Abstract
There is some evidence that early colonization of the intestine affects the composition of the intestinal microbiota after weaning. In the present study, the effect of prebiotics administered from the first day of life on fecal counts of bifidobacteria and lactobacilli were studied during and after the administration of the prebiotics. In this double-blind, randomized, placebo-controlled, explorative study, 20 newborns of hepatitis C virus-infected mothers who decided not to breast feed due to their concerns regarding their plasma viral load were randomly assigned to either a formula with 8 g/L of a specific prebiotic mixture (short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides, ratio 9:1) or a formula containing the same amount of maltodextrin (placebo). Clinical examination including anthropometric measurements, microbiological analysis of fecal samples, and blood leukocyte population analysis were performed at birth and 3, 6, and 12 mo age. At the age of 12 mo, hepatitis B vaccine-specific IgG serum titers (Hepatitis B virus surface antibodies) were also measured. Prebiotic supplementation resulted in more fecal bifidobacteria (P < 0.0001) and lactobacilli (P = 0.0044) compared with the placebo group. These differences between the groups were maintained during the second half of the first year without any prebiotic supplementation. There was no influence of the different diets on anthropometric data or the measured immunological variables. The data from this small explorative study indicate that early colonization of the intestine might have long-lasting effects on the composition of the intestinal microbiota.
Introduction
There is increasing evidence that the intestinal microbiota plays a crucial role in postnatal development of the immune system (1–3). There is broad consensus that breastfeeding is essential and is the best option for optimal development of the intestinal microbiota, resulting in a healthy and balanced immune system (2). Consequently, many attempts have been made to influence the intestinal microbiota composition of bottle-fed infants toward that found during breastfeeding. Most of these experiences exist for prebiotics (4, 5) and probiotics (6). More recently, a combination of both, so-called synbiotics (7) or fermented milk (8, 9), have also been used as potential treatments to achieve a bifidogenic effect in formula-fed infants.
Oligosaccharides are the main prebiotic factor in human milk (10–13). Because their structure is very complex, they are not yet available for the production of infant formulas. Thus, research activities have focused on the development of nonhuman milk oligosaccharides as an alternative (14). Several compounds of different structures have been identified and investigated (5).
Since 2002, several infant formulas containing a specific mixture of short-chain galacto-oligosaccharides (scGOS)7 and long-chain fructo-oligosaccharides (lcFOS) have been on the market (15). Studies in infants with a familial history of atopy/allergy fed such prebiotic formulae demonstrated a reduced risk of atopic dermatitis accompanied by an antiallergic antibody profile in plasma (16, 17). Similarly, antiallergic effects have been demonstrated in animals sensitized with ova albumin (18) or cow milk proteins (19, 20). In these latter animal studies, it was demonstrated that so-called regulatory T-cells were involved (20), indicating that the inhibition of allergic symptoms were not only due to Th2 damping and/or Th1 stimulation. In addition, a reduction in infectious episodes was observed in infants at risk of developing allergy/atopy (21) as well as in those without such risk (22). In summary, the experimental data (23) as well as the results of studies in humans (5) suggest an immune-modulating effect of this prebiotic mixture (24).
For ethical reasons, a certain period of breastfeeding has to be accepted. Because the early imprinting of intestinal microbiota is heavily influenced by early breastfeeding (25), it was of particular interest to obtain data from infants fed the formula from the first day of life. Particular interest was given to the weaning period and the second half of the first year of life to evaluate the stability of the fecal counts of bifidobacteria and lactobacilli after intervention.
In addition, there is still a debate about which biomarkers are really relevant for the evaluation of a normal balanced “healthy” immune system (26). As explorative biomarkers, leukocyte populations and lymphocyte subpopulations, total IgE levels, and hepatitis B virus antibody titers in response to hepatitis B vaccination were examined in this group of infants.
Therefore, this explorative study was initiated to evaluate the influence of a specific prebiotic mixture administered from the first day of life on the development of intestinal microbiota in formula-fed infants with a focus on fecal counts of bifidobacteria and lactobacilli during and after intervention with prebiotics; the above-mentioned explorative immune parameters were also examined.
Materials and Methods
Study population and methods.
This randomized, placebo-controlled, double-blinded trial was conducted at the Pediatric Department of the San Paolo Hospital in Milan between January 2002 and December 2005. Infants born from hepatitis C virus (HCV)-infected mothers were eligible for the study. Although it is not the policy of the hospital to recommend formula feeding in these cases, some mothers decided not to breastfeed due to their concerns regarding the relevant plasma HCV viral load before delivery.
The study was approved by the San Paolo Hospital Ethical Committee and written informed consent was obtained from all parents of all enrolled infants.
Inclusion criteria were a gestational age between 37 and 42 wk, a birth weight appropriate for gestational age, and exclusive formula feeding from the first day of life. Prematurity, breastfeeding, major malformations, and HCV infection were exclusion criteria.
Infants who developed metabolic, endocrinologic, and immunologic disorders, lactose intolerance and/or allergy to cow's milk as well as assumption of other pre- or probiotics were planned to be excluded from the study.
The infants were tested for exclusion of vertical HCV infection at birth and at the age of 1 mo. Two negative results of measurement of qualitative plasma HCV viral load were the basis to exclude vertical HCV infection (27).
The infants were randomly assigned to receive a regular bovine milk formula (Aptamil, Milupa) either supplemented with 8 g/L prebiotics or a similar quantity of maltodextrin as placebo (Milupa) from birth to 6 mo of life. During the second half-year of life, the infants received regular bovine milk-based, follow-on formula without any supplementation.
The prebiotic mixture used in the study consisted of scGOS derived from enzymatic synthesis from lactose and lcFOS separated from chicory inulin in a ratio of 9:1 (15).
The infants underwent clinical examination at birth and 3, 6, and 12 mo of age. During this examination, anthropometric measurements (weight, length, head circumference) using standard methods (28) and a medical examination were performed. Clinical data concerning infections or antibiotic treatments during the month before each medical examination were registered. Fecal samples were obtained for measurements of counts of bifidobacteria and lactobacilli as well as fecal pH.
Additionally, 2 mL venous blood was obtained for routine analysis of leukocyte populations and plasma total IgE titers. At the end of the study, an extra 1 mL blood was obtained for measurement of anti-hepatitis B virus surface specific antibody serum titers (1 mo after the 3rd vaccine dosage was administered).
Fecal counts of bifidobacteria and lactobacilli were measured as previously described (29). Fecal pH was measured by using a Handylab pH meter (Schott Glas) equipped with an Inlab 423 pH electrode (Mettler-Toledo).
Immunophenotyping of peripheral blood lymphocyte T cell subsets was performed by multiparameter flow cytometry on a FACScan flow cytometer (Becton Dickinson).
The IgE titer and specific IgG anti HBs-antigen titer were measured by Electro-Chemi-Luminescence Immuno Assay (ECLIA, Roche Diagnostics).
Statistics.
The results were analyzed on a per protocol basis. Time-balanced randomization was performed with the software RANCODE (IDV Gauting) with a random permuted block size of 4. Data were compared between the 2 formula groups by 2-sided Mann-Whitney U test. For analysis of the fecal counts of bifidobacteria and lactobacilli as the primary outcomes, 95% CI of the mean were calculated using the Wilson score method for each time point. P < 0.05 (2-tailed) was considered significant. Data are presented as mean ± SD or mean (95% CI). The statistical analysis was performed using SAS (SAS Enterprise Guide 4.1) for Windows (SAS Institute).
Results
Twenty of 22 enrolled infants completed the study. One infant (placebo group) failed due to noncompliance and 1 infant discontinued the follow-up for unknown reasons (prebiotic group).
At enrollment, the 2 groups did not differ in terms of mode of delivery, gestational age, gender, weight, length, or head circumference at birth (Table 1).
TABLE 1.
Baseline characteristics of the infants that completed the study1
| Prebiotic group | Control group | |
| n (M/F) | 10 (5/5) | 10 (2/8) |
| Vaginal delivery, n | 10 | 10 |
| Gestational age, wk | 39.1 ± 0.8 | 39.1 ± 1.3 |
| Weight at birth, kg | 3.32 ± 0.7 | 3.29 ± 0.34 |
| Length at birth, cm | 50.2 ± 2.7 | 51.2 ± 1.9 |
| Head circumference at birth, cm | 33.4 ± 0.9 | 33.9 ± 1.0 |
| Bifidobacteria, CFU/g stool | 5.5 (4.8–6.3) | 5.6 (5.0–6.3) |
| Lactobacilli, CFU/g stool | 4.6 (4.0–5.2) | 4.8 (4.2–5.4) |
Data are mean ± SD or mean (95% CI).
All infants had a normal weight gain, length growth, and head circumference increment, and there were no differences between the groups for all measurements (Table 2). None of the infants underwent any antibiotic treatment or suffered from any clinically relevant infection during the month before each evaluation.
TABLE 2.
Formula consumption, weight, length, head circumference, fecal pH, and fecal counts of bifidobacteria and lactobacilli in infants that received prebiotic or control formula from birth to 6 mo of age1
| Age and Item | Prebiotic group | Control group | P |
| 3 mo | |||
| Formula, mL/d | 767 ± 145 | 779 ± 71.4 | 0.87 |
| Weight, kg | 6.15 ± 0.81 | 6.27 ± 0.71 | 0.82 |
| Length, cm | 63.1 ± 2.9 | 62.1 ± 2.4 | 0.88 |
| Head circumference, cm | 39.6 ± 2.8 | 40.6 ± 1.3 | 0.36 |
| Bifidobacteria, CFU/g stool | 9.1 (8.7–9.5) | 6.4 (5.8–7.0) | 0.0014 |
| Lactobacilli, CFU/g stool | 6.7 (6.1–7.3) | 5.2 (4.6–5.9) | 0.0125 |
| Fecal pH | 5.24 ± 0.32 | 6.25 ± 0.51 | 0.0006 |
| 6 mo | |||
| Formula, mL/d | 622 ± 223 | 654 ± 234 | 0.94 |
| Weight, kg | 8.99 ± 0.85 | 7.97 ± 1.03 | 0.47 |
| Length, cm | 69.0 ± 2.6 | 69.0 ± 3.1 | 0.88 |
| Head circumference, cm | 43.2 ± 1.0 | 43.4 ± 1.4 | 0.59 |
| Bifidobacteria, CFU/g stool | 9.4 (8.9–10.0) | 7.3 (6.9–7.7) | 0.0014 |
| Lactobacilli, CFU/g stool | 6.9 (6.3–7.5) | 5.5 (5.0–6.0) | 0.0054 |
| Fecal pH | 5.15 ± 0.39 | 6.19 ± 0.54 | 0.0011 |
| 12 mo | |||
| Formula, mL/d | 310 ± 105 | 374 ± 156 | 0.44 |
| Weight, kg | 10.9 ± 1.26 | 9.78 ± 1.21 | 0.08 |
| Length, cm | 78.5 ± 4.2 | 77.4 ± 5.0 | 0.42 |
| Head circumference, cm | 46.0 ± 1.0 | 46.9 ± 1.9 | 0.30 |
| Bifidobacteria, CFU/g stool | 8.9 (8.6–9.3) | 7.1 (6.8–7.4) | 0.0016 |
| Lactobacilli, CFU/g stool | 6.7 (6.2–7.2) | 5.4 (5.1–5.8) | 0.0042 |
| Fecal pH | 5.88 ± 0.33 | 6.20 ± 0.58 | 0.30 |
Data are mean ± SD or mean (95% CI).
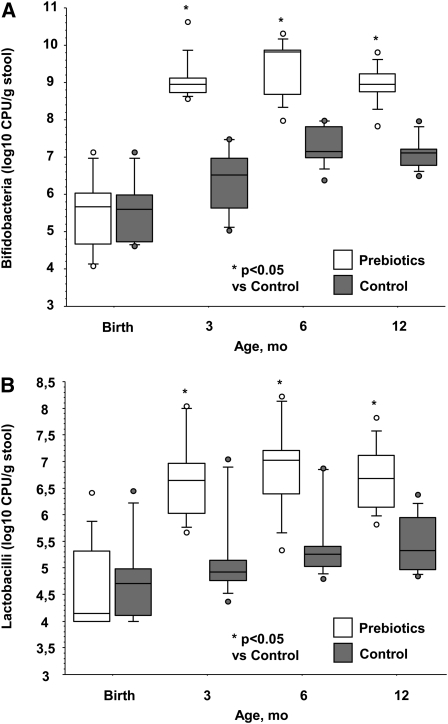
The prebiotic supplementation influenced the number of bifidobacteria and lactobacilli in the feces (P < 0.005). The counts of bifidobacteria and lactobacilli increased during the first 3 mo in both groups (P < 0.0001) and remained stable afterwards (P > 0.05). Starting from the 3 mo of age evaluation, the counts of both bifidobacteria and lactobacilli were higher in the prebiotic group (P < 0.013). This difference persisted even after discontinuation of the prebiotic supplementation at 7 mo of age (Fig. 1;Table 2).
FIGURE 1.
Bifidobacteria (A) and lactobacilli (B) counts in fecal samples from infants that received prebiotic or control formula from birth to 6 mo of age. Data are shown in box plots (medians and 95% CI), n = 10.
There was an influence of the supplementation on fecal pH (P = 0.0005), with significantly higher pH values in the control group (Table 2). Except for lymphocyte T CD3+ titers at 12 mo of age, which were lower in the control group (P = 0.017), the groups did not differ at each evaluation in the measured leukocyte populations (Supplemental Table 1). The total IgE titers increased from 3 mo of age to the end of the study (P = 0.03). The diet did not influence the IgE levels in serum (P = 0.27). At 12 mo of age, the anti-hepatitis B virus surface antibody titers did not differ between the feeding groups (data not shown). Both formulas were well tolerated with no adverse effects recorded.
Discussion
In this study, the prebiotic mixture of scGOS:lcFOS in a ratio of 9:1 significantly increased numbers of fecal bifidobacteria and lactobacilli, as found in several studies (5). The interesting finding in this study is that the early imprinted intestinal microbiota remained relatively stable even after weaning. This is in line with a study using classical microbiological techniques (30) as well as a study performed with molecular-based methods (31). Both studies, as in the present study, demonstrated a substantial stability of bifidobacteria after weaning, confirming data from the early 1980s (32).
Apart from the early imprinting of the intestinal microbiota, the genotype of the host could have an important impact on the stability of the intestinal ecosystem as well (33). The present study did not allow us to conclude whether early imprinting or other factors play a role in the observed stability of the number of fecal bifidobacteria and lactobacilli. The hypothesis that the early colonization might have an effect is supported by the observation that differences in early colonization between infants born by caesarean and vaginal delivery (34) can have long-lasting effects as well (35, 36). A similar observation was reported by Jernberg et al. (37) in infants treated with antibiotics. The impact of the treatment persisted up to 2 y. Furthermore, Roger et al. (38) reported a greater diversity of Bifidobacterium populations in breast-fed than in formula-fed infants, an effect persisting up to 18 mo of age. On the other hand, Rinne et al. (39) studied the effect of early administration of probiotics and could not find any significant interference with composition or quantity of gut microbiota.
In the present study, only culturing methods were used to quantify bifidobacteria and lactobacilli, which is a limitation of the study (40). However, direct comparison of the technique used in the study with the FISH technique demonstrated nearly similar results at a concentration of 8 g prebiotics/L formula (24).
In animal experiments (19), the scGOS:lcFOS mixture has shown immune modulation capacity. These experimental data are in line with the observation that in infants with a familiar history of atopy/allergy, the same prebiotic mixture induced a significant reduction in allergy-related symptoms at 6 mo of age (17). This effect was still seen at an age of 2 y (41), indicating an immune-modulating and -imprinting capacity of the prebiotics used.
In the present study, there was no effect on different leukocyte populations that might indicate that these parameters are not suitable to investigate developmental aspects of the immune system. Raes et al. (42) also found no significant effect of the same prebiotic mixture on basal blood immune variables. However, in the same cohort, fecal secretory IgA titer was significantly higher in infants fed the supplemented formula (43), indicating an effect of the specific prebiotic mixture on the mucosal immune system.
The vaccine-specific antibody production or T cell function are classified as markers with high suitability in terms of biological relevance, sensitivity, and practical feasibility (26). In the present study, no influence of the prebiotic supplement on hepatitis B vaccination response could be observed. This might be partially related to methodological aspects such as the optimal timing to measure vaccination response during infancy. The vaccine response is very robust. For this reason, subtle effects induced by prebiotic diets might be difficult to detect. If the infants were vaccinated using suboptimal vaccination protocols as is done in animal models (18), it might be possible to demonstrate vaccination improvement. For ethical reasons, suboptimal vaccination in infants is not possible.
More clinical trials are needed to identify predictive biomarkers in human infants that describe the development of the immune system. One example might be Ig-free light chain, which predicts several immune disorders in humans, including infants. Very recent data already indicate that this biomarker can be analyzed easily in plasma from infants and that this biomarker predicts the onset of allergic disorders such as atopic dermatitis, which can significantly be affected by the prebiotic diet. Thus, in future studies, these new biomarkers for immune development should be analyzed (20).
In summary, the data from this small explorative study indicate that early colonization of the intestine might have long-lasting effects on the composition of the intestinal microbiota. This must be confirmed in larger studies additionally using molecular techniques of characterization of intestinal microbiota.
Supplementary Material
Acknowledgments
M.G., F.S., G. Boehm, J.J., and E.R. designed the research; F.S., E.S., and G. Banderali conducted the research; F.S., G. Boehm, and J.J. analyzed the data; F.S., G. Boehm, J.J., and E.R. wrote the paper; and M.G. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by Danone Research, Centre for Specialised Nutrition.
Supplemental Table 1 is available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: HCV, hepatitis C virus; lcFOS, long-chain fructo-oligosaccharide; scGOS, short-chain galacto-oligosaccharide.
Literature Cited
- 1.Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr. 2005;135:1–4 [DOI] [PubMed] [Google Scholar]
- 2.M’Rabet L, Vos AP, Boehm G, Garssen J. Breast-feeding and its role in early development of the immune system in infants: consequences for health later in life. J Nutr. 2008;138:S1782–90 [DOI] [PubMed] [Google Scholar]
- 3.Rook GAW, Brunet LR. Microbes, immunoregulation and the gut. Gut. 2005;54:317–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson GR, Probeert HM, Van Loo JAE, Rastall RA, Robertfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–75 [DOI] [PubMed] [Google Scholar]
- 5.Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. J Nutr. 2008;138:S1818–28 [DOI] [PubMed] [Google Scholar]
- 6.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–78 [PubMed] [Google Scholar]
- 7.Collins MD, Gibson GR. Probiotics, prebiotics and synbiotics: approaches for the nutritional modulation of microbial ecology. Am J Clin Nutr. 1999;69:S1052–7 [DOI] [PubMed] [Google Scholar]
- 8.Thibault H, Aubert-Jacquin C, Goulet O. Effects of long-term consumption of a fermented infant formula (with Bifidobacterium breve C50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. J Pediatr Gastroenterol Nutr. 2004;39:147–52 [DOI] [PubMed] [Google Scholar]
- 9.Agostoni C, Goulet O, Kolacek S, Koletzko B, Moreno L, Puntis J, Rigo J, Shamir R, Szajewska H, et al. ESPGHAN Committee on Nutrition. Medical position paper: fermented infant formulae without live bacteria. J Pediatr Gastroenterol Nutr. 2007;44:392–7 [DOI] [PubMed] [Google Scholar]
- 10.Coppa GV, Zampini I, Galeazzi T, Gabrielli O. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38:S291–4 [DOI] [PubMed] [Google Scholar]
- 11.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional and metabolic aspects. Annu Rev Nutr. 2000;20:699–722 [DOI] [PubMed] [Google Scholar]
- 12.Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr. 2006;136:2127–30 [DOI] [PubMed] [Google Scholar]
- 13.Boehm G, Stahl B. Oligosaccharides. In: Functional dairy products Mattila-Sandholm T, editor Cambridge: Woodhead Publ; 2003. p. 203–43 [Google Scholar]
- 14.Rivero-Urgell M, Santamaria-Orleans A. Oligosaccharides: application in infant food. Early Hum Dev. 2001;65:S43–52 [DOI] [PubMed] [Google Scholar]
- 15.Boehm G, Fanaro S, Jelinek J, Stahl B, Marini A. Prebiotic concept for infant nutrition. Acta Paediatr Suppl. 2003;91 Suppl 441:64–7 [DOI] [PubMed] [Google Scholar]
- 16.Moro G, Arsanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Hoffen E, Ruiter B, Faber J, M’Rabet L, Knol EF, Stahl B, Arslanoglu S, Moro G, Boehm G, et al. A specific mixture of short chain galacto-oligosaccharides and long chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at risk for allergy. Allergy. 2009;64:484–7 [DOI] [PubMed] [Google Scholar]
- 18.Vos AP, van Esch B, M’Rabet L, Folkerts G, Garssen J. Dietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in mice. Int Immunopharmacol. 2007;7:1582–7 [DOI] [PubMed] [Google Scholar]
- 19.Schouten B, van Esch BCAM, Hofman GA, van Doorn SA, Knol J, Nauta AJ, Garssen J, Willemsen LEM, Knippels LMJ. Cow’s milk allergic symptoms are reduced in mice fed dietary synbiotics during oral sensitization with whey. J Nutr. 2009;139:1398–403 [DOI] [PubMed] [Google Scholar]
- 20.Schouten B, van Esch BCAM, van Thuijl AOJ, Blokhuis BRJ, Kormelink TG, Hofman GA, Moro GE, Boehm G, Arslanoglu S, et al. Contribution of IgE and immunoglobulin free light chain in the allergic reaction to cow’s milk protein. J Allergy Clin Immunol. 2010;125: 1308–14 [DOI] [PubMed] [Google Scholar]
- 21.Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protect formula fed infants against infections during the first 6 months of life. J Nutr. 2007;137:2420–4 [DOI] [PubMed] [Google Scholar]
- 22.Bruzzese E, Volpicelli M, Squeglia V, Bruzzese D, Salvini F, Bisceglia M, Lionetti P, Cinquetti M, Iacono G, et al. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infection: an observational study. Clin Nutr. 2009;28:156–61 [DOI] [PubMed] [Google Scholar]
- 23.Vos AP, M’Rabet L, Stahl B, Boehm G, Garssen J. Immune modulatory effects and potential working mechanisms of orally applied non-digestible carbohydrates. Crit Rev Immunol. 2007;27:97–140 [DOI] [PubMed] [Google Scholar]
- 24.Boehm G, Jelinek J, Knol J, M’Rabet L, Stahl B, Vos P, Garssen J. Prebiotic and immune responses. J Pediatr Gastroenterol Nutr. 2004;39:S772–3 [DOI] [PubMed] [Google Scholar]
- 25.Penders J, This C, Vink C, Stelma FF, Snijders B, Kummeling I, van de Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21 [DOI] [PubMed] [Google Scholar]
- 26.Albers R, Antoine JM, Bourdet-Sicard R, Calder PC, Gleeson M, Lesourd B, Samartin S, Sanderson IR, van Loo J, et al. Markers to measure immunomodulation in human nutrition intervention studies. Br J Nutr. 2005;94:452–81 [DOI] [PubMed] [Google Scholar]
- 27.Pembrey L, Newell ML, Tovo PA. the EPHN Collaborators. The management of HCV infected pregnant women and their children: European paediatric HCV Network. J Hepatol. 2005;43:515–25 [DOI] [PubMed] [Google Scholar]
- 28.Johnson TS, Engstrom JL, Gelhar DK. Intra- and interexaminer reliability of anthropometric measurements of term infants. J Pediatr Gastroenterol Nutr. 1997;24:497–505 [DOI] [PubMed] [Google Scholar]
- 29.Fanaro S, Vigi V, Chierici R, Boehm G. Fecal flora measurements of breast fed infants using an integrated transport and culturing system. Acta Paediatr. 2003;92:634–5 [DOI] [PubMed] [Google Scholar]
- 30.Amarri S, Benatti F, Callegari ML, Shahkhalili Y, Chauffard F, Rochat F, Acheson KJ, Hager C, Benyacoub J, et al. Changes of gut microbiota and immune markers during the complementary feeding period in healthy breast-fed infants. J Pediatr Gastroenterol Nutr. 2006;42:488–95 [DOI] [PubMed] [Google Scholar]
- 31.Magne F, Hachelaf W, Suau A, Boudraa G, Mangin I, Touhami M, Bouziane-Nedjadi K, Pochart P. A longitudinal study of infant faecal microbiota during weaning. FEMS Microbiol Ecol. 2006;58:563–71 [DOI] [PubMed] [Google Scholar]
- 32.Stark PL, Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol. 1982;15:189–203 [DOI] [PubMed] [Google Scholar]
- 33.Stewart JA, Chadwick VS, Murray A. Investigations into influence of host geneticson the predominant eubacteria in the faecal microflora of children. J Med Microbiol. 2005;54:1239–42 [DOI] [PubMed] [Google Scholar]
- 34.Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Caesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008;138:S1796–800 [DOI] [PubMed] [Google Scholar]
- 35.Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora inhealthy infants born by different methods of delivery: permanent changes in intestinal flora after caesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25 [DOI] [PubMed] [Google Scholar]
- 36.Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota in seven years old children. Gut. 2004;53:1388–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jernberg C, Löfmark S, Edlund C, Jansson K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56–66 [DOI] [PubMed] [Google Scholar]
- 38.Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology. 2010;156:3329–41 [DOI] [PubMed] [Google Scholar]
- 39.Rinne M, Kalliomäki M, Salminen S, Isolauri E. Probiotic intervention in the first months of life: short-term effects on gastrointestinal symptoms and long-term effects on gut microbiota. J Pediatr Gastroenterol Nutr. 2006;43:200–5 [DOI] [PubMed] [Google Scholar]
- 40.Morelli L. Postnatal development of intestinal microflora as influenced by infant nutrition. J Nutr. 2008;138:S1791–5 [DOI] [PubMed] [Google Scholar]
- 41.Arslanoglu S, Moro G, Schmitt J, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the allergy associated symptoms and infections during the first two years of life. J Nutr. 2008;138:1091–5 [DOI] [PubMed] [Google Scholar]
- 42.Raes M, Scholtens PA, Alliet P, Hensen K, Jongen H, Boehm G, Vandenplas Y, Rummens JL. Exploration of basal immune parameters in healthy infants receiving an infant milk formula supplemented with prebiotics. Pediatr Allergy Immunol. 2010;21: e377–85 [DOI] [PubMed] [Google Scholar]
- 43.Scholtens PAMJ, Alliet P, Raes M, Alles MS, Kroes H, Boehm G, Knippels LMJ, Knol J, Vandenplas Y. Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J Nutr. 2008;138:1141–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.