous system. In the dark, the activity of the parasympathetic nervous system is greatly reduced; therefore, the pupil diameter is determined by the activity of the sympathetic nervous system.52,99 When the patient, while in a complete dark environment, displays an increase in pupil diameter, this should be indicative of a relative unopposed hyper-activity of the sympathetic system.47
The results of the current study investigating the pupil response after a physical therapeutic thrust manipulation targeting the T3–T4 segment showed that the mean pupil diameter in the placebo group exhibited a significant decrease in diameter (P = 0.022), which would indicate an increase in parasympathetic activity or a decrease in activity of the sympathetic system. The mean pupil diameter of the manipulation group did not show a statistical significant change, which would indicate that, the balance between the sympathetic and parasympathetic nervous system remained the same following the manipulation. The findings of this study were not consistent with the findings of Gibbons49 who showed that suboccipital thrust manipulation resulted in a change in pupil response to light, indicating an increased activity of the sympathetic nervous system or Driscoll and Hall101, who demonstrated that cervical manipulation had a direct effect on the autonomic nervous system. Therefore, the original hypothesis that a non-specific manipulation would directly cause an increased activity of the sympathetic system could not be confirmed with the findings of this study and additional research seems warranted to further explore this hypothesis.
There was no statistical significant difference in VAS change scores following the intervention between the placebo and thrust manipulation groups (P = 0.961). This is not consistent with the findings of Cleland et al.,23 who showed that a subject group of neck patients responded to a thoracic manipulation with an immediate overall reduction of their neck pain. The difference in findings might be based on the fact the mean duration of neck pain symptoms for the subjects, in the current study, was 24.3 months compared to the 12–13 weeks of duration of symptoms in the Cleland study.23 Additionally, the subjects in the Cleland study23 underwent an average of three manipulations compared to only one in this study and the manipulations in the Cleland study23 were directed at restricted segments, which was not the case in this study. The findings of this study could be an indication that thrust manipulation in the upper thoracic spine is less effective in reducing pain in the more chronic patient group versus the more acute patient group.
Pain is the result of the activity of the dorsal horn inter-neurons,14,102–104 which will determine the accumulative affect of the efferent input. This inter-neuron activity is not yet fully understood;105 however, there are many neuro-chemicals, such as endorphins, Substance P, serotonin, and GABA, which could affect the activity of these inter-neurons.47,90,106 The descending pathways from the cranial structures can result in an inhibiting or facilitating effect on the segmental inter-neurons.57,102,107–114 It has been shown that spinal manipulative techniques affect inter-neural activity at the spinal segments influencing the descending central pathways,115,116 and also have an effect at the cortical level.117 In a state of central sensitization, or chronic pain, it appears that there is a change in processing of nociceptive information in time.9 There will be a lowering of threshold for excitation of neurons in the spinal cord, which will result in an increased central discharge on relatively normal effective inputs and inputs that previously did not exceed the neuron threshold.10 This lowering of the threshold of the spinal inter-neurons might be the result of the fact that the descending inhibiting pathways from the cranial structures has reached the exhaustion state,118 which could explain why the patient population of this study did not respond to a thoracic manipulation with an immediate overall reduction of pain. How the descending pathways and inter-neuron activity are regulated is still not understood and should be the focus of future multidisciplinary research.
There were a few limitations to this study. First, this study evaluated only the immediate response of a single manipulation on the autonomic nervous system; therefore, no conclusions can be drawn regarding the long-term effects. This is similar to previous studies evaluating the direct effect of manipulation on the central nervous system.58,59 The main rationale for this was the inability to re-create the same basic level of autonomic function within the subjects to obtain valid repeated measures. Follow-up studies should evaluate the long-term effect of repeated manipulation on the autonomic nervous system and how this system might change when patient’s overall complaints reduce. In addition, only one physical therapist performed the manipulation technique, which may limit generalizability of the results.
Conclusion
This study measured the immediate short-term effect on the autonomic nervous system of a physical therapeutic thrust manipulation targeting the T3–T4 segment. The results of this study demonstrated no significant change in pupil diameter following manipulation, which would indicate that the balance between the sympathetic and parasympathetic nervous system activity remained the same. There was a significant reduction in sympathetic nervous system activity in the placebo group. This study did not show a statistical difference in the subject’s pain perception when comparing the effects of either the manipulation or a placebo intervention. This suggests that thrust manipulation was not effective in reducing pain in the chronic neck pain subjects of this study. Future studies should investigate the effect of manipulation on inter-neuron activity at different stages of chronicity. Additionally, research needs to further evaluate the effect of segmental specific versus non-specific manipulation on the autonomic nervous system.
References
- 1.Boswell MV, Shah RV, Everett CK, Sehgal N, Brown AM, Abdi S, et al. Interventional techniques in the management of chronic spinal pain: evidence-based practice guidelines. Pain Physician 2005;8:1–47 [PubMed] [Google Scholar]
- 2.Manchikanti L, Singh V, Pampati V, Damron K, Beyer C, Barnhill R. Is there correlation of facet joint pain in lumbar and cervical spine? An evaluation of prevalence in combined chronic low back and neck pain. Pain Physician 2002;5:365–72 [PubMed] [Google Scholar]
- 3.Gross AR, Hoving JL, Haines TA, Goldsmith CH, Kay T, Aker P, et al. A Cochrane review of manipulation and mobilization for mechanical neck disorders. Spine 2004;29:1541–48 [DOI] [PubMed] [Google Scholar]
- 4.Gross AR, Kay T, Hondras M, Goldsmith C, Haines T, Peloso P, et al. Manual therapy for mechanical neck disorders: a systematic review. Man Ther 2002;7:131–49 [DOI] [PubMed] [Google Scholar]
- 5.Tseng Y, Wang W, Chen W, Hou T, Chen T, Lieu F. Predictors for immediate responders to cervical manipulation in patients with neck pain. Man Ther 2006;11:306–315 [DOI] [PubMed] [Google Scholar]
- 6.Manchikanti L, Manchikanti K, Cash K, Singh V, Giordano J. Age-related prevalence of facet-joint involvement in chronic neck and low back pain. Pain Physician 2008;11:67–75 [PubMed] [Google Scholar]
- 7.Manchikanti L, Manchikanti KN, Manchukonda R, Cash KA, Damron KS, Pampati V, et al. Evaluation of lumbar facet joint nerve blocks in the management of chronic low back pain: preliminary report of a randomized, double-blind controlled trial: clinical trial NCT00355914. Pain Physician 2007;10:425–40 [PubMed] [Google Scholar]
- 8.Najm WI, Seffinger MI, Mishra SA, Dickerson VM, Adams A, Reinsch S, et al. Content validity of manual spinal palpatory exams: a systematic review. BMC Musculoskelet Disord 2003;3:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staud R, Vierck C, Cannon R, Mauderli A, Price D. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 2001;91:165–75 [DOI] [PubMed] [Google Scholar]
- 10.Woolf C, Doubell T. The pathophysiology of chronic pain-increased sensitivity to low threshold Abeta-fibre inputs. Curr Opin Neurobiol 1994;4:525–34 [DOI] [PubMed] [Google Scholar]
- 11.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther 2009;14:531–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bialosky JE, Bishop MD, Robinson ME, Zeppieri GJr, George SZ. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther 2009;89:1292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George SZ, Bishop MD, Bialosky JE, Zeppieri GJr, Robinson ME. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord 2006;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Cranenburgh B. Inleiding in the toegepaste neurowetenschappen. Vol. 1. Lochem: Uitgeversmaatchappij de Tijdstroom; 1989 [Google Scholar]
- 15.Bialosky JE, Bishop MD, Robinson ME, George SZ. The relationship of the audible pop to hypoalgesia associated with high-velocity, low-amplitude thrust manipulation: a secondary analysis of an experimental study in pain-free participants. J Manipulative Physiol Ther 2010;33:117–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paris S. S1 course notes 2000. Chicago, IL [Google Scholar]
- 17.Winkel D. Diagnosis and treatment of the spine. Gaithersburg, MD: Aspen Publishers, Inc; 1996 [Google Scholar]
- 18.Van der El A, Lunacies P, Wagemaker A. Manuele therapies. Rotterdam: Manuel; 1993 [Google Scholar]
- 19.Cantu R, Grodin A. Myofascial manipulation, theory and clinical application. 2nd ed. Gaithersburg, MD: Aspen Publications; 2001 [Google Scholar]
- 20.Kerry R, Taylor A, Mitchell J, McCarthy C, Brew J. Manual therapy and cervical arterial dysfunction, directions for the future: a clinical perspective. J Man Manip Ther 2008;16:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Iglesias J, Fernandez-De-Las-Penas C, Cleland J, Alburquerque-Sedin F, Palomeque-del-Cerro L, Mendez-Sanchez R. Inclusion of thoracic spine thrust manipulation into an electro-therapy/thermal program for the management of patients with acute mechanical neck pain: a randomized clinical trial. Man Ther 2009;14:306–13 [DOI] [PubMed] [Google Scholar]
- 22.Astin J, Ernst E. The effectiveness of spinal manipulation for the treatment of headache disorders: a systematic review of randomized clinical trials. Cephalagia 2002;22:617–23 [DOI] [PubMed] [Google Scholar]
- 23.Cleland JA, Childs JD, McRae M, Palmer JA, Stowell T. Immediate effects of thoracic manipulation in patients with neck pain: a randomized clinical trial. Man Ther 2005;10:127–35 [DOI] [PubMed] [Google Scholar]
- 24.Dishman J, Greco D, Burke J. Motor-evoked potentials recorded from lumbar erector spinae muscles: a study of corticospinal excitability changes associated with spinal manipulation. J Manipulative Physiol Ther 2008;31:258–69 [DOI] [PubMed] [Google Scholar]
- 25.Benarroch E. Pain-autonomic interactions. Neurol Sci 2006;27(Suppl. 2):S130–3 [DOI] [PubMed] [Google Scholar]
- 26.Barman S, Wurster R. Interaction of descending spinal sympathetic pathways and afferent nerves. Am J Physiol 1978;234:H223–9 [DOI] [PubMed] [Google Scholar]
- 27.Cortelli P, Pierangeli G. Chronic pain-autonomic interactions. Neurol Sci 2003;24:S68–70 [DOI] [PubMed] [Google Scholar]
- 28.Benarroch E. Pain–autonomic interactions: a selective review. Clin Auton Res 2001;11:343–9 [DOI] [PubMed] [Google Scholar]
- 29.Zusman M. Forebrain-mediated sensitization of central pain pathways: “non-specific” pain and a new image for MT. Man Ther 2002;7:80–8 [DOI] [PubMed] [Google Scholar]
- 30.Sato A. Somato-sympathetic reflex discharges evoked through supramedullary pathways. Pflugers Arch 1972;332:117–26 [PubMed] [Google Scholar]
- 31.Sato A. Neural mechanisms of autonomic responses elicted by somatic stimulation. Neurosci Behav Physiol 1997;27:610–21 [DOI] [PubMed] [Google Scholar]
- 32.Schwartzman R, Alexander G, Grothusen J. Pathophysiology of complex regional pain syndrome. Expert Rev Neurother 2006;6:669–81 [DOI] [PubMed] [Google Scholar]
- 33.Cleland J, Glynn P, Whiteman J, Eberhart S, MacDonald C, Childs JD. Short-term effects of thrust versus nonthrust mobilization/manipulation directed at the throracic spine in patients with neck pain: a randomized clinical trial. Phys Ther 2007;87:431–40 [DOI] [PubMed] [Google Scholar]
- 34.Krauss J, Creighton D, Ely J, Podlewska-Ely J. The immediate effects of upper thoracic translatoric spinal manipulation on cervical pain and range of motion: a randomized clinical trial. J Man Manip Ther 2008;16:93–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-De-Las-Penas C, Palomeque-del-Cerro L, Rodriquez-Blanco C, Gomez-Conesa A, Miangolarra-Page J. Changes in neck pain and active range of motion after a single throracic spine manipulation in subjects presenting with mechanical neck pain: a case series. J Manipulative Physiol Ther 2007;30:312–20 [DOI] [PubMed] [Google Scholar]
- 36.Childs J, Cleland J, Elliot J, Deydre T, Wainner R, Whitman J, et al. Neck pain: clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopaedic section of the american physical therapy association. J Orthop Sports Phys Ther 2008;38:A1–34 [DOI] [PubMed] [Google Scholar]
- 37.Huijbregts P. Clinical prediction rules: time to sacrifice the holy cow of specificity? J Man Manip Ther 2007;15:5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cleland J, Fritz J, Whiteman J, Childs JD, Palmer JA. The use of a lumbar spine manipulation technique by physical therapists in patients who satisfy a clinical prediction rule: a case series. J Orthop Sports Phys Ther 2006;36:209–14 [DOI] [PubMed] [Google Scholar]
- 39.Flynn T, Fritz J, Whiteman J, Wainner R, Magel J, Rendeiro D, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine 2002;27:2835–43 [DOI] [PubMed] [Google Scholar]
- 40.Cleland J, Childs J, Fritz J, Whiteman J, Eberhart S. Development of a clinical prediction rule for guiding treatment of a subgroup of patients with neck pain: use of thoracic spine manipulation, exercise, and patient education. Phys Ther 2007;87:9–23 [DOI] [PubMed] [Google Scholar]
- 41.Mink A, ter Veen H, Vorselaars J. Extremiteiten, functie-onderzoek en manuele therapy. Utrecht: Bohn, Scheltema & Holkema; 1990 [Google Scholar]
- 42.Lucini D, Di Fede G, Parati G, Pagani M. Impact of chronic psychosocial stress on autonomic cardiovascular reulation in otherwise healthy subjects. Hypertension 2005;46:1201–6 [DOI] [PubMed] [Google Scholar]
- 43.Maguire AM, Craig ME, Craighead A, Chan AK, Cusumano JM, Hing SJ, et al. Autonomic nerve testing predicts the development of complications. Diabetes Care 2007;30:77–82 [DOI] [PubMed] [Google Scholar]
- 44.Vicenzino B, Collins D, Wright T. Sudomotor changes induced by neural mobilisation techniques in asymptomatic subjects. J Man Manip Ther 1994;2:66–74 [Google Scholar]
- 45.Butler D. The sensitive nervous system. Adelaide, SA: Noigroup Publications; 2000 [Google Scholar]
- 46.Menck J, Requejo S, Kulig K. Thoracic spine dysfunction in upper extremity complex regional pain syndrome type I. J Orthop Sports Phys Ther 2000;30:401–9 [DOI] [PubMed] [Google Scholar]
- 47.Bernards J, Bouman L. Fysiologie van de mens. Utrecht: Bohn, Scheltema & Holkema; 1988 [Google Scholar]
- 48.Elenkov I, Wilder R, Chrousos G, Viziz E. The sympathetic nerve-an integrative interfase between two supersystems: the brain and the immune system. Pharmacol Rev 2000;52:595–638 [PubMed] [Google Scholar]
- 49.Gibbons P, Gosling C, Holmes M. Short-term effects of cervical manipulation on edge light pupil cycle time: a pilot study. J Manipulative Physiol Ther 2000;23:465–9 [DOI] [PubMed] [Google Scholar]
- 50.Oostendorp R. Functionele Vertebrobasilaire Insufficientie. Nijmegen: Drukkerij Leijn; 1988 [Google Scholar]
- 51.Filipe J, Falcao-Reis F, Castro-Correia, Barros H. Assessment of autonomic function in high level athletes by pupillometry. Auton Neurosci 2003;104:66–72 [DOI] [PubMed] [Google Scholar]
- 52.Pfeifer MA, Cook D, Brodsky J, Tice D, Parrish D, Reenan A, et al. Quantitative evaluation of sympathetic and parasympathetic control of iris function. Diabetes Care 1982;5:518–28 [DOI] [PubMed] [Google Scholar]
- 53.Bertinotti L, Pietrini U, del Rosso A, Casale R, Colangelo N, Zoppi M, et al. The use of pupillometry in joint and connective tissue diseases. Ann NY Acad Sci 2002;966:446–55 [DOI] [PubMed] [Google Scholar]
- 54.Bitsios P, Prettyman R, Szabadi E. Changes in autonomic function with age: a study of pupillary kinetics in healthy young and old people. Age Ageing 1996;25:432–8 [DOI] [PubMed] [Google Scholar]
- 55.Capao Filipe J, Falcao-Reis F, Castro-Correia J, Barros H. Assessment of autonomic function in high level athletes by pupillometry. Auton Neurosci 2003;104:66–72 [DOI] [PubMed] [Google Scholar]
- 56.Fotiou F, Fountoulakis K, Goulas A, Alexopoulos L, Palikaras A. Automated standardized pupillometry with optical method for purposes of clinical practice and research. Clin Physiol 2000;20:336–47 [DOI] [PubMed] [Google Scholar]
- 57.Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manuipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain 1996;68:69–74 [DOI] [PubMed] [Google Scholar]
- 58.George S, Bishop M, Bialosky J, Zeppieri G, Robinson M. Immediate effects of spinal manipulation on theral pain sensitivity: an experimental study. BMC Musculoskelet Disord 2006;7:1471–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskelet Disord 2008;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cleland J, Flynn T, Childs JD, Eberhart S. The audible pop from thoracic spine thrust manipulation and its relation to short-term outcomes in patients with neck pain. J Man Manip Ther 2007;15:143–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elliott A, Smith B, Penny K, Smith W, Chambers W. The epidemiology of chronic pain in the community. Lancet 1999;354:1248–52 [DOI] [PubMed] [Google Scholar]
- 62.Ries A. Minimally Clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analogue Scale. COPD 2005;2:105–10 [DOI] [PubMed] [Google Scholar]
- 63.Bird S, Dickson E. Clinically significant changes in pain along the Visual Analoge Scale. Ann Emerg Med 2001;38:639–43 [DOI] [PubMed] [Google Scholar]
- 64.Jensen M. The validity and reliability of pain measures in adults with cancer. J Pain 2003;4:2–21 [DOI] [PubMed] [Google Scholar]
- 65.Jensen M, Connie C, Brugger A. Postsurgical pain outcome assessment. Pain 2002:99;101–9 [DOI] [PubMed] [Google Scholar]
- 66.Jensen M, Turner J, Romano J, Fisher L. Comparative reliability and validity of chronic pain intensity measures. Pain 1999;83:157–62 [DOI] [PubMed] [Google Scholar]
- 67.Gallagher E, Liebman M, Bijur P. Prosepctive validation of clinically important changes in pain severity measured on a visual analoge scale. Ann Emerg Med 2001;38:633–8 [DOI] [PubMed] [Google Scholar]
- 68.Bijur P, Silver W, Gallagher E. Reliabilty of the Visual Analog Scale for measurement of acute pain. Acad Emerg Med 2001;8:1153–7 [DOI] [PubMed] [Google Scholar]
- 69.Harle D, Wolffsohn J, Evans B. The pupillary light reflex in migraine. Ophthalmic Physiol Opt 2005;25:240–5 [DOI] [PubMed] [Google Scholar]
- 70.Dutsch M, Hilz M, Raunhut U, Solomon J, Neundorfer B, Axelrod F. Sympathetic and parasympathetic pupillary dysfunction in familial dysautonomia. J Neurol Sci 2002;195:77–83 [DOI] [PubMed] [Google Scholar]
- 71.Boxer Walcher B, Krueger R. Agreement and repeatability of infrared pupillometry and the comparison method. Ophthalmology 1999;106:319–23 [DOI] [PubMed] [Google Scholar]
- 72.Boxer Walcher B, Krueger R. Agreement and repeatability of pupillometry using videokeratography and infrared devices. J Cataract Refract Surg 2000;26:35–40 [DOI] [PubMed] [Google Scholar]
- 73.Levy D, Rowley D, Abraham R. Portable infrared pupilloemtry using Pupilscan: relation to somatic and autonomic nerve function in diabetes mellitus. Clin Auton Res 1992;2:335–41 [DOI] [PubMed] [Google Scholar]
- 74.Meeker M, Du R, Bachetti C, Larson M, Holland M, Manley G. Pupil examination: validity and clinical utility of an automated pupillometer. J Neurosci Nurs 2005;37:34–40 [PubMed] [Google Scholar]
- 75.Piha S, Halonen J-P. Infrared pupillometry in the assessment of autonomic function. Diabetes Res Clin Pract 1994;26:61–6 [DOI] [PubMed] [Google Scholar]
- 76.Pop M, Payette Y, Santoriello E. Comparison of the pupil card and pupillometer in measuring pupil size. J Cataract Refract Surg 2002;28:281–8 [DOI] [PubMed] [Google Scholar]
- 77.Twa M, Bailey M, Hayes J, Bullimore M, McOptom Estimation of pupil size by digital photography. J Cataract Refract Surg 2004;30:381–9 [DOI] [PubMed] [Google Scholar]
- 78.Boev AN, Fountas KN, Karampelas I, Machinis TG, Feltes C, Okosun I, et al. Quantitative pupillometry: normative data in healthy pediatric volunteers. J Neurosurg 2005;103:496–500 [DOI] [PubMed] [Google Scholar]
- 79.Taylor WR, Chen JW, Meltzer H, Gennarelli TA, Kelbch C, Knowlton S, et al. Quantitative pupillometry, a new technology: normative data and preliminary observations in patients with acute head injury. J Neurosurg 2003;98:205–13 [DOI] [PubMed] [Google Scholar]
- 80.Mylius V, Braune H, Schepelmann K. Dysfunction of the pupillary light reflex following migraine headache. Clin Auton Res 2003;13:16–21 [DOI] [PubMed] [Google Scholar]
- 81.Neil HA, Smith SA. A simple clinical test of pupillary autonomic function, correlation with cardiac autonomic function tests in diabetes. Neuro-ophtalmology 1989;9:237–42 [Google Scholar]
- 82.Giakoumaki S, Hourdaki E, Grinakis V, Theou K, Bitsios P. Effects of peripheral sympatehtic blockade with dapiprazole on the fear-inhibited light reflex. J Psychopharmacol 2005;19:139–48 [DOI] [PubMed] [Google Scholar]
- 83.Miciele G, Tassorelli C, Martignoni E, Marcheselli S, Rossi F, Nappi G. Further characterization of autonomic involvement in multiple system atrophy: a pupillometric study. Funct Neurol 1995;10:273–80 [PubMed] [Google Scholar]
- 84.Brismee JM, Gipson D, Ivie D, Lopez A, Moore M, Matthijs O, et al. Interrater reliability of a passive physiological intervertebral motion test in the mid-thoracic spine. J Manipulative Physiol Ther 2006;29:368–73 [DOI] [PubMed] [Google Scholar]
- 85.Vleeming A, Winkel D, Meijer O. Weke delen aandoeningen van het bewegingsapparaat. Vol. 1. Utrecht/Antwerpen: Bohn, Scheltema & Holkema; 1984 [Google Scholar]
- 86.Hartman L. Handbook of osteopathic technique. 3rd ed. Cheltenham: Stamley Thornes Ltd; 1997 [Google Scholar]
- 87.Cleland J, Selleck B, Stowell T. Short-term effects of thoracic manipulation on lower trapezius muscle strength. J Man Manip Ther 2004;12:82–90 [Google Scholar]
- 88.Cronk B. How to use SPSS: a step-by-step guide to analysis and interpretation. 2nd ed. Los Angeles, CA: Pyrczak Publishing; 2002 [Google Scholar]
- 89.Portney L, Watkins M. Foundations of clinical research, application to practice. 2nd ed. Saddle River, NJ: Prentice-Hall; 2000 [Google Scholar]
- 90.Ma Y-T, Ma M, Cho ZH. Biomedical acupuncture for pain management; an integrative approach. St Louis, MO: Elsevier Churchill Livingstone; 2005 [Google Scholar]
- 91.Smith S, Vale W. The role of the hypothalamic–pituitary–adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 2006;8:383–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Merritt S, Keegan A, Mercer P. Artifact management in pupillometry. Nurs Res 1994;43:56–9 [PubMed] [Google Scholar]
- 93.Yang L, Niemann C, Larson M. Mechansim of pupillary reflex dilation in awake volunteers and in organ donors. Anesthesiology 2003;99:1281–6 [DOI] [PubMed] [Google Scholar]
- 94.Ellermeier W, Westphal W. Gender differences in pain ratings and pupil reactions to painful pressure stimuli. Pain 1995;61:435–9 [DOI] [PubMed] [Google Scholar]
- 95.Oka S, Chapman R, Kim B, Nahajima I, Shimizu O, Oi Y. Pupil dilation response to noxious stimulation: effect of varying nitrous oxide concentration. Clin Neurophysiol 2007;118:2016–24 [DOI] [PubMed] [Google Scholar]
- 96.Wilson S, Amling J, Floyd S, McNair N. Determining interrater reliability of nurses’ assessments of pupillary size and reaction. J Neurosci Nurs 1988;20:189–92 [DOI] [PubMed] [Google Scholar]
- 97.Hreidarsson A. Pupil size in insulin-dependent diabetes: relationship to duration, metabolic control, and long-term manifestations. Diabetes 1982;31:442–8 [DOI] [PubMed] [Google Scholar]
- 98.Loewenfeld I, Newsome D. Iris mechanics. I. Influance of pupil size on dynamics of pupillary movements. Am J Ophthalmol 1971;71:347–62 [DOI] [PubMed] [Google Scholar]
- 99.Bakes A, Bradshaw M, Szabadi E. Attentuation of the pupillary light reflex in anxious patients. Br J Clin Pharmacol 1990;30:377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawasaki A. Physiology, assessment, and disorders of the pupil. Curr Opin Opthalmol 1999;10:394–400 [DOI] [PubMed] [Google Scholar]
- 101.Driscoll M, Hall M. Effects of spinal manipulative therapy on autonomic activity and the cardiovascular system: a case study using the electrocardiogram and arterial tonometry. J Manipulative Physiol Ther 2000;23:545–50 [DOI] [PubMed] [Google Scholar]
- 102.Rhudy J, Williams A, McCabe K, Thu M, Nguyen V, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology 2005;42:579–87 [DOI] [PubMed] [Google Scholar]
- 103.Rhudy J, Williams A, McCabe K, Rambo P, Russell J. Emotional modulation of spinal nociception and pain: the impact of predictable noxious stimulation. Pain 2006;126:221–33 [DOI] [PubMed] [Google Scholar]
- 104.Coutaux A, Adam F, Willer J, Le Bars D. Hyperalgesia and allodynia: peripheral mechanisms. Joint Bone Spine 2005;72:359–71 [DOI] [PubMed] [Google Scholar]
- 105.Watkins L, Milligan E, Maier S. Spinal cord glia: new players in pain. Pain 2001;93:201–05 [DOI] [PubMed] [Google Scholar]
- 106.Dung H, Chen S. Bipolar nature of pain. Taipei: Yi Hsien Publishing Co, ltd; 2008 [Google Scholar]
- 107.Zusman M. Spinal manipulative therapy: review of some proposed mechanisms, and a new hypothesis. Australian J Physiother 1986;32:89–99 [DOI] [PubMed] [Google Scholar]
- 108.Dubner R, Ren K. Endogenous mechanisms of sensory modulation. Pain 1999;Suppl. 6:S45–53 [DOI] [PubMed] [Google Scholar]
- 109.Slater H, Vicenzino B, Wright A. “Sympathetic slump”: the effects of a noval manual therapy technique on peripheral sympatehtic nervous system function. J Man Manip Ther 1994;2:156–62 [Google Scholar]
- 110.Skyba D, Radhakrishnan R, Rohlwing J, Wright A, Sluka K. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opiod or GABA receptors in the spinal cord. Pain 2003;106:159–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vicenzino B, Cartwright T, Collins D, Wright A. Cardiovascular and respiratory changes produced by lateral glide mobilization of the cervical spine. Man Ther 1998;3:67–71 [Google Scholar]
- 112.Vicenzino B, Collins D, Benson H, Wright A. An investigation of the interrelationship between manipulative-induced hypoalgesia and sympathoexcitation. J Manipulative Physiol Ther 1998;21:448–53 [PubMed] [Google Scholar]
- 113.Paungmali A, O'Leary S, Souvlis T, Vicenzino B. Hypoalgesic and sympathoexcitatory effects of mobilization with movement for lateral epicondylalgia. Phys Ther 2003;83:374–83 [PubMed] [Google Scholar]
- 114.Fernandez-De-Las-Penas C, Perez-De-Heredia M, Brea-Rivero M, Miangolarra-Page J. Immediate effects on pressure pain threshold following a single cervical spine manipulation in healthy subjects. J Orthop Sports Phys Ther 2007;37:325–9 [DOI] [PubMed] [Google Scholar]
- 115.Taylor H, Murphy B. Altered Sensorimotor integration with cervical spine manipulation. J Manipulative Physiol Ther 2008;31:116–25 [DOI] [PubMed] [Google Scholar]
- 116.Herzog W, Scheele D, Conway P. Electromyographic responses of back and limb muscles associated with spinal manipulative therapy. Spine 1999;24:146–53 [DOI] [PubMed] [Google Scholar]
- 117.Haavik-Taylor H, Murphy B. Cervical spine manipulation alters sensorimotor integration: a somatosensory evoked potential study. Clin Neurophysiol 2007;118:391–402 [DOI] [PubMed] [Google Scholar]
- 118.Millan M. Descending control of pain. Prog Neurobiol 2002;66:355–474 [DOI] [PubMed] [Google Scholar]